Abstract
Background
Maternal smoking in pregnancy is associated with adverse health outcomes in children, including cancers; underlying mechanisms may include epigenetic modifications. Using Illumina’s 450K array, we previously identified differential DNA methylation related to maternal smoking during pregnancy at 26 CpG sites (CpGs) in 10 genes in newborn cord bloods from the Norwegian Mother and Child Cohort Study (MoBa). Whether these methylation signals in newborns reflect in utero exposure only or possibly epigenetic inheritance of smoking-related modifications is unclear.
Methods
We therefore evaluated the impact of the timing of mother’s smoking (before or during pregnancy using cotinine measured at 18 weeks gestation), the father’s smoking before conception, and the grandmother’s smoking during her pregnancy with the mother on methylation at these 26 CpGs in 1,042 MoBa newborns. We used robust linear regression, adjusting for covariates, applying Bonferroni correction.
Results
The strongest and only statistically significant associations were observed for sustained smoking by the mother during pregnancy through at least gestational week 18 (p<1.6×10−5 for all 26 CpGs). We observed no statistically significant differential methylation due to smoking by the mother prior to pregnancy or that ceased by week 18, father’s smoking before conception, or grandmother’s smoking while pregnant with the mother.
Conclusions
Differential methylation at these CpGs in newborns appears to reflect sustained in utero exposure rather than epigenetic inheritance.
Impact
Smoking cessation in early pregnancy may negate effects on methylation. Analyses of maternal smoking during pregnancy and offspring health outcomes, including cancer, limited to ever smoking might miss true associations.
Keywords: Epigenetic, inheritance, methylation, prenatal, smoking
Introduction
Maternal smoking during pregnancy is associated with many adverse health outcomes in children including certain cancers such as childhood leukemia, lymphoma, and others (1). Recent evidence suggests that the underlying mechanisms behind detrimental effects of maternal smoking may involve epigenetic modifications such as DNA methylation (2–6). We previously reported associations between maternal smoking during pregnancy (measured objectively using cotinine in maternal plasma samples taken at gestational week 18) and differential DNA methylation in cord blood from newborns in the Norwegian Mother and Child Cohort Study (MoBa) (3). Using the Illumina Infinium HumanMethylation450 Beadchip (450K) we identified epigenome-wide statistically significant associations at 26 CpGs mapping to 10 genes. The genes included AHRR and CYP1A1 that are key members of the aryl hydrocarbon receptor pathway well known to be involved in biologic response to polyaromatic hydrocarbons in tobacco smoke. We also identified novel genes not previously recognized as playing a role in the response to tobacco smoke including genes involved in development (GFI1, MYO1G, CNTNAP2 and RUNX1) and other processes (HLA-DPB2, ENSG00000225718, EXT1, and TTC7B). We replicated our findings in an independent U.S. birth cohort. The methylation differences at these 26 CpGs were not seen in the range of cotinine consistent with secondhand smoke exposure of the mother. Of note, differential methylation in AHRR, GFI1, MYO1G, and CNTNAP2 have also been associated with smoking in adults (7–10). Methylation differences in AHRR related to smoking in adults have been observed in lung as well as blood (9) confirming that these findings do not reflect shifts in cell types due to smoking.
Multigenerational health effects from in utero exposure to smoking and other toxicants have been proposed in a few epidemiologic studies (11, 12). Our observation that DNA methylation at birth at these 26 CpGs is related to having a mother who smoked during pregnancy raises several questions about when and how these changes might occur. There is considerable interest in the possibility that environmental exposures such as smoking result in epigenetic effects that can be transmitted from one generation to the next but there is no direct evidence in humans (13). This mechanism has been referred to as transgenerational epigenetic inheritance (via the gametes) (14) and implies epigenetic alterations to gametes that escape reprogramming after fertilization.
One possible scenario for the inheritance of smoking-related methylation is that the mother’s smoking prior to becoming pregnant impacts the epigenome of the ovum that gives rise to the child. If this occurs, we might observe that offspring methylation is associated with the mother’s smoking before pregnancy, even if the mother stopped smoking before becoming pregnant. In a similar way, the father’s smoking before conception could impact the epigenome of the sperm that gave rise to the study child. If so, we might observe that offspring methylation is associated with smoking by the father prior to conception.
Another scenario for the inheritance of the smoking-related methylation changes could be that smoking by the study child’s grandmother, when she was pregnant with the mother, impacts the epigenome of the developing ovum that gave rise to the study child. If so, we would expect the grandmother’s smoking while pregnant with the mother to be associated with the study child’s methylation at birth, independently of the mother’s smoking during her pregnancy.
Alternatively, it is possible that the methylation differences at birth related to maternal smoking primarily reflect the in utero exposure. If so, it is relevant to ask whether early exposure (smoking very early in pregnancy followed by cessation) is sufficient or whether sustained exposure through pregnancy is needed.
To address these questions we performed new statistical analyses for the 26 CpGs where we observed methylation differences at birth related to maternal smoking during pregnancy. We examined the impact of the timing of the mother’s smoking (before or during pregnancy), the father’s smoking prior to conception, and the grandmother’s smoking in her pregnancy with the mother on methylation at these 26 CpGs.
Materials and Methods
Study population
Participants in the current analysis were selected from a substudy of the Norwegian Mother and Child Cohort Study (MoBa) (15, 16) that evaluated the association between maternal plasma folate during pregnancy and childhood asthma status at 3 years of age (17). Umbilical cord blood samples were collected and frozen at birth at −80 degrees Celsius and maternal plasma samples were collected at approximately gestational week 18. All biological material was obtained from the biobank of the MoBa study (16). DNA methylation in cord blood was measured in 1,068 singleton births using the Illumina Infinium HumanMethylation450 Beadchip (450K). We previously analyzed 1,062 of these participants who had complete data for maternal plasma cotinine and covariates to evaluate the association between maternal smoking during pregnancy and DNA methylation in cord blood (3). In the current study, we analyzed the 1,042 participants who had DNA methylation measurements and data for maternal cotinine, self-reported smoking behavior before and during pregnancy, and covariates. Smoking information for the grandmother was reported as unknown or missing for 114 participants, leaving 928 of 1,042 for analyses with that variable. Analyses of father’s smoking information included 1,035 participants with data for that variable (7 missing). The MoBa study has been approved by the Regional Committee for Ethics in Medical Research, the Norwegian Data Inspectorate and the Institutional Review Board of the National Institute of Environmental Health Sciences, USA, and written informed consent was provided by all participants.
Methylation measurements
Details of the 450K methylation measurements and quality control were previously published (3) and are described in detail in the supplementary text of that paper. Briefly, bisulfite conversion was performed using the EZ-96 DNA Methylation kit (Zymo Research Corporation, Irvine, CA) and DNA methylation was measured at 485,577 CpGs in cord blood using Illumina’s Infinium HumanMethylation450 BeadChip (18, 19). Illumina’s GenomeStudio® Methylation module version 1.0 (Illumina Inc., San Diego, CA, 2012) was used to calculate the methylation level at each CpG as the beta-value (β=intensity of the methylated allele (M)/(intensity of the unmethylated allele (U) + intensity of the methylated allele (M) + 100)) (18). The laboratory analysis was designed to minimize potential batch effects; the bisulfite conversion and methylation measurements including reruns were completed in less than one month on a single machine. Variables representing chip (12 samples), chip set (four contiguous chips or half of a plate), and plate (96 samples) included as covariates in statistical models did not influence the results and thus were not included in the final models.
For the current analysis, we present results accounting for the two different probe designs by applying the intra-array normalization strategy Beta Mixture Quantile dilation (BMIQ) (20). The 26 CpGs evaluated in this analysis did not include any underlying SNPs in the probe sequence as detailed previously in the supplementary text (3).
Timing of mother’s smoking
Information about smoking by the mother, father, and grandmother was reported by the mother on questionnaires completed at different time points in pregnancy (15, 16) (Supplementary Figure 1). For the mother, cotinine, a biomarker of smoking, was measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) (21) in plasma collected at approximately gestational week 18. Cotinine values above 56.8 nmol/L were used to indicate that a mother was smoking at this time point (22). We classified mother’s smoking into four categories using her report of smoking during pregnancy, and cotinine values: never smoked, quit before pregnancy, smoked during pregnancy but quit by 18 weeks, and smoked through gestational week 18. Quitting by 18 weeks was defined by mother’s report plus having a cotinine value below 56.8 nmol/L.
Father’s smoking
Father’s smoking is an important source of secondhand tobacco smoke exposure to the mother. However, in our previous analysis, maternal cotinine levels consistent with secondhand smoke exposure alone (0 to 56.8 nmol/L) were not associated with differential methylation at the 26 CpGs we evaluate here (3). Nonetheless, the father’s smoking prior to pregnancy could possibly influence methylation in the sperm that could be passed to the offspring. We classified the father’s smoking prior to pregnancy using the mother’s response to the question, “Did the baby’s father smoke before you became pregnant?”
Grandmother’s smoking and combined mother’s and grandmother’s smoking
The grandmother’s smoking was determined by the mother’s response to the following question on a questionnaire administered in early pregnancy, “Did your mother smoke when she was pregnant with you?” The response choices were “Yes”, “No”, or “Don’t know.” Mother’s report of the grandmother’s smoking during pregnancy has been validated in a previous publication reporting an association between the grandmother smoking in her pregnancy with the mother and a lower birth weight of the mother (23). We created a categorical variable to jointly classify mother and grandmother smoking during pregnancy into four groups: neither smoked, only grandmother smoked in her pregnancy, only mother smoked in her pregnancy, and both smoked in their pregnancies. For this variable the mother’s smoking in pregnancy was determined solely based on a cotinine value above 56.8 nmol/L.
Statistical analysis
As in our previous publication (3) we used robust linear regression to account for potential outliers or heteroskedasticity (24). However, in the current analysis, rather than logratios, we used the actual methylation betas because the results were nearly identical and coefficients are easily interpretable as the incremental change in methylation at each probe between the categories compared.
We ran four separate models to examine the association between each smoking variable and methylation in newborns for the 26 CpGs we previously found to be associated with maternal smoking in pregnancy at Bonferroni-corrected epigenome-wide statistical significance (480,000 tests, p-value < 1×10−7). The smoking variables evaluated were the timing of the mother’s smoking (quit before pregnancy, quit by 18 weeks, or smoked through 18 weeks relative to never smoked), father smoking prior to the pregnancy (yes compared to no), and the combined mother and grandmother smoking (mother only, grandmother only, or both smoked relative to neither smoking). To further tease apart the effects of grandmother and mother smoking, we also compared smoking by both grandmother and mother in pregnancy to smoking only by the mother.
All models were adjusted for maternal age, maternal education, and parity. For the analysis of father’s smoking before the mother’s pregnancy we additionally adjusted for whether the mother was smoking during pregnancy. The number of cigarettes per day that the mother reported smoking during pregnancy was not a confounder and not included in the final models. Sex of the child would not be expected to be associated with smoking behavior of the mother, father, or grandmother before the birth of the child and also did not affect results so was not included. After our previous publication, a method was published to evaluate potential confounding by differential cell counts in whole blood (25). A reference dataset of cord blood, which would be most applicable to our data, is not available. Therefore we used the reference dataset of 6 adult men (26) to implement this method (25) and adjustment for estimated cell counts did not alter our results so we present results without this adjustment. We applied Bonferroni correction for 26 tests (26 CpGs evaluated) adjusting the level of significance from 0.05 to 0.0019. We also note the CpGs considered statistically significant after additional Bonferroni correction for the four models run, adjusting the level of significance to 0.05/(26*4)=0.00048. These statistical analyses were performed using R (27).
Results
Study population
Of the 1,042 mothers, 50% had never smoked, 22% quit before pregnancy, 15% quit early in pregnancy, and 13% smoked through gestational week 18 (Table 1). Among the mothers who quit by 18 weeks, 80% reported quitting by 6 weeks or earlier and 95% reported that they quit by 10 weeks or earlier. Father’s smoking prior to pregnancy was reported for 31% of study newborns (Table 1). The frequencies for the combined classification of grandmother and mother smoking are also reported in Table 1.
Table 1.
Descriptive characteristics of the study populationa
| Variable | Category | N (%) |
|---|---|---|
| Timing of mother’s smoking during pregnancy b | Never | 520 (49.9) |
| Quit before pregnancy | 230 (22.1) | |
| Quit during pregnancy by 18 weeks | 156 (15.0) | |
| Smoked through gestational week 18 | 136 (13.1) | |
| Combined grandmother’s and mother’s smoking during their pregnancies c (N=928) | Neither grandmother nor mother smoked | 607 (65.4) |
| Only grandmother smoked | 204 (22.0) | |
| Only mother smoked | 57 (6.1) | |
| Both grandmother and mother smoked | 60 (6.5) | |
| Father’s smoking prior to the mother’s pregnancy (N=1,035) | Yes | 317 (30.6) |
| No | 718 (69.4) | |
| Sex of the child | Male | 556 (53.4) |
| Female | 486 (46.6) | |
| Maternal age | <25 | 124 (11.9) |
| 25–30 | 504 (48.4) | |
| >30 | 414 (39.7) | |
| Maternal education | Less than high school | 75 (7.2) |
| High school degree | 336 (32.2) | |
| Some college | 464 (44.5) | |
| 4 years of college or more | 167 (16.0) | |
| Parity | 0 | 439 (42.1) |
| 1 | 425 (40.8) | |
| 2 | 135 (13.0) | |
| 3+ | 43 (4.1) |
N=1,042 individuals in the study population. Grandmother’s smoking missing for 114 and father’s smoking missing for 7 individuals.
Determined by mother’s self-report and mother’s plasma cotinine measured during pregnancy at approximately gestational week 18, where cotinine values above 56.8 nmol/L indicate smoking.
“Their pregnancies” reflects the grandmother’s pregnancy with the study mother and the mother’s pregnancy with the study newborn whose cord blood DNA methylation we measured.
The results for models unadjusted and adjusted for covariates were very similar. Thus, we present only the adjusted model results.
Timing of the mother’s smoking
Figure 1 shows graphically that the largest differences in mean methylation level corresponded to having a mother who smoked through gestational week 18, relative to never smoking whereas the mean methylation differed very little between never, quit before, and quit during pregnancy smoking categories.
Figure 1.
Mean DNA methylation level for each probe by timing of mother smoking (before and during pregnancy) category. For clarity, only CpGs in genes with multiple CpGs showing consistent direction of effect are displayed (AHRR cg23067299 is not displayed).
Table 2 and the supplementary volcano plot (Supplementary Figure 2) provide the model results for the analysis of methylation differences across the timing of mother smoking categories. Relative to never smoking, former smoking by the mother (quitting before pregnancy) and smoking in pregnancy that stopped by 18 weeks had minimal effects on cord blood methylation that were not statistically significant for any of the 26 CpGs evaluated (Table 2). In contrast, smoking by the mother through at least gestational week 18 had a much stronger association with methylation, relative to never smoking, with regression coefficients ranging from −0.149 to 0.084. Relative to never smoking, the median regression coefficient (across all 26 CpGs) for smoking through gestational week 18 was 4 fold higher than the median regression coefficient for smoking that stopped by week 18 and 8 fold higher than the median regression coefficient for stopping before pregnancy. Only smoking through 18 weeks in pregnancy was statistically significantly associated with differential methylation in cord blood (p-values < 1.64×10−4 for all 26 CpGs, Table 2, Supplementary Figure 2). These associations retain statistical significance if additional Bonferroni correction for four models run is applied (p-value < 4.8×10−4). As expected, our most statistically significant finding from our epigenome-wide analysis (3), AHRR cg05575921, was one of the most statistically significant findings for smoking through gestational week 18 relative to never smoking (regression coefficient = −0.074, standard error = 0.008, p-value=9.70×10−22). All of the methylation differences due to smoking are in the same direction as in our original report (3). Models including an additional adjustment for the amount of cigarettes smoked per day reported by the mother in pregnancy (17 week questionnaire) gave similar results.
Table 2.
Differential methylation in cord blood DNA in relation to the timing of the mother’s smokinga
| Chrb | Gene | Distance to Genec | CpG | Positiond | Quit before pregnancy vs. never smokers | Quit during pregnancy by 18 weeks vs. never smokers | Smoked through gestational week 18 vs. never smokers | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefe | SEf | p-value | Coef | SE | p-value | Coef | SE | p-value | |||||
| 1 | GFI1 | 3688 | cg10399789 | 92945668 | 0.001 | 0.004 | 0.867 | −0.008 | 0.005 | 0.091 | −0.041 | 0.006 | 2.19E-10 |
| 1 | GFI1 | 3224 | cg09662411 | 92946132 | 0.002 | 0.005 | 0.654 | −0.017 | 0.007 | 0.016 | −0.082 | 0.009 | 7.47E-19 |
| 1 | GFI1 | 3169 | cg06338710 | 92946187 | −0.002 | 0.004 | 0.581 | −0.011 | 0.006 | 0.046 | −0.063 | 0.009 | 2.02E-13 |
| 1 | GFI1 | 2656 | cg18146737 | 92946700 | 0.005 | 0.006 | 0.439 | −0.021 | 0.008 | 0.011 | −0.117 | 0.012 | 4.21E-23 |
| 1 | GFI1 | 2531 | cg12876356 | 92946825 | 0.006 | 0.007 | 0.415 | −0.020 | 0.010 | 0.046 | −0.134 | 0.012 | 1.62E-27 |
| 1 | GFI1 | 2321 | cg18316974 | 92947035 | 0.001 | 0.003 | 0.810 | −0.010 | 0.004 | 0.016 | −0.071 | 0.008 | 2.93E-18 |
| 1 | GFI1 | 1768 | cg09935388 | 92947588 | 0.010 | 0.008 | 0.227 | −0.025 | 0.010 | 0.014 | −0.149 | 0.013 | 7.74E-32 |
| 1 | GFI1 | 1395 | cg14179389 | 92947961 | 0.004 | 0.007 | 0.568 | −0.011 | 0.008 | 0.155 | −0.087 | 0.007 | 2.07E-35 |
| 5 | AHRR | 19617 | cg23067299 | 323907 | −0.002 | 0.004 | 0.569 | 0.000 | 0.004 | 1.000 | 0.030 | 0.005 | 1.77E-09 |
| 5 | AHRR | 64157 | cg03991871 | 368447 | −0.005 | 0.002 | 0.035 | 0.000 | 0.003 | 0.906 | −0.021 | 0.003 | 4.23E-10 |
| 5 | AHRR | 69088 | cg05575921 | 373378 | −0.003 | 0.003 | 0.343 | −0.004 | 0.004 | 0.302 | −0.074 | 0.008 | 9.70E-22 |
| 5 | AHRR | 95070 | cg21161138 | 399360 | −0.006 | 0.003 | 0.033 | −0.004 | 0.004 | 0.320 | −0.031 | 0.004 | 9.79E-13 |
| 6 | HLA-DPB2 | 11549 | cg11715943 | 33091841 | −0.003 | 0.003 | 0.411 | 0.003 | 0.004 | 0.364 | −0.019 | 0.004 | 2.99E-06 |
| 7 | MYO1G | 16417 | cg19089201 | 45002287 | −0.002 | 0.002 | 0.473 | 0.001 | 0.002 | 0.562 | 0.013 | 0.002 | 4.08E-08 |
| 7 | MYO1G | 16218 | cg22132788 | 45002486 | −0.002 | 0.003 | 0.509 | 0.002 | 0.003 | 0.589 | 0.023 | 0.003 | 3.80E-14 |
| 7 | MYO1G | 15968 | cg04180046 | 45002736 | −0.001 | 0.004 | 0.747 | 0.001 | 0.005 | 0.896 | 0.061 | 0.007 | 1.39E-19 |
| 7 | MYO1G | 15785 | cg12803068 | 45002919 | −0.002 | 0.008 | 0.768 | 0.007 | 0.008 | 0.364 | 0.084 | 0.009 | 2.09E-20 |
| 7 | ENSG00000225718 | 198306 | cg04598670 | 68697651 | −0.007 | 0.006 | 0.236 | −0.014 | 0.008 | 0.076 | −0.045 | 0.007 | 8.88E-10 |
| 7 | CNTNAP2 | 854 | cg25949550 | 145814306 | 0.001 | 0.001 | 0.327 | −0.002 | 0.001 | 0.129 | −0.016 | 0.001 | 1.01E-39 |
| 8 | EXT1 | −33821 | cg03346806 | 119157879 | −0.004 | 0.003 | 0.214 | 0.004 | 0.004 | 0.322 | −0.014 | 0.004 | 1.64E-04 |
| 14 | TTC7B | 274756 | cg18655025 | 91008005 | −0.002 | 0.002 | 0.266 | 0.002 | 0.002 | 0.297 | −0.011 | 0.003 | 5.31E-05 |
| 15 | CYP1A1 | −1266 | cg05549655 | 75019143 | −0.004 | 0.004 | 0.272 | −0.004 | 0.004 | 0.338 | 0.036 | 0.006 | 2.63E-10 |
| 15 | CYP1A1 | −1374 | cg22549041 | 75019251 | −0.012 | 0.008 | 0.156 | −0.011 | 0.010 | 0.278 | 0.065 | 0.011 | 1.57E-08 |
| 15 | CYP1A1 | −1406 | cg11924019 | 75019283 | −0.004 | 0.005 | 0.440 | −0.005 | 0.006 | 0.433 | 0.039 | 0.006 | 1.43E-09 |
| 15 | CYP1A1 | −1425 | cg18092474 | 75019302 | −0.007 | 0.007 | 0.336 | −0.011 | 0.009 | 0.238 | 0.060 | 0.010 | 2.05E-09 |
| 21 | RUNX1 | 1746 | cg12477880 | 36259241 | −0.001 | 0.004 | 0.838 | 0.004 | 0.006 | 0.528 | 0.038 | 0.008 | 9.25E-07 |
N=1,042 individuals analyzed. Categorized using a combination of mother’s self-report and mother’s plasma cotinine measured during pregnancy at approximately gestational week 18, where cotinine values above 56.8 nmol/L indicate smoking. All analyses adjusted for maternal age, maternal education, and parity.
Chromosome;
Distance (nucleotides) from CpG to transcription start site of the nearest gene;
Chromosomal position based on NCBI human reference genome assembly Build 37.3;
Regression coefficient;
Standard error for regression coefficient. CpGs with p-values reaching a Bonferroni-corrected statistical significance threshold of 0.05/26=0.0019 are noted in bold.
Father’s smoking
We did not observe any statistically significant differences in DNA methylation related to smoking by the father (Supplementary Table 1). Effect estimates were small (coefficients ranged from −0.011 to 0.009).
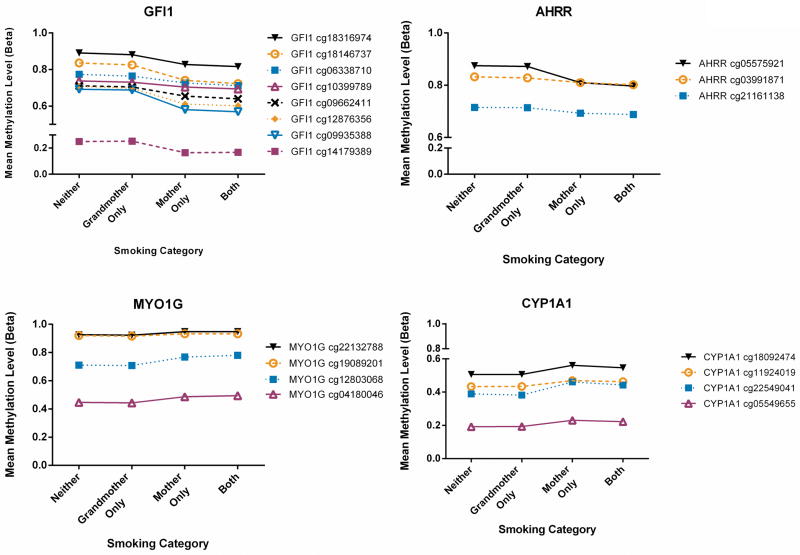
Grandmother’s smoking during pregnancy with the mother alone and in combination with the mother’s smoking during pregnancy
Figure 2 shows graphically that the largest differences in the mean methylation level occurred when only the mother smoked, relative to either only the grandmother smoked or neither the mother nor grandmother smoked. In comparison, the mean methylation levels differed very little between neither and grandmother only, and between mother only and both grandmother and mother smoking during pregnancy categories.
Figure 2.
Mean DNA methylation level for each probe by the combined grandmother and mother smoking category. For clarity, only CpGs in genes with multiple CpGs showing consistent direction of effect are displayed (AHRR cg23067299 is not displayed).
Table 3 and the supplementary volcano plot (Supplementary Figure 3) display the model results for the analysis of methylation differences across the combined mother and grandmother smoking categories. We observed no statistically significant association for grandmother’s smoking alone, relative to no smoking by either the grandmother or the mother, with methylation at any of the 26 CpGs (Table 3, Supplementary Figure 3) and the effect sizes were small (ranging from −0.007 to 0.004) (Table 3). Much larger effect sizes (regression coefficients ranging from −0.137 to 0.075) were observed for only the mother smoking relative to no smoking by either the grandmother or the mother (Table 3). Relative to no smoking by either the grandmother or the mother, the median regression coefficient (across all 26 CpGs) for smoking by the mother only was 12 fold higher than the median regression coefficient for smoking by the grandmother only. The associations remain statistically significant for 23 of the 26 CpGs if additional Bonferroni correction for four models run is applied (p-value < 4.8×10−4). For the combined grandmother and mother smoking analyses, additional adjustment for the amount of cigarettes smoked per day reported by the mother in pregnancy (17 week questionnaire) gave similar results.
Table 3.
Differential methylation in cord blood DNA in relation to the combined grandmother’s and mother’s smoking during their pregnanciesa
| Chrb | Gene | CpG | Only grandmother smoked in her pregnancy vs. neither smoked | Only mother smoked in her pregnancy vs. neither smoked | Both grandmother and mother smoked in their pregnancies vs. neither smoked | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefc | SEd | p-value | Coef | SE | p-value | Coef | SE | p-value | |||
| 1 | GFI1 | cg10399789 | −0.004 | 0.004 | 0.339 | −0.042 | 0.009 | 5.47E-06 | −0.039 | 0.010 | 1.14E-04 |
| 1 | GFI1 | cg09662411 | −0.001 | 0.006 | 0.824 | −0.075 | 0.012 | 6.89E-10 | −0.082 | 0.013 | 5.01E-10 |
| 1 | GFI1 | cg06338710 | −0.005 | 0.004 | 0.260 | −0.056 | 0.010 | 7.61E-08 | −0.065 | 0.013 | 6.84E-07 |
| 1 | GFI1 | cg18146737 | −0.003 | 0.007 | 0.624 | −0.104 | 0.017 | 4.70E-10 | −0.117 | 0.017 | 1.50E-11 |
| 1 | GFI1 | cg12876356 | −0.006 | 0.008 | 0.438 | −0.127 | 0.017 | 1.45E-14 | −0.133 | 0.019 | 2.90E-12 |
| 1 | GFI1 | cg18316974 | −0.003 | 0.003 | 0.444 | −0.067 | 0.010 | 1.66E-10 | −0.070 | 0.014 | 2.91E-07 |
| 1 | GFI1 | cg09935388 | −0.003 | 0.008 | 0.692 | −0.137 | 0.016 | 1.79E-18 | −0.147 | 0.019 | 2.48E-14 |
| 1 | GFI1 | cg14179389 | 0.004 | 0.006 | 0.495 | −0.083 | 0.010 | 3.29E-18 | −0.079 | 0.010 | 1.45E-16 |
| 5 | AHRR | cg23067299 | −0.003 | 0.004 | 0.450 | 0.025 | 0.007 | 2.94E-04 | 0.025 | 0.006 | 6.61E-05 |
| 5 | AHRR | cg03991871 | −0.004 | 0.002 | 0.116 | −0.021 | 0.005 | 2.18E-05 | −0.020 | 0.005 | 3.71E-05 |
| 5 | AHRR | cg05575921 | −0.002 | 0.003 | 0.514 | −0.057 | 0.011 | 2.67E-07 | −0.074 | 0.009 | 1.18E-16 |
| 5 | AHRR | cg21161138 | −0.001 | 0.003 | 0.777 | −0.026 | 0.005 | 1.70E-06 | −0.027 | 0.007 | 1.29E-04 |
| 6 | HLA-DPB2 | cg11715943 | −0.007 | 0.003 | 0.028 | −0.019 | 0.006 | 9.76E-04 | −0.018 | 0.005 | 4.85E-04 |
| 7 | MYO1G | cg19089201 | −0.002 | 0.002 | 0.337 | 0.010 | 0.003 | 6.78E-04 | 0.015 | 0.003 | 1.00E-06 |
| 7 | MYO1G | cg22132788 | −0.0002 | 0.003 | 0.935 | 0.021 | 0.004 | 2.70E-08 | 0.023 | 0.004 | 4.17E-09 |
| 7 | MYO1G | cg04180046 | 0.001 | 0.004 | 0.882 | 0.050 | 0.009 | 2.74E-08 | 0.062 | 0.008 | 4.61E-14 |
| 7 | MYO1G | cg12803068 | 0.003 | 0.007 | 0.685 | 0.071 | 0.012 | 7.89E-09 | 0.086 | 0.012 | 4.63E-13 |
| 7 | ENSG00000225718 | cg04598670 | −0.00004 | 0.006 | 0.995 | −0.038 | 0.009 | 4.05E-05 | −0.036 | 0.011 | 9.81E-04 |
| 7 | CNTNAP2 | cg25949550 | −0.001 | 0.001 | 0.600 | −0.014 | 0.002 | 1.31E-17 | −0.016 | 0.002 | 4.30E-23 |
| 8 | EXT1 | cg03346806 | −0.001 | 0.003 | 0.732 | −0.022 | 0.005 | 6.19E-06 | −0.007 | 0.006 | 1.95E-01 |
| 14 | TTC7B | cg18655025 | −0.002 | 0.002 | 0.388 | −0.014 | 0.004 | 2.83E-04 | −0.007 | 0.004 | 8.20E-02 |
| 15 | CYP1A1 | cg05549655 | 0.001 | 0.004 | 0.838 | 0.043 | 0.008 | 3.41E-08 | 0.034 | 0.009 | 1.08E-04 |
| 15 | CYP1A1 | cg22549041 | −0.007 | 0.008 | 0.436 | 0.075 | 0.014 | 1.57E-07 | 0.057 | 0.018 | 1.50E-03 |
| 15 | CYP1A1 | cg11924019 | 0.001 | 0.005 | 0.789 | 0.043 | 0.007 | 4.37E-09 | 0.040 | 0.011 | 1.39E-04 |
| 15 | CYP1A1 | cg18092474 | 0.001 | 0.007 | 0.877 | 0.068 | 0.012 | 4.79E-09 | 0.059 | 0.017 | 4.51E-04 |
| 21 | RUNX1 | cg12477880 | −0.002 | 0.004 | 0.700 | 0.026 | 0.010 | 1.17E-02 | 0.054 | 0.011 | 1.16E-06 |
N=928 individuals analyzed. “Their pregnancies” indicates the grandmother’s pregnancy with the study mother and the mother’s pregnancy with the study newborn whose cord blood DNA methylation we measured. All analyses adjusted for maternal age, maternal education, and parity.
Chromosome;
Regression coefficient;
Standard error for regression coefficient. CpGs with p-values reaching a Bonferroni-corrected statistical significance threshold of 0.05/26=0.0019 are noted in bold.
We also compared the effects of both the grandmother and mother smoking to only the mother smoking. If the mother smoked, the additional effect of grandmother smoking in her pregnancy with the mother was minimal and not statistically significant (Supplementary Table 2, Supplementary Figure 4).
Discussion
We recently reported effects of maternal smoking during pregnancy on DNA methylation in newborn cord blood at epigenome-wide statistical significance (p-value < 1×10−7) for 26 CpGs across 10 loci using the Illumina 450K array (3). In the current paper, we extend our analysis to investigate fundamental questions in epigenetics: inheritance and persistence of exposure effects. We looked for evidence of epigenetic inheritance by evaluating the impact of the mother’s and father’s smoking prior to pregnancy and the maternal grandmother’s smoking in her pregnancy with the mother and on DNA methylation in newborn cord blood. Each of these exposure conditions might potentially alter methylation status of ova or sperm and if effects persisted through fertilization and embryonic development, these might be detected in the cord blood. Our findings do not support epigenetic inheritance. Rather, the methylation differences we observed at birth in relation to maternal smoking appear to reflect in utero exposure.
We found that the effects of in utero exposure on newborn methylation at these 26 loci were much stronger when the mother smoked past 18 weeks in pregnancy than when she quit earlier in pregnancy. Methylation at these loci in newborns with mothers who quit smoking earlier in pregnancy was nearly indistinguishable from those whose mothers never smoked. Thus sustained exposure through at least 18 weeks in pregnancy appears to be required to observe the effects on DNA methylation at birth. Our findings in newborns are in agreement with results from studies of adults suggesting that smoking-related epigenetic effects are stronger for current than former smoking (9, 10).
The prevalence of smoking in our study population is comparable to the larger MoBa population. Kvalvik et al. measured maternal plasma cotinine concentrations at approximately gestational week 18 in a larger sample of 2,997 women in the MoBa study which partially overlaps with the current sample (28). The prevalence of daily or occasional smoking during pregnancy in this larger sample based on self-report and cotinine concentrations > 30 nmol/L is 13%, the same as in our smaller population. A ban on smoking in restaurants and bars went into effect in Norway in June 2004 resulting in decreased prevalence of smoking (29). Among all 19,140 MoBa women delivering singleton births between 2002 and 2004 (the date of birth range for our sample), smoking in pregnancy was reported by approximately 11% of mothers. However, cotinine was not available for most participants and Kvalvik et al. (30) found that the smoking proportion incorporating cotinine is slightly higher than that obtained from self-report in MoBa. The prevalence of grandmaternal smoking (23% grandmother only) and paternal smoking prior to conception (31%) in these 19,140 MoBa subjects was also similar to the prevalence in our smaller sample (22% and 31% respectively). The percentage of women that stopped smoking during pregnancy was 16% among the 19,140 MoBa subjects and 15% in our sample. Among mothers who reported that they stop smoking during pregnancy, the median gestational week of quitting was 5.0 (IQR 4–9) in the both the 19,140 MoBa subjects and our sample.
Cotinine measurements were available only at approximately gestational week 18, so we could not objectively confirm reports on the 30 week questionnaire of quitting later in pregnancy. Among the 136 women smoking at gestational week 18 based on cotinine, only 8 (6%) reported that they quit smoking later in pregnancy; excluding these 8 women yielded similar results.
This study cannot address mechanisms for the apparent need for sustained exposure (defined in this study as through at least 18 weeks gestation) in utero to identify methylation differences at birth. However, one can speculate that methylation differences reflect a cellular response to exposure that enables fetal cells, such as hematopoietic progenitors, to be more resistant to toxins in tobacco smoke. If this occurs, these cells may have a selective advantage for survival in the presence of exposure to Ah receptor ligands such as polyaromatic hydrocarbons. However, when exposure is not persistent, proliferation of progenitor cells without these epigenetic changes might be favored.
If mothers who were able to quit smoking early in pregnancy smoke less than mothers who continued to smoke, it is possible that this lower amount of smoking accounts for why we see no significant effect of quitting early in pregnancy. However, adjustment for the number of cigarettes smoked per day reported by the mother early in pregnancy did not materially alter the associations. The 136 women who smoked through at least gestational week 18 were disproportionately light smokers (median cigarettes per day=4, IQR=2–10) and thus we had little power to observe a dose response. However, we observed a statistically significant association between cigarettes smoked per day and methylation at 5 of the 8 GFI1 CpGs and 2 of the 4 AHRR CpGs, adjusting for maternal age, parity, and education and applying Bonferroni correction (p<0.0019).
Our previous publication included the Newborn Epigenetics Study (NEST) as a replication dataset to confirm our findings (3). We were not able to use those data for replication of the current analyses because information on the grandmother’s smoking during pregnancy with the mother, the mother’s past smoking, mother cotinine data, and the father’s past smoking was not available.
Subsequent to our original paper, a wide range of preprocessing methods for the Illumina 450K data, including background correction, normalization, and transformation have been published. In addition to the BMIQ performed for this analysis, we evaluated various normalization methods in this dataset and observed little impact on the results (31).
Environmental stimuli, including smoking, can affect the cell type composition of blood. In our previous report, we had evaluated possible confounding of our findings by smoking-related shifts in cell type by evaluating methylation at the top CpGs in two major cell pools – mononuclear cells (mostly lymphocytes) and granulocytes (mostly polymorphonuclear leukocytes) (3). The methylation differences by smoking were in all cases larger than methylation differences between these two major pools suggesting that the results were not due to confounding by smoking-related shifts in differential cell counts. Subsequent to submission of our publication (3), a method for statistical adjustment for cell type differences was published (25) and implementing this did not alter our results for the 26 CpGs. Of note, Shenker et al. confirmed findings in blood for AHRR by showing differential methylation at AHRR by smoking in the lung. Shenker et al. also reported consistent methylation levels for AHRR cg05775921 across 6 different cell type pools (9). These various lines of evidence make it unlikely that our previously published findings reflect smoking-related cell type differences.
There is little information about the functional impact of the methylation differences that we observed, many of which have been replicated in studies of adult smokers (7–10). However, for one of the top hits in our analysis and several studies of adult smoking, AHRR cg05575921, Zeilinger et al. found methylation-specific protein binding patterns (10). In addition, Shenker et al. (9) observed decreases in AHRR expression related to AHRR methylation.
We use the term epigenetic inheritance to indicate epigenetic modifications in the germline that escape meiotic resetting during gametogenesis (32), where meiotic resetting is the DNA methylation erasure that takes place just after fertilization (epigenetic reprogramming) (33). Other than imprinting, the data on epigenetic inheritance in humans is sparse. The epigenetic inheritance effects that we evaluated here might be more precisely determined by comparing DNA methylation status in relation to smoking across the three generations– grandparents, parents, and the study child. Unfortunately, we do not have these extensive data. However, even with such direct cross-generation data, interpretation could be challenging because of known methylation changes with age (34) as well as distinguishing between the effects of personal smoking in adults and their in utero exposure. We do believe that our data in newborns at different levels of exposure bring relevant evidence to bear on this difficult issue. Our findings do not support the inheritance of these epigenetic marks across generations or from embryonic to somatic cells.
Our results may have relevance to the design of epidemiologic studies of maternal smoking and childhood leukemia or other cancers. Recent studies of childhood leukemia have not identified associations between maternal smoking during pregnancy and childhood leukemia (35–38). However maternal smoking was classified in these studies based on any smoking during pregnancy. In our study more than half of the women who reported smoking in the early part of pregnancy had quit by week 18, confirmed by cotinine measurements. Although we do not know that the methylation changes that we observed predispose to the development of childhood leukemia, our results indicate that a potential biomarker of in utero exposure to maternal smoking in pregnancy is only seen with sustained exposure (through at least 18 weeks gestation). We note that several of the genes differentially methylated in relation to smoking in our data are plausibly involved in normal or disordered hematopoiesis including, RUNX1 (39), MYO1G (40), GFI1 (41) and AHRR (42). The fact that methylation changes that we observed require sustained exposure suggests that epidemiologic studies of leukemia or other childhood cancers or health outcomes may miss true associations if data on maternal smoking are limited to ever smoking during the pregnancy.
Supplementary Material
Acknowledgments
We are grateful to the participating families in Norway who take part in this ongoing cohort study. We thank Rolf Erik Kolstad, Arild Sunde, and Kari Harbak from the MoBa Biobank, and Huiling Li, Gary Pittman, Laura Wharey, and Kevin Gerrish from the NIEHS for expert technical assistance. We acknowledge Shuangshuang Dai of SRA International, Inc., Jianping Jin and David Shore of Westat, and Huiling Li of the NIEHS, for computing support.
Financial support: B.R. Joubert, S.J. London, D.A. Bell, S.D. Peddada are supported by the Intramural Research Program of the NIH, NIEHS (Z01-ES-49019, Z01-ES-046008). S.E. Håberg and W. Nystad are supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS (contract no NO-ES-75558), NIH/NINDS (grant no.1 UO1 NS 047537-01), and the Norwegian Research Council/FUGE (grant no. 151918/S10). R.M. Nilsen is supported by the Faculty of Medicine and Dentistry, University of Bergen, Norway. Ø. Midttun is employed at Bevital A/S, Norway.
Footnotes
None of the authors has any actual or potential competing financial interests.
References
- 1.Office of the Surgeon General. The health consequences of involuntary exposure to tobacco smoke : a report of the Surgeon General. Rockville, MD: U.S. Dept. of Health and Human Services, Public Health Service, Office of the Surgeon General; 2006. [Google Scholar]
- 2.Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 2009;180:462–7. doi: 10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joubert BR, Haberg SE, Nilsen RM, Wang X, Vollset SE, Murphy SK, et al. 450K epigenome-wide scan identifies differential DNA methylation in newborns related to maternal smoking during pregnancy. Environ Health Perspect. 2012;120:1425–31. doi: 10.1289/ehp.1205412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suter M, Ma J, Harris AS, Patterson L, Brown KA, Shope C, et al. Maternal tobacco use modestly alters correlated epigenome-wide placental DNA methylation and gene expression. Epigenetics. 2011;6 doi: 10.4161/epi.6.11.17819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KW, Pausova Z. Cigarette smoking and DNA methylation. Front Genet. 2013;4:132. doi: 10.3389/fgene.2013.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flom JD, Ferris JS, Liao Y, Tehranifar P, Richards CB, Cho YH, et al. Prenatal smoke exposure and genomic DNA methylation in a multiethnic birth cohort. Cancer Epidemiol Biomarkers Prev. 2011;20:2518–23. doi: 10.1158/1055-9965.EPI-11-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monick MM, Beach SR, Plume J, Sears R, Gerrard M, Brody GH, et al. Coordinated changes in AHRR methylation in lymphoblasts and pulmonary macrophages from smokers. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:141–51. doi: 10.1002/ajmg.b.32021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philibert RA, Beach SR, Brody GH. Demethylation of the aryl hydrocarbon receptor repressor as a biomarker for nascent smokers. Epigenetics. 2012;7:1331–8. doi: 10.4161/epi.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shenker NS, Polidoro S, van Veldhoven K, Sacerdote C, Ricceri F, Birrell MA, et al. Epigenome-wide association study in the European Prospective Investigation into Cancer and Nutrition (EPIC-Turin) identifies novel genetic loci associated with smoking. Hum Mol Genet. 2013;22:843–51. doi: 10.1093/hmg/dds488. [DOI] [PubMed] [Google Scholar]
- 10.Zeilinger S, Kuhnel B, Klopp N, Baurecht H, Kleinschmidt A, Gieger C, et al. Tobacco smoking leads to extensive genome-wide changes in DNA methylation. PLoS One. 2013;8:e63812. doi: 10.1371/journal.pone.0063812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller LL, Henderson J, Northstone K, Pembrey M, Golding J. Do grandmaternal smoking patterns influence the aetiology of childhood asthma? Chest. 2013 doi: 10.1378/chest.13-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjostrom M, et al. Sex-specific, male-line transgenerational responses in humans. European journal of human genetics : EJHG. 2006;14:159–66. doi: 10.1038/sj.ejhg.5201538. [DOI] [PubMed] [Google Scholar]
- 13.Grossniklaus U, Kelly B, Ferguson-Smith AC, Pembrey M, Lindquist S. Transgenerational epigenetic inheritance: how important is it? Nat Rev Genet. 2013;14:228–35. doi: 10.1038/nrg3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daxinger L, Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat Rev Genet. 2012;13:153–62. doi: 10.1038/nrg3188. [DOI] [PubMed] [Google Scholar]
- 15.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35:1146–50. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 16.Ronningen KS, Paltiel L, Meltzer HM, Nordhagen R, Lie KK, Hovengen R, et al. The biobank of the Norwegian Mother and Child Cohort Study: a resource for the next 100 years. Eur J Epidemiol. 2006;21:619–25. doi: 10.1007/s10654-006-9041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Håberg SE, London SJ, Nafstad P, Nilsen RM, Ueland PM, Vollset SE, et al. Maternal folate levels in pregnancy and asthma in children at age 3 years. J Allergy Clin Immunol. 2011;127:262–4. 4 e1. doi: 10.1016/j.jaci.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bibikova M, Barnes B, Tsan C, Ho V, Klotzle B, Le JM, et al. High density DNA methylation array with single CpG site resolution. Genomics. 2011;98:288–95. doi: 10.1016/j.ygeno.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Sandoval J, Heyn HA, Moran S, Serra-Musach J, Pujana MA, Bibikova M, et al. Validation of a DNA methylation microarray for 450,000 CpG sites in the human genome. Epigenetics. 2011;6:692–702. doi: 10.4161/epi.6.6.16196. [DOI] [PubMed] [Google Scholar]
- 20.Teschendorff AE, Marabita F, Lechner M, Bartlett T, Tegner J, Gomez-Cabrero D, et al. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics. 2013;29:189–96. doi: 10.1093/bioinformatics/bts680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Midttun O, Hustad S, Ueland PM. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2009;23:1371–9. doi: 10.1002/rcm.4013. [DOI] [PubMed] [Google Scholar]
- 22.Shaw GM, Carmichael SL, Vollset SE, Yang W, Finnell RH, Blom H, et al. Mid-pregnancy cotinine and risks of orofacial clefts and neural tube defects. J Pediatr. 2009;154:17–9. doi: 10.1016/j.jpeds.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Cupul-Uicab LA, Baird DD, Skjaerven R, Saha-Chaudhuri P, Haug K, Longnecker MP. In utero exposure to maternal smoking and women’s risk of fetal loss in the Norwegian Mother and Child Cohort (MoBa) Hum Reprod. 2011;26:458–65. doi: 10.1093/humrep/deq334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox J, Weisberg S. An R companion to applied regression. 2. Thousand Oaks, Calif: SAGE Publications; 2011. [Google Scholar]
- 25.Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics. 2012;13:86. doi: 10.1186/1471-2105-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinius LE, Acevedo N, Joerink M, Pershagen G, Dahlen SE, Greco D, et al. Differential DNA methylation in purified human blood cells: implications for cell lineage and studies on disease susceptibility. PLoS One. 2012;7:e41361. doi: 10.1371/journal.pone.0041361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2010. [Google Scholar]
- 28.Kvalvik LG, Nilsen RM, Skjaerven R, Vollset SE, Midttun O, Ueland PM, et al. Self-reported smoking status and plasma cotinine concentrations among pregnant women in the Norwegian Mother and Child Cohort Study. Pediatr Res. 2012;72:101–7. doi: 10.1038/pr.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braverman MT, Aaro LE, Hetland J. Changes in smoking among restaurant and bar employees following Norway’s comprehensive smoking ban. Health promotion international. 2008;23:5–15. doi: 10.1093/heapro/dam041. [DOI] [PubMed] [Google Scholar]
- 30.Kvalvik LG, Nilsen RM, Skjaerven R, Vollset SE, Midttun O, Ueland PM, et al. Self-reported smoking status and plasma cotinine concentrations among pregnant women in the Norwegian Mother and Child Cohort Study. Pediatric research. 2012;72:101–7. doi: 10.1038/pr.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu MC, Joubert BR, Kuan PF, Haberg SE, Nystad W, Peddada SD, et al. A systematic assessment of normalization approaches for the Infinium 450k methylation platform. Epigenetics. 2013;9 doi: 10.4161/epi.27119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez-Diaz E, Jorda M, Peinado MA, Rivero A. Epigenetics of host-pathogen interactions: the road ahead and the road behind. PLoS Pathog. 2012;8:e1003007. doi: 10.1371/journal.ppat.1003007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monk M, Boubelik M, Lehnert S. Temporal and regional changes in DNA methylation in the embryonic, extraembryonic and germ cell lineages during mouse embryo development. Development. 1987;99:371–82. doi: 10.1242/dev.99.3.371. [DOI] [PubMed] [Google Scholar]
- 34.Florath I, Butterbach K, Muller H, Bewerunge-Hudler M, Brenner H. Cross-sectional and longitudinal changes in DNA methylation with age: an epigenome-wide analysis revealing over 60 novel age-associated CpG sites. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klimentopoulou A, Antonopoulos CN, Papadopoulou C, Kanavidis P, Tourvas AD, Polychronopoulou S, et al. Maternal smoking during pregnancy and risk for childhood leukemia: a nationwide case-control study in Greece and meta-analysis. Pediatric blood & cancer. 2012;58:344–51. doi: 10.1002/pbc.23347. [DOI] [PubMed] [Google Scholar]
- 36.Metayer C, Zhang L, Wiemels JL, Bartley K, Schiffman J, Ma X, et al. Tobacco smoke exposure and the risk of childhood acute lymphoblastic and myeloid leukemias by cytogenetic subtype. Cancer Epidemiol Biomarkers Prev. 2013;22:1600–11. doi: 10.1158/1055-9965.EPI-13-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milne E, Greenop KR, Scott RJ, Bailey HD, Attia J, Dalla-Pozza L, et al. Parental prenatal smoking and risk of childhood acute lymphoblastic leukemia. Am J Epidemiol. 2012;175:43–53. doi: 10.1093/aje/kwr275. [DOI] [PubMed] [Google Scholar]
- 38.Slater ME, Linabery AM, Blair CK, Spector LG, Heerema NA, Robison LL, et al. Maternal prenatal cigarette, alcohol and illicit drug use and risk of infant leukaemia: a report from the Children’s Oncology Group. Paediatr Perinat Epidemiol. 2011;25:559–65. doi: 10.1111/j.1365-3016.2011.01229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuzuki S, Seto M. Expansion of functionally defined mouse hematopoietic stem and progenitor cells by a short isoform of RUNX1/AML1. Blood. 2012;119:727–35. doi: 10.1182/blood-2011-06-362277. [DOI] [PubMed] [Google Scholar]
- 40.Pierce RA, Field ED, Mutis T, Golovina TN, Von Kap-Herr C, Wilke M, et al. The HA-2 minor histocompatibility antigen is derived from a diallelic gene encoding a novel human class I myosin protein. J Immunol. 2001;167:3223–30. doi: 10.4049/jimmunol.167.6.3223. [DOI] [PubMed] [Google Scholar]
- 41.van der Meer LT, Jansen JH, van der Reijden BA. Gfi1 and Gfi1b: key regulators of hematopoiesis. Leukemia. 2010;24:1834–43. doi: 10.1038/leu.2010.195. [DOI] [PubMed] [Google Scholar]
- 42.Zudaire E, Cuesta N, Murty V, Woodson K, Adams L, Gonzalez N, et al. The aryl hydrocarbon receptor repressor is a putative tumor suppressor gene in multiple human cancers. J Clin Invest. 2008;118:640–50. doi: 10.1172/JCI30024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.