Introduction
Cardiac sarcoidosis is an unusual cause of refractory ventricular arrhythmias and heart failure. Catheter ablation of ventricular tachycardia (VT) secondary to cardiac sarcoidosis has been reported with good success, but these tachycardias are occasionally epicardial (1). We report the gross and microscopic pathological examination of a patient who underwent an epicardial ablation for recurrent VT in the setting of cardiac sarcoidosis, aided by electrocardiographic imaging (ECGI) (2,3).
Case Report
45 year old white male presented with two episodes of syncope. A 30-day event monitor demonstrated sustained monomorphic VT. Initial echocardiogram revealed an ejection fraction of 29% with postero-lateral dyskinesis. Cardiac catheterization revealed no significant coronary artery disease. Cardiac biopsy revealed no granulomata; there was no mediastinal adenopathy; and the ACE level was normal. Cardiac MRI demonstrated patchy, predominately subepicardial enhancement centered in the lateral wall of the left ventricle with adjacent foci of nodular transmural enhancement. While in the hospital, he continued to have episodes of sustained VT, and elected to undergo an electrophysiology study with the goal of curative ablation. During the study, two VT morphologies were induced, one with a right bundle block (RBBB) morphology, a right superior axis, and cycle length (CL) of 466 ms, the other with a RBBB morphology, a right inferior axis and a CL of 500 ms. These were targeted for ablation. An ICD was placed, and mexiletine, 150 mg three times daily, was initiated.
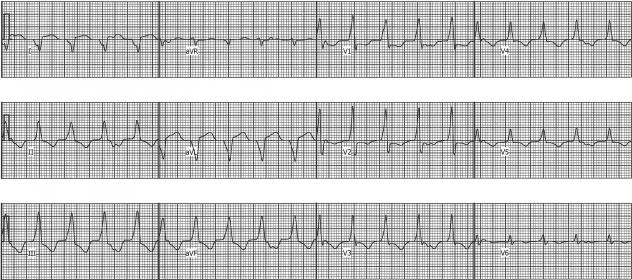
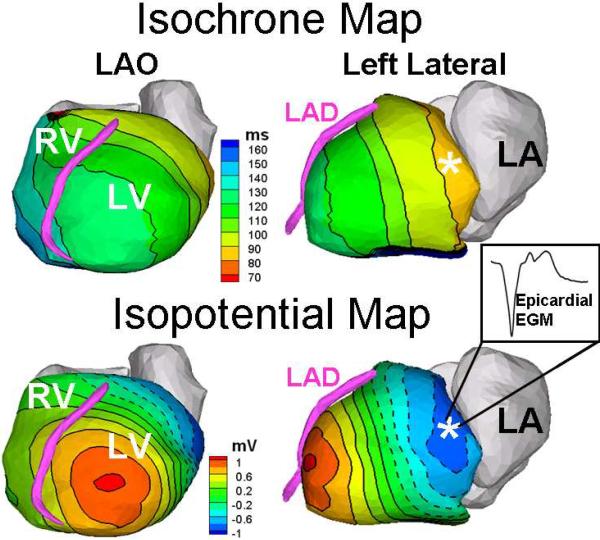
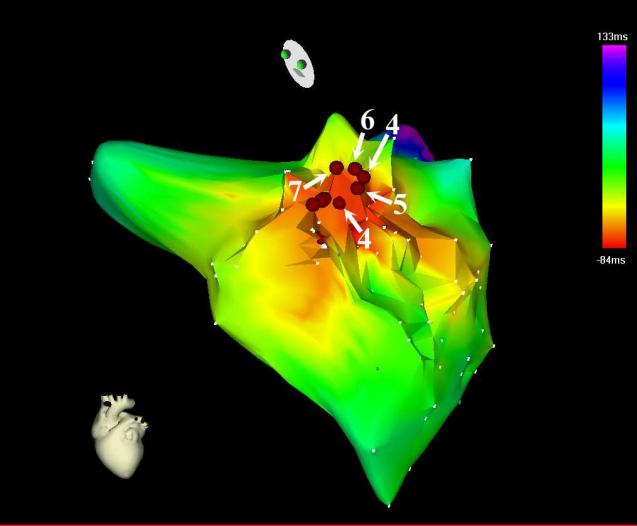
Seven months later, he experienced recurrent VT, with a different morphology (Figure 1). A “pseudo-delta” wave (a gradual onset of the QRS prior to the more rapid deflection) was seen in the precordial leads, suggesting an epicardial site of origin (4). ECGI localized the VT focus to the basal anterolateral aspect of the left ventricle (Figure 2). ECGI-reconstructed electrograms from this site were of Q-wave morphology, without an initial positive deflection, consistent with epicardial origin (3). During electrophysiology study, the pericardial space was accessed using the method of Sosa, et al (5). A bariatric epidural needle and a 0.38 inch guide wire were used to access the pericardial space. An 8 Fr sheath was placed, and an open irrigated ablation catheter (Thermocool, Biosense-Webster) was inserted. Non-sustained monomorphic VT with a RBBB morphology and inferior axis occurred spontaneously. Electroanatomic mapping (CARTO, Biosense-Webster), activation mapping, and pace mapping localized the VT to the basal anterolateral aspect of the LV, corresponding closely to the site seen on ECGI. The epicardial electroanatomic map is shown in Figure 3. Coronary angiography was performed to assure the site was a safe distance from any epicardial coronary arteries. Pacing at the site of planned ablation captured the phrenic nerve; therefore the pericardium was insufflated with air, which prevented prhenic nerve stimulation. Seven 1 minute radiofrequency energy applications were delivered. The maximum power settings were 30–50 W. The average power delivered was 29, 29, 39, 40, 40, 39, and 39 W. The 4th through the 7th lesions appeared to have the most effect on the VT. After the 4th radiofrequency energy application, the ventricular rhythm became much less frequent, though there was a episode of a slow (cycle length 1243; 48 bpm) ventricular rhythm with the same morphology as the VT. The ventricular rhythm did not recur after the 5th application, and all ventricular ectopy ceased during the 6th application. After ablation, there were no further sustained ventricular arrhythmias, despite aggressive programmed stimulation. No complications occurred.
Figure 1.
Ventricular tachycardia with a RBBB morphology and right superior axis and VA dissociation. Note the “pseudo-delta” wave in lead V1 is suggesting an epicardial site of origin.
Figure 2.
ECGI isochrones and potential maps localizing the VT origin to the basal antero-lateral surface of the LV. Note the Q-wave morphology of the ECGI-reconstructed epicardial electrogram (EGM)at that site [inset], indicating an epicardial source. The asterisk in the isochrone map marks the site of earliest activation; the asterisk in the potential map is the local potential minimum associated with earliest activation.
Figure 3.
CARTO electroanatomic activation maps in Left Anterior Oblique Cranial orientation. The radiofrequency energy applications are represented by the red spheres. Lesions 4–7 (labeled) affected the ventricular tachycardia. (Two points were collected during the 4th application).
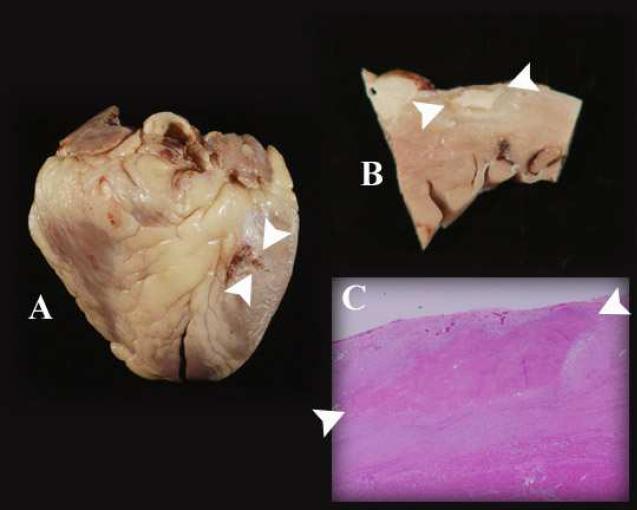
Several months later, the patient was admitted to the hospital for progressive congestive heart failure, refractory to aggressive medical treatment. There was also VT of a different morphology than seen previously. He underwent successful cardiac transplantation, and the explanted heart was retained for pathological examination. Gross examination revealed an ablation lesion at the expected site of ablation. The lesion measured 3.0 cm × 1.5 cm with a maximum depth of 3 mm. Using 2 mm as an estimated average depth, the calculated lesion volume is 0.9 cm3. Microscopic examination revealed noncaseating granulomas in the background of fibrosis and chronic inflammation with minimal myocyte necrosis, consistent with sarcoidosis. Microscopic examination of the site of ablation also revealed ghosts of multinucleate giant cells, indicating that the ablated area was affected by the sarcoidosis (Figure 4).
Figure 4.
Gross, sectioned, and microscopic examination of the heart displays the epicardial ablation lesions. In each panel, the ablation lesions are indicated by the two white arrowheads. A: The lesions are seen at the basal anterolateral aspect of the whole heart. The lesions to the left of the arrowheads were superficial, as demonstrated in panel B. B: Section through the area ablation, revealing dense scar of ablation surrounded by scar from sarcoidosis. The depth of the lesion is 2 mm. C: The microscopic cross sectional examination shows that the ablation lesion is in an area affected by sarcoidosis. There is normal myocardium at the lower border of the panel.
Conclusion
To our knowledge, this is the first example of the gross and microscopic pathological examination of a site of epicardial ablation for cardiac sarcoidosis. The fact that the patient underwent cardiac transplantation afforded the opportunity to prove that the lesions were in the expected area based on both non-invasive and CARTO imaging, and that the lesions were of sufficient depth to ablate the affected tissue. ECGI imaging proved useful in localizing this epicardial VT.
Acknowledgments
Grant Support This study was supported by NIH – National Heart, Lung and Blood Institute Grants HL33343 and HL49054 to Yoram Rudy. Yoram Rudy is the Frank Saigh Distinguished Professor at Washington University.
Abbreviations
- VT
Ventricular Tachycardia
- ECGI
Non-invasive electrocardiographic imaging
- RBBB
Right bundle branch block
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Yoram Rudy co-chairs the scientific advisory board and owns equity in CardioInsight Technologies (CIT). CIT does not support any research conducted by Dr Rudy, including that presented here.
References
- 1.Jefic D, Joel B, Good E, et al. Role of radiofrequency catheter ablation of ventricular tachycardia in cardiac sarcoidosis: report from a multicenter registry. Heart Rhythm. 2009;6:189–195. doi: 10.1016/j.hrthm.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 2.Ramanathan C, Ghanem RN, Jia P, Ryu K, Rudy Y. Noninvasive electrocardiographic imaging for cardiac electrophysiology and arrhythmia. Nature Medicine. 2004;10:422–428. doi: 10.1038/nm1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramanathan C, Jia P, Ghanem R, Ryu K, Rudy Y. Activation and repolarization of the normal human heart under complete physiological conditions. Proceedings of the National Academy of Sciences USA. 2006;103:6309–6314. doi: 10.1073/pnas.0601533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berruezo A, Mont L, Nava S, Chueca E, Barholomay E, Brugada J. Electrocardiographic recognition of the epicardial origin of ventricular tachycardias. Circulation. 2004;109:1842–1847. doi: 10.1161/01.CIR.0000125525.04081.4B. [DOI] [PubMed] [Google Scholar]
- 5.Sosa E, Scanavacca M. Percutaneous pericardial access for mapping and ablation of epicardial ventricular tachycardia. Circulation. 2007;115:e542–44. doi: 10.1161/CIRCULATIONAHA.107.701623. [DOI] [PubMed] [Google Scholar]