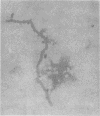
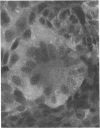
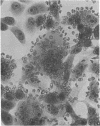
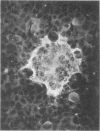
Full text
PDF





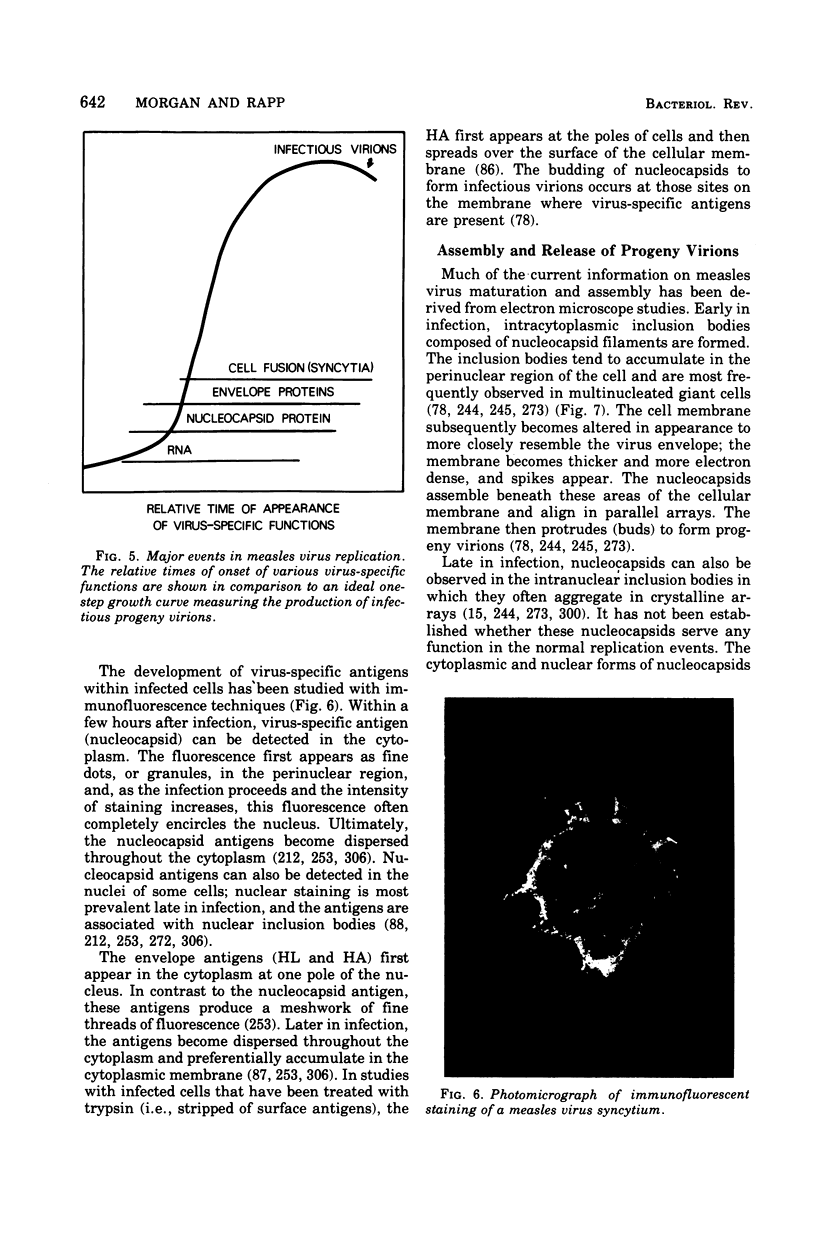

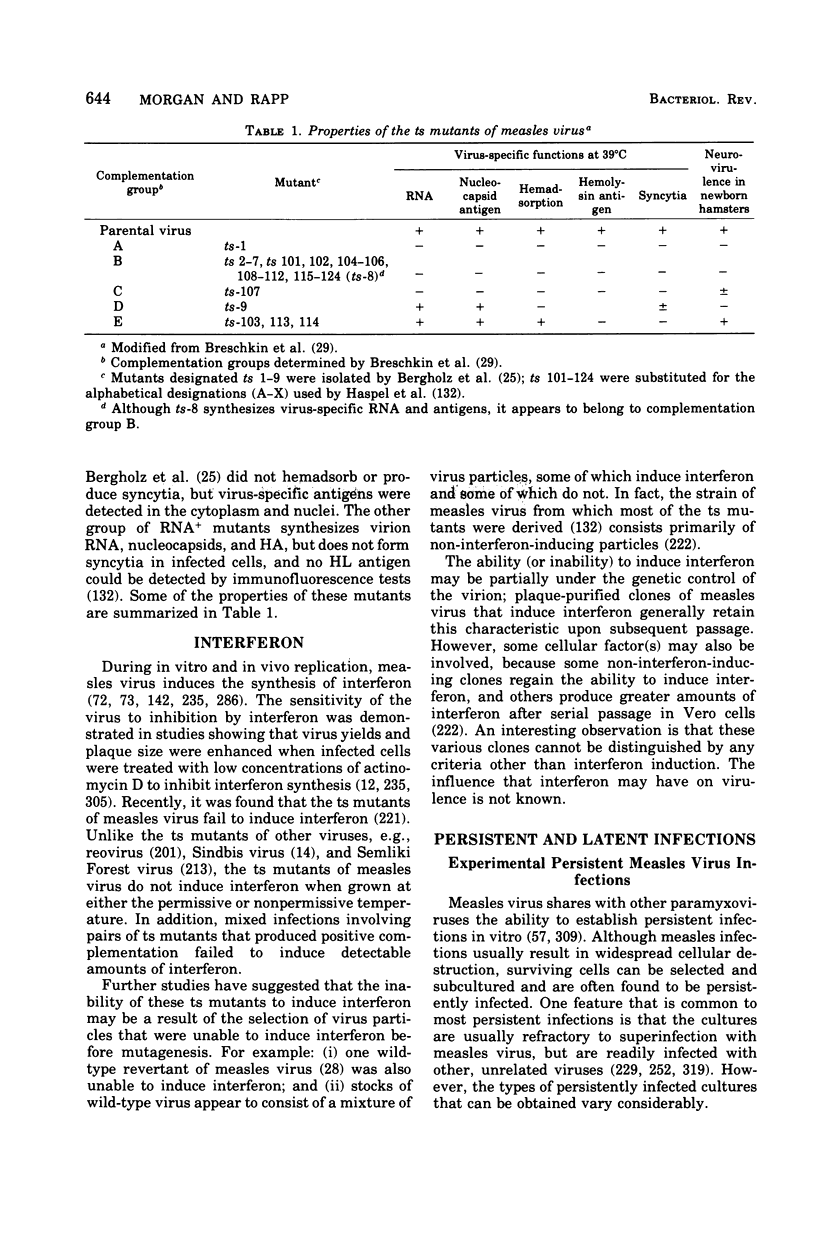






















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS J. M., IMAGAWA D. T. Measles antibodies in multiple sclerosis. Proc Soc Exp Biol Med. 1962 Dec;111:562–566. doi: 10.3181/00379727-111-27855. [DOI] [PubMed] [Google Scholar]
- ANDERSON C. D., ATHERTON J. G. EFFECT OF ACTINOMYCIN D ON MEASLES VIRUS GROWTH AND ON INTERFERON PRODUCTION. Nature. 1964 Aug 8;203:671–671. doi: 10.1038/203671a0. [DOI] [PubMed] [Google Scholar]
- Adams J. M., Baird C., Filloy L. Inclusion bodies in measles encephalitis. JAMA. 1966 Jan 24;195(4):290–298. [PubMed] [Google Scholar]
- Adams J. M. Clinical pathology of measles encephalitis and sequelae. Neurology. 1968 Jan;18(1 Pt 2):52–57. doi: 10.1212/wnl.18.1_part_2.052. [DOI] [PubMed] [Google Scholar]
- Ahmed A., Strong D. M., Sell K. W., Thurman G. B., Knudsen R. C., Wistar R., Jr, Grace W. R. Demonstration of a blocking factor in the plasma and spinal fluid of patients with subacute sclerosing panencephalitis. I. Partial characterization. J Exp Med. 1974 Apr 1;139(4):902–924. doi: 10.1084/jem.139.4.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicardi J., Goutieres F., Arsenio-Nunes M. L., Lebon P. Acute measles encephalitis in children with immunosuppression. Pediatrics. 1977 Feb;59(2):232–239. [PubMed] [Google Scholar]
- Albrecht P., Burnstein T., Klutch M. J., Hicks H. T., Ennis F. A. Subacute sclerosing panencephalitis: experimental infection in primates. Science. 1977 Jan 7;195(4273):64–66. doi: 10.1126/science.831255. [DOI] [PubMed] [Google Scholar]
- Albrecht P., Schumacher H. P. Neurotropic properties of measles virus in hamsters and mice. J Infect Dis. 1971 Jul;124(1):86–93. doi: 10.1093/infdis/124.1.86. [DOI] [PubMed] [Google Scholar]
- Albrecht P., Shabo A. L., Burns G. R., Tauraso N. M. Experimental measles encephalitis in normal and cyclophosphamide-treated rhesus monkeys. J Infect Dis. 1972 Aug;126(2):154–161. doi: 10.1093/infdis/126.2.154. [DOI] [PubMed] [Google Scholar]
- Alekberova Z. S., Parfanovich M. I., Nasonova V. A., Zhdanov V. M. Molecular pathogenesis of systemic lupus erythematosus. Arch Virol. 1975;47(2):109–121. doi: 10.1007/BF01320551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter M., Cendrowski W. Multiple sclerosis and childhood infections. Neurology. 1976 Mar;26(3):201–204. doi: 10.1212/wnl.26.3.201. [DOI] [PubMed] [Google Scholar]
- Arbeter A. M., Arthur J. H., Blakeman G. J., McIntosh K. Measles immunity: reimmunization of children who previously received live measles vaccine and gamma globulin. J Pediatr. 1972 Oct;81(4):737–741. doi: 10.1016/s0022-3476(72)80094-5. [DOI] [PubMed] [Google Scholar]
- Atkins G. J., Lancashire C. L. The induction of interferon by temperature-sensitive mutants of Sindbis virus: its relationship to double-stranded RNA synthesis and cytopathic effect. J Gen Virol. 1976 Feb;30(2):157–165. doi: 10.1099/0022-1317-30-2-157. [DOI] [PubMed] [Google Scholar]
- BAKER R. F., GORDON I., RAPP F. Electron-dense crystallites in nuclei of human amnion cells infected with measles virus. Nature. 1960 Mar 12;185:790–791. doi: 10.1038/185790a0. [DOI] [PubMed] [Google Scholar]
- BECH V. THE MEASLES EPIDEMIC IN GREENLAND IN 1962. Arch Gesamte Virusforsch. 1965;16:53–56. doi: 10.1007/BF01253792. [DOI] [PubMed] [Google Scholar]
- BRODY J. A., OVERFIELD T., HAMMES L. M. DEPRESSION OF THE TUBERCULIN REACTION BY VIRAL VACCINES. N Engl J Med. 1964 Dec 17;271:1294–1296. doi: 10.1056/NEJM196412172712505. [DOI] [PubMed] [Google Scholar]
- Baker R. F., Gordon I., Stevenson D. Electron microscope study of hemadsorption in measles virus infection. Virology. 1965 Nov;27(3):441–445. doi: 10.1016/0042-6822(65)90129-7. [DOI] [PubMed] [Google Scholar]
- Barbanti-Brodano G., Oyanagi S., Katz M., Koprowski H. Presence of 2 different viral agents in brain cells of patients with subacute sclerosing panencephalitis. Proc Soc Exp Biol Med. 1970 May;134(1):230–236. doi: 10.3181/00379727-134-34765. [DOI] [PubMed] [Google Scholar]
- Baringer J. R., Griffith J. F. Experimental measles virus encephalitis. A light, phase, fluorescence, and electron microscopic study. Lab Invest. 1970 Sep;23(3):335–346. [PubMed] [Google Scholar]
- Barkin R. M. Measles mortality. Analysis of the primary cause of death. Am J Dis Child. 1975 Mar;129(3):307–309. doi: 10.1001/archpedi.1975.02120400019004. [DOI] [PubMed] [Google Scholar]
- Barry D. W., Sullivan J. L., Lucas S. J., Dunlap R. C., Albrecht P. Acute and chronic infection of human lymphoblastoid cell lines with measles virus. J Immunol. 1976 Jan;116(1):89–98. [PubMed] [Google Scholar]
- Bass J. W., Halstead S. B., Fischer G. W., Podgore J. K., Pearl W. R., Schydlower M., Wiebe R. A., Ching F. M. Booster vaccination with further live attenuated measles vaccine. JAMA. 1976 Jan 5;235(1):31–34. [PubMed] [Google Scholar]
- Baublis J. V., Payne F. E. Measles antigen and syncytium formtion in brain cell cultures from subacute sclerosing panencephalitis (SSPE). Proc Soc Exp Biol Med. 1968 Nov;129(2):593–597. doi: 10.3181/00379727-129-33377. [DOI] [PubMed] [Google Scholar]
- Bergholz C. M., Kiley M. P., Payne F. E. Isolation and characterization of temperature-sensitive mutants of measles virus. J Virol. 1975 Jul;16(1):192–202. doi: 10.1128/jvi.16.1.192-202.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitfeld V., Hashida Y., Sherman F. E., Odagiri K., Yunis E. J. Fatal measles infection in children with leukemia. Lab Invest. 1973 Mar;28(3):279–291. [PubMed] [Google Scholar]
- Breschkin A. M., Haspel M. V., Rapp F. Neurovirulence and induction of hydrocephalus with parental, mutant, and revertant strains of measles virus. J Virol. 1976 May;18(2):809–811. doi: 10.1128/jvi.18.2.809-811.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breschkin A. M., Rapp F., Payne F. E. Complementation analysis of measles virus temperature-sensitive mutants. J Virol. 1977 Jan;21(1):439–441. doi: 10.1128/jvi.21.1.439-441.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky A. L. Atypical measles. Severe illness in recipients of killed measles virus vaccine upon exposure to natural infection. JAMA. 1972 Dec 11;222(11):1415–1416. doi: 10.1001/jama.222.11.1415. [DOI] [PubMed] [Google Scholar]
- Brody J. A., Detels R., Sever J. L. Measles-antibody titres in sibships of patients with subacute sclerosing panencephalitis and controls. Lancet. 1972 Jan 22;1(7743):177–178. doi: 10.1016/s0140-6736(72)90572-7. [DOI] [PubMed] [Google Scholar]
- Brody J. A., Detels R. Subacute sclerosing panencephalitis: a zoonosis following aberrant measles. Lancet. 1970 Sep 5;2(7671):500–501. doi: 10.1016/s0140-6736(70)90116-9. [DOI] [PubMed] [Google Scholar]
- Brody J. A. Epidemiology of multiple sclerosis and a possible virus aetiology. Lancet. 1972 Jul 22;2(7769):173–176. doi: 10.1016/s0140-6736(72)91340-2. [DOI] [PubMed] [Google Scholar]
- Brody J. A., Sever J. L., Edgar A., McNew J. Measles antibody titers of multiple sclerosis patients and their siblings. Neurology. 1972 May;22(5):492–499. doi: 10.1212/wnl.22.5.492. [DOI] [PubMed] [Google Scholar]
- Brown P., Cathala F., Gajdusek D. C., Gibbs C. J., Jr Measles antibodies in the cerebrospinal fluid of patients with multiple sclerosis. Proc Soc Exp Biol Med. 1971 Jul;137(3):956–961. doi: 10.3181/00379727-137-35704. [DOI] [PubMed] [Google Scholar]
- Burnet F. M. Measles as an index of immunological function. Lancet. 1968 Sep 14;2(7568):610–613. doi: 10.1016/s0140-6736(68)90701-0. [DOI] [PubMed] [Google Scholar]
- Burnstein T., Jacobsen L. B., Zeman W., Chen T. T. Persistent infection of BSC-1 cells by defective measles virus derived from subacute sclerosing panencephalitis. Infect Immun. 1974 Dec;10(6):1378–1382. doi: 10.1128/iai.10.6.1378-1382.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser F. Side reaction to measles vaccination suggesting the Arthus phenomenon. N Engl J Med. 1967 Aug 3;277(5):250–251. doi: 10.1056/NEJM196708032770507. [DOI] [PubMed] [Google Scholar]
- Bussell R. H., Waters D. J., Seals M. K. Measles, canine distemper and respiratory syncytial virions and nucleocapsids. A comparative study of their structure, polypeptide and nucleic acid composition. Med Microbiol Immunol. 1974;160(2-3):105–124. doi: 10.1007/BF02121718. [DOI] [PubMed] [Google Scholar]
- Byington D. P., Castro A. E., Burnstein T. Adaptation to hamsters of neurotropic measles virus from subacute sclerosing panencephalitis. Nature. 1970 Feb 7;225(5232):554–555. doi: 10.1038/225554b0. [DOI] [PubMed] [Google Scholar]
- Byington D. P., Johnson K. P. Experimental subacute sclerosing panencephalitis in the hamster: correlation of age with chronic inclusion-cell encephalitis. J Infect Dis. 1972 Jul;126(1):18–26. doi: 10.1093/infdis/126.1.18. [DOI] [PubMed] [Google Scholar]
- Byington D. P., Johnson K. P. Subacute sclerosing panencephalitis (SSPE) agent in hamsters. II. The neuropathology of acute and chronic infections. Exp Mol Pathol. 1973 Jun;18(3):345–356. doi: 10.1016/0014-4800(73)90030-0. [DOI] [PubMed] [Google Scholar]
- Byington D. P., Johnson K. P. Subacute sclerosing panencephalitis virus in immunosuppressed adult hamster. Lab Invest. 1975 Jan;32(1):91–97. [PubMed] [Google Scholar]
- CASCARDO M. R., KARZON D. T. MEASLES VIRUS GIANT CELL INDUCING FACTOR (FUSION FACTOR). Virology. 1965 Jun;26:311–325. doi: 10.1016/0042-6822(65)90279-5. [DOI] [PubMed] [Google Scholar]
- Carp R. I., Licursi P. C., Merz G. S. Multiple sclerosis-induced reduction in the yield of a mouse cell line. Infect Immun. 1975 Apr;11(4):737–741. doi: 10.1128/iai.11.4.737-741.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp R. I., Licursi P. C., Merz P. A., Merz G. S. Decreased percentage of polymorphonuclear neutrophils in mouse peripheral blood after inoculation with material from multiple sclerosis patients. J Exp Med. 1972 Sep 1;136(3):618–629. doi: 10.1084/jem.136.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp R. I., Merz G. S., Licursi P. C. Reduced cell yields of mouse cell line cultures after exposure to homogenates of multiple sclerosis tissues. Infect Immun. 1974 Jun;9(6):1011–1015. doi: 10.1128/iai.9.6.1011-1015.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C., Black F. L., Schluederberg A. Nucleus-associated RNA in measles virus-infected cells. Biochem Biophys Res Commun. 1973 Sep 5;54(1):411–416. doi: 10.1016/0006-291x(73)90937-6. [DOI] [PubMed] [Google Scholar]
- Carter C., Schuluederberg A., Black F. L. Viral RNA synthesis in measles virus-infected cells. Virology. 1973 Jun;53(2):379–383. doi: 10.1016/0042-6822(73)90217-1. [DOI] [PubMed] [Google Scholar]
- Chen T. T., Watanabe I., Zeman W., Mealey J., Jr Subacute sclerosing panencephalitis: propagation of measles virus from brain biopsy in tissue culture. Science. 1969 Mar 14;163(3872):1193–1194. doi: 10.1126/science.163.3872.1193. [DOI] [PubMed] [Google Scholar]
- Cherry J. D., Feigin R. D., Lobes L. A., Jr, Hinthorn D. R., Shackelford P. G., Shirley R. H., Lins R. D., Choi S. C. Urban measles in the vaccine era: a clinical, epidemiologic, and serologic study. J Pediatr. 1972 Aug;81(2):217–230. doi: 10.1016/s0022-3476(72)80287-7. [DOI] [PubMed] [Google Scholar]
- Cherry J. D., Feigin R. D., Lobes L. A., Jr, Shackelford P. G. Atypical measles in children previously immunized with attenuated measles virus vaccines. Pediatrics. 1972 Nov;50(5):712–717. [PubMed] [Google Scholar]
- Cherry J. D., Feigin R. D., Shackelford P. G., Hinthorn D. R., Schmidt R. R. A clinical and serologic study of 103 children with measles vaccine failure. J Pediatr. 1973 May;82(5):802–808. doi: 10.1016/s0022-3476(73)80070-8. [DOI] [PubMed] [Google Scholar]
- Cho C. T., Lansky L. J., D'Souza B. J. Panencephalitis following measles vaccination. JAMA. 1973 May 28;224(9):1299–1299. [PubMed] [Google Scholar]
- Chou S. M. Myxovirus-like structures in a case of human chronic polymyositis. Science. 1967 Dec 15;158(3807):1453–1455. doi: 10.1126/science.158.3807.1453. [DOI] [PubMed] [Google Scholar]
- Christian C. L., Phillips P. E. Viruses and autoimmunity. Am J Med. 1973 May;54(5):611–620. doi: 10.1016/0002-9343(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Holmes K. V., Dales S., Choppin P. W. An electron microscopic study of moderate and virulent virus-cell interactions of the parainfluenza virus SV5. Virology. 1966 Nov;30(3):411–426. doi: 10.1016/0042-6822(66)90119-x. [DOI] [PubMed] [Google Scholar]
- Connolly J. H., Allen I. V., Hurwitz L. J., Millar J. H. Measles-virus antibody and antigen in subacute sclerosing panencephalitis. Lancet. 1967 Mar 11;1(7489):542–544. doi: 10.1016/s0140-6736(67)92117-4. [DOI] [PubMed] [Google Scholar]
- Connolly J. H., Haire M., Hadden D. S. Measles immunoglobulins in subacute sclerosing panencephalitis. Br Med J. 1971 Jan 2;1(5739):23–25. doi: 10.1136/bmj.1.5739.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka E., Ruzicska P., Koch A. S. Chromosome aberrations in cells infected with various strains of measles virus. Acta Microbiol Acad Sci Hung. 1975;22(1):41–44. [PubMed] [Google Scholar]
- Cutler R. W., Merler E., Hammerstad J. P. Production of antibody by the central nervous system in subacute sclerosing panencephalitis. Neurology. 1968 Jan;18(1 Pt 2):129–132. doi: 10.1212/wnl.18.1_part_2.129. [DOI] [PubMed] [Google Scholar]
- DE MAEYER E., ENDERS J. F. An interferon appearing in cell cultures infected with measles virus. Proc Soc Exp Biol Med. 1961 Jul;107:573–578. doi: 10.3181/00379727-107-26692. [DOI] [PubMed] [Google Scholar]
- DEMAEYER E., ENDERS J. F. GROWTH CHARACTERISTICS, INTERFERON PRODUCTION AND PLAQUE FORMATION WITH DIFFERENT LINES OF EDMONSTON MEASLES VIRUS. Arch Gesamte Virusforsch. 1965;16:151–160. doi: 10.1007/BF01253804. [DOI] [PubMed] [Google Scholar]
- DEMEIO J. L. Measles virus hemolysin. Virology. 1962 Mar;16:342–344. doi: 10.1016/0042-6822(62)90256-8. [DOI] [PubMed] [Google Scholar]
- Davies J. W., Portner A., Kingsbury D. W. Synthesis of Sendai virus polypeptides by a cell-free extract from wheat germ. J Gen Virol. 1976 Oct;33(1):117–123. doi: 10.1099/0022-1317-33-1-117. [DOI] [PubMed] [Google Scholar]
- Dawson J. R. Cellular Inclusions in Cerebral Lesions of Lethargic Encephalitis. Am J Pathol. 1933 Jan;9(1):7–16.3. [PMC free article] [PubMed] [Google Scholar]
- Dayan A. D. Encephalitis due to simultaneous infection by herpes simplex and measles viruses. J Neurol Sci. 1971 Nov;14(3):315–323. doi: 10.1016/0022-510x(71)90220-6. [DOI] [PubMed] [Google Scholar]
- Dayan A. D., Gostling J. V., Greaves J. L., Stevens D. W., Woodhouse M. A. Evidence of a pseudomyxovirus in the brain in subacute sclerosing leucoencephalitis. Lancet. 1967 May 6;1(7497):980–981. doi: 10.1016/s0140-6736(67)92360-4. [DOI] [PubMed] [Google Scholar]
- De Jong J. C., Winkler K. C. The multiplication of measles virus in human amnion cells in vitro. J Gen Virol. 1970 Apr;7(1):13–17. doi: 10.1099/0022-1317-7-1-13. [DOI] [PubMed] [Google Scholar]
- Detels R., Brody J. A., McNew J., Edgar A. H. Further epidemiological studies of subacute sclerosing panencephalitis. Lancet. 1973 Jul 7;2(7819):11–14. doi: 10.1016/s0140-6736(73)91946-6. [DOI] [PubMed] [Google Scholar]
- Doi Y., Sanpe T., Nakajima M., Okawa S., Koto T. Properties of a cytopathic agent isolated from a patient with subacute sclerosing panencephalitis in Japan. Jpn J Med Sci Biol. 1972 Oct;25(5):321–333. doi: 10.7883/yoken1952.25.321. [DOI] [PubMed] [Google Scholar]
- Dreyer E. O., Muldiyarov P. Y., Nassonova V. A., Alekberova Z. S. Endothelial inclusions and "nuclear bodies" in systemic lupus erythematosus. Ann Rheum Dis. 1973 Sep;32(5):444–449. doi: 10.1136/ard.32.5.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M., Barbosa L. H., Hamilton R., Sever J. L. Comparison between productive and latent subacute sclerosing panencephalitis viral infection in vitro. An electron microscopic and immunoperoxidase study. Lab Invest. 1974 Mar;30(3):241–250. [PubMed] [Google Scholar]
- Dubois-Dalcq M., Barbosa L. H. Immunoperoxidase stain of measles antigen in tissue culture. J Virol. 1973 Oct;12(4):909–918. doi: 10.1128/jvi.12.4.909-918.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunmire C., Ruckdeschel J. C., Mardiney M. R., Jr Suppression of in vitro lymphocyte responsiveness to purified protein derivative by measles virus. A reexploration of the phenomenon. Cell Immunol. 1975 Dec;20(2):205–217. doi: 10.1016/0008-8749(75)90098-2. [DOI] [PubMed] [Google Scholar]
- EHRENGUT W. MEASLES ENCEPHALITIS: AGE DISPOSITION AND VACCINATION. Arch Gesamte Virusforsch. 1965;16:311–314. doi: 10.1007/BF01253828. [DOI] [PubMed] [Google Scholar]
- ENDERS J. F., KATZ S. L., MILOVANOVIC M. V., HOLLOWAY A. Studies on an attenuated measles-virus vaccine. I. Development and preparations of the vaccine: technics for assay of effects of vaccination. N Engl J Med. 1960 Jul 28;263:153–159. doi: 10.1056/NEJM196007282630401. [DOI] [PubMed] [Google Scholar]
- ENDERS J. F., McCARTHY K., MITUS A., CHEATHAM W. J. Isolation of measles virus at autopsy in cases of giant-cell pneumonia without rash. N Engl J Med. 1959 Oct 29;261:875–881. doi: 10.1056/NEJM195910292611801. [DOI] [PubMed] [Google Scholar]
- ENDERS J. F., PEEBLES T. C. Propagation in tissue cultures of cytopathogenic agents from patients with measles. Proc Soc Exp Biol Med. 1954 Jun;86(2):277–286. doi: 10.3181/00379727-86-21073. [DOI] [PubMed] [Google Scholar]
- East J. L., Kingsbury D. W. Mumps virus replication in chick embryo lung cells: properties of ribonucleic acids in virions and infected cells. J Virol. 1971 Aug;8(2):161–173. doi: 10.1128/jvi.8.2.161-173.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman R., Sheridan J., Poskanzer D. C. Multiple sclerosis clustering in a small Massachusetts community, with possible common exposure 23 years before onset. N Engl J Med. 1973 Oct 11;289(15):793–794. doi: 10.1056/NEJM197310112891507. [DOI] [PubMed] [Google Scholar]
- Ehrnst A. C. Characterization of measles virus-specific cytotoxic antibodies by use of a chronically infected cell line. J Immunol. 1975 Mar;114(3):1077–1082. [PubMed] [Google Scholar]
- Ehrnst A., Sundqvist K. G. Polar appearance and nonligand induced spreading of measles virus hemagglutinin at the surface of chronically infected cells. Cell. 1975 Aug;5(4):351–359. doi: 10.1016/0092-8674(75)90053-7. [DOI] [PubMed] [Google Scholar]
- Ehrnst A., Weiner L., Norrby E. Fluctuations and distribution of measles virus antigens in chronically infected cells. Nature. 1974 Apr 19;248(5450):691–693. doi: 10.1038/248691a0. [DOI] [PubMed] [Google Scholar]
- FJELDE A., HOLTERMANN O. A. Chromosome studies in the Hep 2 tissue culture cell line during infection with measles virus. Life Sci. 1962 Dec;1:683–692. doi: 10.1016/0024-3205(62)90135-2. [DOI] [PubMed] [Google Scholar]
- FRANKEL J. W., WEST M. K. Cultivation of measles virus in stable line of human amnion cells. Proc Soc Exp Biol Med. 1958 Apr;97(4):741–742. doi: 10.3181/00379727-97-23865. [DOI] [PubMed] [Google Scholar]
- Feorino P. M., Humphrey D. Mononucleosis-associated subacute sclerosing panencephalitis. Lancet. 1975 Sep 20;2(7934):530–532. doi: 10.1016/s0140-6736(75)90898-3. [DOI] [PubMed] [Google Scholar]
- Field E. J., Cowshall S., Narang H. K., Bell T. M. Viruses in multiple sclerosis? Lancet. 1972 Aug 5;2(7771):280–281. doi: 10.1016/s0140-6736(72)91714-x. [DOI] [PubMed] [Google Scholar]
- Field E. J. Role of viral infection and autoimmunity in aetiology and pathogenesis of multiple sclerosis. Lancet. 1973 Feb 10;1(7798):295–297. doi: 10.1016/s0140-6736(73)91542-0. [DOI] [PubMed] [Google Scholar]
- Finkel A., Dent P. B. Abnormalities in lymphocyte proliferation in classical and atypical measles infection. Cell Immunol. 1973 Jan;6(1):41–48. doi: 10.1016/0008-8749(73)90004-x. [DOI] [PubMed] [Google Scholar]
- Fireman P., Friday G., Kumate J. Effect of measles vaccine on immunologic responsiveness. Pediatrics. 1969 Feb;43(2):264–272. [PubMed] [Google Scholar]
- Flanagan T. D., Menna J. H. Induction of measles virus hemagglutinin in a persistently infected, nonvirogenic line of cells (BGM/MV). J Virol. 1976 Mar;17(3):1052–1055. doi: 10.1128/jvi.17.3.1052-1055.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett E. A., Pringle C. R., Pennington T. H. Events following the infections of enucleate cells with measles virus. J Gen Virol. 1976 Aug;32(2):163–175. doi: 10.1099/0022-1317-32-2-163. [DOI] [PubMed] [Google Scholar]
- Forget P. P., Fernandes J., Begemann P. H. Triglyceride clearing in glycogen storage disease. Pediatr Res. 1974 Feb;8(2):114–119. doi: 10.1203/00006450-197402000-00008. [DOI] [PubMed] [Google Scholar]
- Freeman J. M., Magoffin R. L., Lennette E. H., Herndon R. M. Additional evidence of the relation between subacute inclusion-body encephalitis and measles virus. Lancet. 1967 Jul 15;2(7507):129–131. doi: 10.1016/s0140-6736(67)92965-0. [DOI] [PubMed] [Google Scholar]
- Fulginiti V. A., Eller J. J., Downie A. W., Kempe C. H. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA. 1967 Dec 18;202(12):1075–1080. doi: 10.1001/jama.202.12.1075. [DOI] [PubMed] [Google Scholar]
- Furman P. A., Hallum J. V. RNA-dependent DNA polymerase activity in preparations of a mutant of Newcastle disease virus arising from persistently infected L cells. J Virol. 1973 Sep;12(3):548–555. doi: 10.1128/jvi.12.3.548-555.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS J. L., MILLER H. G., STANTON J. B. Para-infectious encephalomyelitis and related syndromes; a critical review of the neurological complications of certain specific fevers. Q J Med. 1956 Oct;25(100):427–505. [PubMed] [Google Scholar]
- GIBBS F. A., GIBBS E. L., CARPENTER P. R., SPIES H. W. Electroencephalographic abnormality in "uncomplicated" childhood diseases. J Am Med Assoc. 1959 Oct 24;171:1050–1055. doi: 10.1001/jama.1959.03010260006002. [DOI] [PubMed] [Google Scholar]
- GILLESPIE J. H., KARZON D. T. A study of the relationship between canine distemper and measles in the dog. Proc Soc Exp Biol Med. 1960 Dec;105:547–551. doi: 10.3181/00379727-105-26171. [DOI] [PubMed] [Google Scholar]
- GOOD R. A., ZAK S. J. Disturbances in gamma globulin synthesis as experiments of nature. Pediatrics. 1956 Jul;18(1):109–149. [PubMed] [Google Scholar]
- Gerson K. L., Haslam R. H. Subtle immunologic abnormalities in four boys with subacute sclerosing panencephalitis. N Engl J Med. 1971 Jul 8;285(2):78–82. doi: 10.1056/NEJM197107082850203. [DOI] [PubMed] [Google Scholar]
- Gibson P. E., Bell T. M. Persistent infection of measles virus in mouse brain cell cultures infected in vivo. Arch Gesamte Virusforsch. 1972;37(1):45–53. doi: 10.1007/BF01241149. [DOI] [PubMed] [Google Scholar]
- Gould E. A., Cosby S. L., Shirodaria P. V. Salt-dependent haemagglutinating measles virus in S.S.P.E. J Gen Virol. 1976 Oct;33(1):139–142. doi: 10.1099/0022-1317-33-1-139. [DOI] [PubMed] [Google Scholar]
- Gould E. A., Linton P. E. The production of a temperature-sensitive persistent measles virus infection. J Gen Virol. 1975 Jul;28(1):21–28. doi: 10.1099/0022-1317-28-1-21. [DOI] [PubMed] [Google Scholar]
- Grausz H., Earley L. E., Stephens B. G., Lee J. C., Hopper J., Jr Diagnostic import of virus-like particles in the glomerular endothelium of patients with systemic lupus erythematosus. N Engl J Med. 1970 Sep 3;283(10):506–511. doi: 10.1056/NEJM197009032831003. [DOI] [PubMed] [Google Scholar]
- Graziano K. D., Ruckdeschel J. C., Mardiney M. R., Jr Cell-associated immunity to measles (rubeola). The demonstration of in vitro lymphocyte tritiated thymidine incorporation in response to measels complement fixation antigen. Cell Immunol. 1975 Feb;15(2):347–359. doi: 10.1016/0008-8749(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Greenham L. W., Peacock D. B., Hill T. J., Brownell B., Schutt W. H. The isolation of S.S.P.E. measles virus in newborn mice. Arch Gesamte Virusforsch. 1974;44(2):109–120. doi: 10.1007/BF01250219. [DOI] [PubMed] [Google Scholar]
- Griffin D. E., Mullinix J., Narayan O., Johnson R. T. Age dependence of viral expression: comparative pathogenesis of two rodent-adapted strains of measles virus in mice. Infect Immun. 1974 Apr;9(4):690–695. doi: 10.1128/iai.9.4.690-695.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. F., Katz S. L. Subacute sclerosing panencephalitis, laboratory findings in 6 cases. Neurology. 1968 Jan;18(1 Pt 2):98–100. doi: 10.1212/wnl.18.1_part_2.098. [DOI] [PubMed] [Google Scholar]
- Györkey F., Min K. W., Sincovics J. G., Györkey P. Systemic lupus erythematosus and myxovirus. N Engl J Med. 1969 Feb 6;280(6):333–333. doi: 10.1056/nejm196902062800620. [DOI] [PubMed] [Google Scholar]
- HO M., ENDERS J. F. Further studies on an inhibitor of viral activity appearing in infected cell cultures and its role in chronic viral infections. Virology. 1959 Nov;9:446–477. doi: 10.1016/0042-6822(59)90135-7. [DOI] [PubMed] [Google Scholar]
- HSIUNG G. D., MANNINI A., MELNICK J. L. Plaque assay of measles virus on Erythrocebus patas monkey kidney monolayers. Proc Soc Exp Biol Med. 1958 May;98(1):68–70. doi: 10.3181/00379727-98-23943. [DOI] [PubMed] [Google Scholar]
- Haas E. J., Wendt V. E. Atypical measles 14 years after immunization. JAMA. 1976 Aug 30;236(9):1050–1050. [PubMed] [Google Scholar]
- Hall W. W., Martin S. J., Gould E. Defective interfering particles produced during the replication of measles virus. Med Microbiol Immunol. 1974;160(2-3):155–164. doi: 10.1007/BF02121722. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Martin S. J. Purification and characterization of measles virus. J Gen Virol. 1973 May;19(2):175–188. doi: 10.1099/0022-1317-19-2-175. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Martin S. J. Structure and function relationships of the envelope of measles virus. Med Microbiol Immunol. 1974;160(2-3):143–154. doi: 10.1007/BF02121721. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Martin S. J. The biochemical and biological characteristics of the surface components of measles virus. J Gen Virol. 1974 Mar;22(3):363–374. doi: 10.1099/0022-1317-22-3-363. [DOI] [PubMed] [Google Scholar]
- Hall W. W., ter Meulen V. RNA homology between subacute sclerosing panencephalitis and measles viruses. Nature. 1976 Dec 2;264(5585):474–477. doi: 10.1038/264474a0. [DOI] [PubMed] [Google Scholar]
- Hall W. W., ter Meulen V. The effects of actinomycin D on RNA synthesis in measles virus-infected cells. J Gen Virol. 1977 Feb;34(2):391–396. doi: 10.1099/0022-1317-34-2-391. [DOI] [PubMed] [Google Scholar]
- Hamilton R., Barbosa L., Dubois M. Subacute sclerosing panencephalitis measles virus: study of biological markers. J Virol. 1973 Sep;12(3):632–642. doi: 10.1128/jvi.12.3.632-642.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarskjöld B., Norrby E. A comparison between virion RNA of measles virus and some other paramyxoviruses. Med Microbiol Immunol. 1974;160(2-3):99–104. doi: 10.1007/BF02121717. [DOI] [PubMed] [Google Scholar]
- Haspel M. V., Duff R., Rapp F. Experimental measles encephalitis: a genetic analysis. Infect Immun. 1975 Oct;12(4):785–790. doi: 10.1128/iai.12.4.785-790.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel M. V., Duff R., Rapp F. Isolation and preliminary characterization of temperature-sensitive mutants of measles virus. J Virol. 1975 Oct;16(4):1000–1009. doi: 10.1128/jvi.16.4.1000-1009.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel M. V., Knight P. R., Duff R. G., Rapp F. Activation of a latent measles virus infection in hamster cells. J Virol. 1973 Oct;12(4):690–695. doi: 10.1128/jvi.12.4.690-695.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel M. V., Rapp F. Measles virus: an unwanted variant causing hydrocephalus. Science. 1975 Feb 7;187(4175):450–451. doi: 10.1126/science.1111114. [DOI] [PubMed] [Google Scholar]
- Henle G., Koldovsky U., Koldovsky P., Henle W., Ackermann R., Haase G. Multiple sclerosis-associated agent: neutralization of the agent by human sera. Infect Immun. 1975 Dec;12(6):1367–1374. doi: 10.1128/iai.12.6.1367-1374.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson T. E., Brody J. A., Sever J. L., Dyken M. L., Cannon J. Measles antibody titers in multiple sclerosis patients, siblings, and controls. JAMA. 1970 Mar 23;211(12):1985–1988. [PubMed] [Google Scholar]
- Herndon R. M., Rena-Descalzi L., Griffin D. E., Coyle P. K. Age dependence of viral expression. Electron microscopic and immunoperoxidase studies of Measles virus replication in mice. Lab Invest. 1975 Nov;33(5):544–553. [PubMed] [Google Scholar]
- Herndon R. M., Rubinstein L. J. Light and electron microscopy observations on the development of viral particles in the inclusions of Dawson's encephalitis (subacute sclerosing panencephalitis). Neurology. 1968 Jan;18(1 Pt 2):8–20. doi: 10.1212/wnl.18.1_part_2.008. [DOI] [PubMed] [Google Scholar]
- Hicks J. T., Albrecht P. Cytolytic, complement-dependent antibodies to measles virus in rhesus monkeys after administration of live or killed virus. J Infect Dis. 1976 Jun;133(6):648–654. doi: 10.1093/infdis/133.6.648. [DOI] [PubMed] [Google Scholar]
- Hilleman M. R., Buynak E. B., Weibel R. E., Stokes J., Jr, Whitman J. E., Jr, Leagus M. B. Development and evaluation of the Moraten measles virus vaccine. JAMA. 1968 Oct 14;206(3):587–590. [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P., Welsh R. M., Oldstone M. B., Kohne D., Lazzarini R., Scolnick E. Long-term persistent vesicular stomatitis virus and rabies virus infection of cells in vitro. J Gen Virol. 1976 Nov;33(2):193–211. doi: 10.1099/0022-1317-33-2-193. [DOI] [PubMed] [Google Scholar]
- Hollinger F. B., Sharp J. T., Lidsky M. D., Rawls W. E. Antibodies to viral antigens in systemic lupus erythematosus. Arthritis Rheum. 1971 Jan-Feb;14(1):1–11. doi: 10.1002/art.1780140102. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., Hamilton R., Traub R., Ley A., Sever J. L. Some characteristics of SSPE measles virus. Proc Soc Exp Biol Med. 1970 May;134(1):17–21. doi: 10.3181/00379727-134-34718. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., London W. T., Jabbour J. T., Zeman W., Sever J. L. Isolation of measles virus from brain cell cultures of two patients with subacute sclerosing panencephalitis. Proc Soc Exp Biol Med. 1969 Oct;132(1):272–277. doi: 10.3181/00379727-132-34196. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., Sever J. L., Zeman W. Subacute sclerosing panencephalitis: isolation of measles virus from a brain biopsy. Nature. 1969 Mar 8;221(5184):974–974. doi: 10.1038/221974a0. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Hamilton R., Wittig B., Fuccillo D. A., Sever J. L., Vernon M. L. Subacute sclerosing panencephalitis: isolation of suppressed measles virus from lymph node biopsies. Science. 1971 Aug 27;173(3999):840–841. doi: 10.1126/science.173.3999.840. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Krebs H., Ley A., Chen T. C., Gilkeson M. R., Sever J. L. Progressive increase in cerebrospinal fluid measles antibody levels in subacute sclerosing panencephalitis. Pediatrics. 1971 Apr;47(4):782–783. [PubMed] [Google Scholar]
- Howe C., Schluederberg A. Neurminidase associated with measles virus. Biochem Biophys Res Commun. 1970 Aug 11;40(3):606–607. doi: 10.1016/0006-291x(70)90946-0. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Huang A. S. Defective interfering viruses. Annu Rev Microbiol. 1973;27:101–117. doi: 10.1146/annurev.mi.27.100173.000533. [DOI] [PubMed] [Google Scholar]
- IMAGAWA D. T., ADAMS J. M. Propagation of measles virus in suckling mice. Proc Soc Exp Biol Med. 1958 Jul;98(3):567–569. doi: 10.3181/00379727-98-24109. [DOI] [PubMed] [Google Scholar]
- Imagawa D. T. Relationships among measles, canine distemper and rinderpest viruses. Prog Med Virol. 1968;10:160–193. [PubMed] [Google Scholar]
- Imamura M., Phillips P. E., Mellors R. C. The occurrence and frequency of type C virus-like particles in placentas from patients with systemic lupus erythematosus and from normal subjects. Am J Pathol. 1976 May;83(2):383–394. [PMC free article] [PubMed] [Google Scholar]
- Iwasaki Y., Koprowski H. Cell to cell transmission of virus in the central nervous system. I. Subacute sclerosing panencephalitis. Lab Invest. 1974 Aug;31(2):187–196. [PubMed] [Google Scholar]
- Jabbour J. T., Duenas D. A., Sever J. L., Krebs H. M., Horta-Barbosa L. Epidemiology of subacute sclerosing panencephalitis (SSPE). A report of the SSPE registry. JAMA. 1972 May 15;220(7):959–962. [PubMed] [Google Scholar]
- Jabbour J. T., Garcia J. H., Lemmi H., Ragland J., Duenas D. A., Sever J. L. Subacute sclerosing panencephalitis. A multidisciplinary study of eight cases. JAMA. 1969 Mar 24;207(12):2248–2254. doi: 10.1001/jama.207.12.2248. [DOI] [PubMed] [Google Scholar]
- Janda Z., Norrby E., Marusyk H. Neurotropism of measles virus variants in hamsters. J Infect Dis. 1971 Dec;124(6):553–564. doi: 10.1093/infdis/124.6.553. [DOI] [PubMed] [Google Scholar]
- Jenis E. H., Knieser M. R., Rothouse P. A., Jensen G. E., Scott R. M. Subacute sclerosing panencephalitis. Immunoultrastructural localization of measles-virus antigen. Arch Pathol. 1973 Feb;95(2):81–89. [PubMed] [Google Scholar]
- Johannes R. S., Sever J. L. Subacute sclerosing panencephalitis. Annu Rev Med. 1975;26:589–601. doi: 10.1146/annurev.me.26.020175.003105. [DOI] [PubMed] [Google Scholar]
- Johnson K. P., Byington D. P. Subacute sclerosing panencephalitis (SSPE) agent in hamsters. I. Acute giant cell encephalitis in newborn animals. Exp Mol Pathol. 1971 Dec;15(3):373–379. doi: 10.1016/0014-4800(71)90044-x. [DOI] [PubMed] [Google Scholar]
- Johnson K. P., Norrby E. Subacute sclerosing panencephalitis (SSPE) agent in hamsters. 3. Induction of defective measles infection in hamster brain. Exp Mol Pathol. 1974 Oct;21(2):166–178. doi: 10.1016/0014-4800(74)90087-2. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Johnson K. P. Hydrocephalus as a sequela of experimental myxovirus infections. Exp Mol Pathol. 1969 Feb;10(1):68–80. doi: 10.1016/0014-4800(69)90049-5. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Johnson K. P. Hydrocephalus following viral infection: the pathology of aqueductal stenosis developing after experimental mumps virus infection. J Neuropathol Exp Neurol. 1968 Oct;27(4):591–606. [PubMed] [Google Scholar]
- Johnson R. T. Subacute sclerosing panencephalitis. J Infect Dis. 1970 Feb;121(2):227–230. doi: 10.1093/infdis/121.2.227. [DOI] [PubMed] [Google Scholar]
- Joseph B. S., Cooper N. R., Oldstone M. B. Immunologic injury of cultured cells infected with measles virus. I. role of IfG antibody and the alternative complement pathway. J Exp Med. 1975 Apr 1;141(4):761–774. [PMC free article] [PubMed] [Google Scholar]
- Joseph B. S., Lampert P. W., Oldstone M. B. Replication and persistence of measles virus in defined subpopulations of human leukocytes. J Virol. 1975 Dec;16(6):1638–1649. doi: 10.1128/jvi.16.6.1638-1649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B. S., Oldstone M. B. Antibody-induced redistribution of measles virus antigens on the cell surface. J Immunol. 1974 Oct;113(4):1205–1209. [PubMed] [Google Scholar]
- Joseph B. S., Oldstone M. B. Immunologic injury in measles virus infection. II. Suppression of immune injury through antigenic modulation. J Exp Med. 1975 Oct 1;142(4):864–876. doi: 10.1084/jem.142.4.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B. S., Perrin L. H., Oldstone M. B. Measurement of virus antigens on the surface of HeLa cells persistently infected with wild type and vaccine strains of measles virus by radioimmune assay. J Gen Virol. 1976 Mar;30(3):329–337. doi: 10.1099/0022-1317-30-3-329. [DOI] [PubMed] [Google Scholar]
- KATZ S. L. IMMUNIZATION WITH LIVE ATTENUATED MEASLES VIRUS VACCINES: FIVE YEARS' EXPERIENCE. Arch Gesamte Virusforsch. 1965;16:222–230. doi: 10.1007/BF01253813. [DOI] [PubMed] [Google Scholar]
- KEMPE C. H., FULGINITI V. A. THE PATHOGENESIS OF MEASLES VIRUS INFECTION. Arch Gesamte Virusforsch. 1965;16:103–128. doi: 10.1007/BF01253798. [DOI] [PubMed] [Google Scholar]
- KOHN A. Haemadsorption by measles syncytia. Nature. 1962 Mar 17;193:1088–1089. doi: 10.1038/1931088a0. [DOI] [PubMed] [Google Scholar]
- KRUGMAN S., GILES J. P., JACOBS A. M., FRIEDMAN H. Studies with a further attenuated live measles-virus vaccine. Pediatrics. 1963 Jun;31:919–928. [PubMed] [Google Scholar]
- Katz M., Käckell Y. M., Müller D., ter Meulen V., Koprowski H. Immunohistological, microscopical and neurochemical studies on encephalitides. 7. Subacute sclerosing panencephalitis. Susceptibility of dogs to the virus, and characterization of host response. Acta Neuropathol. 1973 Jun 26;25(1):81–88. doi: 10.1007/BF00686860. [DOI] [PubMed] [Google Scholar]
- Katz M., Oyanagi S., Koprowski H. Subacute sclerosing panencephalitis: structures resembling myxovirus nucleocapsids in cells cultured from brain. Nature. 1969 May 31;222(5196):888–890. doi: 10.1038/222888a0. [DOI] [PubMed] [Google Scholar]
- Katz M., Rorke L. B., Masland W. S., Brodano G. B., Koprowski H. Subacute sclerosing panencephalitis: isolation of a virus encephalitogenic for ferrets. J Infect Dis. 1970 Feb;121(2):188–195. doi: 10.1093/infdis/121.2.188. [DOI] [PubMed] [Google Scholar]
- Katz M., Rorke L. B., Masland W. S., Koprowski H., Tucker S. H. Transmission of an encephalitogenic agent from brains of patients with subacute sclerosing panencephalitis to ferrets. Preliminary report. N Engl J Med. 1968 Oct 10;279(15):793–798. doi: 10.1056/NEJM196810102791503. [DOI] [PubMed] [Google Scholar]
- Kawano K., Miller L., Kimmelstiel P. Virus-like structures in lupus erythematosus. N Engl J Med. 1969 Nov 27;281(22):1228–1229. doi: 10.1056/NEJM196911272812207. [DOI] [PubMed] [Google Scholar]
- Kiley M. P., Payne F. E. Evidence of precursors of defective measles virus. Med Microbiol Immunol. 1974;160(2-3):91–97. doi: 10.1007/BF02121716. [DOI] [PubMed] [Google Scholar]
- Kiley M. P., Payne F. E. Replication of measles virus: continued synthesis of nucleocapsid RNA and increased synthesis of mRNA in the presence of cycloheximide. J Virol. 1974 Oct;14(4):758–764. doi: 10.1128/jvi.14.4.758-764.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. W. Cell-free translation of paramyxovirus messenger RNA. J Virol. 1973 Nov;12(5):1020–1027. doi: 10.1128/jvi.12.5.1020-1027.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. W. Paramyxovirus replication. Curr Top Microbiol Immunol. 1972;59:1–33. doi: 10.1007/978-3-642-65444-2_1. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W., Portner A., Darlington R. W. Properties of incomplete Sendai virions and subgenomic viral RNAs. Virology. 1970 Dec;42(4):857–871. doi: 10.1016/0042-6822(70)90335-1. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W. The molecular biology of paramyxoviruses. Med Microbiol Immunol. 1974;160(2-3):73–83. doi: 10.1007/BF02121714. [DOI] [PubMed] [Google Scholar]
- Knight P., Duff R., Rapp F. Latency of human measles virus in hamster cells. J Virol. 1972 Nov;10(5):995–1001. doi: 10.1128/jvi.10.5.995-1001.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakofsky D. Isolation and characterization of Sendai virus DI-RNAs. Cell. 1976 Aug;8(4):547–555. doi: 10.1016/0092-8674(76)90223-3. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Spahr P. F., Koprowski H. Comparison of 6-94 virus and Sendai virus RNA by RNA-RNA hybridization. J Virol. 1974 Apr;13(4):935–936. doi: 10.1128/jvi.13.4.935-936.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldovsky U., Koldovsky P., Henle G., Henle W., Ackermann R., Haase G. Multiple sclerosis-associated agent: transmission to animals and some properties of the agent. Infect Immun. 1975 Dec;12(6):1355–1366. doi: 10.1128/iai.12.6.1355-1366.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., Barbanti-Brodano G., Katz M. Interaction between papova-like virus and paramyxovirus in human brain cells: a hypothesis. Nature. 1970 Mar 14;225(5237):1045–1047. doi: 10.1038/2251045a0. [DOI] [PubMed] [Google Scholar]
- Kreth H. W., Kaeckell Y. M., ter Meulen V. Cellular immunity in SSPE patients. Med Microbiol Immunol. 1974;160(2-3):191–199. doi: 10.1007/BF02121726. [DOI] [PubMed] [Google Scholar]
- Krugman S. Measles immunization: new recommendation. JAMA. 1977 Jan 24;237(4):366–366. doi: 10.1001/jama.237.4.366. [DOI] [PubMed] [Google Scholar]
- Krugman S. Present status of measles and rubella immunization in the United States: a medical progress report. J Pediatr. 1977 Jan;90(1):1–12. doi: 10.1016/s0022-3476(77)80755-5. [DOI] [PubMed] [Google Scholar]
- LABOCCETTA A. C., TORNAY A. S. MEASLES ENCEPHALITIS. REPORT OF 61 CASES. Am J Dis Child. 1964 Mar;107:247–255. [PubMed] [Google Scholar]
- Lai M. H., Joklik W. K. The induction of interferon by temperature-sensitive mutants of reovirus, UV-irradiated reovirus, and subviral reovirus particles. Virology. 1973 Jan;51(1):191–204. doi: 10.1016/0042-6822(73)90379-6. [DOI] [PubMed] [Google Scholar]
- Laitinen O., Vesikari T., Vaheri A. Virus antibody levels in rheumatoid arthritis and systemic lupus erythematosus. Acta Med Scand. 1972 Jul-Aug;192(1-2):37–40. doi: 10.1111/j.0954-6820.1972.tb04775.x. [DOI] [PubMed] [Google Scholar]
- Landrigan P. J., Witte J. J. Neurologic disorders following live measles-virus vaccination. JAMA. 1973 Mar 26;223(13):1459–1462. [PubMed] [Google Scholar]
- Lehrich J. R., Katz M., Rorke L. B., Barbanti-Brodano G., Koprowski H. Subacute sclerosing panencephalitis. Encephalitis in hamsters produced by viral agents isolated from human brain cells. Arch Neurol. 1970 Aug;23(2):97–102. doi: 10.1001/archneur.1970.00480260003001. [DOI] [PubMed] [Google Scholar]
- Lennette E. H., Magoffin R. L., Freeman J. M. Immunologic evidence of measles virus as an etiologic agent in subacute sclerosing panencephalitis. Neurology. 1968 Jan;18(1 Pt 2):21–29. doi: 10.1212/wnl.18.1_part_2.021. [DOI] [PubMed] [Google Scholar]
- Lerman S. J., Gold E. Measles in children previously vaccinated against measles. JAMA. 1971 May 24;216(8):1311–1314. [PubMed] [Google Scholar]
- Levenshtam M. A., Radyshich N. S., Zakharchenko E. M., Shroit I. G. Chromosomal aberrations in human amniotic cells before the completion of a single growth cycle of measles vaccine virus L-16. Acta Virol. 1969 Sep;13(5):401–406. [PubMed] [Google Scholar]
- Lewandowski L. J., Lief F. S., Verini M. A., Pienkowski M. M., ter Meulen V., Koprowski H. Analysis of a viral agent isolated from multiple sclerosis brain tissue: characterization as a parainfluenzavirus type 1. J Virol. 1974 May;13(5):1037–1045. doi: 10.1128/jvi.13.5.1037-1045.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidin-Janson G., Strannegård O. Two cases of Guillain-Barré syndrome and encephalitis after measles. Br Med J. 1972 Jun 3;2(5813):572–572. doi: 10.1136/bmj.2.5813.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnemann C. C., Hegg M. E., Rotte T. C., Phair J. P., Schiff G. M. Measles IgM response during reinfection of previously vaccinated children. J Pediatr. 1973 May;82(5):798–801. doi: 10.1016/s0022-3476(73)80069-1. [DOI] [PubMed] [Google Scholar]
- Linnemann C. C., Jr Measles vaccine: immunity, reinfection and revaccination. Am J Epidemiol. 1973 Jun;97(6):365–371. doi: 10.1093/oxfordjournals.aje.a121517. [DOI] [PubMed] [Google Scholar]
- Llanes-Rodas R., Liu C. A study of measles virus infection in tissue culture cells with particular reference to the development of intranuclear inclusion bodies. J Immunol. 1965 Nov;95(5):840–845. [PubMed] [Google Scholar]
- Lomniczi B., Burke D. C. Interferon production by temperature-sensitive mutants of Semliki Forest virus. J Gen Virol. 1970 Jul;8(1):55–68. doi: 10.1099/0022-1317-8-1-55. [DOI] [PubMed] [Google Scholar]
- Lucas C. J., Brouwer R., Feltkamp T. E., ten Veen J. H., van Loghem J. J. Measles antibodies in sera from patients with autoimmune diseases. Lancet. 1972 Jan 15;1(7742):115–116. doi: 10.1016/s0140-6736(72)90679-4. [DOI] [PubMed] [Google Scholar]
- Luster M. I., Armen R. C., Hallum J. V., Leslie G. A. Measles virus-specific IgD antibodies in patients with subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1297–1299. doi: 10.1073/pnas.73.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELLMAN W. J., WETTON R. Depression of the tuberculin reaction by attenuated measles virus vaccine. J Lab Clin Med. 1963 Mar;61:453–458. [PubMed] [Google Scholar]
- MILOVANOVIC M. V., ENDERS J. F., MITUS A. Cultivation of measles virus in human amnion cells and in developing chick embryo. Proc Soc Exp Biol Med. 1957 May;95(1):120–127. doi: 10.3181/00379727-95-23140. [DOI] [PubMed] [Google Scholar]
- MITUS A., ENDERS J. F., CRAIG J. M., HOLLOWAY A. Persistence of measles virus and depression of antibody formation in patients with giant-cell pneumonia after measles. N Engl J Med. 1959 Oct 29;261:882–889. doi: 10.1056/NEJM195910292611802. [DOI] [PubMed] [Google Scholar]
- MITUS A., ENDERS J. F., EDSALL G., HOLLOWAY A. MEASLES IN CHILDREN WITH MALIGNANCY PROBLEMS AND PREVENTION. Arch Gesamte Virusforsch. 1965;16:331–337. doi: 10.1007/BF01253832. [DOI] [PubMed] [Google Scholar]
- Macintyre E. H., Armstrong J. A. Fine structural changes in human astrocyte carrier lines for measles virus. Nature. 1976 Sep 16;263(5574):232–234. doi: 10.1038/263232a0. [DOI] [PubMed] [Google Scholar]
- Marx J. L. Autoimmune disease: new evidence about lupus. Science. 1976 Jun 11;192(4244):1089–1150. doi: 10.1126/science.192.4244.1089. [DOI] [PubMed] [Google Scholar]
- Marx P. A., Jr, Pridgen C., Kingsbury D. W. Location and abundance of poly (A) sequences in Sendai virus messenger RNA molecules. J Gen Virol. 1975 May;27(2):247–250. doi: 10.1099/0022-1317-27-2-247. [DOI] [PubMed] [Google Scholar]
- Matumoto M. Multiplication of measles virus in cell cultures. Bacteriol Rev. 1966 Mar;30(1):152–176. doi: 10.1128/br.30.1.152-176.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maugh T. H., 2nd Multiple sclerosis: two or more viruses may be involved. Science. 1977 Feb 25;195(4280):768–771. doi: 10.1126/science.195.4280.768. [DOI] [PubMed] [Google Scholar]
- McFarland H. F. The effect of measles virus infection on T and B lymphocytes in the mouse. I. Suppression of helper cell activity. J Immunol. 1974 Dec;113(6):1978–1983. [PubMed] [Google Scholar]
- McKimm J., Rapp F. Inability of measles virus temperature-sensitive mutants to induce interferon. Virology. 1977 Jan;76(1):409–415. doi: 10.1016/0042-6822(77)90312-9. [DOI] [PubMed] [Google Scholar]
- McKimm J., Rapp F. Variation in ability of measles virus plaque progeny to induce interferon. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3056–3059. doi: 10.1073/pnas.74.7.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menna J. H., Collins A. R., Flanagan T. D. Characterization of an in vitro persistent-state measles virus infection: establishment and virological characterization of the BGM/MV cell line. Infect Immun. 1975 Jan;11(1):152–158. doi: 10.1128/iai.11.1.152-158.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menna J. H., Collins A. R., Flanagan T. D. Characterization of an in vitro persistent-state measles virus infection: species characterization and interference in the BGM/MV cell line. Infect Immun. 1975 Jan;11(1):159–163. doi: 10.1128/iai.11.1.159-163.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa T., Sakuma T., Kuwajima S., Yamamoto T. K., Iida H. Characterization of measles viruses in establishment of persistent infections in human lymphoid cell line. J Gen Virol. 1976 Dec;33(3):361–379. doi: 10.1099/0022-1317-33-3-361. [DOI] [PubMed] [Google Scholar]
- Minagawa T. Studies on the persistent infection with measles virus in HeLa cells. I. Clonal analysis of cells of carrier cultures. Jpn J Microbiol. 1971 Jul;15(4):325–331. doi: 10.1111/j.1348-0421.1971.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Minagawa T., Yamada M. Studies on the persistent infection with measles virus in HeLa cells. 3. Immunolysis of cells in carrier state by anti-measles sera. Jpn J Microbiol. 1971 Jul;15(4):341–350. doi: 10.1111/j.1348-0421.1971.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Mirchamsy H., Rapp F. Role of interferon in replication of virulent and attenuated strains of measles virus. J Gen Virol. 1969 Jun;4(4):513–522. doi: 10.1099/0022-1317-4-4-513. [DOI] [PubMed] [Google Scholar]
- Morgan C., Howe C. Structure and development of viruses as observed in the electron microscope. IX. Entry of parainfluenza I (Sendai) virus. J Virol. 1968 Oct;2(10):1122–1132. doi: 10.1128/jvi.2.10.1122-1132.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle W. E., Choppin P. W. A comparison of the polypeptides of four measles virus strains. Virology. 1977 May 15;78(2):463–474. doi: 10.1016/0042-6822(77)90123-4. [DOI] [PubMed] [Google Scholar]
- Mountcastle W. E., Compans R. W., Caliguiri L. A., Choppin P. W. Nucleocapsid protein subunits of simian virus 5, Newcastle disease virus, and Sendai virus. J Virol. 1970 Nov;6(5):677–684. doi: 10.1128/jvi.6.5.677-684.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle W. E., Compans R. W., Lackland H., Choppin P. W. Proteolytic cleavage of subunits of the nucleocapsid of the paramyxovirus simian virus 5. J Virol. 1974 Nov;14(5):1253–1261. doi: 10.1128/jvi.14.5.1253-1261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORRBY E. C., FALKSVEDEN L. G. SOME GENERAL PROPERTIES OF THE MEASLES VIRUS HEMOLYSIN. Arch Gesamte Virusforsch. 1964 Mar 13;14:474–486. doi: 10.1007/BF01555079. [DOI] [PubMed] [Google Scholar]
- NORRBY E. C., MAGNUSSON P., FALKSVEDEN L. G., GROENBERG M. SEPARATION OF MEASLES VIRUS COMPONENTS BY EQUILIBRIUM CENTRIFUGATION IN CSCL GRADIENTS. II. STUDIES ON THE LARGE AND THE SMALL HEMAGGLUTININ. Arch Gesamte Virusforsch. 1964 Mar 13;14:462–473. doi: 10.1007/BF01555078. [DOI] [PubMed] [Google Scholar]
- NORRBY E. Hemagglutination by measles virus. 4. A simple procedure for production of high potency antigen for hemagglutination-inhibition (HI) tests. Proc Soc Exp Biol Med. 1962 Dec;111:814–818. doi: 10.3181/00379727-111-27930. [DOI] [PubMed] [Google Scholar]
- NORRBY E. Hemagglutination by measles virus. I. The production of hemagglutinin in tissue culture and the influence of different conditions on the hemagglutinating system. Arch Gesamte Virusforsch. 1962;12:153–163. doi: 10.1007/BF01258126. [DOI] [PubMed] [Google Scholar]
- NORRBY E. Hemagglutination by measles virus. II. Properties of the hemagglutinin and of the receptors on the erythrocytes. Arch Gesamte Virusforsch. 1962;12:164–172. doi: 10.1007/BF01258127. [DOI] [PubMed] [Google Scholar]
- NORRBY E. SEPARATION OF MEASLES VIRUS COMPONENTS BY EQUILIBRIUM CENTRIFUGATION IN CSCL GRADIENTS. I. CRUDE AND TWEEN AND ETHER TREATED CONCENTRATED TISSUE CULTURE MATERIAL. Arch Gesamte Virusforsch. 1964;14:306–318. doi: 10.1007/BF01555823. [DOI] [PubMed] [Google Scholar]
- Nagata I., Kimura Y., Ito Y., Tanaka T. Temperature-sensitive phenomenon of viral maturation observed in BHK cells persistently infected with HVJ. Virology. 1972 Aug;49(2):453–461. doi: 10.1016/0042-6822(72)90497-7. [DOI] [PubMed] [Google Scholar]
- Nakai M., Imagawa D. T. Electron microscopy of measles virus replication. J Virol. 1969 Feb;3(2):187–197. doi: 10.1128/jvi.3.2.187-197.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai T., Shand F. L., Howatson A. F. Development of measles virus in vitro. Virology. 1969 May;38(1):50–67. doi: 10.1016/0042-6822(69)90127-5. [DOI] [PubMed] [Google Scholar]
- Narang H. K., Field E. J. Paramyxovirus like tubules in multiple sclerosis biopsy material. Acta Neuropathol. 1973;25(4):281–290. doi: 10.1007/BF00691756. [DOI] [PubMed] [Google Scholar]
- Norrby E. C., Magnusson P. Some morphological characteristics of the internal component of measles virus. Arch Gesamte Virusforsch. 1965;17(3):443–447. doi: 10.1007/BF01241199. [DOI] [PubMed] [Google Scholar]
- Norrby E. A carrier cell line of measles virus in Lu 106 cells. Arch Gesamte Virusforsch. 1967;20(2):215–224. doi: 10.1007/BF01241275. [DOI] [PubMed] [Google Scholar]
- Norrby E., Gollmar Y. Appearance and persistence of antibodies against different virus components after regular measles infections. Infect Immun. 1972 Sep;6(3):240–247. doi: 10.1128/iai.6.3.240-247.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby E., Gollmar Y. Identification of measles virus-specific hemolysis-inihibiting antibodies separate from hemagglutination-inhibiting antibodies. Infect Immun. 1975 Feb;11(2):231–239. doi: 10.1128/iai.11.2.231-239.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby E., Hammarskjöld B. Structural components of measles virus. Microbios. 1972 Jan;5(17):17–29. [PubMed] [Google Scholar]
- Norrby E. Intracellular accumulation of measles virus nucleocapsid and envelope antigens. Microbios. 1972 Jan;5(17):31–40. [PubMed] [Google Scholar]
- Norrby E., Link H., Olsson J. E. Measles virus antibodies in multiple sclerosis. Comparison of antibody titers in cerebrospinal fluid and serum. Arch Neurol. 1974 Apr;30(4):285–292. doi: 10.1001/archneur.1974.00490340013002. [DOI] [PubMed] [Google Scholar]
- Norrby E. Occurrence of antibodies against envelope components after immunization with formalin inactivated and live measles vaccine. J Biol Stand. 1975;3(4):375–380. doi: 10.1016/0092-1157(75)90062-1. [DOI] [PubMed] [Google Scholar]
- Norton W. L. Endothelial inclusions in active lesions of systemic lupus erythematosus. J Lab Clin Med. 1969 Sep;74(3):369–379. [PubMed] [Google Scholar]
- Numazaki Y., Karzon D. T. Density separable fractions during growth of measles virus. J Immunol. 1966 Oct;97(4):458–469. [PubMed] [Google Scholar]
- O'Brien T. C., Albrecht P., Tauraso N. M., Burns G. R. Properties of a measles virus neuropathic for rhesus monkeys. Arch Gesamte Virusforsch. 1972;39(1):228–239. doi: 10.1007/BF01241545. [DOI] [PubMed] [Google Scholar]
- ODDO F. G., FLACCOMIO R., SINATRA A. "Giant-cell" and "strand-forming" cytopathic effect of measles virus lines conditioned by serial propagation with diluted or concentrated inoculum. Virology. 1961 Apr;13:550–553. doi: 10.1016/0042-6822(61)90288-4. [DOI] [PubMed] [Google Scholar]
- Oddo F. G., Chiarini A., Sinatra A. On the hemagglutinating and hemolytic activity of measles virus variants. Arch Gesamte Virusforsch. 1967;22(1):35–42. doi: 10.1007/BF01240499. [DOI] [PubMed] [Google Scholar]
- Orvell C., Norrby E. Further studies on the immunologic relationships among measles, distemper, and rinderpest viruses. J Immunol. 1974 Dec;113(6):1850–1858. [PubMed] [Google Scholar]
- Osunkoya B. O., Adeleye G. I., Adejumo T. A., Salimonu L. S. Studies on leukocyte cultures in measles. II. Detection of measles virus antigen in human leucocytes by immunofluorescence. Arch Gesamte Virusforsch. 1974;44(4):323–329. doi: 10.1007/BF01251013. [DOI] [PubMed] [Google Scholar]
- Osunkoya B. O., Cooke A. R., Ayeni O., Adejumo T. A. Studies on leukocyte cultures in measles. I. Lymphocyte transformation and giant cell formation in leukocyte cultures from clinical cases of measles. Arch Gesamte Virusforsch. 1974;44(4):313–322. [PubMed] [Google Scholar]
- Oyanagi S., ter Meulen V., Katz M., Koprowski H. Comparison of subacute sclerosing panencephalitis and measles viruses: an electron microscope study. J Virol. 1971 Jan;7(1):176–187. doi: 10.1128/jvi.7.1.176-187.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyanagi S., ter Meulen V., Müller D., Katz M., Koprowski H. Electron microscopic observations in subacute sclerosing panencephalitis brain cell cultures: their correlation with cytochemical and immunocytological findings. J Virol. 1970 Sep;6(3):370–379. doi: 10.1128/jvi.6.3.370-379.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERIES J. R., CHANY C. Studies on measles viral hemagglutination. Proc Soc Exp Biol Med. 1962 Jul;110:477–482. doi: 10.3181/00379727-110-27555. [DOI] [PubMed] [Google Scholar]
- PERIES J. R., CHANY C. [Hemagglutinating and hemolytic activity of the measles virus]. C R Hebd Seances Acad Sci. 1960 Aug 1;251:820–821. [PubMed] [Google Scholar]
- PETRALLI J. K., MERIGAN T. C., WILBUR J. R. CIRCULATING INTERFERON AFTER MEASLES VACCINATION. N Engl J Med. 1965 Jul 22;273:198–201. doi: 10.1056/NEJM196507222730405. [DOI] [PubMed] [Google Scholar]
- Pampiglione G., Griffith A. H., Bramwell E. C. Transient cerebral changes after vaccination against measles. Lancet. 1971 Jul 3;2(7714):5–8. doi: 10.1016/s0140-6736(71)90003-1. [DOI] [PubMed] [Google Scholar]
- Panelius M., Salmi A., Halonen P. E., Kivalo E., Rinne U. K., Penttinen K. Virus antibodies in serum specimens from patients with multiple sclerosis, from siblings, and matched controls. A final report. Acta Neurol Scand. 1973;49(1):85–107. doi: 10.1111/j.1600-0404.1973.tb01281.x. [DOI] [PubMed] [Google Scholar]
- Panelius M., Salmi A., Halonen P. Gel precipitation reactions between measles antigens and sera of patients with multiple sclerosis. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(5):588–592. doi: 10.1111/j.1699-0463.1970.tb04345.x. [DOI] [PubMed] [Google Scholar]
- Parfanovich M., Hammarskjöld B., Norrby E. Synthesis of virus-specific RNA in cells infected with two different variants of measles virus. Arch Gesamte Virusforsch. 1971;35(1):38–44. doi: 10.1007/BF01249750. [DOI] [PubMed] [Google Scholar]
- Parker J. C., Jr, Klintworth G. K., Graham D. G., Griffith J. F. Uncommon morphologic features in subacute sclerosing panencephalitis (SSPE). Report of two cases with virus recovery from one autopsy brain specimen. Am J Pathol. 1970 Nov;61(2):275–292. [PMC free article] [PubMed] [Google Scholar]
- Payne F. E., Baublis J. V. Decreased reactivity of SSPE strains of measles virus with antibody. J Infect Dis. 1973 May;127(5):505–511. doi: 10.1093/infdis/127.5.505. [DOI] [PubMed] [Google Scholar]
- Payne F. E., Baublis J. V., Itabashi H. H. Isolation of measles virus from cell cultures of brain from a patient with subacute sclerosing panencephalitis. N Engl J Med. 1969 Sep 11;281(11):585–589. doi: 10.1056/NEJM196909112811103. [DOI] [PubMed] [Google Scholar]
- Pettay O., Donner M., Halonen H., Palosuo T., Salmi A. Subacute sclerosing panencephalitis: preceding intellectual deterioration and deviant measles serology. J Infect Dis. 1971 Nov;124(5):439–444. doi: 10.1093/infdis/124.5.439. [DOI] [PubMed] [Google Scholar]
- Phillips P. E., Christian C. L. Myxovirus antibody increases in human connective tissue disease. Science. 1970 May 22;168(3934):982–984. doi: 10.1126/science.168.3934.982. [DOI] [PubMed] [Google Scholar]
- Phillips P. E., Christian C. L. Virus antibodies in systemic lupus erythematosus and other connective tissue diseases. Ann Rheum Dis. 1973 Sep;32(5):450–456. doi: 10.1136/ard.32.5.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Blacklow N. R., Grimley P. M., Bellanti J. A. Glomerular microtubules of systemic lupus erythematosus. Lancet. 1970 Nov 21;2(7682):1058–1061. doi: 10.1016/s0140-6736(70)90287-4. [DOI] [PubMed] [Google Scholar]
- Plotkin S. A. Failures of protection by measles vaccine. J Pediatr. 1973 May;82(5):908–911. doi: 10.1016/s0022-3476(73)80107-6. [DOI] [PubMed] [Google Scholar]
- Pollack M. A., Grose C., Friend H. Letter: Measles associated with Bell palsy. Am J Dis Child. 1975 Jun;129(6):747–747. doi: 10.1001/archpedi.1975.02120430079022. [DOI] [PubMed] [Google Scholar]
- Portner A., Bussell R. H. Measles virus ribonucleic acid and protein synthesis: effects of 6-azauridine and cycloheximide on viral replication. J Virol. 1973 Jan;11(1):46–53. doi: 10.1128/jvi.11.1.46-53.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A., Kingsbury D. W. Identification of transcriptive and replicative intermediates in Sendai virus-infected cells. Virology. 1972 Mar;47(3):711–725. doi: 10.1016/0042-6822(72)90561-2. [DOI] [PubMed] [Google Scholar]
- Poser C. M. Recent advances in multiple sclerosis. Med Clin North Am. 1972 Nov;56(6):1343–1362. doi: 10.1016/s0025-7125(16)32325-2. [DOI] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Selection of temperature-sensitive mutants during persistent infection: role in maintenance of persistent Newcastle disease virus infections of L cells. J Virol. 1973 Sep;12(3):481–491. doi: 10.1128/jvi.12.3.481-491.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive mutants isolated from L cells persistently infected with Newcastle disease virus. J Virol. 1972 Feb;9(2):200–206. doi: 10.1128/jvi.9.2.200-206.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive viruses and the etiology of chronic and inapparent infections. J Infect Dis. 1975 Apr;131(4):467–473. doi: 10.1093/infdis/131.4.467. [DOI] [PubMed] [Google Scholar]
- Prineas J. Paramyxovirus-like particles associated with acute demyelination in chronic relapsing multiple sclerosis. Science. 1972 Nov 17;178(4062):760–763. doi: 10.1126/science.178.4062.760. [DOI] [PubMed] [Google Scholar]
- Périer O., Thiry L., Vanderhaeghen J. J., Pelc S. Attempts at experimental transmission and electron microscopic observations in subacute sclerosing panencephalitis. Neurology. 1968 Jan;18(1 Pt 2):138–143. doi: 10.1212/wnl.18.1_part_2.138. [DOI] [PubMed] [Google Scholar]
- RAPP F., GORDON I., BAKER R. F. Observations of measles virus infection of cultured human cells. I. A study of development and spread of virus antigen by means of immunofluorescence. J Biophys Biochem Cytol. 1960 Feb;7:43–48. doi: 10.1083/jcb.7.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F. PLAQUE DIFFERENTIATION AND REPLICATION OF VIRULENT AND ATTENUATED STRAINS OF MEASLES VIRUS. J Bacteriol. 1964 Nov;88:1448–1458. doi: 10.1128/jb.88.5.1448-1458.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSANOFF E. I. Hemagglutination and hemadsorption of measles virus. Proc Soc Exp Biol Med. 1961 Mar;106:563–567. doi: 10.3181/00379727-106-26403. [DOI] [PubMed] [Google Scholar]
- ROSEN L. Hemagglutination and hemagglutination-inhibition with measles virus. Virology. 1961 Jan;13:139–141. doi: 10.1016/0042-6822(61)90042-3. [DOI] [PubMed] [Google Scholar]
- RUSTIGIAN R. A carrier state in HeLa cells with measles virus (Edmonston strain) apparently associated with non-infectious virus. Virology. 1962 Jan;16:101–104. doi: 10.1016/0042-6822(62)90212-x. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Feldman L. A., Sheppard R. D., Barbosa L. H., Bornstein M. B. Subacute sclerosing panencephalitis virus. Observations on a neuroadapted and non-neuroadapted strain in organotypic central nervous system cultures. Lab Invest. 1974 Jul;31(1):42–53. [PubMed] [Google Scholar]
- Raine C. S., Feldman L. A., Sheppard R. D., Bornstein M. B. Subacute sclerosing panencephalitis virus in cultures of organized central nervous tissue. Lab Invest. 1973 May;28(5):627–640. [PubMed] [Google Scholar]
- Raine C. S., Feldman L. A., Sheppard R. D., Bornstein M. B. Ultrastructure of measles virus in cultures of hamster cerebellum. J Virol. 1969 Aug;4(2):169–181. doi: 10.1128/jvi.4.2.169-181.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine C. S., Powers J. M., Suzuki K. Acute multiple sclerosis. Confirmation of "paramyxovirus-like" intranuclear inclusions. Arch Neurol. 1974 Jan;30(1):39–46. doi: 10.1001/archneur.1974.00490310041007. [DOI] [PubMed] [Google Scholar]
- Rand K. H., Emmons R. W., Merigan T. C. Measles in adults. An unforeseen consequence of immunization? JAMA. 1976 Aug 30;236(9):1028–1031. doi: 10.1001/jama.236.9.1028. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Rawls M. L., Chernesky M. A. Analysis of a measles epidemic; possible role of vaccine failures. Can Med Assoc J. 1975 Nov 22;113(10):941–944. [PMC free article] [PubMed] [Google Scholar]
- Rima R. K., Martin S. J. Persistent infection of tissue culture cells by RNA viruses. Med Microbiol Immunol. 1976 Jun 1;162(2):89–119. doi: 10.1007/BF02121320. [DOI] [PubMed] [Google Scholar]
- Robinson W. S. Ribonucleic acid polymerase activity in Sendai virions and nucleocapsid. J Virol. 1971 Jul;8(1):81–86. doi: 10.1128/jvi.8.1.81-86.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S. Sendai virus RNA synthesis and nucleocapsid formation in the presence of cycloheximide. Virology. 1971 Jun;44(3):494–502. doi: 10.1016/0042-6822(71)90362-x. [DOI] [PubMed] [Google Scholar]
- Roman J. M., Simon E. H. Defective interfering particles in monolayer-propagated Newcastle disease virus. Virology. 1976 Jan;69(1):298–303. doi: 10.1016/0042-6822(76)90216-6. [DOI] [PubMed] [Google Scholar]
- Rothfield N. F., Evans A. S., Niederman J. C. Clinical and laboratory aspects of raised virus antibody titres in systemic lupus erythematosus. Ann Rheum Dis. 1973 May;32(3):238–246. doi: 10.1136/ard.32.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckdeschel J. C., Graziano K. D., Mardiney M. R., Jr Additional evidence that the cell-associated immune system is the primary host defense against measles (rubeola). Cell Immunol. 1975 May;17(1):11–18. doi: 10.1016/s0008-8749(75)80002-5. [DOI] [PubMed] [Google Scholar]
- Rustigian R. Persistent infection of cells in culture by measles virus. I. Development and characteristics of HeLa sublines persistently infected with complete virus. J Bacteriol. 1966 Dec;92(6):1792–1804. doi: 10.1128/jb.92.6.1792-1804.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustigian R. Persistent infection of cells in culture by measles virus. II. Effect of measles antibody on persistently infected HeLa sublines and recovery of a HeLa clonal line persistently infected with incomplete virus. J Bacteriol. 1966 Dec;92(6):1805–1811. doi: 10.1128/jb.92.6.1805-1811.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARZ A. J., ANDERSON J. T. IMMUNIZATION WITH A FURTHER ATTENUATED LIVE MEASLES VACCINE. Arch Gesamte Virusforsch. 1965;16:273–278. doi: 10.1007/BF01253822. [DOI] [PubMed] [Google Scholar]
- SCHWARZ A. J. Preliminary tests of a highly attenuated measles vaccine. Am J Dis Child. 1962 Mar;103:386–389. doi: 10.1001/archpedi.1962.02080020398042. [DOI] [PubMed] [Google Scholar]
- ST GEME J. W., Jr EVIDENCE FOR THE NUCLEIC ACID COMPOSITION OF MEASLES VIRUS. Pediatrics. 1964 Jan;33:71–74. [PubMed] [Google Scholar]
- Salmi A. A., Norrby E., Panelius M. Identification of different measles virus-specific antibodies in the serum and cerebrospinal fluid from patients with subacute sclerosing pancencephalitis and multiple sclerosis. Infect Immun. 1972 Sep;6(3):248–254. doi: 10.1128/iai.6.3.248-254.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi A. A., Panelius M., Halonen P., Rinne U. K., Penttinen K. Measles virus antibody in cerebrospinal fluids from patients with multiple sclerosis. Br Med J. 1972 Feb 19;1(5798):477–479. doi: 10.1136/bmj.1.5798.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaff Z., Barry D. W., Grimley P. M. Cytochemistry of tubuloreticular structures in lymphocytes from patients with systemic lupus erythematosus and in cultured human lymphoid cells: comparison to a paramyxovirus. Lab Invest. 1973 Dec;29(6):577–586. [PubMed] [Google Scholar]
- Schluederberg A., Chavanich S., Lipman M. B., Carter C. Comparative molecular weight estimates of measles and subacute sclerosing panencephalitis virus structural polypeptides by simultaneous electrophoresis in acrylamide gel slabs. Biochem Biophys Res Commun. 1974 Jun 4;58(3):647–651. doi: 10.1016/s0006-291x(74)80467-5. [DOI] [PubMed] [Google Scholar]
- Schluederberg A., Chavanich S. The role of the nucleus in measles virus replication. Med Microbiol Immunol. 1974;160(2-3):85–90. doi: 10.1007/BF02121715. [DOI] [PubMed] [Google Scholar]
- Schluederberg A., Lamm S. H., Landrigan P. J., Black F. L. Measles immunity in children vaccinated before one year of age. Am J Epidemiol. 1973 Jun;97(6):402–409. doi: 10.1093/oxfordjournals.aje.a121521. [DOI] [PubMed] [Google Scholar]
- Schluederberg A. Measles virus RNA. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1012–1015. doi: 10.1016/0006-291x(71)90004-0. [DOI] [PubMed] [Google Scholar]
- Schluederberg A., Nakamura M. A salt-dependent hemagglutinating particle from measles-infected cells. Virology. 1967 Oct;33(2):297–306. doi: 10.1016/0042-6822(67)90148-1. [DOI] [PubMed] [Google Scholar]
- Schluederberg A., Williams C. A., Black F. L. Inhibition of measles virus replication and RNA synthesis by actinomycin D. Biochem Biophys Res Commun. 1972 Aug 7;48(3):657–661. doi: 10.1016/0006-291x(72)90398-1. [DOI] [PubMed] [Google Scholar]
- Schneck S. A., Fulginiti V., Leestma J. Measles virus and panencephalitis. Lancet. 1967 Jun 24;1(7504):1381–1382. doi: 10.1016/s0140-6736(67)91793-x. [DOI] [PubMed] [Google Scholar]
- Schumacher H. R., Jr Tubular paramyxovirus-like structures in synovial vascular endothelium. Ann Rheum Dis. 1970 Jul;29(4):445–447. doi: 10.1136/ard.29.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott T. F., Bonanno D. E. Reactions to live-measles-virus vaccine in children previously inoculated with killed-virus vaccine. N Engl J Med. 1967 Aug 3;277(5):248–250. doi: 10.1056/NEJM196708032770506. [DOI] [PubMed] [Google Scholar]
- Sever J. L., Zeman W. Serological studies of measles and subacute sclerosing panencephalitis. Neurology. 1968 Jan;18(1 Pt 2):95–97. doi: 10.1212/wnl.18.1_part_2.095. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Shope T. C., Downs H., Herrmann K. L., Polkowski J. Epidemic measles in a highly vaccinated population. N Engl J Med. 1977 Mar 17;296(11):585–589. doi: 10.1056/NEJM197703172961102. [DOI] [PubMed] [Google Scholar]
- Shaw C. M., Buchan G. C., Carlson C. B. Myxovirus as a possible etiologic agent in subacute inclusion body-encephalitis. N Engl J Med. 1967 Sep 7;277(10):511–515. doi: 10.1056/NEJM196709072771004. [DOI] [PubMed] [Google Scholar]
- Shaw C., Sumi S. M. Nonviral intranuclear filamentous inclusions. Arch Neurol. 1975 Jul;32(7):428–432. doi: 10.1001/archneur.1975.00490490032002. [DOI] [PubMed] [Google Scholar]
- Shearn M. A., Tu W. H., Stephens B. G., Lee J. C. Virus-like structure in Sjögren's syndrome. Lancet. 1970 Mar 14;1(7646):568–569. doi: 10.1016/s0140-6736(70)90804-4. [DOI] [PubMed] [Google Scholar]
- Sheppard R. D., Raine C. S., Burnstein T., Bornstein M. B., Feldman L. A. Cell-associated subacute sclerosing panencephalitis agent studied in organotypic central nervous system cultures: viral rescue attempts and morphology. Infect Immun. 1975 Oct;12(4):891–900. doi: 10.1128/iai.12.4.891-900.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokata K., Nishiyama Y., Ito Y., Kimura Y., Nagata I. Pathogenesis of Sendai virus infection in the central nervous system of mice. Infect Immun. 1976 May;13(5):1497–1502. doi: 10.1128/iai.13.5.1497-1502.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirodaria P. V., Dermott E., Gould E. A. Some characteristics of salt-dependent haemagglutinating measles viruses. J Gen Virol. 1976 Oct;33(1):107–115. doi: 10.1099/0022-1317-33-1-107. [DOI] [PubMed] [Google Scholar]
- Simpson R. W., Iinuma M. Recovery of infectious proviral DNA from mammalian cells infected with respiratory syncytial virus. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3230–3234. doi: 10.1073/pnas.72.8.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkovics J. G., Gyorkey F., Thoma G. W. A rapidly fatal case of systemic lupus erythematosus: structures resembling viral nucleoprotein strands in the kidney and activities of lymphocytes in culture. Tex Rep Biol Med. 1969 Fall;27(3):887–908. [PubMed] [Google Scholar]
- Stanwick T. L., Hallum J. V. Comparison of RNA polymerase associated with Newcastle disease virus and a temperature-sensitive mutant of Newcastle disease virus isolated from persistently infected L cells. J Virol. 1975 Jan;17(1):68–73. doi: 10.1128/jvi.17.1.68-73.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone H. O., Portner A., Kingsbury D. W. Ribonucleic acid transcriptases in Sendai Virions and infected cells. J Virol. 1971 Aug;8(2):174–180. doi: 10.1128/jvi.8.2.174-180.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. L., Barry D. W., Albrecht P., Lucas S. J. Inhibition of lymphocyte stimulation by measles virus. J Immunol. 1975 May;114(5):1458–1461. [PubMed] [Google Scholar]
- Sullivan J. L., Barry D. W., Lucas S. J., Albrecht P. Measles infection of human mononuclear cells. I. Acute infection of peripheral blood lymphocytes and monocytes. J Exp Med. 1975 Sep 1;142(3):773–784. doi: 10.1084/jem.142.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick H. M., Brooks W. H., Roszman T. L., Caldwell D. A heat-stable blocking factor in the plasma of patients with subacute sclerosing panencephalitis. Neurology. 1976 Jan;26(1):84–88. doi: 10.1212/wnl.26.1.84. [DOI] [PubMed] [Google Scholar]
- THorne H. V., Dermott E. Circular and elongated linear forms of measles virus nucleocapsid. Nature. 1976 Dec 2;264(5585):473–474. doi: 10.1038/264473a0. [DOI] [PubMed] [Google Scholar]
- TYLER H. R. Neurological complications of rubeola (measles). Medicine (Baltimore) 1957 May;36(2):147–167. doi: 10.1097/00005792-195705000-00001. [DOI] [PubMed] [Google Scholar]
- Tellez-Nagel I., Harter D. H. Subacute sclerosing leukoencephalitis. I. Clinico-pathological, electron microscopic and virological observations. J Neuropathol Exp Neurol. 1966 Oct;25(4):560–581. doi: 10.1097/00005072-196610000-00005. [DOI] [PubMed] [Google Scholar]
- Tellez-Negal I., Harter D. H. Subacute sclerosing leukoencephalitis: ultrastructure of intranuclear and intracytoplasmic inclusions. Science. 1966 Nov 18;154(3751):899–901. doi: 10.1126/science.154.3751.899. [DOI] [PubMed] [Google Scholar]
- Thormar H., Jervis G. A., Karl S. C., Brown H. R. Passage in ferrets of encephalitogenic cell-associated measles virus isolated from brain of a patient with subacute sclerosing panencephalitis. J Infect Dis. 1973 Jun;127(6):678–685. doi: 10.1093/infdis/127.6.678. [DOI] [PubMed] [Google Scholar]
- Tonietti G., Oldstone M. B., Dixon F. J. The effect of induced chronic viral infections on the immunologic diseases of New Zealand mice. J Exp Med. 1970 Jul 1;132(1):89–109. doi: 10.1084/jem.132.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourtellotte W. W., Parker J. A., Herndon R. M., Cuadros C. V. Subacute sclerosing panencephalitis: brain immunoglobulin-G, measles antibody and albumin. Neurology. 1968 Jan;18(1 Pt 2):117–121. doi: 10.1212/wnl.18.1_part_2.117. [DOI] [PubMed] [Google Scholar]
- Townsend J. J., Baringer J. R., Wolinsky J. S., Malamud N., Mednick J. P., Panitch H. S., Scott R. A., Oshiro L., Cremer N. E. Progressive rubella panencephalitis. Late onset after congenital rubella. N Engl J Med. 1975 May 8;292(19):990–993. doi: 10.1056/NEJM197505082921902. [DOI] [PubMed] [Google Scholar]
- UNDERWOOD G. E. Studies on measles virus in tissue culture. I. Growth rates in various cells and development of a plaque assay. J Immunol. 1959 Aug;83:198–205. [PubMed] [Google Scholar]
- Ueda S., Okuno Y., Hamamoto Y., Oya H. Subacute sclerosing panencephalitis (SSPE): isolation of a defective variant of measles virus from brain obtained at autopsy. Biken J. 1975 Jun;18(2):113–122. [PubMed] [Google Scholar]
- Ueda S., Otsuka T., Okuno Y. Experimental subacute sclerosing panencephalitis (SSPE) in a monkey by subcutaneous inoculation with a defective SSPE virus. Biken J. 1975 Sep;18(3):179–181. [PubMed] [Google Scholar]
- Underwood B., Brown F. Physico-chemical characterisation of rinderpest virus. Med Microbiol Immunol. 1974;160(2-3):125–132. doi: 10.1007/BF02121719. [DOI] [PubMed] [Google Scholar]
- WAKSMAN B. H., BURNSTEIN T., ADAMS R. D. Histologic study of the encephalomyelitis produced in hamsters by a neurotropic strain of measles. J Neuropathol Exp Neurol. 1962 Jan;21:25–49. doi: 10.1097/00005072-196201000-00003. [DOI] [PubMed] [Google Scholar]
- WATERSON A. P., CRUICKSHANK J. G., LAURENCE G. D., KANAREK A. D. The nature of measles virus. Virology. 1961 Nov;15:379–382. doi: 10.1016/0042-6822(61)90370-1. [DOI] [PubMed] [Google Scholar]
- WATERSON A. P. MEASLES VIRUS. Arch Gesamte Virusforsch. 1965;16:57–80. doi: 10.1007/BF01253793. [DOI] [PubMed] [Google Scholar]
- WATERSON A. P. Two kinds of myxovirus. Nature. 1962 Mar 24;193:1163–1164. doi: 10.1038/1931163a0. [DOI] [PubMed] [Google Scholar]
- Watanabe I., Okazaki H. Virus-like structure in multiple sclerosis. Lancet. 1973 Sep 8;2(7828):569–570. doi: 10.1016/s0140-6736(73)92394-5. [DOI] [PubMed] [Google Scholar]
- Waters D. J., Bussell R. H. Isolation and comparative study of the nucleocapsids of measles and canine distemper viruses from infected cells. Virology. 1974 Sep;61(1):74–79. [PubMed] [Google Scholar]
- Waters D. J., Bussell R. H. Polypeptide composition of measles and canine distemper viruses. Virology. 1973 Oct;55(2):554–557. doi: 10.1016/0042-6822(73)90202-x. [DOI] [PubMed] [Google Scholar]
- Waters D. J., Hersh R. T., Bussell R. H. Isolation and characterization of measles nucleocapsid from infected cells. Virology. 1972 Apr;48(1):278–281. doi: 10.1016/0042-6822(72)90138-9. [DOI] [PubMed] [Google Scholar]
- Wear D. J., Rabin E. R., Richardson L. S., Rapp F. Virus replication and ultrastructural changes after induction of encephalitis in mice by measles virus. Exp Mol Pathol. 1968 Dec;9(3):405–417. doi: 10.1016/0014-4800(68)90029-4. [DOI] [PubMed] [Google Scholar]
- Wear D. J., Rapp F. Encephalitis in newborn hamsters after intracerebral injection of attenuated human measles virus. Nature. 1970 Sep 26;227(5265):1347–1348. doi: 10.1038/2271347a0. [DOI] [PubMed] [Google Scholar]
- Wear D. J., Rapp F. Latent measles virus infection of the hamster central nervous system. J Immunol. 1971 Dec;107(6):1593–1598. [PubMed] [Google Scholar]
- Weibel R. E., Buynak E. B., McLean A. A., Hilleman M. R. Long-term follow-up for immunity after monovalent or combined live measles, mumps, and rubella virus vaccines. Pediatrics. 1975 Sep;56(3):380–387. [PubMed] [Google Scholar]
- Weil M. L., Itabashi H., Cremer N. E., Oshiro L., Lennette E. H., Carnay L. Chronic progressive panencephalitis due to rubella virus simulating subacute sclerosing panencephalitis. N Engl J Med. 1975 May 8;292(19):994–998. doi: 10.1056/NEJM197505082921903. [DOI] [PubMed] [Google Scholar]
- Weiner L. B., Corwin R. M., Nieburg P. I., Feldman H. A. A measles outbreak among adolescents. J Pediatr. 1977 Jan;90(1):17–20. doi: 10.1016/s0022-3476(77)80757-9. [DOI] [PubMed] [Google Scholar]
- Weiner L. P., Johnson R. T., Herndon R. M. Viral infections and demyelinating diseases. N Engl J Med. 1973 May 24;288(21):1103–1110. doi: 10.1056/NEJM197305242882106. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Bratt M. A. Polyadenylate sequences on Newcastle disease virus mRNA synthesized in vivo and in vitro. J Virol. 1974 Jun;13(6):1220–1230. doi: 10.1128/jvi.13.6.1220-1230.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. G., Boyd J. F. The effect of measles on the thymus and other lymphoid tissues. Clin Exp Immunol. 1973 Mar;13(3):343–357. [PMC free article] [PubMed] [Google Scholar]
- Winston S. H., Rustigian R., Bratt M. A. Persistent infection of cells in culture by measles virus. 3. Comparison of virus-specific RNA synthesized in primary persistent infection in HeLa cells. J Virol. 1973 Jun;11(6):926–932. doi: 10.1128/jvi.11.6.926-932.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woyciechowska J., Breschkin A. M., Rapp F. Measles virus meningoencephalitis. Immunofluorescence study of brains infected with virus mutants. Lab Invest. 1977 Mar;36(3):233–236. [PubMed] [Google Scholar]
- Wyll S. A., Witte J. J. Measles in previously vaccinated children. An epidemiological study. JAMA. 1971 May 24;216(8):1306–1310. [PubMed] [Google Scholar]
- Yamanouchi K., Kobune F., Fukuda A., Hayami M., Shishido A. Comparative immunofluorescent studies on measles, canine distemper, and rinderpest viruses. Immunofluorescence of measles, distemper, and rinderpest viruses. Arch Gesamte Virusforsch. 1970;29(1):90–100. doi: 10.1007/BF01253884. [DOI] [PubMed] [Google Scholar]
- Yeager A. S., Davis J. H., Ross L. A., Harvey B. Measles immunization. Successes and failures. JAMA. 1977 Jan 24;237(4):347–351. [PubMed] [Google Scholar]
- Yeh J. Characterization of virus-specific RNAs from subacute sclerosing panencephalitis virus-infected CV-1 cells. J Virol. 1973 Nov;12(5):962–968. doi: 10.1128/jvi.12.5.962-968.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J., Iwasaki Y. Isolation and characterization of subacute sclerosing panencephalitis virus nucleocapsids. J Virol. 1972 Dec;10(6):1220–1227. doi: 10.1128/jvi.10.6.1220-1227.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Quagliana D. O. Temperature-sensitive mutants isolated from hamster and canine cell lines persistently infected with Newcastle disease virus. J Virol. 1975 Nov;16(5):1332–1336. doi: 10.1128/jvi.16.5.1332-1336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Quagliana D. O. Temperature-sensitive mutants of vesicular stomatitis virus are conditionally defective particles that interfere with and are rescued by wild-type virus. J Virol. 1976 Jul;19(1):102–107. doi: 10.1128/jvi.19.1.102-107.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman W., Kolar O. Reflections on the etiology and pathogenesis of subacute sclerosing panencephalitis. Neurology. 1968 Jan;18(1 Pt 2):1–7. doi: 10.1212/wnl.18.1_part_2.1. [DOI] [PubMed] [Google Scholar]
- Zhdanov V. M. Integration of viral genomes. Nature. 1975 Aug 7;256(5517):471–473. doi: 10.1038/256471a0. [DOI] [PubMed] [Google Scholar]
- Zhdanov V. M., Parfanovich M. I. Integration of measles virus nucleic acid into the cell genome. Arch Gesamte Virusforsch. 1974;45(3):225–234. doi: 10.1007/BF01249685. [DOI] [PubMed] [Google Scholar]
- Ziff M. Viruses and the connective tissue diseases. Ann Intern Med. 1971 Dec;75(6):951–958. doi: 10.7326/0003-4819-75-6-951. [DOI] [PubMed] [Google Scholar]
- Zweiman B. In vitro effects of measles virus on proliferating human lymphocytes. J Immunol. 1971 May;106(5):1154–1158. [PubMed] [Google Scholar]
- Zweiman B., Pappagianis D., Maibach H., Hildreth E. A. Effect of measles immunization on tuberculin hypersensitivity and in vitro lymphocyte reactivity. Int Arch Allergy Appl Immunol. 1971;40(6):834–841. doi: 10.1159/000230466. [DOI] [PubMed] [Google Scholar]
- ter Meulen V., Katz M., Käckell Y. M., Barbanti-Brodano G., Koprowski H., Lennette E. H. Subacute sclerosing panencephalitis: in-vitro characterization of viruses isolated from brain cells in culture. J Infect Dis. 1972 Jul;126(1):11–17. doi: 10.1093/infdis/126.1.11. [DOI] [PubMed] [Google Scholar]
- ter Meulen V., Katz M., Müller D. Subacute sclerosing panencephalitis: a review. Curr Top Microbiol Immunol. 1972;57:1–38. doi: 10.1007/978-3-642-65297-4_1. [DOI] [PubMed] [Google Scholar]
- ter Meulen V., Martin S. J. Genesis and maintenance of a persistent infection by canine distemper virus. J Gen Virol. 1976 Sep;32(3):431–440. doi: 10.1099/0022-1317-32-3-431. [DOI] [PubMed] [Google Scholar]
- ter Meulen V., Müller D., Käckell Y., Katz M., Meyermann R. Isolation of infectious measles virus in measles encephalitis. Lancet. 1972 Dec 2;2(7788):1172–1175. doi: 10.1016/s0140-6736(72)92595-0. [DOI] [PubMed] [Google Scholar]
- ter Meulen V. Pathogenetic aspects of measles virus infections. Med Microbiol Immunol. 1974;160(2-3):165–172. doi: 10.1007/BF02121723. [DOI] [PubMed] [Google Scholar]