Abstract
Background
Electroporation uses an electric field to induce membrane pores that can transfer macromolecules into target cells. Modulation of electrical parameters leads to irreversible electroporation (IRE) which is being developed for tissue ablation. We seek to evaluate if the application of IRE may induce a lower electric field in the periphery where reversible electroporation may occur, facilitate gene transfer of a granulocyte macrophage colony-stimulating factor (GM-CSF) plasmid, and produce its biological response.
Methods
Yorkshire pigs underwent laparotomy, and IRE of the liver was performed during hepatic arterial infusion of 1 or 7 mg of a naked human GM-CSF plasmid. The serum, liver, lymph nodes, and bone marrow were harvested for analysis.
Results
After IRE and plasmid infusion, human GM-CSF level rose from undetectable to 131 pg/ml in the serum at 24 hours after. The liver demonstrated an ablation zone surrounded by an immune infiltrate that had greater macrophage intensity than when treated with IRE or plasmid infusion alone. This dominance of macrophages was dose-dependent. Distant effects of GM-CSF were found in the bone marrow where proliferating myeloid cells rose from 14% to 25%.
Conclusion
IRE facilitated gene transfer of the GM-CSF plasmid and brought about a local and systemic biological response. This technique holds potential for tumor eradication and immunotherapy of residual cancer.
Keywords: ablation, cancer therapy, gene therapy, immunotherapy, liver cancer, solid tumor
Introduction
Surgical resection is the gold standard for cure of solid tumors, yet only a fraction of patients have resectable tumors and many have recurrence 1, 2. Patients with unresectable disease or recurrence will die of cancer. Thus, new techniques are continuously sought to address the treatment of unresectable tumors and eliminate the microscopic residual disease which is the culprit for recurrence.
Electroporation is the technique of inducing cell membrane permeability via an electric field. It has been utilized in the laboratory for years to transfer macromolecules into target cells. Transfer of various anti-tumor genes via reversible electroporation led to tumor regression in animal models 3-5, and preliminary results of phase I and II clinical trials are encouraging that reversible electroporation with immune gene plasmids may be used for cancer therapy 6-8.
Recently, the electrical parameters have been modulated with a higher electric field and increased number of electric pulses to produce irreversible electroporation (IRE). IRE causes permanent pores in the cell membrane and thus cell death over days. The utility of IRE for tissue ablation has been observed in a variety of tissues including the liver, pancreas, and prostate 9-12. A non-thermal ablation technique, IRE can ablate tissue adjacent to a large vessel without a heat sink effect, and as proteins are not denatured by heat, surrounding structures like bile ducts, nerves, and large vessels appear less sensitive to injury 11, 12. IRE has been successful in tumor ablation in animal models 13-15 and is under investigation in man with early studies showing a largest diameter of ablation of about 3 cm in pancreatic and liver tumors16, 17.
Our laboratory is interested in the interaction of IRE and reversible electroporation with the goal of developing combined cancer therapy through ablation and gene transfer. Our earlier proof of principle study with a marker gene plasmid for green fluorescent protein demonstrated successful uptake of a gene plasmid with IRE18. We hypothesize that when IRE is applied, the central area undergoes necrosis. The electric field decreases as it radiates out and enables reversible electroporation and the uptake of gene plasmids at the periphery of the area of ablation. Thus, while the zone of irreversible electroporation occurs between and surrounding the probes, a zone of reversible electroporation may occur outside the zone of irreversible electroporation in the periphery. Our objective in this study is to evaluate whether the application of IRE may induce a lower electric field in the periphery where reversible electroporation may occur, facilitate gene transfer of a granulocyte macrophage colony-stimulating factor (GM-CSF) plasmid, and produce its biological response.
Methods
GM-CSF plasmid
A naked human GM-CSF DNA plasmid was constructed from the pING vector with a selection gene for kanamycin resistance and under the control of a cytomegalovirus promoter 19. Human GM-CSF has high sequence and amino acid homology with porcine GM-CSF and cross-reacts in pigs 20. Escherichia coli DH5α was transfected with the GM-CSF plasmid by heat shock and cultured at 37°C in Lysogeny Broth media with kanamycin. The DNA plasmid was isolated from the bacteria with a Qiagen plasmid kit (Qiagen, Valencia, CA), and the concentration determined through the ND-1000 spectrophotometer (NanoDrop, Wilmington, DE). To verify the presence and functionality of the isolated plasmid, human colorectal cancer HCT8 cells were transfected with the isolated plasmid using lipofectamine (Invitrogen, Carlsbad, CA). Twenty-four hours after, the media was analyzed for human GM-CSF cytokine with an ELISA (eBioscience, San Diego, CA).
IRE system and parameters
IRE was delivered through the Nanoknife system (Angiodynamics, Queensbury, NY) via dual monopolar probes spaced 1.5 cm apart with an active tip exposure of 1 cm. A synchronization unit timed the delivery of IRE pulses to the refractory period of the cardiac cycle to prevent arrhythmias. The electrical parameters were an electric field of 1500 V/cm, a pulse length of 100 microseconds, a total of 90 pulses, and a pulse interval of 100 milliseconds.
Animal procedure
All animal work was performed with approval from the Institutional Animal Care and Use Committee at Memorial Sloan-Kettering Cancer Center. Yorkshire pigs weighing 50-55 kg (Archer Farms, Darlington, MD) were obtained and received food and water ad libitum. Prior to surgery, the pigs were sedated with tiletamine/zolazepam (2.2mg/kg) to collect blood from an ear or mammary vein, and collect bone marrow via a core needle bone marrow biopsy in the tibia. Buprenorphine (0.01mg/kg) and local bupivicaine (1 ml of 0.25%) were given for pain control for the bone marrow biopsy.
For the survival surgery, pigs were sedated with tiletamine/zolazepam (4.4mg/kg) and given buprenorphine (0.01 mg/kg) and glycopyrrolate (0.007 mg/kg). Each pig was intubated and maintained on isoflurane (1.5-3%). A laparotomy was performed, and a small wedge of liver and a lymph node near the liver were resected via electrocautery for negative controls. The common hepatic artery was cannulated with a 20-gauge angiocatheter, and a Harvard PHD Ultra syringe pump (Harvard Apparatus, Holliston, MA) was used to infuse the DNA solution composed of 7 mg GM-CSF plasmid in 30 ml normal saline. An initial loading infusion of 5 ml at 1.67 ml/min for 3 minutes was followed by an infusion of the remaining 25 ml at 0.93 ml/min for 27 minutes. Pancuronium (0.15 mg/kg) was administered intravenously prior to IRE to prevent muscle spasms, and IRE ablation was performed in three areas on each of the left medial and lateral lobes during plasmid infusion. With the exception of the pig with an experiment time point of 6 hours, the abdomen was closed in layers using Vicryl sutures and Vetbond skin glue (3M, St. Paul, MN), and the pig was recovered from surgery with close postoperative monitoring. A fentanyl patch (25 mcg) and meloxicam (0.4 mg/kg) were given for pain control, and cephalexin (10 mg/kg) was also administered. For tissue harvest, pigs were euthanized with Euthasol (pentobarbital sodium and phenytoin sodium, 1 ml/4.5 kg).
Treatment with IRE and 7 mg GM-CSF plasmid infusion was tested with tissue harvest at 6, 24, and 48 hours. One pig received only 1 mg of GM-CSF plasmid with IRE to test for dose response, and one pig had just saline infusion as a control; tissues for these pigs were harvested at 48 hours (Table 1).
Table 1. Treatment Conditions of Pigs.
| IRE | GM-CSF | Time Point |
|---|---|---|
| Yes | 7 mg | 6 hour |
| Yes | 7 mg | 24 hours |
| Yes | 7 mg | 48 hours |
| Yes | 1 mg (low-dose) | 48 hours |
| Yes | 0 mg (control) | 48 hours |
Specimen Analysis
Blood was submitted for chemistry and complete blood panel lab tests. A separate sample ofblood was centrifuged at 6000 rpm to separate out the serum which was stored frozen at -80°C until analysis with the human GM-CSF ELISA.
Liver specimens were collected from the ablated left lobes and the unablated right lobes which received the plasmid infusion only. One part was flash frozen for storage, homogenized with T-PER reagent (Thermo Scientific, Rockford, IL) and the MM300 Mixer Mill (Qiagen, Valencia, CA), and analyzed with the human GM-CSF ELISA. The other part was fixed in 10% neutral buffered formalin, processed routinely, and stained with hematoxylin and eosin (H&E) for histopathological evaluation. The perihepatic lymph nodes were processed and analyzed similarly to the liver.
Additionally, lymph nodes were processed for flow cytometry. After mechanical homogenization through a 100 μm filter, lymphocytes were suspended in phosphate buffer solution with 1% fetal bovine serum and fixed with BD Cytofix fixation buffer (BD, San Jose, CA). Lymph node samples for the same pig were stained together with mouse anti-porcine CD172a-PE antibody (Southern Biotech, Birmingham, AL) for a population rich in neutrophils, monocytes, and dendritic cells. These samples were also stained with a mouse IgG1-PE antibody (BD, San Jose, CA) as an isotype control and analyzed on the BD FACSCalibur flow cytometer (BD, San Jose, CA).
Bone marrow specimens were made into smears on slides and stained with Wright-Giemsa stain. Three hundred cells were morphologically categorized as part of the myeloid or erythroid lines and of the mature or proliferating pools for each line by a pathologist specialized in bone marrow evaluation.
Results
GM-CSF Plasmid
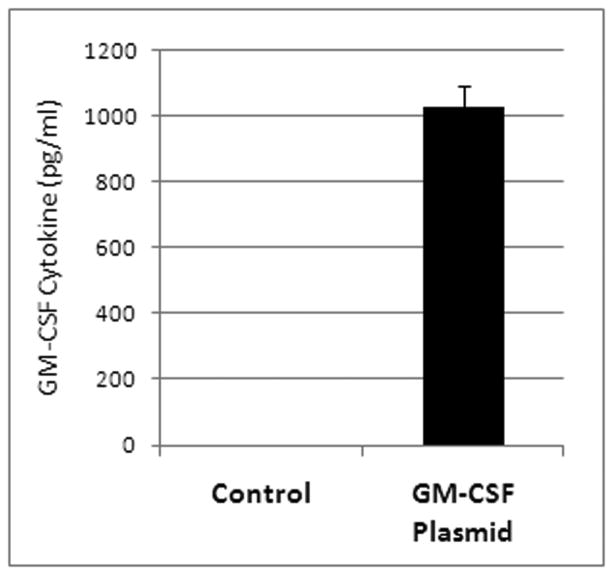
HCT8 cells transfected with the isolated plasmid produced human GM-CSF cytokines. After 24 hours, the cytokine level had risen to 1029 pg/ml on the ELISA (Figure 1). Cells that were given lipofectamine without any plasmid produced no human GM-CSF.
Figure 1. Cells transfected with the isolated plasmid.
Media of HCT8 cells transfected with the isolated plasmid via lipofectamine showed high levels of the GM-CSF cytokine whereas control transfection with no DNA resulted in no GM-CSF.
IRE Ablation
IRE ablation was performed (Figure 2) without any adverse events, and the pigs recovered from surgery unremarkably. The chemistry panel at 24 and 48 hours after IRE did not show any significant rise in liver enzymes, bilirubin, or alkaline phosphatase (Table 2). On histological evaluation, IRE ablated areas of the liver demonstrated a well demarcated zone of necrosis characterized by dissociation of hepatocytes, cytoplasmic hypereosinophilia, and nuclear pyknosis. There was also sinusoidal congestion and hemorrhage in the ablated tissue.
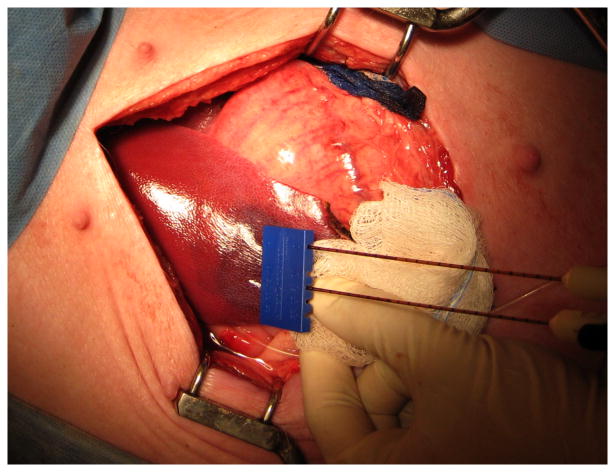
Figure 2. IRE ablation in the liver.
Dual probes spaced 1.5 cm apart with a tip exposure of 1 cm were used to deliver the electric pulses of IRE at 1500 V/cm into the liver. A dark red area where IRE ablation has taken place can be seen grossly.
Table 2. Chemistry Labs 24 hours after IRE.
| AST (U/L) [9-113] |
ALT (U/L) [32-84] |
Alkaline Phosphatase (U/L) [26-362] |
Bilirubin (mg/dl) [0-0.3] |
|
|---|---|---|---|---|
| Preop | 43 | 43 | 184 | 0.3 |
| 24 hr post-tx | 229 | 51 | 199 | 0.3 |
| 48 hr post-tx | 94 | 48 | 124 | 0.1 |
Gene Transfer
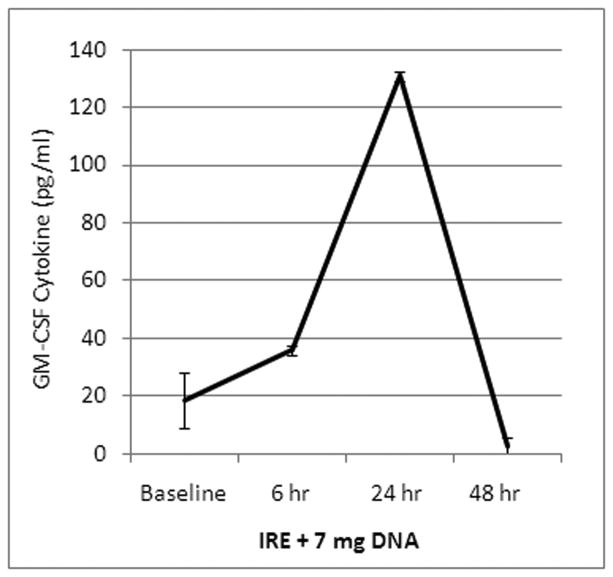
Human GM-CSF levels were not detectable in the serum at baseline. After treatment with IRE and plasmid infusion, the level of human GM-CSF rose to 131 pg/ml at 24 hours then fell back down by 48 hours (Figure 3). This demonstrated that the GM-CSF plasmid was taken up and expressed. GM-CSF was undetectable in the liver and lymph nodes.
Figure 3. Human GM-CSF in the blood.
After treatment with IRE and 7 mg of GM-CSF plasmid infusion, the levels of human GM-CSF levels rose and peaked at 24 hours then fell.
Biological Response
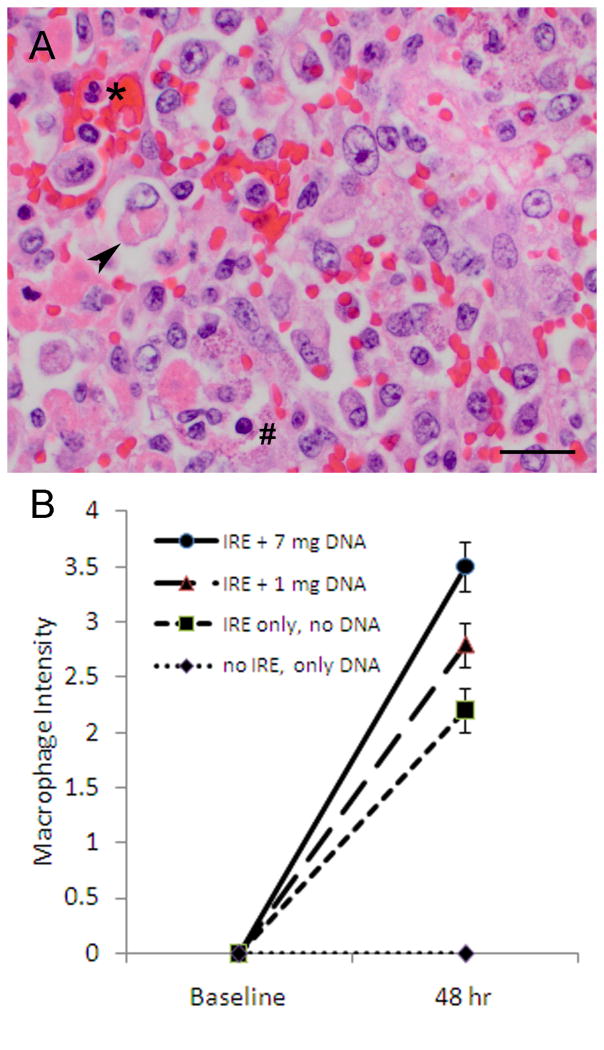
The negative control liver resected before treatment was normal on H&E stain. The right liver specimens that received only the GM-CSF plasmid infusion but were not ablated were also normal without any changes. Liver specimens that were ablated with IRE but received just saline without any plasmid showed a distinct ablation zone with a mild peripheral immune response characterized by small numbers of neutrophils and macrophages. Combined treatment with IRE and GM-CSF plasmid infusion resulted in a marked immune infiltrate that surrounded the ablation zone (Figure 4). Upon closer examination, this immune response consisted predominantly of macrophages (Figure 5A). When graded in a blinded fashion on an intensity scale from 0 for no macrophages to 4 for severe macrophage infiltration, there was a significant dose-dependent increase in macrophages from liver treated with IRE only, to liver treated with IRE and 1 mg GM-CSF plasmid infusion, to liver treated with IRE and 7 mg GM-CSF plasmid infusion (Figure 5B)(Pearson chi-square=10.83, P=0.029).
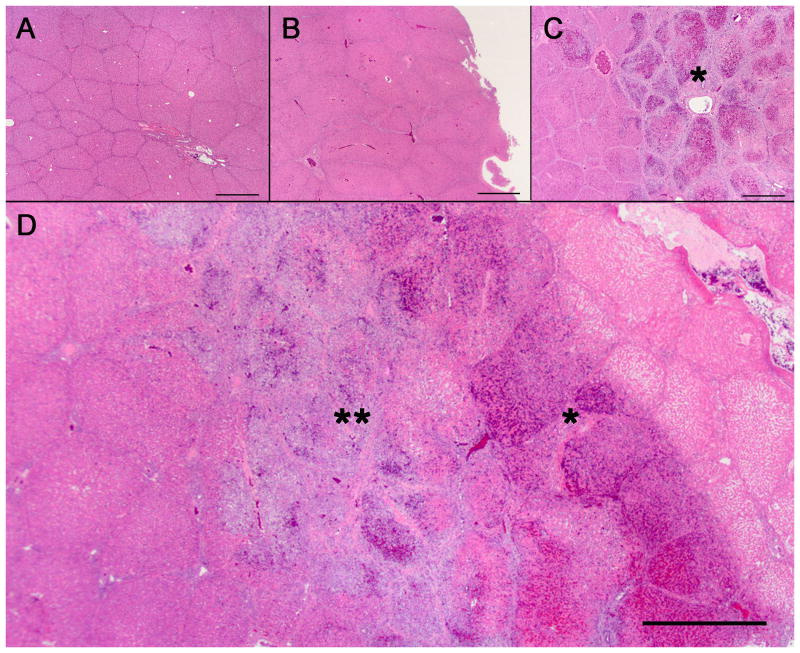
Figure 4. Immune infiltrate surrounding the ablation zone.
(A) Normal untreated liver and (B) liver that received the GM-CSF plasmid infusion but not IRE did not have any immune infiltrate. (C) Liver that was treated with IRE but received saline infusion with no DNA showed an ablation zone (asterisk). (D) Liver with treatment with both IRE and GM-CSF plasmid infusion demonstrated an ablation zone (asterisk) that was surrounded by a prominent immune infiltrate (double asterisk). H&E stain. Scale bar represents 1 mm.
Figure 5. Macrophage response in the liver.
(A) After treatment with IRE and GM-CSF plasmid infusion, an immune infiltrate surrounding the ablation zone was present and composed of few neutrophils (asterisk), few lymphocytes (hash mark), and florid macrophages which are large and mononuclear. Highlighted is a macrophage (arrowhead) which has phagocytosed necrotic debris. H&E stain. Scale bar represents 20 μm. (B) When graded on a scale from 0=none to 4=severe, the macrophage intensity in the immune infiltrate was highest in livers treated with IRE and infusion of 7 mg GM-CSF plasmid.
Lymph nodes treated with IRE and GM-CSF plasmid infusion had moderate follicular lymphoid hyperplasia, eosinophils and macrophages on H&E stain. These characteristics were similar to that with IRE ablation only.
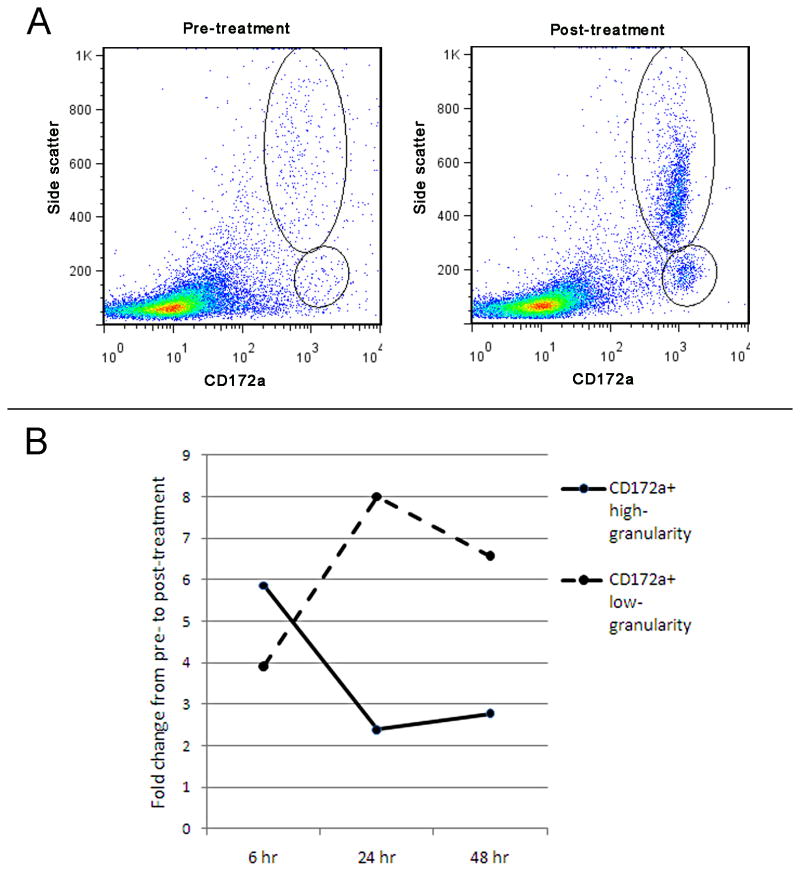
On flow cytometry, two distinct populations of CD172a+ cells emerged after treatment. One population was CD172a+ with high side scatter, which represented high granularity and likely neutrophils; the other population was CD172+ with low side scatter which represented low granularity and likely monocytes and dendritic cells (Figure 6). Within each pig, these two populations were higher after treatment than before treatment. The increase in these populations after treatment were also greater in pigs receiving IRE and GM-CSF plasmid infusion than in control pigs receiving IRE only (Table 3).
Figure 6. Flow cytometry of lymph nodes for immune cells.
(A) Representative image from treatment with IRE and GM-CSF plasmid infusion after 6 hours showed an increase in the CD172a+, high-granularity population representing neutrophils, and the CD172a+, low-granularity population representing monocytes and dendritic cells. (B) The fold change from pre-treatment to post-treatment of the immune cells in the lymph nodes is depicted over time. An early rise in the CD172a+, high-granularity population, which is consistent with neutrophils appearing first in an inflammatory response, is followed by a rise in the CD172a+, low-granularity population which would represent the appearance of monocytes and dendritic cells. In control pigs receiving IRE but no plasmid, the 48 hour response has an only 1.4 fold increase in both the CD172a+, high-granularity and CD172a+, low-granularity populations.
Table 3. Flow Cytometry of Lymph Nodes.
| Tx Group | CD172a+, high granularity | CD172a+, low granularity | ||||||
|---|---|---|---|---|---|---|---|---|
| Absolute % | Fold change | Change from control | Absolute % | Fold change | Change from control | |||
| Pre-tx | Post-tx | Pre-tx | Post-tx | |||||
| IRE & GM-CSF plasmid, 6 hr | 1.4 | 8.0 | 5.86 | 0.5 | 2 | 3.92 | ||
| IRE & GM-CSF plasmid, 24 hr | 0.7 | 1.7 | 2.39 | 0.2 | 1.5 | 8.00 | ||
| IRE & GM-CSF plasmid, 48 hr | 1.7 | 4.8 | 2.77 | 1.97 | 0.3 | 1.8 | 6.57 | 3.12 |
| Control - IRE only, 48 hr | 1.2 | 1.7 | 1.40 | 0.7 | 1.4 | 2.11 | ||
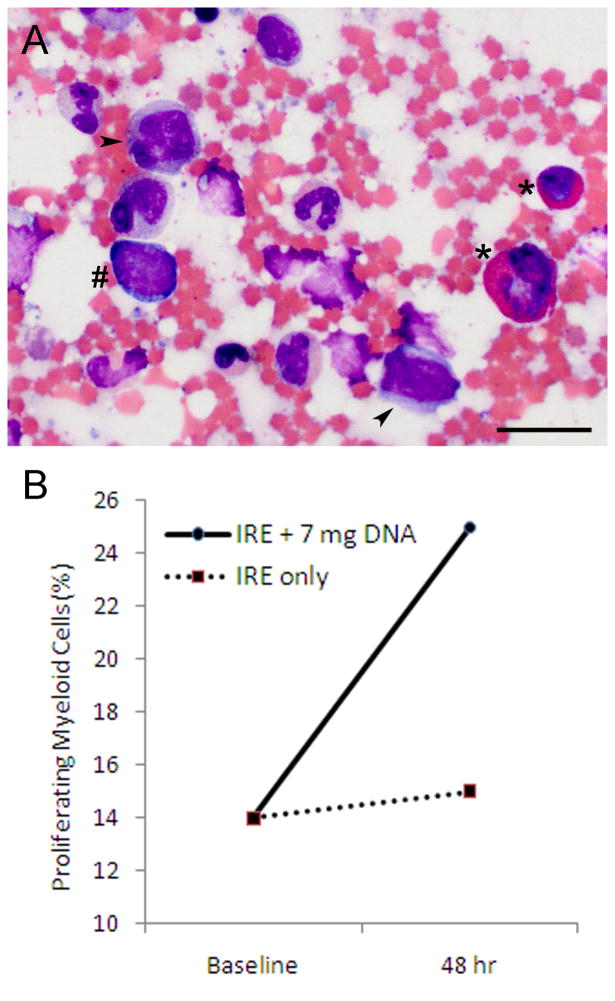
Bone marrow smears exhibited myeloblasts, promyelocytes, and myelocytes (Figure 7A). The pool of proliferating myeloid cells increased from 14% at baseline to 25% at 48 hours after treatment with IRE and GM-CSF plasmid infusion (Figure 7B). When treated with IRE only, the pool of proliferating myeloid cells remained the same at 15%.
Figure 7. Bone marrow response.
(A) Bone marrow smears after treatment with IRE and GM-CSF plasmid infusion exhibited myeloblasts (hash mark), promyelocytes (arrowhead) and myelocytes (asterisk). Wright-Giemsa stain. Scale bar represents 20 μm. (B) The pool of proliferating myeloid cells increased after combined treatment with IRE and plasmid infusion but remained about the same with IRE only.
Discussion
While surgical resection or ablation can grossly reduce tumor burden, adjuvant immunotherapy can help treat microscopic residual disease and prevent recurrence. Such microscopic residual disease is viable cancer cells that persist despite treatment with ablation or resection and usually reside in the periphery of the treated area. Reversible electroporation with gene transfer of immune plasmids bolstered the immune response and induced tumor regression in animal models of colon cancer, hepatocellular carcinoma, and melanoma 3, 4, 18as well as preclinical and clinical studies with patients with melanoma 6-8. Our lab and institution have had favorable experiences with the GM-CSF plasmid for immunotherapy. We have shown that virally-mediated gene transfer of the GM-CSF plasmid inhibited tumor growth, ameliorated symptoms like weight loss, and protected against a second inoculation of cancer cells even in the immunosuppressive setting of a hepatectomy 21-23. The human GM-CSF DNA plasmid used in this present study has been shown to be safe in a phase I/II clinical trial with melanoma patients 19. These findings make the GM-CSF plasmid an excellent candidate for this study, which follows our previous proof of principle study with a marker gene plasmid for green fluorescent protein where IRE did enable uptake of a gene plasmid 18.
This study shows that IRE can mediate gene transfer of a naked DNA plasmid and elicit its biological response. The human GM-CSF plasmid was taken up and expressed such that there was human GM-CSF cytokine in the serum and an intense macrophage-dominant immune infiltrate surrounding the ablation zone in the liver. We believe that as the IRE pulses are applied, the electric field is high in the center, resulting in cell killing, but diminishes as it radiates out. This enables reversible electroporation to occur in the viable cells in the periphery, leading to uptake and expression of the gene plasmid. Despite the absence of a foreign substance or tumor, the immune infiltrate and macrophage intensity in the liver was greater with the combined treatment of IRE and GM-CSF plasmid infusion than with IRE alone. As macrophages are antigen-presenting cells that stimulate an immune response, we anticipate that with the addition of an antigen, the immune response would be even more intense and be antigen-specific. Furthermore, our study found that the effects of gene transfer with IRE extended beyond the sites immediately around the ablation zone. The GM-CSF was expressed and entered the systemic circulation, enabling its effects to reach the bone marrow where it increased the pool of proliferating myeloid cells.
Further investigation is needed in several areas. The serum levels of human GM-CSF initially rose but fell back by at 48 hours. This limitation may be due to increased clearance of human GM-CSF which is still a foreign substance to pigs. It could also be due to problems at the gene level like silencing of the promoter or loss of the plasmid. Human GM-CSF was not found in the liver, and a possible explanation is efflux by hepatocytes which are well equipped with transporters for shuttling molecules in and out. Flow cytometry showed that lymph nodes treated with IRE and GM-CSF plasmid infusion had a two-fold increase in the CD172a+ high-granularity population which is rich in neutrophils compared to treatment with IRE only; similarly, there was a three-fold increase in the CD172a+ low-granularity population which is rich in monocytes and dendritic cells. The time frame of these changes is consistent with an early immune response in which immature dendritic cells take up antigen at the site of injury, mature, and migrate to the lymph nodes to prime further immune response. We believe that this effect may be even more robust in the setting of tumor ablation, leading to an antigen-specific immune response. Further characterization of the dendritic cell response would help understand the effects of gene transfer with GM-CSF on anti-tumor immunity. Future experiments in a tumor model with an antigen will be necessary to test the ability of this treatment to stimulate, amplify, and make specific any immune response from gene transfer of the GM-CSF plasmid. Control studies will be needed to check for any confounding immunologic effect from an empty plasmid vector devoid of the target gene but still with the cytomegalovirus promoter. Finally, future directions of this research will be clinical trials in cancer patients to determine if pairing an infusion of GM-CSF plasmid with IRE ablation can more thoroughly treat the tumor and prevent recurrence.
Gene transfer from IRE ablation is an attractive technique of interest for multiple reasons. It is simple, does not require any viral vectors, and is not limited to dividing cells. Compared to regional hydrodynamic gene transfer 24, 25, electroporation-mediated gene transfer requires only a small volume and a low infusion rate, and does not require outflow obstruction, thus conferring a low risk of acute cardiovascular overload and hepatic swelling. While gene transfer can be paired with radiofrequency ablation and high intensity focused ultrasound ablation 26, 27, such gene transfer requires a viral vector, and protein denaturation would diminish the availability of tumor antigens. On the other hand, combined treatment with IRE ablation and gene transfer of immune plasmids would create an opportunistic context for cancer therapy where a paracrine immune response is in close proximity to the intact antigens of the ablated target. This type of treatment is especially attractive for highly immunogenic types of cancers in patients who are otherwise not surgical candidates or whose tumors are unresectable.
In conclusion, our study demonstrates that IRE is not only a novel ablative technique in the liver, but also enhances gene transfer of a naked GM-CSF plasmid and produces a biological response in the liver, lymph nodes, and bone marrow. This strategy holds promise for combined destruction of tumor and microscopic treatment of residual cancer.
Acknowledgments
The authors wish to thank Dr. Kelley Penraat for her expertise in bone marrow evaluation. We also express gratitude to the veterinarian technicians and Jacqueline Candelier at the Research Animal Resource Center for excellent animal care and technical assistance in support of this project.
References
- 1.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–18. doi: 10.1097/00000658-199909000-00004. discussion 18-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229(6):790–9. doi: 10.1097/00000658-199906000-00005. discussion 9-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heller LC, Ugen K, Heller R. Electroporation for targeted gene transfer. Expert Opin Drug Deliv. 2005;2(2):255–68. doi: 10.1517/17425247.2.2.255. [DOI] [PubMed] [Google Scholar]
- 4.Tamura T, Sakata T. Application of in vivo electroporation to cancer gene therapy. Curr Gene Ther. 2003;3(1):59–64. doi: 10.2174/1566523033347462. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita YI, Shimada M, Hasegawa H, Minagawa R, Rikimaru T, Hamatsu T, et al. Electroporation-mediated interleukin-12 gene therapy for hepatocellular carcinoma in the mice model. Cancer Res. 2001;61(3):1005–12. [PubMed] [Google Scholar]
- 6.Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008;26(36):5896–903. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horton HM, Lalor PA, Rolland AP. IL-2 plasmid electroporation: from preclinical studies to phase I clinical trial. Methods Mol Biol. 2008;423:361–72. doi: 10.1007/978-1-59745-194-9_28. [DOI] [PubMed] [Google Scholar]
- 8.Heller LC, Heller R. Electroporation gene therapy preclinical and clinical trials for melanoma. Curr Gene Ther. 2010;10(4):312–7. doi: 10.2174/156652310791823489. [DOI] [PubMed] [Google Scholar]
- 9.Charpentier KP, Wolf F, Noble L, Winn B, Resnick M, Dupuy DE. Irreversible electroporation of the pancreas in swine: a pilot study. HPB (Oxford) 2010;12(5):348–51. doi: 10.1111/j.1477-2574.2010.00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee EW, Chen C, Prieto VE, Dry SM, Loh CT, Kee ST. Advanced hepatic ablation technique for creating complete cell death: irreversible electroporation. Radiology. 2010;255(2):426–33. doi: 10.1148/radiol.10090337. [DOI] [PubMed] [Google Scholar]
- 11.Onik G, Mikus P, Rubinsky B. Irreversible electroporation: implications for prostate ablation. Technol Cancer Res Treat. 2007;6(4):295–300. doi: 10.1177/153303460700600405. [DOI] [PubMed] [Google Scholar]
- 12.Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality--clinical implications. Technol Cancer Res Treat. 2007;6(1):37–48. doi: 10.1177/153303460700600106. [DOI] [PubMed] [Google Scholar]
- 13.Al-Sakere B, Andre F, Bernat C, Connault E, Opolon P, Davalos RV, et al. Tumor ablation with irreversible electroporation. PLoS One. 2007;2(11):e1135. doi: 10.1371/journal.pone.0001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y, Zhang Y, Klein R, Nijm GM, Sahakian AV, Omary RA, et al. Irreversible electroporation therapy in the liver: longitudinal efficacy studies in a rat model of hepatocellular carcinoma. Cancer Res. 2010;70(4):1555–63. doi: 10.1158/0008-5472.CAN-09-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neal RE, 2nd, Singh R, Hatcher HC, Kock ND, Torti SV, Davalos RV. Treatment of breast cancer through the application of irreversible electroporation using a novel minimally invasive single needle electrode. Breast Cancer Res Treat. 2010;123(1):295–301. doi: 10.1007/s10549-010-0803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kingham TP, Karkar AM, D'Angelica MI, Allen PJ, Dematteo RP, Getrajdman GI, et al. Ablation of perivascular hepatic malignant tumors with irreversible electroporation. J Am Coll Surg. 2012;215(3):379–87. doi: 10.1016/j.jamcollsurg.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 17.Martin RC, 2nd, McFarland K, Ellis S, Velanovich V. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J Am Coll Surg. 2012;215(3):361–9. doi: 10.1016/j.jamcollsurg.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Au JT, Wong J, Mittra A, Carpenter S, Haddad D, Carson J, et al. Irreversible electroporation is a surgical ablation technique that enhances gene transfer. Surgery. 2011;150(3):474–9. doi: 10.1016/j.surg.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perales MA, Yuan J, Powel S, Gallardo HF, Rasalan TS, Gonzalez C, et al. Phase I/II study of GM-CSF DNA as an adjuvant for a multipeptide cancer vaccine in patients with advanced melanoma. Mol Ther. 2008;16(12):2022–9. doi: 10.1038/mt.2008.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inumaru S, Takamatsu H. cDNA cloning of porcine granulocyte-macrophage colony-stimulating factor. Immunol Cell Biol. 1995;73(5):474–6. doi: 10.1038/icb.1995.74. [DOI] [PubMed] [Google Scholar]
- 21.Delman KA, Zager JS, Bennett JJ, Malhotra S, Ebright MI, McAuliffe PF, et al. Efficacy of multiagent herpes simplex virus amplicon-mediated immunotherapy as adjuvant treatment for experimental hepatic cancer. Ann Surg. 2002;236(3):337–42. doi: 10.1097/00000658-200209000-00010. discussion 42-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karpoff HM, D'Angelica M, Blair S, Brownlee MD, Federoff H, Fong Y. Prevention of hepatic tumor metastases in rats with herpes viral vaccines and gamma-interferon. J Clin Invest. 1997;99(4):799–804. doi: 10.1172/JCI119226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malhotra S, Kim T, Zager J, Bennett J, Ebright M, D'Angelica M, et al. Use of an oncolytic virus secreting GM-CSF as combined oncolytic and immunotherapy for treatment of colorectal and hepatic adenocarcinomas. Surgery. 2007;141(4):520–9. doi: 10.1016/j.surg.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alino SF, Herrero MJ, Noguera I, Dasi F, Sanchez M. Pig liver gene therapy by noninvasive interventionist catheterism. Gene Ther. 2007;14(4):334–43. doi: 10.1038/sj.gt.3302873. [DOI] [PubMed] [Google Scholar]
- 25.Fabre JW, Grehan A, Whitehorne M, Sawyer GJ, Dong X, Salehi S, et al. Hydrodynamic gene delivery to the pig liver via an isolated segment of the inferior vena cava. Gene Ther. 2008;15(6):452–62. doi: 10.1038/sj.gt.3303079. [DOI] [PubMed] [Google Scholar]
- 26.Moonen CT. Spatio-temporal control of gene expression and cancer treatment using magnetic resonance imaging-guided focused ultrasound. Clin Cancer Res. 2007;13(12):3482–9. doi: 10.1158/1078-0432.CCR-07-0204. [DOI] [PubMed] [Google Scholar]
- 27.Saito K, Araki K, Reddy N, Guang W, O'Malley BW, Jr, Li D. Enhanced local dendritic cell activity and tumor-specific immunoresponse in combined radiofrequency ablation and interleukin-2 for the treatment of human head and neck cancer in a murine orthotopic model. Head Neck. 2011;33(3):359–67. doi: 10.1002/hed.21456. [DOI] [PubMed] [Google Scholar]