Abstract
In the epidemiological literature, the fetal origins hypothesis associated with David J. Barker posits that chronic, degenerative conditions of adult health, including heart disease and type 2 diabetes, may be triggered by circumstance decades earlier, in utero nutrition in particular. Economists have expanded on this hypothesis, investigating a broader range of fetal shocks and circumstances and have found a wealth of later-life impacts on outcomes including test scores, educational attainment, and income, along with health. In the process, they have provided some of the most credible observational evidence in support of the hypothesis. The magnitude of the impacts is generally large. Thus, the fetal origins hypothesis has not only survived contact with economics, but has flourished.
In the late 1950s epidemiologists believed that a fetus was a “perfect parasite” that was “afforded protection from nutritional damage that might be inflicted on the mother” [Susser and Stein, 1994]. The placenta was regarded as a “perfect filter, protecting the fetus from harmful substances in the mother’s body and letting through helpful ones” [Landro, 2010]. Nonchalance extended to prenatal nutrition. During the 1950s and 1960s, women were strongly advised against gaining too much weight during pregnancy [Paul, 2010]. During the baby boom, “pregnant women were told it was fine to light up a cigarette and knock back a few drinks” [Landro, 2010]. Roughly half of US mothers reported smoking in pregnancy in 1960 [Aizer and Stroud, 2009].
But what if the nine months in utero are one of the most critical periods in a person’s life, shaping future abilities and health trajectories – and thereby the likely path of earnings? This paper reviews the growing literature on the so-called “fetal origins” hypothesis. The most famous proponent of this hypothesis is David J. Barker, a British physician and epidemiologist, who has argued that inadequate nutrition in utero “programs” the fetus to have metabolic characteristics that can lead to future disease [Barker, 1992]. For example, Barker argued that individuals starved in utero are more likely to become overweight as adults, and that they are more likely to suffer from diseases associated with obesity including cardiovascular problems and diabetes.
The fetal origins hypothesis combines several key ideas. First, the effects of fetal conditions are persistent. Second, the health effects can remain latent for many years – typically heart disease does not emerge as a problem until middle age, for example. Third, the hypothesized effects reflect a specific biological mechanism, fetal “programming,” possibly through effects of the environment on the epigenome, which are just beginning to be understood. The epigenome can be conceived of as a series of switches that cause various parts of the genome to be expressed – or not. The period while the fetus is in utero may be particularly important for setting these switches [Petronis, 2010].
The fetal origins hypothesis also broadened the conventional focus on health behaviors of adults, such as smoking, exercise, and diet, to include earlier environmental factors that might affect the well-being of the fetus. The hypothesis has been controversial. Much of the early evidence was mainly correlational, and did not effectively address potential confounders. Perhaps surprisingly, some of the most convincing evidence in favor of a broader version of the hypothesis comes from recent work in economics. Economists have also been active in demonstrating that various environmental factors can have negative impacts on the developing fetus, even at levels previously thought harmless. Later-life impacts extend to “bread and butter” economic outcomes, including educational attainment and wages.
This paper offers an overview of the epidemiological literature and Barker’s place within it, a more detailed discussion of the contribution from economics, and some promising directions for future research. Clearly, a full acceptance of the fetal origins hypothesis idea would have radical implications for individual decisions and policy alike, suggesting for example, that the optimum time to intervene to improve children’s life chances is before they are born, and perhaps before mothers even realize that they are pregnant.
Epidemiology
Effects Apparent at Birth
Although isolated examples of the adverse health consequences of events in utero had been recorded earlier (such as congenital rubella syndrome, which was first recognized in 1941 by Norman Gregg)1 the thalidomide episode in the late 1950s and early 1960s was a watershed event in establishing the importance of the in utero period. Thalidomide was licensed in 1957 and widely prescribed to pregnant women for morning sickness until 1961, when it was identified as the cause of an epidemic of severe birth defects such as missing arms and legs [Mcbride, 1961, Von Lenz and Knapp, 1962]. As Dr. Philip Landrigan (cited in Roan [2007]), Chair of the Department of Community and Preventive Medicine at Mount Sinai School of Medicine in New York City, has said, “Thalidomide was the first episode that made the medical profession and public realize that the placenta is not some sort of impervious barrier.”
There were other examples of fetal exposures which could lead to severe and permanent abnormalities at birth. In 1973, Kenneth Jones and David Smith described a pattern of anomalies common in children of alcoholic mothers, and labeled it “fetal alcohol syndrome.” The facial features and behaviors typical of fetal alcohol syndrome had been recognized for a long time, but had been attributed to heredity. For example, Goddard [1912]’s monograph on the Kallikak family is subtitled “A Study in the Heredity of Feeble-Mindedness.” Karp et al. [1995] were the first to suggest that the characteristics of this family could be well explained by alcoholism and fetal alcohol syndrome.
Latent effects
A distinct strand of medical and epidemiological literature develops the more subtle idea that events in utero could alter the infant in a way that would lead to later disease, even in infants who were apparently healthy at birth. Kermack et al. [1934] explored the idea that the long-term effects of health shocks might remain latent for long periods of time. A series of studies of the Dutch “Hunger Winter” of 1944 set out to test this hypothesis. This famine was precipitated by the Nazi occupation of the Netherlands, in which many Dutch were reduced to eating tulip bulbs in a desperate attempt to forestall starvation.
Nutrition in the Netherlands had been adequate up to October 1944; however, the Nazis had occupied the country and cut off food shipments after that date. By November 1944, official rations had fallen below 1000 dietary calories per day, and by April 1945, they were down to 500 calories per day. The famine was known to have affected fertility, weight gain during pregnancy, maternal blood pressure, and infant birth weight. Stein et al. [1975] examined the relationship between prenatal exposure to famine and the conscription records of over 400,000 18 year-old men. Famine exposure was defined using birth date and place of birth. No effects were found on IQ scores, but the obesity rate was doubled among those who had been exposed in the first trimester [Ravelli, Stein, and Susser, 1976]. A limitation of this study was that it dealt with 18 year olds who were too young to provide any evidence about any effects of fetal origins on the types of chronic degenerative conditions that typically manifest themselves later in life.
Clearly, the idea of latent health effects stemming from the prenatal period preceded the work of David Barker.2 However, Barker brought three important perspectives to the table. First, he is credited with the specific hypothesis that “events early in life might be linked to such chronic degenerative diseases as non-insulin dependent diabetes mellitus (NIDDM) and cardiovascular disease (CVD), which do not usually appear until mid-life or later” [Rasmussen, 2001]. Second, he obtained and analyzed new data, through no small amount of old-fashioned “shoe leather” [Freedman, 1991]. For example, his sample of 16,000 men and women born between 1911 and 1930 in Hertfordshire, near London “came to light as a result of the Medical Research Council’s systematic search of the archives and records offices of Britain” [Barker, 1995]. Finally, Barker brought an effective proselytizing zeal that helped make his name synonymous with the fetal origins hypothesis.
In contrast to the natural experiment employed in studying the “Hunger Winter,” Barker’s empirical work was essentially correlational. For example, in Barker and Osmond [1986], he analyzed extracts from Britain’s Office of Censuses and surveys all death certificates from 1968 to 1978. The basic analysis involved presenting correlation coefficients between adult mortality rates by geographic area and the 1921 to 1925 infant mortality rates for the same area. There are no controls for confounders, nor is there any discussion of how partial correlations might differ from the raw correlations presented. Rather, it is blandly stated that: “Other environmental influences suspected as causes of ischemic diseases [that is, diseases related to reduced supply of blood to a certain organ, like the heart] …are not known to have the same geographical variations as past infant mortality.” Moreover, congenital and infectious causes of infant death show similar correlations with adult mortality, whereas one might have expected the latter to have weaker effects under the fetal origins hypothesis. Barker [1995] presents two basic crosstabulations between birthweight categories and later-life morbidity/mortality and comments that “the associations between birth measurements and coronary risk factors are found to be unchanged after even the most potent adult determinants of risk are allowed for” but without further elaboration about how these controls are dealt with.
This approach provoked sharp criticism. For example, Paneth and Susser [1995] write:
Indeed, it is easy to see the barrage of papers from Barker’s group as an inductionist’s delight. Example is piled on example, each somewhat consistent with the hypothesis but none seriously testing it… What is missing in this work so far is the rigorous testing by rejections and exclusions–that is, by deliberate attempts at refutation.
Their more specific complaints are that anthropometric measures like low birth weight are weak indicators of fetal nutrition, and that the observed relationships between anthropometrics and future health outcomes could reflect many other potential confounders. Nevertheless, Barker has shown a genius for synthesizing and explicating the fetal origins hypothesis in a way that has grasped the imagination of both scientists and lay people.
Subsequent studies used data from national Dutch psychiatric registries and found an increase in schizophrenia among those affected by the famine in utero [Hoek, Brown, and Susser, 1998]. This finding has been replicated for the Chinese famine of 1959–61 [St. Clair et al., 2005], but not for famines associated with the seige of Leningrad [Stanner et al., 1997], nor the 1866–68 famine in Finland [Kannisto, Christensen, and Vaupel, 1997]. However, in both Leningrad and Finland, mortality was high and conditions “so severe it is possible that only the healthy individuals survived” [Rasmussen, 2001]. For example in Finland the infant mortality rate was 40% [Kannisto, Christensen, and Vaupel, 1997]. The possible bias in estimated “fetal effects” due to this type of extreme selection is discussed further below. In the Netherlands, the affected cohort has now been followed to age 59, and significant changes in fat deposition have been found for women (though not for men), and on blood pressure for both men and women [Stein et al., 2006b,a].
How latent?
In recent years, many studies have shown that birth weight can be affected by a variety of subtle and less subtle shocks during the fetal period: see Currie [2011], Almond and Currie [2011] for surveys of this literature. However, although birth weight is the most widely available and most studied measure of fetal health, it may not be a particularly comprehensive or sensitive measure, and the exclusive focus on birth weight may have retarded recognition of the importance of fetal effects. In the seminal study by Stein et al. [1975], cohorts who were exposed to famine during the first half of pregnancy had relatively normal birth weight but later showed more evidence of health effects such as incipient heart disease. In contrast, cohorts affected later in pregnancy suffered greater reductions in birth weight but were healthier in later life. Thus, “One of the important observations from the Dutch Hunger Winter Study was that intrauterine exposures that have long- lasting consequences for adult health do not necessarily result in altered birth weight” [Schulz, 2010].3
That said, there has been no convergence on an alternative, superior metric to birth weight. Of course, it may not be surprising that is hard to find a single comprehensive measure of latent fetal health impacts at the time of birth. Nevertheless, it is problematic for what Douglas Miller of the University of California-Davis has referred to as the “science fiction” problem in analyzing the fetal origins hypothesis. Absent time-travel, we have to wait a generation for the effects of a prenatal intervention of interest to be observed in adulthood. A reliable metric for health at birth would obviate the need for time-travel. Otherwise, we are compelled to analyze historical shocks, which may have limited comparability to present conditions.
An ideal measure would be sensitive for fetal insults at all stages of pregnancy, be easy to measure, and be available for all mothers (or at least a large sample of mothers) in a cohort. Barker observes that: “…from studies of children it should be possible to develop better biochemical markers of fetal under-nutrition” [Barker, 1995]. However, to our knowledge none have been suggested. Most recently, Barker has advocated using placental shape as a marker. But as Gluckman and Hanson [2005, p. 58] caution, “We have to be extremely careful not to view body size and shape at birth as measures of developmental outcome….”
The lack of an ideal measure of fetal health has not prevented economists from addressing the fetal origins hypothesis. This may be because economists are accustomed to considering variables to be latent – like the potential wages of non-workers. On a practical level, economists’ focus on identification strategies has often enabled them to sidestep the question of finding a better measure of fetal health.
Conceptual Framework
One reason economists have become interested in the fetal origins hypothesis is that it holds important implications for the modeling of human capital development. In the now-standard Grossman [1972] model of the development of health capital, the equation of motion of the health stock is often written as:
where It represents investments in health capital and δ represents the depreciation rate. This workhorse model builds in the “fade out” of early-life health shocks. That is, if health capital depreciates, then the effects of shocks to health capital must also depreciate over time, so that events further in the past will have less important effects than more recent events. As Smith [1999] observed, “How could short exposures matter if health is a stock?”4
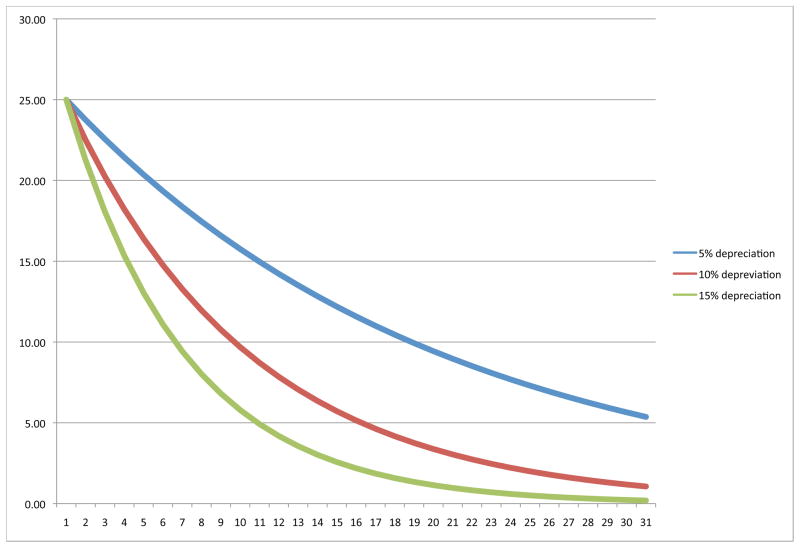
Figure 1 shows how persistent a 25 percent negative shock to the birth endowment would be given alternative annual depreciation rates δ. Even under the lowest annual depreciation rate of 5 percent, half of the endowment shock is gone by the mid-teen years. For the higher depreciation rates of 10 and 15 percent, we would be hard-pressed to detect any lingering effects of the shock after age 30.
Figure 1.
Shock persistence by age: Grossman framework
Heckman [2007] outlines a capacity formation model that is well-suited to consider fetal origins. A basic insight is to use a conventional constant elasticity of substitution production function to produce health, but to consider the inputs to be health investments at different developmental stages of childhood. In the simplest two-input model, instead of having capital and labor inputs, we could have investments in utero and those occurring during the rest of childhood:
One implication of these models is that the effects of prenatal investment shocks may be non-monotonic in age. Thus, they liberate us from the “fade out” of early shocks inherent in the Grossman formulation [Almond and Currie, 2011]. The potential for complementarities between prenatal shocks and subsequent investments underscores the persistent importance of a “good start”.5
Furthermore, “dynamic complementarities” would imply that investments in period t are more productive when there is a high level of capability in period t − 1 [Heckman, 2007]. Second, “self-productivity” implies that higher levels of capacity in one period create higher levels of capacity in future periods (which is noteworthy when capacity is multidiemnsional, e.g. baseline health facilitates cognition). Cunha and Heckman [2008] and Cunha, Heckman, and Schennach [2010] simulate models of this type and suggest that capabilities measured in early childhood can explain much of the variation in adult accomplishments. The intuition is that early shocks have had a longer time to feed through the dynamic system, while late shocks have not yet had time to dissipate. Currie et al. [2009] provide some empirical evidence consistent with this hypothesized time pattern, finding evidence that health at birth, health in early childhood, and health in the late teen years have larger effects on educational attainment and use of welfare than health in early adolescence. To date, there has been no similar empirical exercise considering the impact of fetal shocks.
Before turning to empirical evidence from economics on the fetal origins hypothesis, it is important to consider what studies of fetal origins can actually hope to measure. For example, if exposing a fetus to rubella at a specific time in pregnancy results in deafness, then it might be reasonable to view this as a “biological” effect. However, the long-term impacts of being born with Fetal Alcohol Syndrome may be very different depending on whether or not the child is institutionalized, or whether the child is “mainstreamed” at school. At a more subtle level, parents may try to help children overcome disabilities, or they may decide to focus their efforts on siblings without disability (see Behrman, Rosenzweig, and Taubman [1994], Almond, Edlund, and Palme [2009], Datar, Kilburn, and Loughran [2010], Bharadwaj, Eberhard, and Neilson [2010], Adhvaryu and Nyshadham [2011].) Hence, when examining longer-term outcomes, it is important to keep in mind that these can represent both biological and social factors [Royer, 2009, Almond and Currie, 2011]. Even in the short term, people may act to mitigate the effects of shocks. For example, in a famine, family members might try to give a pregnant woman a larger share of their food rations. Such actions would make it harder to draw inferences based on average food rations.
Empirical Contributions from Economics
Barker’s fetal origins hypothesis has spawned a large and growing empirical literature both within health economics and more broadly [Currie, 2009]. Development economics has been especially active in exploring the fetal origins hypothesis; examples include [Schultz and Strauss, 2008] and observational studies by Bleakley [2007], Chen and Zhou [2007], Field et al. [2009], Maccini and Yang [2009], Cutler et al. [2010], Bhalotra and Rawlings [2011]. Furthermore, environmental economics [Deschenes, Greenstone, and Guryan, 2009], economic history [Fogel and Costa, 1997], family economics [Bono and Ermisch, 2009] and even economic growth [Birchenall, 2007] have been influenced by the fetal origins hypothesis. We will not attempt to summarize this voluminous literature. Instead, we will describe four thematic contributions economists have made to the fetal origins hypothesis: 1) analysis of non-health endpoints; 2) improved identification strategies; 3) consideration of relatively mild and more varied prenatal exposures; and 4) formalizing the tension between “scarring” and selective mortality in ecological data.
Non-Health Endpoints
Currie and Hyson [1999] broke new ground in economics by exploring whether fetal origins effects might extend not just to measures of health, but also to measures of human capital measures. Using the British National Child Development Survey, they found that low birth weight children (children with birth weight less than 2,500 grams) were more than 25 percent less likely to pass English and math O-level tests (equivalent to high school exit exams), and were also less likely to be employed at age 33. While this study was primarily descriptive, the finding that test scores were lower in low birth weight children was surprising as epidemiologists had routinely posited fetal “brain sparing” mechanisms, whereby adverse in utero conditions were parried through a placental triage that prioritized neural development over the body (for example, Scherjon et al. [1996]).
Since then, a substantial empirical literature has developed analyzing within-family (and within-twin-pair) differences in birth outcomes and their relationship to educational attainment, often using large samples of data from birth registries. Although as discussed above, birth weight is a relatively crude measure of fetal health, such studies typically find significant effects of birth weight on educational and/or labor force outcomes, as in Currie and Moretti [2007], Oreopoulos et al. [2008], Black et al. [2007] and Royer [2009]. For example, Black, Devereux, and Salvanes [2007] find a 10 percent increase in birth weight increases high school graduation by 1.2 percent, IQ (of men) by 1.2 percent, earnings by .9 percent, and height by .3 percent.
Besides human capital and labor market outcomes, various other outcomes have been found to respond to in utero shocks, as well. For example, marital status is negatively impacted by early childhood exposure to the 1959–61 China famine [Almond, Edlund, Li, and Zhang, 2010a, Brandt, Siow, and Vogel, 2008]. Welfare dependency is higher following in utero shocks [Almond, 2006, Oreopoulos et al., 2008, Nilsson, 2008] and neighborhood characteristics in adulthood are also compromised [Almond, 2006].
Improving Identification
Economists have risen to the challenge posed by Paneth and Susser [1995] of subjecting the fetal origins hypothesis to “rigorous testing by rejections and exclusions–that is, by deliberate attempts at refutation.” The fact that the hypothesis applies to a well defined developmental period lends itself to “severe” hypothesis testing (see Dinardo [2007] and references therein). Cohorts potentially affected by a particular shock in utero can be compared to cohorts born just before or just after the shock. The fetal origins hypothesis offers the prediction that later-life health outcomes should be worse only for those cohorts whose pregnancies overlapped with the shock.6
To be sure, in some contexts it can be challenging to disentangle the in utero effects of the fetal origins hypothesis from the effects of shocks that occur in early childhood. For a fetal shock, we often have sharp comparisons between the affected cohort in the 10 months before birth, and two other cohorts: the cohort that was about to be conceived when the shock occurred (and is therefore too young to be affected) and the cohort that was already born at the time of the shock (and is therefore too old to be affected prenatally). In contrast, it may be difficult to define such sharp comparisons for developmental periods after birth. For example, suppose that air pollution has negative effects on the developing fetus, but also harms young children. The effects on very young children might increase with age as the children spend more time outdoors. The effects may eventually decrease with age as the children mature and their metabolisms slow (children are thought to be particularly sensitive to pollution because their systems are still developing and they have rapid metabolisms). A smooth change in the effects of air pollution with age will make it difficult to disentangle the effects of pollution from other age and period effects. However, given a shock to pollution it may still be possible to find a sharp contrast, for example, between the affected cohort and children who were not yet conceived at the time of the shock.7 Alternatively, many “severe tests” of the fetal origins hypothesis can be interpreted as capturing the additional effect of fetal exposure on top of those stemming from (smoother) early-childhood effects.
However, seeking to quantify in utero effects through comparisons with those born just earlier or later gives rise to several problems. First, most birth cohorts are neither exposed to a identifiable shock in utero, nor were born just before or just after such a shock (and thus cannot serve as good controls). Rather than looking at all the data on births, the researcher is immediately pushed to looking at particular episodes in which an identifiable shock occurred and then attempting to draw defensibly generalizable conclusions from these episodes. Second, we need to be able to link data on adult outcomes to data on the affected cohorts. But many prominent data sets, such as the Current Population Survey, do not include information on where someone was born or precise date of birth. As a result, many interesting and policy-relevant experiments linked to a certain time and place may never be analyzed.
Economists have been creative in linking large-sample cross-sectional date-sets back to ecological conditions around the time of birth. Most often, they have used information on when and where a respondent was born to link that person back to in utero health conditions. This has enabled economists to consider historical events featuring relatively well-defined start and/or end points. Economists have also shown a willingness to focus on exogenous sources of variation in fetal health that are well measured at the aggregate level, but not at the individual level.
In this spirit, the 1918 Influenza Pandemic offers an appealing identification strategy because it was unexpected, short, and surprisingly arbitrary in whom it affected [Almond, 2006]. While the infection status of individual women is unobserved, roughly one one-third of those born in early 1919 had mothers who contracted influenza while pregnant. As a control group, we can consider those born in early 1918, who had essentially zero prenatal exposure to the 1918 Pandemic. Furthermore, we can use variation across US States in the severity of the Pandemic to construct a second, difference-ind-differences estimate of the Pandemic’s effect (using information on state of birth from the US Census.). Both approaches yield large estimates of long-term effects. Despite the brevity of the health shock, children of infected mothers were about 20% more likely to be disabled and experienced wage decreases of 5% or more, as well as reduced educational attainment [Almond, 2006].8,9
Banerjee, Duflo, Postel-Vinay, and Watts [2010] consider the 19th century blight to French vineyards from the phylloxera insect. During the late 1800s, phylloxera spread slowly from the southern coast of France northward, affecting production and thereby income in different regions in different years. They use data on military recruits at age 20 for each Department in each year to assign the phylloxera conditions in the year of birth and examine effects on the recruits. Children born to wine-growing families and born in the years and regions affected by the crisis were .5 to .9 centimeters shorter in adulthood, which is large relative to the 2 centimeter secular increase in heights over the 19th century. In contrast, no long-term impacts were detected for female life expectancy.
Likewise, Field, Robles, and Torero [2009] evaluate the rollout of a prenatal shock in Tanzania during the 1980s, but here a beneficial one: iodine supplementation during pregnancy. Moreover, maternal supplementation is thought to remain effective for two years, so delays in re-supply constitute a second part of the identification strategy. The authors’ data further enable them to consider siblings of those exposed to the sporadic iodization efforts, thereby removing the effect of selective take-up by families. They find large and robust educational impacts – on average about a half a year of schooling, with larger improvements for girls. Health, by contrast, appeared unaffected. In view of the low cost of iodine supplementation and persistence of iodine deficiency in poor countries, the authors conclude that prenatal supplementation offers an efficient, cost-effective means of improving human capital.
Finally, Almond and Mazumder [2010] consider diurnal fasting during pregnancy, using the fact that Ramadan shifts forward approximately 11 days each year. With cohorts born across multiple birth years, Ramadan’s effect can be disentangled from seasonal effects on health [Doblhammer and Vaupel, 2001]. Furthermore, Almond and Mazumder [2010] leverage the fact that away from the equator, the Ramadan fast is longer during summer months (as it is defined by the hours of daylight). A point of departure from previous analyses of Ramadan is to not consider the decision to fast itself as exogenous, or at least conditionally independent. Indeed, Almond and Mazumder [2010] do not observe whether mothers fasted, and instead construct an Intent to Treat estimate based on whether a child was in utero during Ramadan. Interestingly, Almond and Mazumder [2010] find no evidence that Muslims time births relative to Ramadan’s occurrence. Nevertheless, being exposed to Ramadan in early pregnancy has large health effects. Diurnal fasting in early pregnancy increases the likelihood of adult disability by over 20% among Uganda’s Muslims and Iraqis, with substantially larger effects for mental/learning disabilities. The fact that Ramadan is also a relatively mild health shock leads us to consider other more commonly-experienced exposures.
Mild and Non-nutritional Early-life Exposures
Economists have utilized the power of large-sample datasets to detect effects of relatively mild fetal insults. This extension is key as exposure to relatively mild pathogens is common. Hence, estimates of the effects of mild exposures may be more relevant to policy then estimates of the effects of disasters. Because the immediate mortality and economic disruption from the 1918 Flu or the China famine are sufficient to imply that any reasonable measure to prevent such catastrophes is likely to pass a cost-benefit calculation, showing that there was additional damage to fetal health from these disasters merely “makes the rubble bounce.” However, a practical danger in focusing on mild exposures is that identification may suffer. Economists have been skillful at analyzing relatively mild exposures (compared to famine and pandemic) while leveraging the sharp predictions of the fetal origins hypothesis regarding timing of pregnancy relative to shocks.
Infectious disease offers one example. Infections were mentioned in early work by Barker, but this idea was subsequently neglected in the epidemiological literature [Finch, 2007]. Infections can affect fetal health by diverting maternal energy towards fighting infection, by restricting food intake, or through negative sequelae of the body’s own inflammatory response [Crimmins and Finch, 2006]. Examples of economic investigations exploiting variations in infectious disease include seasonal variation in infections [Costa and Lahey, 2005], improvements across U.S. states in reducing the burden of infectious disease [Case and Paxson, 2009] (typhoid and measles), and early-life malaria exposure in the U.S. South [Barreca, 2010]. Despite considering seasonal and endemic infections (cf pandemics), these effects can still be large. For example, Barreca [2010] estimates that schooling falls approximately half a year with ten additional malaria deaths per 100,000 and that early-life malaria “can account for as much as 25 percent of the difference in long-term educational attainment between cohorts born in malaria afflicted states” and non-afflicted areas in the early 20th Century US.
Pollution is another commonly experienced and potentially important source of fetal health insults. Recent research shows that low-level exposures to such everyday contaminants as automobile exhaust and cigarette smoke have negative effects on fetal health [Currie, 2011]. Yet there has been little research to date linking fetal exposures to future outcomes. An exception is Sanders [2010], who builds on Chay and Greenstone [2003] who treated the recession of the early 1980s as a natural experiment that reduced pollution (just as the current recession has reduced the production of greenhouse gases). He finds that high school test scores in Texas improved for the cohort in utero at the time.
Finally, a number of interesting papers consider the effects of economic shocks around the time of birth on fetal health. Here, health in adulthood tends to be the focus (rather than human capital), and findings are less consistent than in the studies of nutrition and infection described above. One problem may be that the shocks are more diffuse in terms of timing, so that comparisons are less sharp. A second issue is that the mechanism is not entirely clear. Presumably economic downturns could affect fetal health through multiple pathways including effects on nutrition, smoking, drinking, stress and stress-related disease. As one example, Van Den Berg et al. [2006] found that those born during economic downturns in the Netherlands lived “a few years less” than those born during a boom. In contrast, Cutler et al. [2007] use data from the Health and Retirement Survey to look for long-term effects on the health of cohorts born during the Dustbowl era of 1930s. They do not find any effects, but this may be a case where the timing of the shock is somewhat diffuse. Baten, Crayen, and Voth [2007] related variations in grain prices in the decade of birth to adult numeracy using an ingenious measure based on “age heaping” in the British Censuses between 1851 and 1881. Persons who are more numerate are less likely to round their ages to multiples of 5 or 10. They find that children born in decades with high grain prices were less numerate by this index.
The fact that the estimated effects of milder fetal health shocks often remain large suggests that it may be possible to go further and consider still milder and more common exposures. For example, there is considerable interest in the possible effects of maternal stress on infant health, e.g. Aizer, Stroud, and Buka [2009]. Stoecker [2010] examines the impact of extreme cold during pregnancy and finds some small but statistically significant negative effects on adult test scores in vulnerable populations.
Fetal Origins versus Culling
Adverse events experienced in utero may do more than “scar” survivors. These events may also increase fetal mortality as well as early life mortality rates. Survivors exposed to these circumstance are thus a potentially selected sample, where selection is endogenous to the same adverse event as the “scarring” effect. The challenge to inference posed by selective mortality has been recognized both in epidemiology and economics.
A first pass at this issue is to consider the direction and scale of the selective mortality. Because mortality tends to remove those in poor health, survivors of negative fetal events are generally positively selected. Thus, in order to be detected, the negative scarring effects have to be sufficiently strong among the survivors to overwhelm the positive effect of selection. Hence, estimates of the effects of fetal health shocks are generally conservative when the shock also increases mortality. It may be possible to use the estimated increase in the mortality rate to bound the magnitude of the bias from selective mortality. For example, if neonatal mortality increases by 2 percentage points due to an adverse in utero event, and we assume that this 2 percent would have had, for example, the best (and alternatively, worst) outcomes observed among survivors, this would yield lower and upper bounds on scarring. A much larger scope for selective mortality may exist if fetal mortality is affected.
The scope for selective mortality is generally larger in the more extreme natural experiments, such as those provided by famines or pandemics. It may also be more important in situations where baseline health is poor, and a relatively modest shock can have accentuated mortality effects. The latter case is relatively likely in developing countries or with historical episodes in developed countries.
Economists have modeled this mortality selection process. For example, Almond [2006] assumes that unobserved, unidimensional health is sufficient to survive to adulthood if it remains above some mortality threshold. An early life shock can raise mortality by either reducing the mean of population health, or by causing the mortality threshold to increase (This could occur if the health care infrastructure deteriorates during the health shock). To the extent that some of the former effect is permanent and stronger than the effect of an increased mortality threshold, we will observe damage among survivors in accordance with the fetal origins hypothesis. In addition to the severity of the health insult, the fraction of the population that is unaffected or asymptomatic will also determine mortality rates.10 Almond also shows how infection rates, the key exogenous variable in this type of study, can be recovered from aggregate mortality rates without making assumptions on the distribution of health – which by definition is unobserved.
Bozzoli, Deaton, and Quintana-Domeque [2009] consider the tension between culling and scarring effects of infection in determining health. They use adult height as a measure of health, arguing that in populations it is a sensitive index of the disease environment (conditional on nutrition). One innovative feature of their work is that they consider both relatively developed, low mortality countries and higher mortality developing countries. A second is that they show how much further it is possible to get in terms of inference if we assume that health is distributed normally. Their basic finding is that in developing countries, the net effect of culling versus scarring is to make survivors taller, while the reverse is found for developing countries with high mortality rates.
Neither Almond [2006] nor Bozzoli, Deaton, and Quintana-Domeque [2009] allow the early-childhood mortality threshold to increase with the virulence of the disease outbreak. This could occur if the health-care infrastructure deteriorates during the health shock (for example, perhaps because health practitioners also get sick). To the extent that this effect increases early-life mortality, a negative bias on later-life outcomes remains and future work might explore the importance of relaxing this assumption.
Future Research
Despite the proliferation of research on the fetal origins hypothesis, a host of promising avenues for future research remain. We highlight five.
First, many papers have documented that a wide array of natural experiments had effects on newborn health, but the long-term effects of these experiments have not been determined, e.g. Currie and Gruber [1996] or Hoynes, Page, and Stevens [2009]. With appropriate data, such analyses constitute low-hanging fruit. There have been a few pioneering attempts to follow the long-term effects of interventions in early childhood on long-term outcomes. For example, Garces, Thomas, and Currie [2002] and Ludwig and Miller [2007] consider the long-term effects of Head Start, while Chetty et al. [2010] and Muennig, Johnson, and Wilde [2011] examine the long-term effects of assignment to different Kindergarten classrooms in the Tennessee STAR experiment. But studies that consider long-term effects of real-world policies (rather than rare disasters) touching fetal health have been relatively rare. Short of conducting actual policy evaluations, if economists can identify the effects of less extreme episodes on long-term outcomes, then this will be extremely useful for policy.
Second, one of the most radical implications of the fetal origins hypothesis may be that one can best help children (throughout their life course) by helping their mothers. That is, we should be focusing on pregnant women, or perhaps even women of child-bearing age if the key period turns out to be so early in pregnancy that many women are unaware of the pregnancy [Almond and Mazumder, 2010]. Such pre-emptive targeting would constitute a radical departure from current policies that steer nearly all healthcare resources to the sick, i.e. the “pound of cure” approach. That said, the existing evidence is not sufficient to allow us to rank the cost-effectiveness of interventions targeted at women against more traditional interventions targeted at children, adolescents, or adults. For example, broadening the target population to women who might get pregnant would reduce the set of policies which are cost effective. Moreover, there are relatively few “shovel-ready” policies that have been shown to affect fetal health at the population level. Prenatal iodine supplementation [Field, Robles, and Torero, 2009], Food Stamps [Almond, Hoynes, and Schanzenbach, 2011], and the Supplemental Feeding Program for Women, Infants, and Children (WIC) are important exceptions.
A third area for future research involves the integration of work on son preference with work on fetal origins. Prior to the advent of prenatal sex determination, girls were protected from gender bias prior to birth, both in terms of its effect on birth outcomes but also in terms of its spectrum of fetal effects.11 With the diffusion of technologies that allow determining the sex of the fetus earlier, such protection has eroded [Lhila and Simon, 2008]. Dahl and Moretti [2008] find, for example, that among mothers who have ultrasounds, those carrying girls are less likely to be married at the time of the birth. On the other hand, increased sex-selective abortion may imply that families who carry a girl to term may be less biased against girls, which should improve postnatal investments. The initial evidence suggests that health investments respond to sex determination and sex selection. In India, Hu and Schlosser [2010] find that fewer girls are born and that they are less likely to be malnourished. In contrast, Bharadwaj and Nelson [2010] find that Indian mothers pregnant with sons have more prenatal care visits and tetanus shots than girls. In China, Almond, Li, and Meng [2010b] find no change in investment measures, but that neonatal mortality, an indicator of prenatal environment, increases for girls after ultrasound becomes available. A new chapter in the literature on fetal origins will consider the ramifications of sex discrimination that can now commence in utero.
Fourth, future work on the fetal origins hypothesis should consider the rising obesity rates in both developed and developing countries. Maternal obesity has been associated with poor birth outcomes, and with premature birth in particular [McDonald et al., 2010]. Currie and Ludwig [2010] show that higher maternal weight gain during pregnancy is related to a higher probability of birth weight over 4000 grams, which is also thought to lead to a greater probability of obesity, diabetes, and heart disease later in life. To date, there has been little explanation of how increased obesity may affect the fetus and thus have long-term health effects. Gluckman and Hanson [2005, p. xii] observe that: “for the first time in our evolutionary history, humans now inhabit an environment in which we have not evolved to live.” One alarming possibility is that the trends in obesity may erode the historical gains in cohort health caused by the reduced disease burden and improved nutrition.
Finally, a major limitation in current research is our inability to make quantitative predictions as to how much a given fetal insult will affect later life outcomes. To this end, it will be important to uncover which measures of maternal health, health at birth, or early childhood health best capture incipient fetal origins effects. At present, this is a open question: to a surprising degree pregnancy remains a “black box”. Obviously, doctors and epidemiologists may need to take the lead in devising new measures. Promising possibilities include research in epigenetics which aims to decipher when and how various genes are switched “on” and “off” and, as a fallback, better measures of proximate maternal factors such as stress levels or maternal nutrition. Progress in measurement will help delineate mechanisms by which fetal origins effects are generated. Economists can help by evaluating whether such measures indeed lie along the casual pathway (in epidemiology, measurement is often treated as a stand-in for credible identification). Progress on measurement will not only allow fetal origins research to move beyond its qualitative predictions, but also to construct stronger falsification tests.
Footnotes
We thank David Autor, Chad Jones, Climent Quintana-Domeque, John List, Bhashkar Mazumder, Kathleen Rasmussen, and Timmothy Taylor for comments and Christine Pal for research assistance.
Mothers infected with Rubella in the first trimester of pregnancy have a 50 percent chance of delivering a baby with a problem such as cataracts, deafness, or heart disease [Dunn, 2007].
In the preface to The Fetal Matrix, Gluckman and Hanson [2005] observe that: “most ideas in science just seem to evolve – no single person is the real inventor – rather multiple groups of researchers converge on a problem, offering thoughts and insights and experimental data, and the theory gradually develops.” More pointedly, Lynch and Smith [2005] refer to Barker and Osmond [1986] as “the most influential replication” of Forsdahl [1977 Forsdahl [1978].
Kelly [2010] notes a similar disconnect between birth weight and fetal origins effects for the the 1957 “Asian Flu” in Britain.
If investments in all periods subsequent to the shock are affected by the shock, then prenatal exposures could be important for adult health in the Grossman [1972] framework. However, the fetal origins literature posits an important and persistent biological effect of the prenatal period — that is, holding investments fixed.
However, the constant elasticity of substitution production function employed does not impose complementarity, but rather allows a the full range of elasticities of substitution from Leontieff through perfect substitutability, see Heckman [2007], Almond and Currie [2011].
The qualitative predictions of the fetal origins hypothesis are stronger than the quantitative predictions, a point we return to in the “Future Research” section.
On the other hand, if the one-off shock affects the composition of births, this comparison may be less clean than considering effects among those already conceived at the time of the shock.
Brown [2011] argues that parental characteristics may have deteriorated for the in utero cohort. Insofar as cohort differences are concerned (US regional variation is not analyzed), when Brown [2011] controls for a vector of parental characteristics in the National Longitudinal Study sample, damage estimates are qualitatively similar and statistically indistinguishable from those without this adjustment.
Nelson [2010], Kelly [2010], Neelsen and Stratmann [2010] and Lin [2011] find similar long-term damage for prenatal exposure to pandemic influenza in Brazil, Britain, Switzerland, and Taiwan, respectively.
Thanks to Larry Katz for making this point.
Gender discrimination in postnatal investments has been well documented, e.g. Das Gupta [1987].
Contributor Information
Douglas Almond, Email: da2152@columbia.edu.
Janet Currie, Email: janet.currie@columbia.edu.
References
- Adhvaryu Achyuta R, Nyshadham Anant. Endowments and investments within the household: Evidence from iodine supplementation in tanzania. Yale University; Apr, 2011. manuscript. [Google Scholar]
- Aizer Anna, Stroud Laura. Education, medical knowledge and the evolution of disparities in health. Brown University; Sep, 2009. manuscript. [Google Scholar]
- Aizer Anna, Stroud Laura, Buka Stephen. Maternal stress and child well-being: Evidence from siblings. Brown University; Feb, 2009. manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almond Douglas. Is the 1918 Influenza Pandemic over? Long-term effects of in utero influenza exposure in the post-1940 U.S. population. Journal of Political Economy. 2006 Aug;114(4):672–712. [Google Scholar]
- Almond Douglas, Currie Janet. Human Capital Development Before Age 5. 4b. Elsevier; 2011. Handbook of Labor Economics; pp. 1315–1486. [Google Scholar]
- DouglasAlmond Douglas, Mazumder Bhashkar. Health capital and the prenatal environment: The effect of maternal fasting during pregnancy. Working Paper 14428, National Bureau of Economic Research, May 2010. American Economic Journal: Applied Economics forthcoming. [Google Scholar]
- Almond Douglas, Edlund Lena, Palme Mårten. Chernobyl’s subclinical legacy: Prenatal exposure to radioactive fallout and school outcomes in sweden. Quarterly Journal of Economics. 2009;124(4):1729–1772. [Google Scholar]
- Almond Douglas, Edlund Lena, Li Hongbin, Zhang Junsen. In: The Economic Consequences of Demographic Change in East Asia, NBER-EASE, chapter Long-term Effects of the 1959–61 China Famine: Mainland China and Hong Kong. Ito Takatoshi, Rose Andrew., editors. University of Chicago Press; Chicago: 2010a. pp. 321–350. [Google Scholar]
- Almond Douglas, Li Hongbin, Meng Lingsheng. Son preference and early childhood investments in China. manuscript. School of Economics and Management, Tsinghua University; 2010b. [Google Scholar]
- Almond Douglas, Hoynes Hilary W, Schanzenbach Diane Whitmore. Inside the war on poverty: The impact of food stamps on birth outcomes. Review of Economics and Statistics. 2011;93(2):387–403. [Google Scholar]
- Banerjee Abhijit, Duflo Esther, Postel-Vinay Gilles, Watts Tim. Long-run health impacts of income shocks: Wine and phylloxera in nineteenth-century france. Review of Economics and Statistics. 2010;92(4):714–728. [Google Scholar]
- Barker DJ. Fetal origins of coronary heart disease. British Medical Journal. 1995 Jul;311(6998):171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in england and wales. The Lancet. 1986 May;327(8489):1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- Barreca Alan I. The Long-Term Economic Impact of In Utero and Postnatal Exposure to Malaria. J Human Resources. 2010;45(4):865–892. URL http://jhr.uwpress.org/cgi/content/abstract/45/4/865. [Google Scholar]
- Baten Jörg, Crayen Dorothee, Voth Joachim. Economics Working Papers 1120. Department of Economics and Business, Universitat Pompeu Fabra; Oct, 2007. Poor, hungry and stupid: Numeracy and the impact of high food prices in industrializing britain, 1780–1850. URL http://ideas.repec.org/p/upf/upfgen/1120.html. [Google Scholar]
- Behrman Jere R, Rosenzweig Mark R, Taubman Paul. Endowments and the allocation of schooling in the family and in the marriage market: The twins experiment. Journal of Political Economy. 1994;102(6):1131–1174. [Google Scholar]
- Bhalotra Sonia, Rawlings Samantha B. Intergenerational persistence in health in developing countries: The penalty of gender inequality? Journal of Public Economics. 2011 Apr;95(3–4):286–299. URL http://ideas.repec.org/a/eee/pubeco/v95y2011i3-4p286-299.html. [Google Scholar]
- Bharadwaj Prashant, Nelson Leah K. Discrimination begins in the womb: Evidence of sex selective prenatal care. UCSD Economics; 2010. manuscript. [Google Scholar]
- PrashantBharadwaj Prashant, Eberhard Juan, Neilson Christopher. Do initial endowments matter only initially? the persistent effect of birth weight on school achievement. Department of Economics; UC San Diego: Sep, 2010. (University of California at San Diego, Economics Working Paper Series 1562347). [Google Scholar]
- Birchenall Javier. Escaping high mortality. Journal of Economic Growth. 2007;12:351–387. URL http://dx.doi.org/10.1007/s10887-007-9022-2.10.1007/s10887-007-9022-2. [Google Scholar]
- Black Sandra E, Devereux Paul J, Salvanes Kjell G. From the cradle to the labor market? the effect of birth weight on adult outcomes. Quarterly Journal of Economics. 2007 Feb;122(1):409–439. [Google Scholar]
- Bleakley Hoyt. Disease amd development: Evidence from hookworm eradication in the south. The Quarterly Journal of Economics. 2007;122(1):73. doi: 10.1162/qjec.121.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bono Emilia, Ermisch John. Iser working paper 2009–16. Institute for Social and Economic Research; 2009. Birth weight and the dynamics of early cognitive and behavioural development. [Google Scholar]
- Bozzoli Carlos, Deaton Angus S, Quintana-Domeque Climent. Child mortality, income and adult height. Demography. 2009 Nov;46(4):647–669. doi: 10.1353/dem.0.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt Loren, Siow Aloysius, Vogel Carl. Large shocks and small changes in the marriage market for famine born cohorts in china. University of Toronto, Dept. of Economics; 2008. Working Paper 2008-09-08. [Google Scholar]
- Brown Ryan. The 1918 u.s. influenza pandemic as natural experiment, revisited. manuscript. Duke; Feb, 2011. [Google Scholar]
- Case Anne, Paxson Christina. Early Life Health and Cognitive Function in Old Age. American Economic Review Papers and Proceedings. 2009 May;99(2):104–109. doi: 10.1257/aer.99.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chay Kenneth Y, Greenstone Michael. The impact of air pollution on infant mortality: Evidence from the geographic variation in pollution shocks induced by a recession. Quarterly Journal of Economics. 2003 Aug;118(3):1121–1167. [Google Scholar]
- Chen Yuyu, Zhou Li-An. The long term health and economic consequences of 1959–1961 famine in China. Journal of Health Economics. 2007 Jul;26(4):659–681. doi: 10.1016/j.jhealeco.2006.12.006. [DOI] [PubMed] [Google Scholar]
- RajChetty Raj, Friedman John N, Hilger Nathaniel, Saez Emmanuel, Schanzenbach Diane Whitmore, Yagan Danny. Working Paper 16381. National Bureau of Economic Research; Sep, 2010. How does your kindergarten classroom affect your earnings? evidence from project star. [DOI] [PubMed] [Google Scholar]
- Costa Dora L, Lahey Joanna N. Predicting older age mortality trends. Journal of the European Economic Association. 2005 Apr-May;3(2–3):487–493. [Google Scholar]
- Crimmins Eileen M, Finch Caleb E. Infection, inflammation, height, and longevity. Proceedings of the National Academy of Sciences. 2006 Jan;103(2):498–503. doi: 10.1073/pnas.0501470103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha Flavio, Heckman James J. Identifying and Estimating the Technology of Cognitive and Noncognitive Skill Formation. J Human Resources. 2008;43(4):738–782. doi: 10.3368/jhr.43.4.738. Formulating. URL http://jhr.uwpress.org/cgi/content/abstract/43/4/738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha Flavio, Heckman James J, Schennach Susanne M. Estimating the technology of cognitive and noncognitive skill formation. Econometrica. 2010;78(3):883–931. doi: 10.3982/ECTA6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie Janet. Healthy, wealthy, and wise: Is there a causal relationship between child health and human capital development? Journal of Economic Literature. 2009 Mar;XLVII(1):87–122. [Google Scholar]
- Currie Janet. Inequality at birth: Some causes and consequences. American Economic Review Papers and Proceedings. 2011 May;(2):984–990. Ely Lecture. [Google Scholar]
- Currie Janet, Gruber Jonathan. Health insurance eligibility, utilization of medical care, and child health. The Quarterly Journal of Economics. 1996 May;111(2):431–466. [Google Scholar]
- Currie Janet, Hyson Rosemary. Is the impact of shocks cusioned by socioeconomic status? the case of low birth weight. American Economic Review. 1999 May;89(2):245–250. [Google Scholar]
- Currie Janet, Ludwig David. The association between pregnancy weight gain and birthweight: A within-family comparison. The Lancet. 2010 Sep;376(9745):984–990. doi: 10.1016/S0140-6736(10)60751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie Janet, Moretti Enrico. Biology as destiny? short- and long-run determinants of intergenerational transmission of birth weight. Journal of Labor Economics. 2007;25(2):231–264. doi: 10.1086/511377. URL http://www.journals.uchicago.edu/doi/abs/10.1086/511377. [DOI] [Google Scholar]
- Currie Janet, Stabile Mark, Manivong Phongsack, Roos Leslie L. Child health and young adult outcomes. Journal of Health Economics. 2009 forthcoming. [Google Scholar]
- Cutler David, Fung Winnie, Kremer Michael, Singhal Monica, Vogl Tom. Early-life malaria exposure and adult outcomes: Evidence from malaria eradication in india. American Economic Journal: Applied Economics. 2010;2(2):72–94. doi: 10.1257/app.2.2.72. URL http://www.aeaweb.org/articles.php?doi=10.1257/app.2.2.72. [DOI] [Google Scholar]
- Cutler David M, Miller Grant, Norton Douglas M. Evidence on early-life income and late-life health from America’s Dust Bowl era. Proceedings of the National Academy of Sciences. 2007;104(33):13244, 13249. doi: 10.1073/pnas.0700035104. URL http://www.pnas.org/content/104/33/13244.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl Gordon B, Moretti Enrico. The demand for sons. Review of Economic Studies. 2008 Oct;75(4):1085–1120. [Google Scholar]
- Das Gupta Monica. Selective discrimination against female children in rural punjab, india. Population and Development Review. 1987;13(1):77–100. URL http://www.jstor.org/stable/1972121. [Google Scholar]
- Datar Ashlesha, Kilburn Rebecca, Loughran David. Endowments and parental investments in infancy and early childhood. Demography. 2010 Feb;47(1):125–144. doi: 10.1353/dem.0.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes Olivier, Greenstone Michael, Guryan Jonathan. Climate change, birth weight and long run earnings capacity. American Economic Review Papers and Proceedings. 2009 May;99(2) doi: 10.1257/aer.99.2.211. [DOI] [PubMed] [Google Scholar]
- Dinardo John. Interesting questions in Freakonomics. Journal of Economic Literature. 2007 Dec;45(4):973–1000. [Google Scholar]
- Doblhammer Gabriele, Vaupel James W. Lifespan depends on month of birth. Proceedings of the National Academy of Sciences. 2001 Feb;98(5):2934–2939. doi: 10.1073/pnas.041431898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn PM. Perinatal lessons from the past: Sir norman gregg, chm, mc, of sydney (1892–1966) and rubella embryopathy. Archives of Disease in Childhood Fetal and Neonatal Edition. 2007;92(6):F513–F514. doi: 10.1136/adc.2005.091405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field Erica, Robles Omar, Torero Maximo. Iodine deficiency and schooling attainment in tanzania. American Economic Journal: Applied Economics. 2009 Oct;1(4):140–169. [Google Scholar]
- Finch Caleb E. The Biology of Human Longevity. Academic Press (Elsevier); Amsterdam: 2007. [Google Scholar]
- Fogel Robert W, Costa Dora L. A theory of technophysio evolution, with some implications for forecasting population, health care costs, and pension costs. Demography. 1997 Feb;34(1):49–66. [PubMed] [Google Scholar]
- Forsdahl A. Are poor living conditions in childhood and adolescence an important risk factor for arteriosclerotic disease? British Journal of Preventive and Social Medicine. 1977;31(2):91–95. doi: 10.1136/jech.31.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsdahl A. Living conditions in childhood and subsequent development of risk factors for arteriosclerotic heart disease. the cardiovascular survey in finmark 1974–75. Journal of Epidemiology and Community Health. 1978 Mar;32(1):34–37. doi: 10.1136/jech.32.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman David A. Statistical models and shoe leather. Sociological Methodology. 1991;21 [Google Scholar]
- Garces Eliana, Thomas Duncan, Currie Janet. Longer-term effects of head start. American Economic Review. 2002 Sep;92(4):999–1012. [Google Scholar]
- Gluckman Peter, Hanson Mark. The Fetal Matrix: Evolution, Development and Disease. Cambridge University Press; Cambridge: 2005. [Google Scholar]
- Goddard HH. The Kallikak Family: A Study in the Heredity of Feeble-Mindedness. MacMillan; New York: 1912. [Google Scholar]
- Grossman Michael. On the concept of health capital and the demand for health. Journal of Political Economy. 1972:223–255. [Google Scholar]
- Heckman James James. The economics, technology, and neuroscience of human capability formation. PNAS. 2007 Aug 14;104(33):13250–13255. doi: 10.1073/pnas.0701362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek HW, Brown AS, Susser E. The dutch famine and schizophrenia spectrum disorders. Social Psychiatry and Psychiatric Epidemiology. 1998;33:373–379. doi: 10.1007/s001270050068. URL http://dx.doi.org/10.1007/s001270050068.10.1007/s001270050068. [DOI] [PubMed] [Google Scholar]
- Hoynes Hilary W, Page Marianne, Stevens Ann H. Working Paper 15589. National Bureau of Economic Research; Dec, 2009. Is a wic start a better start? evaluating wic’s impact on infant health using program introduction. [Google Scholar]
- Hu Luojia, Schlosser Analia. manuscript. Tel Aviv University; 2010. Does sex-selective abortion improve girl’s well-being? evidence from india. [Google Scholar]
- Kannisto Väinö, Christensen Kaare, Vaupel James W. No increased mortality in later life for cohorts born during famine. American Journal of Epidemiology. 1997 Jun;145(11):987–994. doi: 10.1093/oxfordjournals.aje.a009067. [DOI] [PubMed] [Google Scholar]
- Karp RJ, Qazi QH, Moller KA, Angela WA, Davis JM. Fetal alcohol syndrome at the turn of the century: An unexpected explanation of the kallikak family. Archives of Pediatrics and Adolescent Medicine. 1995;149(1):45–48. doi: 10.1001/archpedi.1995.02170130047010. [DOI] [PubMed] [Google Scholar]
- Kelly Elaine. The scourge of asian flu: in utero exposure to pandemic influenza and the development of a cohort of british children. The Journal of Human Resources. 2010 forthcoming. [Google Scholar]
- Kermack WO, McKendrick AG, McKinlay PL. Death-Rates in Great Britain and Sweden. The Lancet. 1934 Mar 31;:698–703. [Google Scholar]
- Landro Laura. The Wall Street Journal. Sep 9, 2010. Healthy living, for two. [Google Scholar]
- Lhila Aparna, Simon Kosali I. Prenatal health investment decisions: Does the childs sex matter? Demography. 2008 Nov;45(4):885–905. doi: 10.1353/dem.0.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming-JenLin Ming-Jen. Does in utero exposure to illness matter? the 1918 influenza epidemic in taiwan as a natural experiment? manuscript. National Taiwan University; 2011. [DOI] [PubMed] [Google Scholar]
- Ludwig Jens, Miller Douglas L. Does head start improve children’s life chances? evidence from a regression discontinuity design. Quarterly Journal of Economics. 2007 Feb;488(1):159–208. [Google Scholar]
- Lynch John, Smith George Davey. A life course approach to chronic disease epidemiology. Annual Review of Public Health. 2005;26(1):1–35. doi: 10.1146/annurev.publhealth.26.021304.144505. [DOI] [PubMed] [Google Scholar]
- Maccini Sharon, Yang Dean. Under the weather: Health, schooling, and economic consequences of early-life rainfall. American Economic Review. 2009;99(3):1006–26. doi: 10.1257/aer.99.3.1006. URL http://www.aeaweb.org/articles.php?doi=10.1257/aer.99.3.1006. [DOI] [PubMed] [Google Scholar]
- Mcbride WG. Thalidomide and congenital abnormalities. The Lancet. 1961;278(7216):1358–1358. doi: 10.1016/S0140-6736(61)90927-8. [DOI] [Google Scholar]
- McDonald Sarah D, Han Zhen, Mulla Sohail, Beyene Joseph. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ. 2010 Jul;:341. doi: 10.1136/bmj.c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muennig P, Johnson G, Wilde ET. The effect of small class sizes on mortality through age 29: evidence from a multi-center randomized controlled trial. American Journal of Epidemiology. 2011 doi: 10.1093/aje/kwr011. forthcoming. [DOI] [PubMed] [Google Scholar]
- Neelsen Sven, Stratmann Thomas. Long-??term effects of prenatal influenza exposure: evidence from switzerland. manuscript. George Mason University Economics; 2010. [Google Scholar]
- Nelson Richard E. Testing the fetal origins hypothesis in a developing country: evidence from the 1918 influenza pandemic. Health Economics. 2010;19(10):1181–1192. doi: 10.1002/hec.1544. URL http://dx.doi.org/10.1002/hec.1544. [DOI] [PubMed] [Google Scholar]
- Nilsson Peter. Does a pint a day affect your child’s pay? the effect of pre-natal alcohol exposure on adult outcomes. Centre for Microdata Methods and Practice Working Paper CWP22/08, The Institute for Fiscal Studies; Aug, 2008. [Google Scholar]
- Oreopoulos Phil, Stabile Mark, Walld Randy, Roos Leslie. Short, medium, and long-term consequences of poor infant health: An anlysis using siblings and twins. Journal of Human Resources. 2008 Winter;43(1) [Google Scholar]
- Paneth Nigel, Susser Mervyn. Early origins of coronary heart disease: the barker hypothesis. British Medical Journal. 1995 Feb 18;310(2):411–412. doi: 10.1136/bmj.310.6977.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul Annie Murphy. Origins. Free Press; New York: 2010. [Google Scholar]
- Petronis Arturas. Epigenetics as a unifying principle in the aetiology of complex traits and diseases. Nature. 2010 Jun;465:721–727. doi: 10.1038/nature09230. [DOI] [PubMed] [Google Scholar]
- Rasmussen Kathleen Maher. The “fetal origins” hypothesis: Challenges and opportunities for maternal and child nutrition. Annual Review of Nutrition. 2001 Jul;21:73–95. doi: 10.1146/annurev.nutr.21.1.73. [DOI] [PubMed] [Google Scholar]
- Ravelli Gian-Paolo, Stein Zena A, Susser Mervyn W. Obesity in young men after famine exposure in utero and early infancy. The New England Journal of Medicine. 1976 Aug;295(7):349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- Roan Shari. Living for two. The Los Angeles Times; Nov 12, 2007. [Google Scholar]
- Royer Heather. Separated at girth: Us twin estimates of the effects of birth weight. American Economic Journal: Applied Economics. 2009 Jan;1(1):49–85. [Google Scholar]
- Sanders Nicholas J. What doesnt kill you makes you weaker: Prenatal pollution exposure and later educational outcomes. manuscript. Stanford University; 2010. [Google Scholar]
- Scherjon Sicco A, Oosting Hans, Ongerboer de Visser Bram W, deWilded Ton, Zondervan Hans A, Kok Joke H. Fetal brain sparing is associated with accelerated shortening of visual evoked potential latencies during early infancy. American Journal of Obstetrics and Gynecology. 1996 Dec;175(6):1569–1575. doi: 10.1016/s0002-9378(96)70108-4. [DOI] [PubMed] [Google Scholar]
- Paul Schultz T, Strauss John. Hadbook of Development Economics. Vol. 4. North-Holland; Amsterdam: 2008. [Google Scholar]
- Schulz Laura C. The Dutch Hunger Winter and the developmental origins of health and disease. Proceedings of the National Academy of Sciences. 2010;107(39):16757–16758. doi: 10.1073/pnas.1012911107. URL http://www.pnas.org/content/107/39/16757.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith James P. Healthy bodies and thick wallets. Journal of Economic Perspectives. 1999;13(2):145–166. [PMC free article] [PubMed] [Google Scholar]
- StClair David, Xu Mingqing, Wang Peng, Yu Yaqin, Fang Yoorong, Zhang Feng, Zheng Xiaoying, Gu Niufan, Feng Guoyin, Sham Pak, He Lin. Rates of adult schizophrenia following prenatal exposure to the Chinese famines of 1959–1961. Journal of the American Medical Association. 2005 Aug;294(5):557–562. doi: 10.1001/jama.294.5.557. [DOI] [PubMed] [Google Scholar]
- Stanner SA, Blumer K, Andrès C, Lantseva OE, Borodina V, Poteen VV, Yudkin JS. Does malnutrition in utero determine diabestes and coronary heart disease in adulthood? results from the leningrad seige study, a cross sectional study. British Medical Journal. 1997 Nov;315:1342–1348. doi: 10.1136/bmj.315.7119.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein AD, Kahn HS, Rundle A, Zybert PA, van der Pal-de Bruin K, Lumey LH. Anthropometric measures in middle age after exposure to famine during gestation: Evidence from the dutch famine. American Journal of Clinical Nutrition. 2006a;85:869–76. doi: 10.1093/ajcn/85.3.869. [DOI] [PubMed] [Google Scholar]
- Stein AD, Zybert PA, van der Pal-de Bruin K, Lumey LH. Exposure to famine during gestation, size and birth and blood pressure at age 59: Evidence from the dutch famine. European Journal of Epidemiology. 2006b;21(2):759–65. doi: 10.1007/s10654-006-9065-2. [DOI] [PubMed] [Google Scholar]
- Stein Zena, Susser Mervyn, Saenger Gerhart, Marolla Francis. Famine and Human Development: The Dutch Hunger Winter of 1944–1945. Oxford University Press; New York: 1975. [Google Scholar]
- Stoecker Charles. Chill out, mom: The long run impact of cold induced maternal stress in utero. manuscript. UC Davis Economics; 2010. [Google Scholar]
- Susser Mervyn, Stein Zena. Timing in prenatal nutrition: A reprise of the dutch famine study. Nutrition Reviews. 1994 Mar;52(3):84–94. doi: 10.1111/j.1753-4887.1994.tb01395.x. [DOI] [PubMed] [Google Scholar]
- Van Den Berg Gerard J, Lindeboom Maarten, Portrait France. Economic conditions early in life and individual mortality. American Economic Review. 2006 Mar;96(1):290–302. doi: 10.1257/000282806776157740. [DOI] [PubMed] [Google Scholar]
- Von Lenz W, Knapp K. Die thalidomid-embryopathie. Deutsche Medizinishe Wochenschrift. 1962;87(24):1232–1242. doi: 10.1055/s-0028-1111892. [DOI] [PubMed] [Google Scholar]