Abstract
We live in a world imbued with a rich mixture of complex sounds. Successful acoustic communication requires the ability to extract meaning from those sounds, even when degraded. One strategy used by the auditory system is to harness high-level contextual cues to modulate the perception of incoming sounds. An ideal substrate for this process is the massive set of top-down projections emanating from virtually every level of the auditory system. In this review, we provide a molecular and circuit-level description of one of the largest of these pathways: the auditory corticocollicular pathway. While its functional role remains to be fully elucidated, activation of this projection system can rapidly and profoundly change the tuning of neurons in the inferior colliculus. Several specific issues are reviewed. First, we describe the complex heterogeneous anatomical organization of the corticocollicular pathway, with particular emphasis on the topography of the pathway. We also review the laminar origin of the corticocollicular projection and discuss known physiological and morphological differences between subsets of corticocollicular cells. Finally, we discuss recent findings about the molecular micro-organization of the inferior colliculus and how it interfaces with corticocollicular termination patterns. Given the assortment of molecular tools now available to the investigator, it is hoped that his review will help guide future research on the role of this pathway in normal hearing.
Keywords: Inferior colliculus, auditory cortex, corticocollicular, top-down modulation, tonotopic
1. Introduction
Sounds rarely exist in isolation. The temporal features used to extract meaning from sounds evolve over multiple and overlapping time scales. For example, in speech, phonemic cues evolve over milliseconds, syntactic cues over hundreds of milliseconds, and semantic cues over seconds. It is currently not known how are these temporally-discordant streams are integrated. A potential substrate for such processing is the hierarchically-organized, massive set of descending projections found in the auditory system. A specific subset of these projections, the corticocollicular (CC) system, has received substantial attention given its large size and complexity. In addition, a large number of studies have demonstrated that stimulation of the auditory cortex (AC) significantly alters inferior colliculus (IC) response properties across multiple species, including bats (Zhang, Suga and Yan 1997; Yan and Suga 1998), mice (Yan and Ehret 2001; Yan and Ehret 2002; Yan, Zhang and Ehret 2005), ferrets (Bajo et al. 2010), rats (Sun et al. 2007; Anderson and Malmierca 2013) and cats (Mitani, Shimokouchi and Nomura 1983). Despite the wealth of data obtained from these physiological studies, our understanding of the neural circuits underlying these changes remains poor. In other descending pathways, such as the corticothalamic pathway, detailed analyses of heterogeneities in synaptic morphology and physiology have led to insights about the functional roles of this pathway (Reichova and Sherman 2004; Groh et al. 2008; Ojima and Murakami 2011). Similarly, it is likely that molecular and circuit level analyses of the CC pathway will uncover the mechanisms by which the AC influences the IC.
This review addresses unanswered questions emerging from physiological studies involving recordings in the IC after cortical stimulation or silencing. For example, one of the dominant theories of CC function (the “egocentric selection” theory) suggests that the AC can shift the peaks of tuning functions of IC neurons towards the peak of the tuning functions from the cortical source (Suga 2012). In the frequency domain, this theory implies that IC neurons receive excitatory AC input across more than one frequency channel, such that conditioned frequencies away from an IC neuron's characteristic frequency can influence its tuning function. The difference in characteristic frequency between the AC stimulation site and the IC neuron being modulated is quite variable, and can be as much as 10 kHz in the mouse, which corresponds to at least 0.5-1.0 octaves (Yan and Ehret 2002). This difference in characteristic frequencies suggests that individual IC neurons may receive input across a broad range of frequencies, pointing to a substantial degree of convergence in this pathway. In addition, shifts in a frequency tuning curve that involve decreases in responses to sound at the characteristic frequency imply that the CC pathway must involve frequency-specific inhibition of responses to ascending acoustically-driven input. Finally, the overwhelming majority of physiological studies have been done in the central nucleus of the IC (CNIC), while most of the CC pathway has terminations in the non-primary regions of the IC, suggesting a potential role for local-circuit interactions in the expression of corticofugal modulations of IC tuning functions. Other theories besides egocentric selection have been postulated about the role of feedback in sensory systems, such as an involvement in attention (Baluch and Itti 2011) or predictive coding (Bastos et al. 2012). The latter, which may be manifested as stimulus-specific adaptation, has been examined in the CC pathway of rats, and has been shown to be highly heterogeneous. That is, silencing of the AC led to no change (approximately 50% of IC neurons), or to an increase or a decrease in the degree of stimulus-specific adaptation in IC neurons (Anderson and Malmierca 2013), suggesting that the impact of the AC on acoustic responses IC is non-uniform and may depend on which specific subcircuits within the CC system are stimulated or suppressed.
Thus empirical observations of modulation in the CC pathway have generated a series of questions that require answers at the molecular and circuit-level of analysis. However, we have only now begun to understand how the circuits that underlie this pathway are organized. Given recent interest in the role of top-down modulation across cognitive and sensory systems (Boly et al. 2011; Gazzaley and Nobre 2012; Gilbert and Li 2013), and the expansion of optical and molecular tools now available, this review will provide investigators with an integrated view of these projections which may be useful in furthering our understanding of this system at a detailed circuit level. Given recent comprehensive reviews of the physiology of the CC pathway (Mei and Chen 2010; Bajo and King 2011; Suga 2012), this review focuses on molecular and circuit-level analyses.
2. Anatomical considerations
Depending on the species, there are at least 5 distinct AC areas and at least 3 distinct IC areas (many would argue that there are more for both regions), with some of these areas showing tonotopy and others not. Therefore, two core organizational questions should be answered to better understand the function of the CC system. First, which regions of the AC project to which regions of the IC? Second, to what degree is the tonotopic organization of the AC retained in the CC pathway? These questions have been addressed across a range of species and will be summarized below.
Anatomical studies have shown that virtually all regions of the AC, including those with non-tonotopic organization and/or complex response properties, project heavily to the non-primary portions of the ipsilateral IC, primarily to the dorsal cortex (DC) and external cortex or lateral nucleus (in cat), with a minor contralateral projection (See Figure 1 for a summary diagram across species), though this pattern may differ in primates ((Fitzpatrick and Imig 1978), see Figure 1F and discussed further below). For the purposes of this review, we will refer to the external cortex and lateral nucleus of the IC, which are likely homologous structures (Loftus et al. 2008), as the lateral cortex or LC, as proposed by Loftus et al. In addition, we will refer to the dorsal cortex and the pericentral nucleus of the IC (FitzPatrick 1975) as the dorsal cortex (DC). It is important to note, however, that the three-dimensional subnuclear architecture of the IC contains considerably more complexity than this tripartite system would suggest (Morest and Oliver 1984), and whenever possible we include descriptions of projections to additional subdivisions in this review. Evidence of a significant projection to the CNIC has been mixed, as described previously (Malmierca and Ryugo 2011), and will be discussed below.
Figure 1.
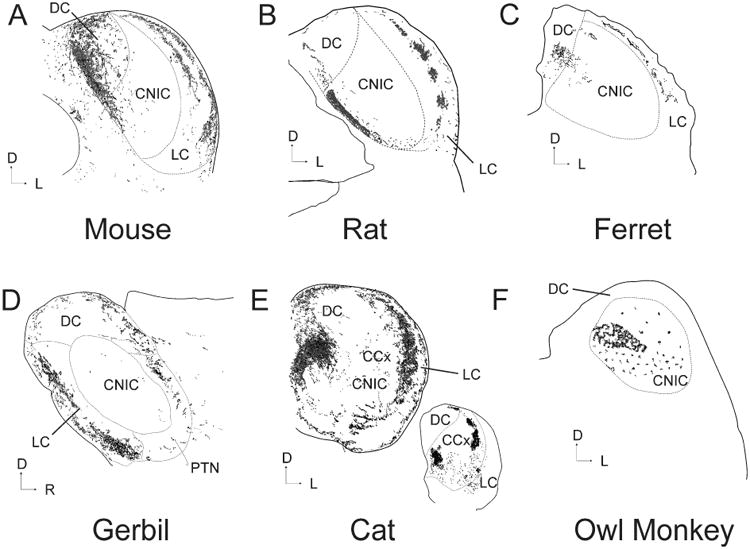
Illustration of the pattern of CC input to the IC after an injection of an anterograde tracer into the primary auditory cortex of six different species. All sections are coronal except D) which is sagittal. Sections redrawn from the following publications: A) Mouse (Torii et al. 2012), B) Rat (Saldaña, Feliciano and Mugnaini 1996), C) Ferret (Bajo et al. 2007), D) Gerbil (Budinger et al. 2013), E) Cat (Winer et al. 1998) and F) Owl Monkey (Fitzpatrick and Imig 1978). Inset in E is a caudal section chosen to illustrate the patchiness of the CC system taken at a point 21% rostral to the caudal pole (100%=anterior-most portion of IC). CCx = caudal cortex of the IC. PTN = paracentral tectal nuclei.
Corticocollicular termination patterns in the IC show high regional and sub-regional specificity. For example, in most species, injections into the primary auditory cortex (A1) or the anterior auditory field (AAF) produce two strips of labeling, generally coplanar with known isofrequency laminae of the IC; one located in the LC and the other in the DC, often encroaching into neighboring regions, such as the CNIC or the caudal cortex (CCx, see Figure 1). There is additional heterogeneity of the CC projection to individual nuclei that has not yet been explored. For example, the projection to the LC appears to contain layer specificity (seen in Figures 1A, B and E), and in rat and cat, appears to show distinct clustering (evident in Figure 1B and E, inset). In addition, in the DC of the cat, the tonotopic areas of A1 and AAF project weakly to layer 1, strongly to layers 2-3 and moderately to layer 4 (Winer et al. 1998). Nonprimary parts of the AC also project prominently to the IC, and show some similarities and differences compared to the projections from A1 and AAF. The nonprimary areas tend to project to the most superficial parts of the LC and DC as well as the intercollicular zone in rat and the rostral pole and intercollicular tegmentum in cat (Andersen, Snyder and Merzenich 1980; Herbert, Aschoff and Ostwald 1991; Winer et al. 1998). This projection to the superficial portions of the IC is an interesting point of divergence from cortical projections to the superior colliculus, where projections from cortical regions sitting higher in the processing hierarchy tend to project to deeper regions of the colliculus (Harting, Updyke and van Lieshout 1992). In addition, as pointed out by Winer et al. (1998), another somewhat surprising finding is that the classically nontonotopically-organized areas such as the insula or temporal cortex have projections to the IC that were as highly focused as those from the primary auditory cortical regions.
The degree to which the CC system retains the tonotopic relationship present in the AC carries significant importance because of the frequency-specific effects of AC stimulation on the IC, as described above. With few exceptions (Andersen, Snyder and Merzenich 1980; Budinger et al. 2013), most anatomical tracing studies of the CC pathway studies did not involve tonotopic mapping of the AC prior to tracer injection, and therefore tonotopic relationships were inferred based on known maps and the examination of whether changes in injection site produce a systematic change in the location of the tracer in the IC, specifically in the CNIC with its known isofrequency laminae. This point of weakness in the literature will likely improve as optical mapping techniques become more commonly used in conjunction with anatomical tracing (Takemoto et al. 2013; Budinger et al. 2013). In an early study by Anderson et al. in the cat (1980), the investigators systematically injected tracer along the electrophysiologically-characterized tonotopic axis of the AC and found a systematic change in the location of label in the IC. Other investigators have seen similar results (Herbert, Aschoff and Ostwald 1991; Saldaña, Feliciano and Mugnaini 1996), though the latter work did not involve electrophysiological verification. There were also some differences in the AC to IC topographic relationship across different subnuclei of the IC. For example, in the rat, injection of retrograde tracer into presumed high- and low-frequency parts of the DC (ventral vs. dorsal, respectively), produced somatic label in the presumed high- and low-frequency parts of Te1 (temporal area 1, a presumed homolog of A1), respectively. However, the retention of point-to-point topography was less evident in LC, which appears to receive a substantial input from area Te2 (temporal area 2, a nonprimary AC region (Herbert, Aschoff and Ostwald 1991)). In addition, Winer et al. (1998) observed substantial divergence and convergence in the CC pathway. They commented that very small injection sites in the AC often produced label across multiple areas of the IC and that each IC area received input from multiple regions of the AC. These data suggest that while there may be a core set of projections that contain point-to-point topography between the AC and IC, there are additional projections that are more diffuse.
Two recent studies highlight heterogeneities in the topographic relationship between neighboring regions of the AC and their projection targets in the IC. Budinger et al. placed dual anterograde tracers into frequency-restricted sites in the gerbil AC and found that the degree of retained local organization varied along the central auditory system. In the corticoreticular and corticothalamic systems, strong point-to-point topography was evident such that high- and low-frequency regions of the AC projected to different regions of the thalamic reticular nucleus and the medial geniculate body. In contrast, high- and low frequency descending projections were observed to strongly overlap in the superior olive. In the IC, the high-frequency CC fibers remain segregated from the low-frequency fibers only in portions of the DC (high-frequency fibers project anterior to low-frequency fibers), but intermixed in other parts of the DC, as well as in the LC and CNIC (Budinger et al. 2013). In the guinea pig, Markovitz et al. placed electrode arrays into the IC and AC to investigate the mapping of CC inputs. They found strong point-to-point topography between AC and IC, though they found the breadth of tuning to be more variable. Certain sites had very sharp tuning, while other sites in the IC received AC inputs across a range of frequencies at least an octave in width (Markovitz, Tang and Lim 2013).
These studies indicate that a tonotopically-organized set of descending projections exists that could support frequency-specific effects of CC stimulation, such as the frequency-specific inhibition required to alter the characteristic frequency of an IC neuron. However, inputs that are necessarily off-CF, which would be needed to produce egocentric selection in the frequency domain, or modulations of responses to sensory features not known to be systematically mapped in the cortex, such as duration tuning (Ma and Suga 2001), may also have a substrate in the convergent non-tonotopic projections from the AC to the IC.
One point of contention in the CC anatomy literature is whether there are descending projections to the central nucleus of the IC, and, if so, are they species-specific? Most studies acknowledge the possibility of projections to the central nucleus, but only in primates. In an early primate study, Fitzpatrick and Imig used injections of tritiated proline to anterogradely label projections from the AC of the owl monkey (Fitzpatrick and Imig 1978). Following injections into A1, labeling in the CNIC appeared as a band of silver grains oriented parallel to known isofrequency contours in the IC (see Figure 1F). Interpretation of these findings depends on the definition of the CNIC. In this case, the medial area labeled as CNIC appears to occupy what is generally considered DC in most anatomical divisions in other species (Andersen, Snyder and Merzenich 1980; Herbert, Aschoff and Ostwald 1991; Saldaña, Feliciano and Mugnaini 1996; Winer et al. 1998). However, modern studies in the same species appear to roughly confirm Fitzpatrick and Imig's subdivisions (Hackett, Takahata and Balaram 2011; Engle et al. 2014). Irrespective of differences in definitions of subdivisions, granting the CNIC space medially it is still clear that most of the projections are to the CNIC and not to the DC and LC, curiously, which are most prevalent in other species (Andersen, Snyder and Merzenich 1980; Herbert, Aschoff and Ostwald 1991; Saldaña, Feliciano and Mugnaini 1996; Winer et al. 1998). This finding may indicate that CC projections differ between primates and other species, such as cats and rats. An important proviso to this interpretation is that it is difficult to distinguish between terminals and fibers using this technique, raising the possibility that the anterograde signals seen in the central nucleus were passing fibers. Since then, multiple studies using modern tracers and electron microscopy have found clear evidence for CC terminals in the CNIC in rats, cats, gerbils, guinea pig and ferrets (Saldaña, Feliciano and Mugnaini 1996; Winer et al. 1998; Bajo and Moore 2005; Bajo et al. 2007; Nakamoto et al. 2013), though generally in lower numbers than in other regions of the IC. In cats, the strongest projections to the central nucleus, come intriguingly from nontonotopic areas of dorsal and intermediate, part of the posterior ectosylvian gyrus. Thus, it appears that an AC to CNIC projection exists in all species studied, though it is generally considerably smaller in size than the projections to the DC or LC. As pointed out previously (Malmierca and Ryugo 2011), the preponderance of CNIC projections compared to DC or LC seen in the primate may be related to species-specific differences, differences in the parcellation of the IC, or tracer-related methodological issues.
3. Laminar sources of corticocollicular input
The specific layers from which corticocollicular projections derive may have functional implications. In other descending systems, such as the corticothalamic system, descending input comes from layer 5 and layer 6, and these neurons are embedded in different cortical subnetworks, have different intrinsic properties, different patterns of projections to the thalamus, and possibly, different roles in shaping thalamic function (Ojima 1994; Reichova and Sherman 2004; Llano and Sherman 2008; Llano and Sherman 2009; Theyel, Llano and Sherman 2010). Corticocollicular neurons arise from layers 5 and 6 of the cortex (Games and Winer 1988; Künzle 1995; Doucet, Molavi and Ryugo 2003; Bajo and Moore 2005; Coomes, Schofield and Schofield 2005; Bajo et al. 2007; Schofield 2009; Slater, Willis and Llano 2013). Probably due to the relative insensitivity of WGA-HRP compared to more modern tracing techniques, there is some discrepancy in the literature with regard to the degree of contribution from layer 6. However, recent investigations using more sensitive tracers have shown that, in addition to the larger subset of layer 5 CC neurons found in older studies, there is a smaller subset, about10%, that arise from layer 6 (Schofield 2009).
Several investigators have examined the details of distribution of retrogradely-labeled CC cells in the AC. For example, Doucet et al. (2003) retrogradely labeled cortical projections to the IC, the superior olivary complex, and the cochlear nucleus using sequential double retrograde labeling. Corticocollicular projections outnumbered cortical projections to the superior olive and cochlear nucleus by at least a factor of 10, confirming that the IC is one of the most strongly cortically-innervated areas of the auditory system. Further, there was a distinct laminar distribution of the IC, superior olive, and cochlear nucleus-projecting neurons within layer 5, suggesting that these three pathways may derive from different populations of cortical neurons. CC neurons were found to occupy all of layer 5, while those neurons projecting to the SOC and CN tend to hug the area between layer 5 and 6. Approximately10-20% of the cells were double labeled, though this may be an underestimate since no attempt to place tracers in tonotopically-aligned regions in the subcortical nuclei. In addition, layer 6 CC neurons were also found to project to the IC and these were located in the most ventral portion of layer 6, abutting the white matter. Only layer 5 CC neurons were found on the contralateral side.
Schofield (2009) confirmed and extended many of these results. Using spatially restricted injections of retrograde tracer to the guinea pig IC, and multiple tracers, two bands of cells were found in the ipsilateral AC, a dense band of cells in layer 5 and a second in deep in layer 6. In general, layer 5 cells were concentrated in the center of the layer, while layer 6 cells were almost exclusively found in the most ventral part of layer 6, bordering the white matter. Layer 6 cells constituted about 10% of the total. Cell morphology in both layers was both pyramidal and nonpyramidal. Only a very small fraction of layer 6 corticollicular projections were found contralaterally, and those that were had morphology typical of layer 5 cells.
More recently, physiological and morphological differences were observed between layer 5 and layer 6 CC cells. Slater et al. (2013) retrogradely prelabeled CC cells in the adult mouse by injecting fluorescent microspheres into the IC. They found that layer 5 CC cells, similar to layer 5 corticothalamic cells, tended to be pyramidal in shape, with a large, tufted apical dendrite, produce either regular spiking patterns or rhythmic bursting when depolarized, and had Ih-mediated rebounds after hyperpolarization. Layer 6 CC cells had expansive and profuse dendritic branching. These dendrites were quite long - some of them reaching 1000 μm in length, making them some of the longest dendrites in the cortex. They did not fire in bursts or have Ih-mediated currents. These data suggest that layer 5 and layer 6 CC neurons receive different sets of inputs from the cortex, and respond to those inputs in different ways (Fig. 2). The specific roles, physiological properties and specific projection patterns of layers 5 and 6 neurons are not yet known, though there are some data suggesting that larger layer 5 cells with tufts extending to layer 1 tend to project to the LC and DC, while smaller pyramidal cells in A1 project to the CNIC (Bajo and Moore 2005). Clearly more work needs to be done to clarify the differences, if any, among the different classes of CC cells relating to their roles in modulation of the IC.
Figure 2.
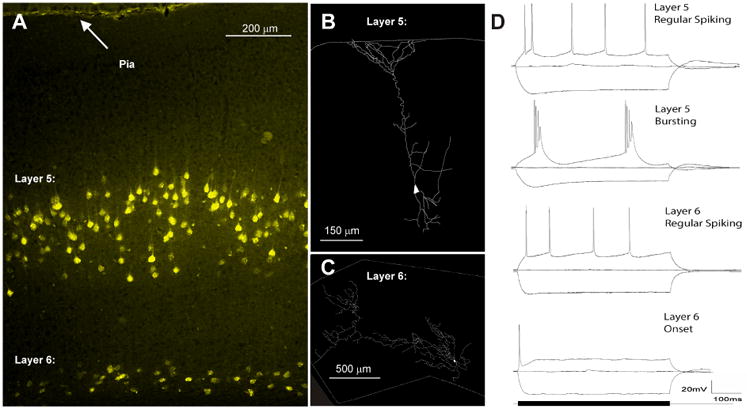
Differences between layer 5 and layer 6 corticocollicular cells. A) Retrogradely labeled layer 5 and layer 6 mouse corticocollicular cells filled with fluoro-gold after IC injection. B) Layer-specific morphological differences between a layer 5 and C) layer 6 corticocollicular cell. D) Layer 5 CC cells show both regular spiking and intrinsic bursting profiles as well as H-current mediated sag and rebound currents not seen in layer 6 (modified from Slater et al. 2013).
4. Molecular and circuit-level considerations
The IC is a complex and heterogeneous structure. It comprises at least 3 subdivisions, contains multiple different cell types which are distinguishable based on morphological and electrophysiological grounds (Morest and Oliver 1984; Faye-Lund and Osen 1985; Peruzzi, Sivaramakrishnan and Oliver 2000; Ahuja and Wu 2007; Malmierca, Blackstad and Osen 2011), and receives a range of ascending acoustically-driven inputs from several different brainstem regions involving glutamatergic, GABAergic and glycinergic synapses (Zhang et al. 1998; Loftus et al. 2004), as well as inputs from neuromodulators such as acetylcholine (Motts and Schofield 2009). Within the CNIC, there are functional zones that receive distinct sets of inputs from brainstem nuclei and which respond in differing ways to synaptic input (Loftus, Bishop and Oliver 2010; Chandrasekaran, Xiao and Sivaramakrishnan 2013), suggesting a level of functional modularity within the IC beyond that suggested by the distribution of cell types. In addition, histochemical approaches have revealed a more complicated compartmental organization within individual regions (Chernock, Larue and Winer 2004). Finally, intra-collicular connectivity within and across subnuclei, which have not been well characterized yet, adds an additional level of complexity to this picture (Saldaña and Merchań 1992; Malmierca et al. 1998; Bajo et al. 1999; Ahuja and Wu 2007). Precisely how the CC system interfaces with this intrinsic organizational heterogeneity of the IC is not yet known. We are only now beginning to answer certain basic questions, such as: What cell types receive CC input? Is CC input excitatory or inhibitory? How does CC input influence intra-collicular processing? Below, we summarize what is known about the interface between the CC system and local cellular and molecular architectures of the IC.
Early investigators had speculated that the CC pathway may be inhibitory (Andersen, Snyder and Merzenich 1980). This was based on physiological findings that CC inputs inhibited sound-induced IC responses (Massopust Jr and Ordy 1962; Amato, La Grutta and Enia 1970). However, electron microscopy of labeled CC synapses in the DC, LC and CNIC all showed asymmetric terminals, and the presence of small round vesicles in CC synaptic boutons that are indicative of excitatory synapses (Saldaña, Feliciano and Mugnaini 1996; Nakamoto et al. 2013). In addition, lesioning of the CC system depressed synaptic release of labeled aspartate, further suggesting that these projections are glutamatergic (Feliciano and Potashner 1995). These data demonstrating that the CC pathway is excitatory coexist with contrary data demonstrating that activation of CC projections can depress responses to sounds and depress spontaneous activity (Massopust Jr and Ordy 1962; Amato, La Grutta and Enia 1970; Syka and Popelář 1984; Bledsoe, Shore and Guitton 2003), can generate IPSPs in IC neurons (Mitani, Shimokouchi and Nomura 1983) and can shift tuning away from previously responded-to sound features (Suga and Ma 2003). These physiological data suggest that the CC pathway must in fact activate inhibitory circuitry as well as excitatory circuitry. One possibility is that the cellular targets of the CC system are inhibitory. However, only approximately 4% of the neurons targeted by the CC pathway are GABAergic (Nakamoto et al. 2013). As discussed by Malmierca and Ryugo (2011), this implies that alternative inhibitory pathways may be involved, such as longer polysynaptic pathways in the IC, non-GABAergic (e.g. glycinergic) pathways, longer-range pathways (e.g. corticobulbar, or cascading projections to the olivocochlear system), or, alternatively that the GABAergic cells by the CC pathway are very highly branched, producing substantial inhibition out of proportion to their cellular numbers (Nakamoto et al. 2013).
To sort through these possibilities, it will be important to identify and classify the cell types in the IC that receive CC input. There are multiple IC cell types that have been defined on morphological, connectional and physiological grounds (Peruzzi, Sivaramakrishnan and Oliver 2000; Ahuja and Wu 2007; Malmierca, Blackstad and Osen 2011). There are data suggesting that both commissural and tectothalamic cells receive axosomatic input from the AC (Coomes Peterson and Schofield 2007; Nakamoto, Sowick and Schofield 2013). However, most of the inputs from the CC system terminate in the neuropil, primarily on distal dendrites and spines (Nakamoto et al. 2013), suggesting that the majority of the cell types receiving CC input and their projection targets have not yet been characterized. Further, the cells that have been shown to receive AC input have not had any physiological characterization. Early data suggests that pause-regular cells in the IC receive CC input (Llano et al. 2014), but more work needs to be done to clarify the cellular targeting of the CC system. One point that appears to be clear is that the CC system uses VGLUT1 transporters (Ito and Oliver 2010). Expression of VGLUT1 is strong in the cerebral cortex, while VGLUT2 is expressed in most every region of the ascending auditory pathway (Hackett, Takahata and Balaram 2011). Although the two are not usually expressed together, coexpression has been reported, but despite the occasional colocalization, there remains a separation of the two at the circuit level (Ito, Bishop and Oliver 2011). In addition, back-labeled CC cells also express VGLUT1, and not VGLUT2 (Ito and Oliver 2010). Together, these results imply that CC cells are glutamatergic and use the VGLUT1 transporter. This distinction is important, since it has been speculated that neurons expressing VGLUT1 may have a higher capacity for plasticity than those expressing VGLUT2, and may recycle faster and support higher firing rates (Fremeau et al. 2004; Fremeau Jr et al. 2004). These data are suggestive of differences in the temporal properties and the potential for plasticity between ‘bottom up’ and ‘top down’ influences on any given IC cell.
4.1. Modularity and layer-specificity of the nonlemniscal IC
An intriguing set of findings by Chernock et al. (2004), revealed regional heterogeneities in the non-primary (and presumably cortico-recipient) portions of the IC. They applied histological approaches to the rat IC and found clearly identifiable modules, identifiable as positive for some combination of several biochemical markers, in layer 2 of the LC as well as in upper layers of the DC. Modules were found with glutamic acid decarboxylase (GAD), parvalbumin and acetylcholinesterase (AChE) staining (see Figs 3A and B). The modules colocalized with nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-d), and cytochrome oxidase indicating that these modules are sites of high metabolic activity. No modularity was reported in immunostaining for glycine, serotonin or calbindin. The border between intra and extramodular neuropil was sharp. Modules in DC, layer 2 of the LC and in the rostral pole were distinguished by a population of GAD-positive somatic staining and a much higher local concentration of terminals than in non-modular parts of these nuclei (Chernock, Larue and Winer 2004). Other studies have shown the presence of such modularity and/or patchiness in the monkey, rat and mouse, though not always commented upon in the text (Paxinos, Watson and Emson 1980; Herbert, Aschoff and Ostwald 1991; Ono, Yanagawa and Koyano 2005; Ito, Bishop and Oliver 2009; Engle et al. 2014), and some unresolved questions remain. For example, Herbert et al. (1991) appear to show NADPH-d staining in the LC that is nonmodular and avoids layer 2, while other studies in rat (e.g. Chernock et al. 2004) and our own unpublished work in mouse (Fig 3C) suggest that NADPH-d shows strong modularity and is present in layer 2.
Figure 3.
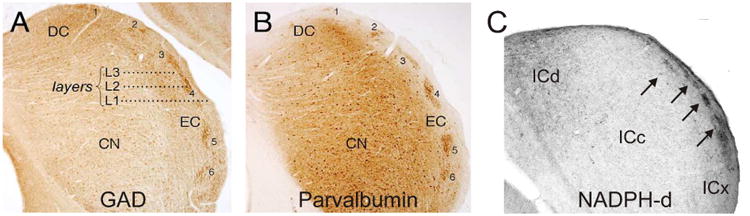
Modular organization of LC, shown in transverse sections of the IC. A) Immunostaining for GAD. B) Immunostaining for parvalbumin. (reproduced from Chernock et al. 2004). C) Staining for NADPH-d in mouse IC (unpublished data, Llano laboratory). Arrows correspond to modules.
The other major source of heterogeneity has to do with layering in the LC. GABAergic and glycinergic neurotransmission play important roles in IC physiology (Palombi and Caspary 1996; Wenstrup and Leroy 2001), and molecular markers for these differ in different regions of the IC. Distinctly higher expression of the vesicular inhibitory amino acid transporter is found in layer 2 of the LC, a result which is consistent with studies showing GAD staining in layer 2 of the LC (Ito, Bishop and Oliver 2011). In addition, for the vesicular glutamate transporters, the labeling of VGLUT1 is densest in the DC and although less dense in the CNIC and LC (Altschuler et al. 2008), the lack of labeling in layer 2 of the LC is remarkable. Given that CC cells likely employ VGLUT1(Ito and Oliver 2010), it is conceivable that the lack of VGLUT1 labeling is due to a paucity CC terminals in this region. In contrast, circumferential axo-somatic VGLUT2 labeling, thought to be only on giant GABAergic tectothalamic neurons (Ito, Bishop and Oliver 2009), was seen in layer 2, not layers 1 and 3 (Altschuler et al. 2008) and appears to be seen preferentially in the extramodular parts of layer 2.
The modularity of the IC takes on potential importance when considered in the context of modularity and target layer specificity of the descending CC system. Several investigators have found that the CC system ends in distinct clusters of terminals in rat and cat (Saldaña, Feliciano and Mugnaini 1996; Winer et al. 1998), as shown in Fig. 1a and b. These data suggest that the CC projections to the LC have a clustered and layer-specific termination pattern that superficially matches layering and clustering seen across a number of molecular markers in the LC. In other neural systems previously thought to be homogenous, such as the patch-matrix organization of the basal ganglia, great progress was made once molecular heterogeneities were mapped onto connectional heterogeneities (Alexander and Crutcher 1990; Parent and Hazrati 1995). Therefore, it will be important to determine whether the descending CC and intrinsic mosaic organization of the LC are aligned, interdigitating, or have some other spatial relationship, and then ultimately to determine the physiological significance of these relationships.
4.2. Potential lemniscal-nonlemniscal IC relationships
In any survey of the CC system that includes both physiology and anatomy, a fundamental problem of synthesis across the two levels of investigation arises. Physiological studies have focused, almost without exception, on the effects of corticofugal modulation in the CNIC, while anatomical studies show that the vast majority of projections are to the DC and LC. Exactly how projections from the AC influence neurons in the DC and LC is not known, and the circuit through which such influences are transferred to the CNIC to cause all the specific changes shown in physiological experiments is not at all known.
Jen et al. tested the hypothesis that inhibitory LC to CNIC projections mediate changes in the CNIC after cortical stimulation (Jen, Sun and Chen 2001). In this study, pairing LC recordings with AC stimulation showed that neurons in the LC increased their responsiveness in terms of auditory spatial response area, frequency response area and rate level functions after AC stimulation. In contrast, stimulation of the AC tended to diminish acoustic responsiveness of CNIC neurons. To examine the potential indirect effects from the AC to the CNIC, neurons in the CNIC were recorded while the LC was stimulated. Stimulation in the LC showed a clear inhibitory effect in 42 out of 42 CNIC neurons studied. Rate intensity functions were drastically reduced, azimuth responsiveness was reduced in all directions, and frequency tuning curves were narrowed. Application of bicuculine to the CNIC elevated rate intensity functions, azimuth responsiveness, and broadened frequency tuning curves but this effect was specific to the low frequency end of the frequency tuning curves. This finding is intriguing, particularly because experiments in the mouse show that there can be very different effects on the high or low-frequency side of tuning curves if the characteristic frequency of stimulated neurons in the AC is higher or lower than the IC neuron (Yan and Ehret 2002). Other possible explanations exist. For example, given the demonstration that local collaterals from CNIC neurons branch to innervate large GABAergic neurons throughout the IC (Ito and Oliver 2014), stimulation of the LC may cause antidromic activation of CNIC neurons that project to the LC. This may have activated local GABAergic neurons, producing the above-described inhibition in the Jen et al. study. Therefore, important questions remain unanswered about the relationship between the CC pathway and local LC-CNIC circuitry.
5. Summary and conclusions
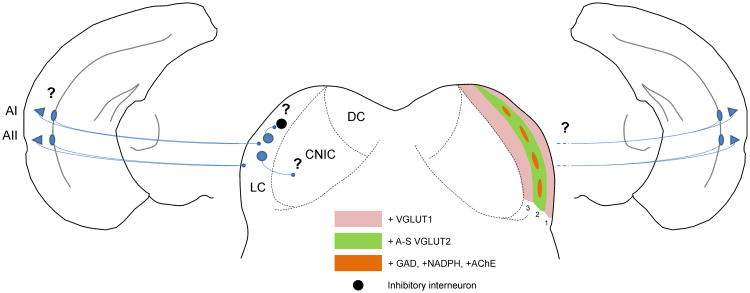
The projections from the AC to the IC are massive and complex. Anatomically, they are derived from all regions of the AC and are highly organized, with components that are tonotopic and other components that are not. In addition, the patterns of CC terminals in the IC appear to either match or complement the intrinsic expression patterns of several molecular markers, such as GAD, NADPH-d, AChE, cytochrome oxidase and parvalbumin, as well as the intrinsic and ascending circuitry to the IC. The descending input to the IC is derived primarily from cortical layer 5 intrinsically bursting and regular spiking pyramidal cells, with a smaller component from deep layer 6, though the distributions of terminals from these two layers and their physiological significance is not yet clear. Finally, although the physiological data suggest that the CC pathway provides both excitation and inhibition onto the IC, most of the anatomical data suggest that the pathway itself is excitatory and synapses primarily on non-GABAergic cells in the IC, at least some of which are tectothalamic projection neurons or commissural neurons that project to the contralateral IC. Finally, the discrepancy between the anatomical data, which show most of the CC terminations in the non-lemniscal regions of the IC, and the physiological data, which show significant effects in the CNIC, remains relatively unexplored, though at least one study is suggestive of an inhibitory LC to CNIC pathway (See Fig. 4 for a summary diagram).
Figure 4.
Diagram summarizing known and unknown circuit and molecular components of the CC pathway. Only the LC is shown in detail for clarity. Left: Layer 5 pyramidal cells (triangles) and layer 6 nonpyramidal cells (ovals) from AI and AII project to the LC. The specific contributions of layers 5 and 6 are not known and denoted with question marks. AII projections are more superficial than AI projections (Herbert, Aschoff and Ostwald 1991; Winer et al. 1998). Intrinsic excitatory cells of the IC are shown in blue. The black cell is a GABAergic neuron. Unknown connections to the CNIC and to GABAergic neurons are denoted with question marks. Right: Mosaic molecular organization of the LC. Layers 1 and 3 stain for VGLUT1. Layer 2 modules are positive for GAD67, AChE and NADPH-d. Layer 2 extramodular regions are positive for circumferential axo-somatic VGLUT2 profiles (A-S VGLUT2), thought to be seen on giant GABAergic tectothalamic neurons (Ito, Bishop and Oliver 2009). The question mark denotes the uncertainty about how the CC projections map onto the molecular heterogeneities across and within layers.
These findings shape an emerging picture of how the AC modifies sensory processing in the IC. Early work on this pathway involved relatively gross measures, such as bulk injection of tracers, electrical stimulation or silencing of the cortex and extracellular recordings in the IC, and revealed an intriguing set of modulatory phenomena. Subsequent work has shown that at virtually every level of this pathway, there is important cell-type or regional heterogeneity that is likely responsible for the wide array of effects seen in the IC after AC manipulation. We also now understand many of the potential indirect ways that the AC may affect the IC, either via the thalamus (Kuwabara and Zook 2000; Winer et al. 2002; Senatorov and Hu 2002; Kuwabara 2012; Llano et al. 2014), cholinergic afferents (Schofield and Motts 2009) or via cascading modulation of lower auditory centers, including at the level of the hair cells (Mulders and Robertson 2000; Xiao and Suga 2002; Liu et al. 2010). Given the emergence of optical and molecular tools now available to the investigator, questions at the micro- and meso-circuit level can now be answered about these pathways. In addition, because of the ubiquitous nature of descending pathways from sensory regions of the cortex, lessons learned about the CC pathway will likely lead to broader insights about top down modulatory pathways in general.
Highlights.
We review recent data describing complexities in the auditory corticocollicular pathway.
Within the auditory cortex, several classes of neurons project to the inferior colliculus.
Corticocollicular axons contain tonotopic and non-tonotopic projections with nonuniform termination patterns.
Molecular heterogeneity exists within the cortical recipient zones of the inferior colliculus.
We provide a framework for advancing our understanding of the corticocollicular sytem.
Acknowledgments
This work emerged out of a class project supervised by Dr. Pritesh Pandya. We thank him for his critical review of an earlier version of this manuscript. D.A.L. was supported under DC012125. We also thank two anonymous reviewers for their insightful comments and suggestions.
Abbreviations
- A1
Primary auditory cortex
- AAF
Anterior auditory field
- AC
Auditory cortex
- AChE
Acetylcholinesterase
- APV
(2R)-amino-5-phosphonovaleric acid; (2R)-amino-5-phosphonopentanoate
- CC
Corticocollicular
- CCx
Caudal cortex
- CF
Characteristic frequency
- CN
Central nucleus of the inferior colliculus
- CNIC
Central nucleus of the inferior colliculus
- D
Dorsal
- DC
Dorsal cortex of the inferior colliculus
- ECIC
External cortex of the inferior colliculus
- GABA
Gamma-amino butyric acid
- GAD
Glutamic acid decarboxylase
- IC
Inferior colliculus
- Ih
Hyperpolarization-activated cation current
- LC
Lateral cortex of IC
- NADPH-d
Nicotinamide adenine dinucleotide phosphate-diaphorase
- PTN
Paracentral tectal nuclei
- SOC
Superior olivary complex
- Te1
Temporal area 1
- VGLUT1
Vesicular Glutamate transporter 1
- VGLUT2
Vesicular Glutamate transporter 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahuja T, Wu S. Intrinsic membrane properties and synaptic response characteristics of neurons in the rat's external cortex of the inferior colliculus. Neuroscience. 2007;145(3):851–865. doi: 10.1016/j.neuroscience.2006.12.031. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends in neurosciences. 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Altschuler RA, Tong L, Holt AG, Oliver DL. Immunolocalization of vesicular glutamate transporters 1 and 2 in the rat inferior colliculus. Neuroscience. 2008;154(1):226–232. doi: 10.1016/j.neuroscience.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato G, La Grutta V, Enia F. The control of acoustic input in the medial geniculate body and inferior colliculus by auditory cortex. Experientia. 1970;26(1):55–56. doi: 10.1007/BF01900389. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Snyder RL, Merzenich MM. The topographic organization of corticocollicular projections from physiologically identified loci in the AI, AII, and anterior auditory cortical fields of the cat. Journal of Comparative Neurology. 1980;191(3):479–494. doi: 10.1002/cne.901910310. [DOI] [PubMed] [Google Scholar]
- Anderson LA, Malmierca MS. The effect of auditory cortex deactivation on stimulus-specific adaptation in the inferior colliculus of the rat. European Journal of Neuroscience. 2013;37(1):52–62. doi: 10.1111/ejn.12018. [DOI] [PubMed] [Google Scholar]
- Bajo VM, King AJ. Cortical modulation of auditory processing in the midbrain. Frontiers in neural circuits. 2011;6:114–114. doi: 10.3389/fncir.2012.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo VM, Merchan MA, Malmierca MS, Nodal FR, Bjaalie JG. Topographic organization of the dorsal nucleus of the lateral lemniscus in the cat. Journal of Comparative Neurology. 1999;407(3):349–366. doi: 10.1002/(sici)1096-9861(19990510)407:3<349::aid-cne4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Bajo VM, Moore DR. Descending projections from the auditory cortex to the inferior colliculus in the gerbil, Meriones unguiculatus. The Journal of Comparative Neurology. 2005;486(2):101–116. doi: 10.1002/cne.20542. [DOI] [PubMed] [Google Scholar]
- Bajo VM, Nodal FR, Bizley JK, Moore DR, King AJ. The ferret auditory cortex: descending projections to the inferior colliculus. Cerebral Cortex. 2007;17(2):475–491. doi: 10.1093/cercor/bhj164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo VM, Nodal FR, Moore DR, King AJ. The descending corticocollicular pathway mediates learning-induced auditory plasticity. Nat Neurosci. 2010;13(2):253–260. doi: 10.1038/nn.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluch F, Itti L. Mechanisms of top-down attention. Trends in neurosciences. 2011;34(4):210–224. doi: 10.1016/j.tins.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Bastos Andre M, Usrey WM, Adams Rick A, Mangun George R, Fries P, Friston Karl J. Canonical Microcircuits for Predictive Coding. Neuron. 2012;76(4):695–711. doi: 10.1016/j.neuron.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe S, Shore SE, Guitton M. Spatial representation of corticofugal input in the inferior colliculus: a multicontact silicon probe approach. Experimental Brain Research. 2003;153(4):530–542. doi: 10.1007/s00221-003-1671-6. [DOI] [PubMed] [Google Scholar]
- Boly M, Garrido MI, Gosseries O, Bruno MA, Boveroux P, Schnakers C, Massimini M, Litvak V, Laureys S, Friston K. Preserved feedforward but impaired top-down processes in the vegetative state. Science. 2011;332(6031):858–862. doi: 10.1126/science.1202043. [DOI] [PubMed] [Google Scholar]
- Budinger E, Brosch M, Scheich H, Mylius J. The subcortical auditory structures in the Mongolian gerbil: II. Frequency-related topography of the connections with cortical field AI. Journal of Comparative Neurology. 2013;521(12):2772–2797. doi: 10.1002/cne.23314. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran L, Xiao Y, Sivaramakrishnan S. Functional architecture of the inferior colliculus revealed with voltage-sensitive dyes. Frontiers in neural circuits. 2013;7 doi: 10.3389/fncir.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernock ML, Larue DT, Winer JA. A periodic network of neurochemical modules in the inferior colliculus. Hearing research. 2004;188(1):12–20. doi: 10.1016/S0378-5955(03)00340-X. [DOI] [PubMed] [Google Scholar]
- Coomes DL, Schofield RM, Schofield BR. Unilateral and bilateral projections from cortical cells to the inferior colliculus in guinea pigs. Brain Research. 2005;1042(1):62–72. doi: 10.1016/j.brainres.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Coomes Peterson D, Schofield BR. Projections from auditory cortex contact ascending pathways that originate in the superior olive and inferior colliculus. Hearing research. 2007;232(1-2):67–77. doi: 10.1016/j.heares.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet J, Molavi D, Ryugo D. The source of corticocollicular and corticobulbar projections in area Te1 of the rat. Experimental Brain Research. 2003;153(4):461–466. doi: 10.1007/s00221-003-1604-4. [DOI] [PubMed] [Google Scholar]
- Engle JR, Gray DT, Turner H, Udell JB, Recanzone GH. Age-related neurochemical changes in the rhesus macaque inferior colliculus. Frontiers in Aging Neuroscience. 2014;6 doi: 10.3389/fnagi.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye-Lund H, Osen KK. Anatomy of the inferior colliculus in rat. Anatomy and embryology. 1985;171(1):1–20. doi: 10.1007/BF00319050. [DOI] [PubMed] [Google Scholar]
- Feliciano M, Potashner SJ. Evidence for a Glutamatergic Pathway from the Guinea Pig Auditory Cortex to the Inferior Colliculus. Journal of Neurochemistry. 1995;65(3):1348–1357. doi: 10.1046/j.1471-4159.1995.65031348.x. [DOI] [PubMed] [Google Scholar]
- FitzPatrick KA. Cellular architecture and topographic organization of the inferior colliculus of the squirrel monkey. Journal of Comparative Neurology. 1975;164(2):185–207. doi: 10.1002/cne.901640204. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick KA, Imig TJ. Projections of auditory cortex upon the thalamus and midbrain in the owl monkey. Journal of Comparative Neurology. 1978;177(4):537–555. doi: 10.1002/cne.901770402. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends in neurosciences. 2004;27(2):98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular Glutamate Transporters 1 and 2 Target to Functionally Distinct Synaptic Release Sites. Science. 2004;304(5678):1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- Games KD, Winer JA. Layer V in rat auditory cortex: Projections to the inferior colliculus and contralateral cortex. Hearing Research. 1988;34(1):1–25. doi: 10.1016/0378-5955(88)90047-0. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Nobre AC. Top-down modulation: bridging selective attention and working memory. Trends in Cognitive Sciences. 2012;16(2):129–135. doi: 10.1016/j.tics.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Li W. Top-down influences on visual processing. Nature Reviews Neuroscience. 2013;14(5):350–363. doi: 10.1038/nrn3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh A, de Kock CPJ, Wimmer VC, Sakmann B, Kuner T. Driver or Coincidence Detector: Modal Switch of a Corticothalamic Giant Synapse Controlled by Spontaneous Activity and Short-Term Depression. J Neurosci. 2008;28(39):9652–9663. doi: 10.1523/JNEUROSCI.1554-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, Takahata T, Balaram P. VGLUT1 and VGLUT2 mRNA expression in the primate auditory pathway. Hearing research. 2011;274(1-2):129–141. doi: 10.1016/j.heares.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harting JK, Updyke BV, van Lieshout DP. Corticotectal projections in the cat: Anterograde transport studies of twenty-five cortical areas. Journal of Comparative Neurology. 1992;324(3):379–414. doi: 10.1002/cne.903240308. [DOI] [PubMed] [Google Scholar]
- Herbert H, Aschoff A, Ostwald J. Topography of projections from the auditory cortex to the inferior colliculus in the rat. Journal of Comparative Neurology. 1991;304(1):103–122. doi: 10.1002/cne.903040108. [DOI] [PubMed] [Google Scholar]
- Ito T, Bishop DC, Oliver DL. Two classes of GABAergic neurons in the inferior colliculus. The Journal of neuroscience. 2009;29(44):13860–13869. doi: 10.1523/JNEUROSCI.3454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Bishop DC, Oliver DL. Expression of glutamate and inhibitory amino acid vesicular transporters in the rodent auditory brainstem. Journal of Comparative Neurology. 2011;519(2):316–340. doi: 10.1002/cne.22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Oliver DL. Origins of glutamatergic terminals in the inferior colliculus identified by retrograde transport and expression ofVGLUT1 and VGLUT2 genes. Auditory neuroanatomy: A sound foundation for sound processing. 2010:33. doi: 10.3389/fnana.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Oliver DL. Local and Commissural IC Neurons Make Axosomatic Inputs on Large GABAergic Tectothalamic Neurons. Journal of Comparative Neurology. 2014 doi: 10.1002/cne.23623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen P, Sun X, Chen Q. An electrophysiological study of neural pathways for corticofugally inhibited neurons in the central nucleus of the inferior colliculus of the big brown bat, Eptesicus fuscus. Experimental Brain Research. 2001;137(3-4):292–302. doi: 10.1007/s002210000637. [DOI] [PubMed] [Google Scholar]
- Künzle H. Regional and Laminar Distribution of Cortical Neurons Projecting to Either Superior or Inferior Colliculus in the Hedgehog Tenrec. Cerebral Cortex. 1995;5(4):338–352. doi: 10.1093/cercor/5.4.338. [DOI] [PubMed] [Google Scholar]
- Kuwabara N. Neuroanatomical technique for studying long axonal projections in the central nervous system: combined axonal staining and pre-labeling in parasagittal gerbil brain slices. Biotechnic & Histochemistry. 2012;87(6):413–422. doi: 10.3109/10520295.2012.688868. [DOI] [PubMed] [Google Scholar]
- Kuwabara N, Zook JM. Geniculo-collicular descending projections in the gerbil. Brain Research. 2000;878(1-2):79–87. doi: 10.1016/s0006-8993(00)02695-0. [DOI] [PubMed] [Google Scholar]
- Liu X, Yan Y, Wang Y, Yan J. Corticofugal modulation of initial neural processing of sound information from the ipsilateral ear in the mouse. PLOS ONE. 2010;5(11):e14038. doi: 10.1371/journal.pone.0014038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano DA, Sherman SM. Evidence for nonreciprocal organization of the mouse auditory thalamocortical-corticothalamic projection systems. The Journal of Comparative Neurology. 2008;507(2):1209–1227. doi: 10.1002/cne.21602. [DOI] [PubMed] [Google Scholar]
- Llano DA, Sherman SM. Differences in Intrinsic Properties and Local Network Connectivity of Identified Layer 5 and Layer 6 Adult Mouse Auditory Corticothalamic Neurons Support a Dual Corticothalamic Projection Hypothesis. Cerebral Cortex. 2009;19(12):2810–2826. doi: 10.1093/cercor/bhp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano DA, Slater BJ, Lesicko AM, Stebbings KA. An auditory colliculothalamocortical brain slice preparation in mouse. Journal of Neurophysiology. 2014;111(1):197–207. doi: 10.1152/jn.00605.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus WC, Bishop DC, Oliver DL. Differential patterns of inputs create functional zones in central nucleus of inferior colliculus. The Journal of neuroscience. 2010;30(40):13396–13408. doi: 10.1523/JNEUROSCI.0338-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus WC, Bishop DC, Saint Marie RL, Oliver DL. Organization of binaural excitatory and inhibitory inputs to the inferior colliculus from the superior olive. Journal of Comparative Neurology. 2004;472(3):330–344. doi: 10.1002/cne.20070. [DOI] [PubMed] [Google Scholar]
- Loftus WC, Malmierca MS, Bishop DC, Oliver DL. The cytoarchitecture of the inferior colliculus revisited: a common organization of the lateral cortex in rat and cat. Neuroscience. 2008;154(1):196–205. doi: 10.1016/j.neuroscience.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Suga N. Corticofugal modulation of duration-tuned neurons in the midbrain auditory nucleus in bats. Proceedings of the National Academy of Sciences. 2001;98(24):14060–14065. doi: 10.1073/pnas.241517098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca MS, Blackstad TW, Osen KK. Computer-assisted 3-D reconstructions of Golgi-impregnated neurons in the cortical regions of the inferior colliculus of rat. Hearing research. 2011;274(1):13–26. doi: 10.1016/j.heares.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Leergaard TB, Bajo VM, Bjaalie JG, Merchán MA. Anatomic evidence of a three-dimensional mosaic pattern of tonotopic organization in the ventral complex of the lateral lemniscus in cat. The Journal of neuroscience. 1998;18(24):10603–10618. doi: 10.1523/JNEUROSCI.18-24-10603.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca MS, Ryugo DK. The auditory cortex. Springer; 2011. Descending connections of auditory cortex to the midbrain and brain stem; pp. 189–208. [Google Scholar]
- Markovitz CD, Tang TT, Lim HH. Tonotopic and localized pathways from primary auditory cortex to the central nucleus of the inferior colliculus. Frontiers in neural circuits. 2013;7 doi: 10.3389/fncir.2013.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massopust LC, Jr, Ordy J. Auditory organization of the inferior colliculi in the cat. Experimental neurology. 1962;6(6):465–477. doi: 10.1016/0014-4886(62)90072-9. [DOI] [PubMed] [Google Scholar]
- Mei H, Chen Q. Neural modulation in inferior colliculus and central auditory plasticity. Frontiers in Biology. 2010;5(2):123–127. [Google Scholar]
- Mitani A, Shimokouchi M, Nomura S. Effects of stimulation of the primary auditory cortex upon colliculogeniculate neurons in the inferior colliculus of the cat. Neuroscience Letters. 1983;42(2):185–189. doi: 10.1016/0304-3940(83)90404-4. [DOI] [PubMed] [Google Scholar]
- Morest DK, Oliver DL. The neuronal architecture of the inferior colliculus in the cat: defining the functional anatomy of the auditory midbrain. Journal of Comparative Neurology. 1984;222(2):209–236. doi: 10.1002/cne.902220206. [DOI] [PubMed] [Google Scholar]
- Motts SD, Schofield BR. Sources of cholinergic input to the inferior colliculus. Neuroscience. 2009;160(1):103–114. doi: 10.1016/j.neuroscience.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders W, Robertson D. Evidence for direct cortical innervation of medial olivocochlear neurones in rats. Hearing research. 2000;144(1):65–72. doi: 10.1016/s0378-5955(00)00046-0. [DOI] [PubMed] [Google Scholar]
- Nakamoto KT, Mellott JG, Killius J, Storey-Workley ME, Sowick CS, Schofield BR. Ultrastructural examination of the corticocollicular pathway in the guinea pig: a study using electron microscopy, neural tracers, and GABA immunocytochemistry. Frontiers in neuroanatomy. 2013;7 doi: 10.3389/fnana.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto KT, Sowick CS, Schofield BR. Auditory cortical axons contact commissural cells throughout the guinea pig inferior colliculus. Hearing research. 2013;306:131–144. doi: 10.1016/j.heares.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojima H. Terminal Morphology and Distribution of Corticothalamic Fibers Originating from Layers 5 and 6 of Cat Primary Auditory Cortex. Cerebral Cortex. 1994;4(6):646–663. doi: 10.1093/cercor/4.6.646. [DOI] [PubMed] [Google Scholar]
- Ojima H, Murakami K. Triadic synaptic interactions of large corticothalamic terminals in non-lemniscal thalamic nuclei of the cat auditory system. Hearing research. 2011;274(1):40–47. doi: 10.1016/j.heares.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Ono M, Yanagawa Y, Koyano K. GABAergic neurons in inferior colliculus of the GAD67-GFP knock-in mouse: electrophysiological and morphological properties. Neuroscience research. 2005;51(4):475–492. doi: 10.1016/j.neures.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Palombi PS, Caspary DM. GABA inputs control discharge rate primarily within frequency receptive fields of inferior colliculus neurons. Journal of Neurophysiology. 1996;75(6):2211–2219. doi: 10.1152/jn.1996.75.6.2211. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Research Reviews. 1995;20(1):91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. Journal of Neuroscience Methods. 1980;3(2):129–149. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- Peruzzi D, Sivaramakrishnan S, Oliver DL. Identification of cell types in brain slices of the inferior colliculus. Neuroscience. 2000;101(2):403–416. doi: 10.1016/s0306-4522(00)00382-1. [DOI] [PubMed] [Google Scholar]
- Reichova I, Sherman SM. Somatosensory Corticothalamic Projections: Distinguishing Drivers From Modulators. J Neurophysiol. 2004;92(4):2185–2197. doi: 10.1152/jn.00322.2004. [DOI] [PubMed] [Google Scholar]
- Saldaña E, Feliciano M, Mugnaini E. Distribution of descending projections from primary auditory neocortex to inferior colliculus mimics the topography of intracollicular projections. The Journal of Comparative Neurology. 1996;371(1):15–40. doi: 10.1002/(SICI)1096-9861(19960715)371:1<15::AID-CNE2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Saldaña E, Merchań MA. Intrinsic and commissural connections of the rat inferior colliculus. The Journal of Comparative Neurology. 1992;319(3):417–437. doi: 10.1002/cne.903190308. [DOI] [PubMed] [Google Scholar]
- Schofield BR. Projections to the inferior colliculus from layer VI cells of auditory cortex. Neuroscience. 2009;159(1):246–258. doi: 10.1016/j.neuroscience.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield BR, Motts SD. Projections from auditory cortex to cholinergic cells in the midbrain tegmentum of guinea pigs. Brain research bulletin. 2009;80(3):163–170. doi: 10.1016/j.brainresbull.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatorov V, Hu B. Extracortical descending projections to the rat inferior colliculus. Neuroscience. 2002;115(1):243–250. doi: 10.1016/s0306-4522(02)00316-0. [DOI] [PubMed] [Google Scholar]
- Slater BJ, Willis AM, Llano DA. Evidence for layer-specific differences in auditory corticocollicular neurons. Neuroscience. 2013;229(0):144–154. doi: 10.1016/j.neuroscience.2012.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N. Tuning shifts of the auditory system by corticocortical and corticofugal projections and conditioning. Neuroscience & Biobehavioral Reviews. 2012;36(2):969–988. doi: 10.1016/j.neubiorev.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N, Ma X. Multiparametric corticofugal modulation and plasticity in the auditory system. Nat Rev Neurosci. 2003;4(10):783–794. doi: 10.1038/nrn1222. [DOI] [PubMed] [Google Scholar]
- Sun X, Xia Q, Lai CH, Shum DKY, Chan YS, He J. Corticofugal modulation of acoustically induced Fos expression in the rat auditory pathway. Journal of Comparative Neurology. 2007;501(4):509–525. doi: 10.1002/cne.21249. [DOI] [PubMed] [Google Scholar]
- Syka J, Popelář J. Inferior colliculus in the rat: neuronal responses to stimulation of the auditory cortex. Neuroscience Letters. 1984;51(2):235–240. doi: 10.1016/0304-3940(84)90557-3. [DOI] [PubMed] [Google Scholar]
- Takemoto M, Hasegawa K, Nishimura M, Song WJ. The insular auditory field receives input from the lemniscal subdivision of the auditory thalamus in mice. Journal of Comparative Neurology. 2013 doi: 10.1002/cne.23491. [DOI] [PubMed] [Google Scholar]
- Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat Neurosci. 2010;13(1):84–88. doi: 10.1038/nn.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M, Hackett TA, Rakic P, Levitt P, Polley DB. EphA Signaling Impacts Development of Topographic Connectivity in Auditory Corticofugal Systems. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenstrup J, Leroy SA. Spectral integration in the inferior colliculus: role of glycinergic inhibition in response facilitation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21(3):RC124–RC124. doi: 10.1523/JNEUROSCI.21-03-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Chernock ML, Larue DT, Cheung SW. Descending projections to the inferior colliculus from the posterior thalamus and the auditory cortex in rat, cat, and monkey. Hearing research. 2002;168(1):181–195. doi: 10.1016/s0378-5955(02)00489-6. [DOI] [PubMed] [Google Scholar]
- Winer JA, Larue DT, Diehl JJ, Hefti BJ. Auditory cortical projections to the cat inferior colliculus. Journal of Comparative Neurology. 1998;400(2):147–174. [PubMed] [Google Scholar]
- Xiao Z, Suga N. Modulation of cochlear hair cells by the auditory cortex in the mustached bat. Nature neuroscience. 2002;5(1):57–63. doi: 10.1038/nn786. [DOI] [PubMed] [Google Scholar]
- Yan J, Ehret G. Corticofugal reorganization of the midbrain tonotopic map in mice. Neuroreport. 2001;12(15):3313–3316. doi: 10.1097/00001756-200110290-00033. [DOI] [PubMed] [Google Scholar]
- Yan J, Ehret G. Corticofugal modulation of midbrain sound processing in the house mouse. European Journal of Neuroscience. 2002;16(1):119–128. doi: 10.1046/j.1460-9568.2002.02046.x. [DOI] [PubMed] [Google Scholar]
- Yan J, Zhang Y, Ehret G. Corticofugal Shaping of Frequency Tuning Curves in the Central Nucleus of the Inferior Colliculus of Mice. Journal of Neurophysiology. 2005;93(1):71–83. doi: 10.1152/jn.00348.2004. [DOI] [PubMed] [Google Scholar]
- Yan W, Suga N. Corticofugal modulation of the midbrain frequency map in the bat auditory system. Nature neuroscience. 1998;1(1):54–58. doi: 10.1038/255. [DOI] [PubMed] [Google Scholar]
- Zhang DX, Li L, Kelly JB, Wu SH. GABAergic projections from the lateral lemniscus to the inferior colliculus of the rat. Hearing research. 1998;117(1):1–12. doi: 10.1016/s0378-5955(97)00202-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Suga N, Yan J. Corticofugal modulation of frequency processing in bat auditory system. Nature. 1997;387(6636):900–903. doi: 10.1038/43180. [DOI] [PubMed] [Google Scholar]