Background: Nox4 is known to be regulated primarily at the transcriptional level and regulates myofibroblast differentiation.
Results: Nox4 protein expression is suppressed by Hic-5 via Cbl-c- and HSP27-mediated ubiquitination and proteasomal degradation.
Conclusion: Nox4 is posttranslationally regulated by Hic-5, and its interacting proteins, Cbl-c and HSP27 regulate myofibroblast differentiation and senescence.
Significance: Prosenescence and profibrotic effects of Nox4 may be mitigated by Hic-5-mediated suppression of its protein expression.
Keywords: Adaptor Protein; Fibrosis; NADPH Oxidase; Senescence; Ubiquitination; Hic-5, Cbl-c, HSP27, Nox4, p16, pRb, UPS
Abstract
Hydrogen peroxide-inducible clone 5 (Hic-5) is a focal adhesion adaptor protein induced by the profibrotic cytokine TGF-β1. We have demonstrated previously that TGF-β1 induces myofibroblast differentiation and lung fibrosis by activation of the reactive oxygen species-generating enzyme NADPH oxidase 4 (Nox4). Here we investigated a potential role for Hic-5 in regulating Nox4, myofibroblast differentiation, and senescence. In normal human diploid fibroblasts, TGF-β1 induces Hic-5 expression in a delayed manner relative to the induction of Nox4 and myofibroblast differentiation. Hic-5 silencing induced constitutive Nox4 expression and enhanced TGF-β1-inducible Nox4 levels. The induction of constitutive Nox4 protein in Hic-5-silenced cells was independent of transcription and translation and controlled by the ubiquitin-proteasomal system. Hic-5 associates with the ubiquitin ligase Cbl-c and the ubiquitin-binding protein heat shock protein 27 (HSP27). The interaction of these proteins is required for the ubiquitination of Nox4 and for maintaining low basal levels of this reactive oxygen species-generating enzyme. Our model suggests that TGF-β1-induced Hic-5 functions as a negative feedback mechanism to limit myofibroblast differentiation and senescence by promoting the ubiquitin-proteasomal system-mediated degradation of Nox4. Together, these studies indicate that endogenous Hic-5 suppresses senescence and profibrotic activities of myofibroblasts by down-regulating Nox4 protein expression. Additionally, these are the first studies, to our knowledge, to demonstrate posttranslational regulation of Nox4.
Introduction
The reactive oxygen species (ROS)2-generating enzyme NADPH oxidase 4 (Nox4) has been implicated in a number of physiological and pathological states (1), including fibrotic disorders and cancer. The regulation of Nox4 expression has been attributed primarily to its transcriptional activation and is, thus, uniquely referred to as a “constitutive” enzyme (2). TGF-β1 is the most widely reported inducer of Nox4 gene transcription and induces sustained levels of hydrogen peroxide (H2O2) (3, 4). Posttranscriptional mechanisms that control Nox4 expression are limited. One study reports a posttranscriptional mechanism involving translation-initiated mRNA destabilization of Nox4 (5). However, to our knowledge, posttranslational mechanisms regulating Nox4 have not been reported.
Hic-5 is a focal adhesion adaptor protein that was identified initially as a TGF-β1- and H2O2-inducible gene (6). Hic-5 has been reported to mediate senescence (7, 8). More recent studies demonstrate that Hic-5 interacts with the small heat shock protein HSP27 (9), which binds ubiquitin (10). Cbl-c has been identified as a new interacting protein of Hic-5. The binding of Hic-5 to Cbl-c leads to an increase in the E3 ubiquitin ligase activity of Cbl-c (11).
HSP27 enhances the degradation of proteins through the ubiquitin-proteasomal system (UPS) (10) and can itself be ubiquitinated (12). Notably, HSP27 regulates TGF-β1 signaling (13, 14) and is known to induce senescence (15). HSP27 is overexpressed in human and experimental lung fibrosis (14). Overexpression of HSP27 in renal tubular epithelial cells protects against oxidative injury (16), and inhibition blocks fibrosis (17). Increased expression of p16, hypophosphorylated-Rb, and HSP27 expression has been observed in senescing human fibroblasts following environmental stress (18). However, the relationship between cellular senescence and fibrogenesis is not well understood.
In this study utilizing lung fibroblasts, we demonstrate that the expression of Nox4 is regulated inversely by Hic-5 and its interacting proteins HSP27 and Cbl-c. The loss of negative regulation of Nox4 at the posttranslational level by ubiquitination may, in part, account for its high expression in fibrotic disorders. Therefore, therapeutic strategies to augment Hic-5-, Cbl-c-, and HSP27-dependent ubiquitination and degradation of Nox4 may prove to be beneficial in progressive fibrotic disorders (19, 20).
EXPERIMENTAL PROCEDURES
Cell Culture
We obtained human diploid fibroblasts (IMR-90 cells, Coriell, Camden, NJ). IMR-90 cells were cultured in DMEM (Invitrogen) supplemented with 10% FBS (Invitrogen), 100 units/ml penicillin, 100 μg/ml streptomycin, and 250 μg/ml Fungizone and were incubated at 37 °C in 5% CO2 and 95% air. 70% confluent cells were serum-starved for 16 h and treated with actinomycin D (0.05 μg/ml) or cycloheximide (1 μg/ml) (Sigma), MG132 (10 μm) (EMD Millipore, Billerica, MA) or bortezomib (100 nm) (LC Chemicals, Boston, MA), and 2 ng/ml TGF-β1 (R&D Systems, Minneapolis, MN) for the indicated time.
Gene Silencing with siRNA
For in vitro RNAi studies, we transfected IMR-90 cells with duplexes targeting Hic-5, Cbl-c, and HSP27 or non-targeting control siRNA (Dharmacon, Lafayette, CO) using Lipofectamine 2000 (Invitrogen). Hic-5 knockdown was performed initially using a pool of four human siRNAs and confirmed using duplex 1 (GGAGCUGGAUAGACUGAUGUU) and duplex 2 (GGACCAGUCUGAAGAUAAGUU). Silencing with both pooled and the individual siRNAs resulted in 90% decreased expression of Hic-5 in cells under basal conditions. Additionally, all results with both duplexes were similar. In this study, we used four pooled human siRNA for in vitro silencing of Cbl-c and the individual human siRNA for HSP27 (GUCUCAUCGGAUUUUGCAGCUU) from Dharmacon.
Overexpression Plasmids (cDNA) and Transient Transfections
Plasmids encoding human Cbl-c and HSP27 were procured from Addgene (Cambridge, MA). Overexpression of Cbl-c and HSP27 plasmid constructs was by transient transfections of IMR-90 cells using the cationic lipid reagent Lipofectamine 2000 according to the instructions of the manufacturer. The optimal ratio of DNA (in micrograms) to Lipofectamine 2000 (in microliters) was determined to be ∼1:2 for IMR-90 cells. Cells were incubated with DNA-lipid complexes in Opti-MEM reduced-serum medium overnight prior to introducing DMEM containing 10% serum for 48 h.
Cloning of the Hic-5 Overexpression Plasmid (Hic-5 cDNA)
The coding region for the human Hic-5 gene was amplified from IMR-90 fibroblasts. Cells were cultured in DMEM supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 1.25 μg/ml Fungizone (Invitrogen) at 37 °C and 5% CO2. Cells were serum-starved for 18 h and subsequently stimulated with 2 ng/ml TGF-β1 for 24 h. RNA was isolated using the RNeasy Miniprep kit (Qiagen, Carlsbad, CA). cDNA was synthesized using SuperScript III cDNA synthesis according to the recommendations of the manufacturer (Invitrogen). 3 ng of cDNA was used as template in a PCR using Phusion polymerase (New England Biolabs, Ipswich, MA). The amplicon was ligated into the pcDNA3.1V5hisB vector (Invitrogen), which had been linearized with EcoRV (New England Biolabs). Colonies were screened for orientation, and the sequence was confirmed (Heflin Center for Genomic Science, University of Alabama at Birmingham). IMR-90 cells were transfected with the control (empty vector) or human Hic-5 plasmid construct as discussed above.
Immunoblotting and Immunoprecipitation
Cells cultured in 6-well plates were washed with cold PBS and lysed using radioimmune precipitation assay buffer (pH 7.5) (Sigma) containing protease inhibitor mixture (EMD Millipore, Billerica, MA), 2 mm sodium vanadate, and sodium fluoride (New England Biolabs). Protein concentration of the cell lysates was determined by BCA protein assay (Pierce). Equal amounts of protein (10 μg) were separated by SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad). The membranes were blocked with 5% nonfat milk powder in TBS (pH 7.5) and incubated overnight at 4 °C with the primary antibody Hic-5 and p16 (BD Transduction Laboratories); Nox4 (Novus Biologicals, LLC, Littleton, CO); α-smooth muscle actin (α-SMA) (American Research Products Inc., Waltham, MA); fibronectin (FN) (Sigma); Cbl-c (Rockland Immunochemicals Inc., Gilbertsville, PA); HSP27 and GAPDH (Abcam, Cambridge, MA); and phospho-Rb, phospho-SMAD3, and SMAD3 (Cell Signaling Technology, Danvers, MA). Immunoprecipitation of cell lysates from control (NT siRNA) and Hic-5-, Cbl-c-, and HSP27-silenced cells treated without or with bortezomib (100 μg protein) were performed with overnight incubation of Nox4 polyclonal antibody at 4 °C, followed by pulldown using anti-rabbit IgG beads (Rockland Immunochemicals Inc.) for 3 h. The proteins were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with anti-Lys48 polyubiquitin antibody (EMD Millipore). Immunoprecipitation with Hic-5 antibody from cell lysates of IMR-90 were immunoblotted with Cbl-c or HSP27 antibody to demonstrate endogenous protein interactions.
Real-time PCR
Cells were washed with PBS. The total RNA was extracted using an RNeasy mini kit (Qiagen) according to the instructions of the manufacturer. Total RNA was reverse-transcribed to cDNA using a random hexamer and Superscript III first strand synthesis kit (Invitrogen). Expression levels of Hic-5, Nox4, and β-actin mRNAs were determined by using specific primer as follows: Hic-5, GACTTCCTGCAGCTGTTCG (forward) and AAGTGGTTCTCGCACAACG (reverse); Nox4, CTGGAGGAGCTGGCTCGCCAACGAAG (forward) and GTGATCATGAGGAATAGCACCACCACCATGCAG (reverse); and actin, CACCCTGAAGTACCCCATCGA (forward) and CTCCTTAATGTCACGCACGATTTC (reverse). The real-time PCR reactions were performed in a 7300 real-time PCR system (Applied Biosystems, Grand Island, NY) using a SYBR Green-based real-time PCR assay with SYBR Green PCR master mix (Applied Biosystems).
Statistical Analysis
We made statistical comparisons with GraphPad software (Prism 5) using one-way analysis of variance followed by a Bonferroni test.
RESULTS
Hic-5 and Nox4 are Up-regulated during TGF-β1-induced Myofibroblast Differentiation
TGF-β1 is known to induce myofibroblast differentiation (21) while up-regulating Hic-5 (6) and Nox4 expression (22) in lung fibroblasts. However, the precise relationships between these effects of TGF-β1 are unknown. We examined the effect of TGF-β1 in normal human diploid lung fibroblasts (IMR-90) on the expression of these proteins. TGF-β1 induced a time-dependent increase in the expression of Hic-5 and Nox-4 in parallel with the up-regulation of α-SMA, a marker of myofibroblast differentiation (Fig. 1).
FIGURE 1.
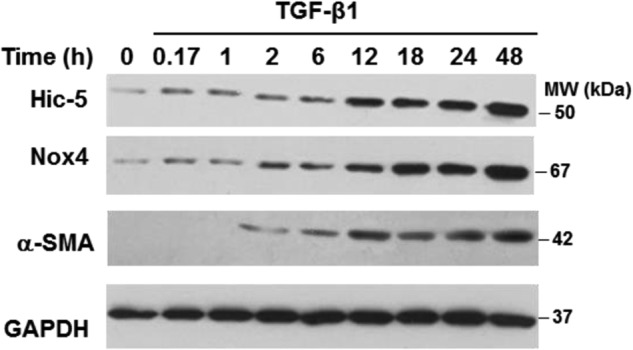
TGF-β1 induces expression of Hic-5 and Nox4 in lung fibroblasts. IMR-90 cells were stimulated with 2 ng/ml of TGF-β1 for the indicated times. Cells were lysed, and levels of Hic-5, Nox4, α-SMA, and GAPDH were determined by Western blotting. Representative results are shown. MW, molecular weight.
Hic-5 Negatively Regulates Nox4 Expression, Myofibroblast Differentiation, and Senescence
To determine whether Hic-5 regulates Nox4 and myofibroblast differentiation/senescence, we silenced Hic-5 with RNAi. Silencing of Hic-5 induced expression of Nox4, α-SMA, and fibronectin, both in the presence and absence of TGF-β1 (Fig. 2A). This constitutive activation was associated with the induced expression of the markers of senescence p16 and hypophosphorylated Rb. TGF-β1-induced myofibroblast differentiation and senescence were augmented under conditions of Hic-5 silencing. Furthermore, silencing of Hic-5 did not inhibit the TGF-β1-induced activation of SMAD3 (Fig. 2B). Collectively, these data indicate that Hic-5 functions as a negative regulator of Nox4, myofibroblast differentiation, and senescence independently of SMAD3 signaling.
FIGURE 2.
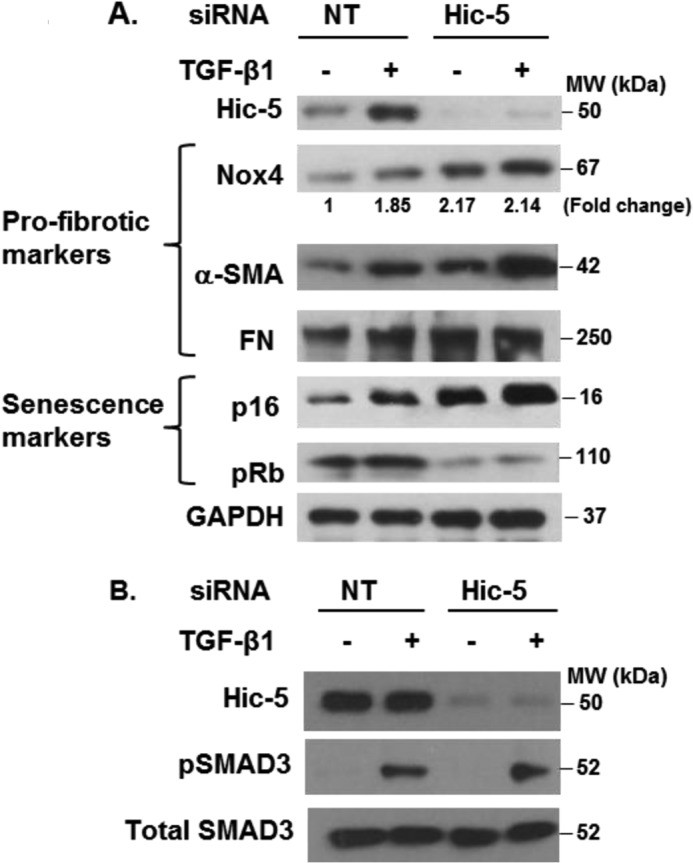
Hic-5 is a negative regulator of senescence and profibrotic genes. IMR-90 cells silenced in Hic-5 or control (NT siRNA) cells were serum-starved and treated without or with 2 ng/ml of TGF-β1 for 24 h (A) or 30 min (B). Levels of Hic-5; GAPDH; the profibrotic markers Nox4, α-SMA, and FN; the senescence markers p16 and pRb; pSMAD3; and SMAD3 were determined by Western blotting. Representative results are shown. MW, molecular weight.
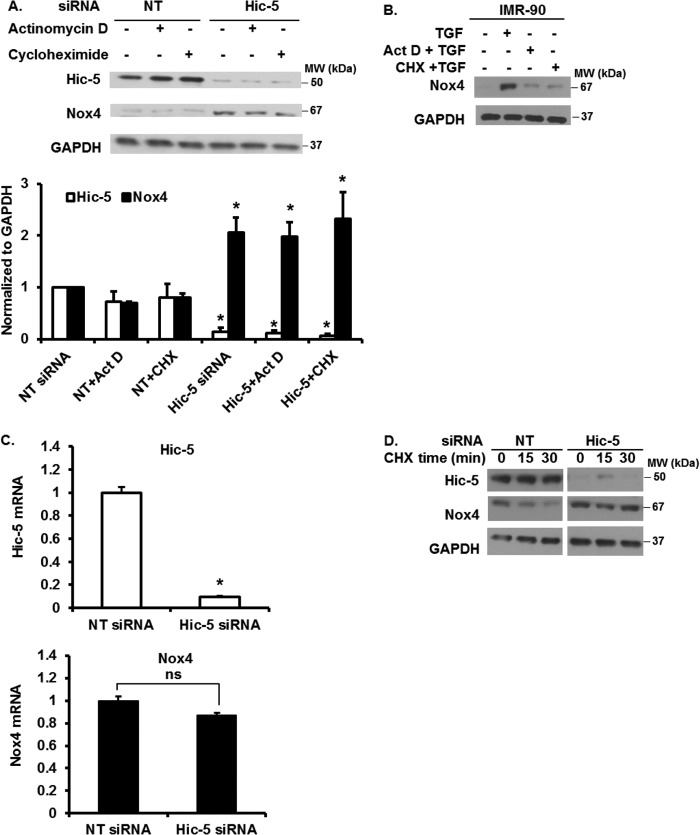
Hic-5 Regulation of Nox4 Is Independent of Transcription and Translation
Next, we investigated mechanisms for the negative regulation of Nox4 by Hic-5. Cells expressing endogenous Hic-5 (NT, non-targeting siRNA) and Hic-5-silenced cells were treated with actinomycin D (an inhibitor of transcription) and cycloheximide (an inhibitor of translation), and the effects on Nox4 were assessed. The up-regulation of Nox4 in Hic-5 silenced cells was not affected by blocking transcription or translation (Fig. 3A), suggesting potential posttranslational mechanisms for this effect. To confirm that actinomycin D and cycloheximide were effective at these doses, we treated IMR-90 cells with TGF-β1 in the absence or presence of these inhibitors and assessed the expression of Nox4. Nox4 expression induced by TGF-β1 was inhibited by actinomycin D and cycloheximide (Fig. 3B), consistent with prior data indicating a role of gene transcription in this TGF-β1 response (3, 23). Next, we assessed the steady-state levels of Nox4 mRNA in Hic-5-silenced cells. Knockdown of Hic-5 did not result in an increase in Nox4 mRNA expression (Fig. 3C). Together, these data indicate that the up-regulation of Nox4 protein expression is not related to the activation of Nox4 gene transcription or protein translation. To determine Nox4 protein stability, we treated control (NT siRNA) and Hic-5-silenced cells with cycloheximide. We observed a marked reduction in steady-state levels of Nox4 protein in NT cells by 30 min, whereas Nox4 expression was sustained in Hic-5-silenced cells. Thus, Nox4 was stabilized with Hic-5 knockdown (Fig. 3D).
FIGURE 3.
Transcription and translational mechanisms do not regulate Nox4 expression. A, IMR-90 cells silenced in Hic-5 or control (NT siRNA) cells were serum-starved and treated without or with 0.05 μg/ml of actinomycin D or 1 μg/ml of cycloheximide for 2 h. Cells were lysed, and the levels of Hic-5, Nox4, and GAPDH were determined by Western blotting. Top panel, representative Western blot data. Bottom panel, densitometry analysis results of the Western blot data from three independent experiments. *, p < 0.05, significantly different from the corresponding control (NT siRNA) (n = 3). Data are mean ± S.E. MW, molecular weight. B, IMR-90 cells were serum-starved and treated without or with TGF-β1 and in the presence or absence of actinomycin D (Act D) or cycloheximide (CHX) for 24 h. Cells were lysed, and the levels of Nox4 and GAPDH were determined by Western blotting. C, Hic-5 and Nox4 mRNA levels in IMR-90 cells silenced in Hic-5 or control (NT siRNA) cells were determined by real-time PCR (n = 3). Data are mean ± S.E. *, p < 0.005, significantly different from the control (NT siRNA); ns, non-significant. D, IMR-90 cells silenced in Hic-5 or control (NT siRNA) cells were serum-starved and treated with cycloheximide for the indicated times. Cells were lysed, and the levels of Hic-5, Nox4, and GAPDH were determined by Western blotting.
Hic-5 Mediates Ubiquitination and Proteasomal Degradation of Nox4
On the basis of our data suggesting posttranslational mechanisms in Nox4 regulation by Hic-5, we investigated the role of the UPS system. We focused on the detection of Lys48 ubiquitination because ubiquitin chains conjugated to this Lys residue target proteins for proteasomal degradation, whereas Lys63 ubiquitination is involved in the recruitment of DNA repair enzymes, cell signaling, and endocytosis (24). Control cells (NT siRNA) treated with the UPS inhibitors MG132 or bortezomib demonstrated higher steady-state levels of Nox4 expression, suggesting that Nox4 is regulated by the UPS (Fig. 4A). Interestingly, the constitutive increase in Nox4 in Hic-5-silenced cells was only marginally augmented in the presence of UPS inhibitors (Fig. 4A). These data suggest that the UPS is responsible for maintaining low levels of Nox4 under basal conditions and that this may be dependent on constitutive Hic-5 expression.
FIGURE 4.
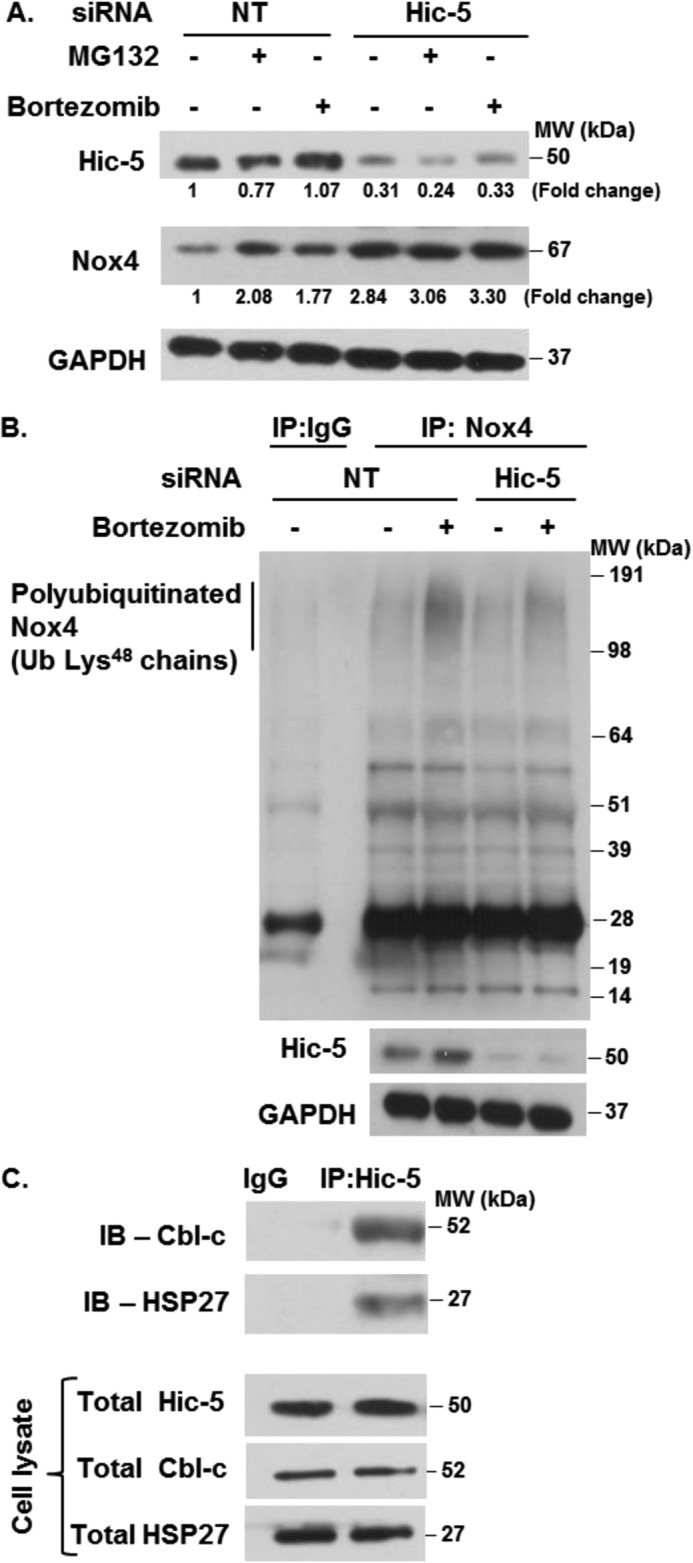
Hic-5 regulates the ubiquitin-proteasomal degradation of Nox4. A, IMR-90 cells silenced in Hic-5 or control (NT siRNA) cells were serum-starved and treated with 10 μm of MG132 or 100 nm bortezomib for 2 h. Cells were lysed, and the levels of Hic-5, Nox4, and GAPDH were determined by Western blotting. MW, molecular weight. B, IMR-90 cells silenced in Hic-5 or control (NT siRNA) cells were serum-starved and treated without or with 100 nm bortezomib for 2 h. Cells were lysed, and immunoprecipitation (IP) was performed as described under “Experimental Procedures.” The levels of polyubiquitinated Nox4 and total protein levels of Hic-5 and GAPDH were determined by Western blotting. Ub, ubiquitin. C, IMR-90 cells were lysed, and immunoprecipitations were performed as described under “Experimental Procedures.” The levels of Cbl-c and HSP27 in immunoprecipitated and total cell lysates were determined by Western blotting. Representative results are shown (n = 3). IB, immunoblot.
Next, we determined whether Hic-5 mediates Nox4 polyubiquitination. Polyubiquitination of Nox4 was detected in cells pretreated with the proteasomal inhibitor bortezomib. This was reduced notably in Hic-5 knockdown cells (Fig. 4B). This suggests that Nox4 is polyubiquitinated under basal conditions and that Hic-5 is, at least in part, involved in the polyubiquitination of Nox4.
Hic-5 Interacts with Cbl-c and HSP27 in Lung Fibroblasts
Hic-5 is known to associate with Cbl-c, an E3 ubiquitin ligase (11), and with HSP27, a ubiquitin-binding protein (9). We assessed whether these interactions are required for the polyubiquitination and degradation of Nox4. First, we examined whether Hic-5 interacts with Cbl-c and HSP27 in lung fibroblasts. Immunoprecipitation studies confirmed the interaction of these endogenous proteins (Fig. 4C), supporting the possibility that they may be involved in Nox4 polyubiquitination.
Cbl-c and HSP27 Are Required for Nox4 Polyubiquitination and Degradation
To determine whether Cbl-c and HSP27 were necessary to mediate Nox4 polyubiquitination, we independently silenced Cbl-c and HSP27 under conditions of proteasomal inhibition and assessed the levels of Nox4 polyubiquitination. Nox4 polyubiquitination was decreased markedly when Cbl-c (Fig. 5A) or HSP27 (Fig. 5B) was silenced. Next, we determined whether knockdown of these Nox4 ubiquitination-promoting proteins controls the steady-state levels of Nox4 protein. Silencing of Cbl-c (Fig. 5C) or HSP27 (Fig. 5D) resulted in increased constitutive levels of Nox4 protein. This increase in Nox4 protein was associated with an increase in the myofibroblast differentiation markers α-SMA and fibronectin as well the senescence markers p16 and hypophosphorylated Rb (Fig. 5, C and D). These data suggest that the Hic-5-interacting proteins Cbl-c and HSP27 are required for Nox4 polyubiquitination and protein stability, which are critical in the control of myofibroblast differentiation and senescence.
FIGURE 5.
The Hic-5-interacting proteins Cbl-c and HSP27 regulate the ubiquitin-proteasomal degradation of Nox4 and senescence. A and B, IMR-90 cells silenced in Cbl-c or HSP27 and control (NT siRNA) cells were treated without or with 100 nm bortezomib for 2 h. Cells were lysed, and immunoprecipitation (IP) was performed as described under “Experimental Procedures.” The levels of polyubiquitinated Nox4 and total protein levels of Cbl-c and HSP27 were determined by Western blotting. MW, molecular weight; Ub, ubiquitin. C and D, the levels of Cbl-c, Nox4, α-SMA, FN, p16, pRb, and GAPDH in total cell lysates of IMR-90 cells silenced in Cbl-c or HSP27 and control (NT siRNA) cells were determined by Western blotting. Representative results are shown.
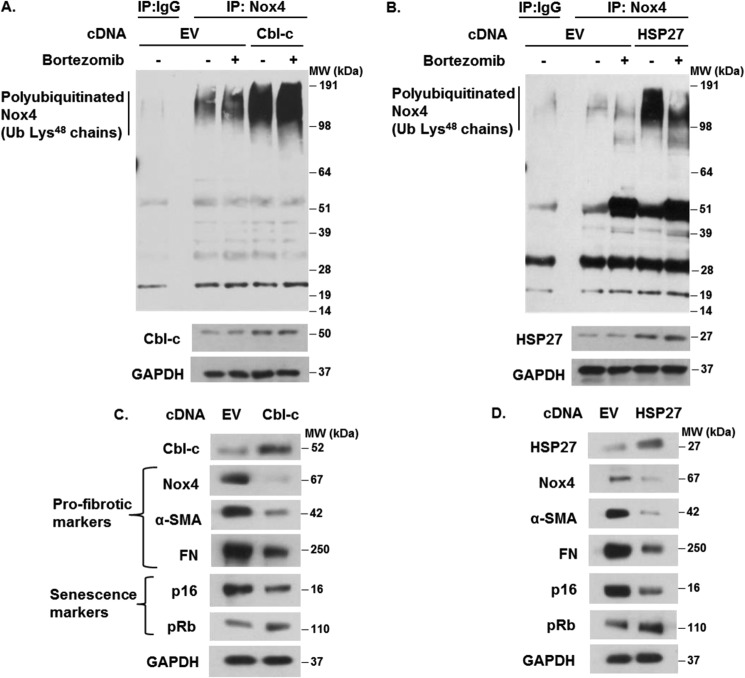
Overexpression of Cbl-c and HSP27 Enhances Nox4 Polyubiquitination and Degradation and Inhibits Myofibroblast Differentiation and Senescence
Next, we tested the hypothesis that overexpression of Cbl-c or HSP27 increases the ubiquitination and proteasomal degradation of Nox4, leading to the inhibition of myofibroblast differentiation and senescence. Overexpression of Cbl-c (Fig. 6A) or HSP27 (Fig. 6B) mediated an increase in Lys48-linked polyubiquitination of Nox4, an effect that was appreciable even in the absence of the proteasomal inhibitor bortezomib. We were unable to independently overexpress Hic-5 because cells appeared to undergo apoptotic cell death (data not shown). Cbl-c or HSP27 overexpression also mediated a decrease in constitutive levels of Nox4 in association with the decreased expression of markers of myofibroblast differentiation and senescence (Figs. 6, C and D). These data further support a role for Cbl-c and HSP27 in regulating Nox4 protein stability via the UPS system and in modulating myofibroblast differentiation and senescence.
FIGURE 6.
Overexpression of Cbl-c and HSP27 enhances the ubiquitin-proteasomal degradation of Nox4 and inhibits senescence in lung fibroblasts. A and B, IMR-90 cells were transfected with Cbl-c or HSP27 and control (empty vector, EV) cDNA plasmids. After transfection, cells were serum-starved and treated without or with 100 nm bortezomib for 2 h. Cells were lysed, and immunoprecipitation (IP) was performed as described under “Experimental Procedures.” The levels of polyubiquitinated Nox4 and total protein levels of Cbl-c and HSP27 were determined by Western blotting. MW, molecular weight; Ub, ubiquitin. C and D, the levels of Cbl-c, Nox4, α-SMA, FN, p16, pRb, and GAPDH in total cell lysates of IMR-90 cells transfected with control (EV), Cbl-c, or HSP27 cDNA plasmids were determined by Western blotting. Representative results are shown.
DISCUSSION
The ROS-generating enzyme Nox4 has been implicated in a number of fibrotic disorders involving diverse organ systems, including the lungs (22). The profibrotic cytokine TGF-β1 induces the expression of Nox4, primarily at the level of gene transcription (25–27). However, mechanisms that “turn off” this profibrotic pathway and maintain low basal levels of protein expression in fibrogenic cells are not well defined. In this report, we studied the regulation of the ROS-generating enzyme Nox4 by the focal adhesion protein Hic-5. In contrast to our initial hypothesis that Hic-5 mediates fibroblast senescence, our studies implicate Hic-5 as a negative regulator of myofibroblast differentiation and senescence. Indeed, Hic-5 silencing led to constitutive increases in the expression of Nox4, with parallel increases in markers of myofibroblast differentiation and senescence. We demonstrate that Hic-5, along with its interacting proteins Cbl-c and HSP27, mediates Nox4 ubiquitination, which leads to its degradation by the UPS. This is the first demonstration of UPS-mediated degradation of Nox4 by Hic-5 and its interacting proteins Cbl-c and HSP27 (Fig. 7).
FIGURE 7.
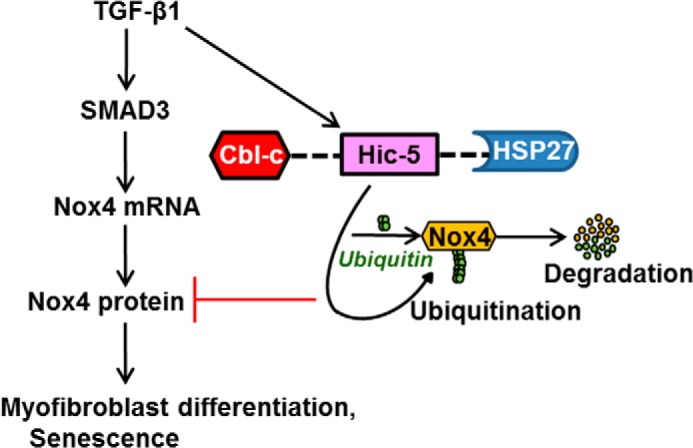
Signaling mechanisms by which Hic-5 regulates the UPS degradation of Nox4, myofibroblast activation, and senescence. TGF-β1 induces the expression of Hic-5. Hic-5 does not regulate TGF-β1-induced SMAD3 activation to induce Nox4 transcription and translation. The Hic-5·Cbl-c·HSP27 complex is required for the ubiquitin-proteasomal degradation of Nox4 and blocks myofibroblast differentiation (α-SMA and FN accumulation) and induction of senescence genes (p16 and hypophosphorylated Rb).
Hic-5 has been proposed initially to be a transcription factor involved in senescence, inducing a senescent phenotype in non-tumorigenic human immortalized cells compared with mortal cells (7). In our study, silencing of endogenous Hic-5 promoted, rather than suppressed, cellular senescence, which, on the basis of our model, may be an indirect effect through effects on Nox4. Recent studies support the concept that Nox4 promotes cellular senescence (28–31).
Hic-5, induced by TGF-β1 stimulation, is a novel marker for the smooth muscle (32) and fibroblast contractile phenotype (33). A previous study reported that autocrine production of TGF-β1 up-regulates Hic-5 expression in pathogenic fibroblasts (34). In this study, TGF-β1 up-regulated Hic-5, α-SMA, and extracellular matrix expression in hypertrophic scar fibroblasts compared with normal adult fibroblasts (35). Silencing of Hic-5 in hypertrophic scar fibroblasts resulted in decreased TGF-β1 production, expression of α-SMA, collagen contraction, and extracellular matrix synthesis. In our study, we did not find evidence for enhanced TGF-β1 signaling, as evidenced by Smad3 phosphorylation in Hic-5-silenced cells (data not shown). Additionally, there was no change in the steady-state levels of Nox4 mRNA under these conditions, suggesting that the effects on Nox4 protein expression were independent of gene regulation. We noted that the expression of α-SMA and FN “tracked” with the expression of Nox4. Although this might imply posttranslational regulation of these proteins by Hic-5, a more plausible explanation is that Nox4 coregulates the expression of these myofibroblast markers, as we have shown previously (22).
Aging is associated with increased incidence of idiopathic pulmonary fibrosis. Recently defined “hallmarks of aging” include genomic instability, loss of proteostasis, and cellular senescence (36). Molecular chaperones such as Hic-5 and HSP27 are involved in protein folding under both stressed and non-stressed conditions. HSP27 is a molecule with pleiotropic characteristics involved in different roles in normal and pathological cells. HSP7 stabilizes protein folding and suppress protein aggregation (37, 38). Similarly, Hic-5 has been reported to function as a chaperone, regulating diverse functions such as growth and differentiation (39–41) in a cell- or tissue-specific manner. The biochemical interaction between HSP27 and Hic-5 has been demonstrated in rat kidney glomeruli (9). Additionally, stress-inducible HSP27 binds chains of ubiquitin and promotes ubiquitination (10). Recent studies demonstrate that the HSP-induced stimulation of proteasome activity could be mediated by interactions with its cochaperones (42, 43). Similar observations have been demonstrated with Hic-5, indicating a correlation between Hic-5, HSP27, and proteasome activation (9). One study demonstrated the novel interaction of the E3 ubiquitin ligase Cbl-c with Hic-5 (11). Polyubiquitin chains of varying length are attached to proteins before degradation. Lys48 ubiquitin chains conjugate to target proteins for proteasomal degradation. We observed decreases in the accumulation of polyubiquitinated Nox4 with silencing of Hic-5, Cbl-c, and HSP27. Conversely, overexpressing these proteins or inhibition of the proteasome increased the ubiquitination of Nox4 and decreased the expression of senescence and profibrotic genes. Our study is the first to demonstrate that Hic-5 and its interacting proteins Cbl-c and HSP27 negatively regulate the expression of this ROS-generating enzyme.
In addition to uncovering a novel regulatory mechanism for controlling the expression/activity of Nox4, our study has several important implications. First, Nox4 has been referred to as a constitutive enzyme that is regulated at the mRNA level (2). Our studies provide the first evidence for Nox4 regulation at the protein level and a potential additional control over indiscriminant ROS production. Secondly, Hic-5 induction by TGF-β1 may serve to limit myofibroblasts from undergoing an irreversible senescence program. Indeed, we have shown that myofibroblast differentiation may represent an uncommitted senescent phenotype that is capable of dedifferentiation (44). Recently, our laboratory has demonstrated that genetic or pharmacological targeting of Nox4 in aged mice with persistent fibrosis attenuated the senescent myofibroblast phenotype (31). Additional studies will be required to determine whether sustained Nox4 expression results in a loss of cellular plasticity and irreversible senescence of myofibroblasts. Finally, loss of Hic-5 by genetic or epigenetic mechanisms may be sufficient to derepress Nox4 expression in pathologic states of fibrosis or cancer associated with high constitutive levels of Nox4. Therapeutic strategies to induce or activate Hic-5 may prove to be beneficial in pathological conditions of Nox4 up-regulation.
Acknowledgments
We thank Kimberly White, Ashish Kurundkar, and Thomas Hock for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants T32 HL105346 (to L. P. D.) and P01 HL114470 (to V. J. T.).
- ROS
- reactive oxygen species
- UPS
- ubiquitin-proteasomal system
- α-SMA
- α-smooth muscle actin
- FN
- fibronectin
- NT
- non-targeting.
REFERENCES
- 1. Bedard K., Krause K. H. (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87, 245–313 [DOI] [PubMed] [Google Scholar]
- 2. Serrander L., Cartier L., Bedard K., Banfi B., Lardy B., Plastre O., Sienkiewicz A., Fórró L., Schlegel W., Krause K. H. (2007) NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem. J. 406, 105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thannickal V. J., Fanburg B. L. (1995) Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor β 1. J. Biol. Chem. 270, 30334–30338 [DOI] [PubMed] [Google Scholar]
- 4. von Löhneysen K., Noack D., Wood M. R., Friedman J. S., Knaus U. G. (2010) Structural insights into Nox4 and Nox2: motifs involved in function and cellular localization. Mol. Cell. Biol. 30, 961–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peshavariya H., Jiang F., Taylor C. J., Selemidis S., Chang C. W., Dusting G. J. (2009) Translation-linked mRNA destabilization accompanying serum-induced Nox4 expression in human endothelial cells. Antioxid. Redox Signal. 11, 2399–2408 [DOI] [PubMed] [Google Scholar]
- 6. Shibanuma M., Mashimo J., Kuroki T., Nose K. (1994) Characterization of the TGF β 1-inducible hic-5 gene that encodes a putative novel zinc finger protein and its possible involvement in cellular senescence. J. Biol. Chem. 269, 26767–26774 [PubMed] [Google Scholar]
- 7. Shibanuma M., Mochizuki E., Maniwa R., Mashimo J., Nishiya N., Imai S., Takano T., Oshimura M., Nose K. (1997) Induction of senescence-like phenotypes by forced expression of hic-5, which encodes a novel LIM motif protein, in immortalized human fibroblasts. Mol. Cell. Biol. 17, 1224–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang J., Zhang L. X., Meltzer P. S., Barrett J. C., Trent J. M. (2000) Molecular cloning of human Hic-5, a potential regulator involved in signal transduction and cellular senescence. Mol. Carcinog. 27, 177–183 [PubMed] [Google Scholar]
- 9. Jia Y., Ransom R. F., Shibanuma M., Liu C., Welsh M. J., Smoyer W. E. (2001) Identification and characterization of hic-5/ARA55 as an hsp27 binding protein. J. Biol. Chem. 276, 39911–39918 [DOI] [PubMed] [Google Scholar]
- 10. Parcellier A., Schmitt E., Gurbuxani S., Seigneurin-Berny D., Pance A., Chantôme A., Plenchette S., Khochbin S., Solary E., Garrido C. (2003) HSP27 is a ubiquitin-binding protein involved in I-κBα proteasomal degradation. Mol. Cell. Biol. 23, 5790–5802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ryan P. E., Kales S. C., Yadavalli R., Nau M. M., Zhang H., Lipkowitz S. (2012) Cbl-c ubiquitin ligase activity is increased via the interaction of its RING finger domain with a LIM domain of the paxillin homolog, Hic 5. PLoS ONE 7, e49428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun Y., Zhou M., Fu D., Xu B., Fang T., Ma Y., Chen J., Zhang J. (2011) Ubiquitination of heat shock protein 27 is mediated by its interaction with Smad ubiquitination regulatory factor 2 in A549 cells. Exp. Lung. Res. 37, 568–573 [DOI] [PubMed] [Google Scholar]
- 13. Kato K., Tokuda H., Adachi S., Matsushima-Nishiwaki R., Yamauchi J., Natsume H., Minamitani C., Mizutani J., Otsuka T., Kozawa O. (2011) Role of heat shock protein 27 in transforming growth factor-β-stimulated vascular endothelial growth factor release in osteoblasts. Int. J. Mol. Med. 27, 423–428 [DOI] [PubMed] [Google Scholar]
- 14. Wettstein G., Bellaye P. S., Kolb M., Hammann A., Crestani B., Soler P., Marchal-Somme J., Hazoume A., Gauldie J., Gunther A., Micheau O., Gleave M., Camus P., Garrido C., Bonniaud P. (2013) Inhibition of HSP27 blocks fibrosis development and EMT features by promoting Snail degradation. FASEB J. 27, 1549–1560 [DOI] [PubMed] [Google Scholar]
- 15. Chen J. H., Stoeber K., Kingsbury S., Ozanne S. E., Williams G. H., Hales C. N. (2004) Loss of proliferative capacity and induction of senescence in oxidatively stressed human fibroblasts. J. Biol. Chem. 279, 49439–49446 [DOI] [PubMed] [Google Scholar]
- 16. Komatsuda A., Wakui H., Oyama Y., Imai H., Miura A. B., Itoh H., Tashima Y. (1999) Overexpression of the human 72 kDa heat shock protein in renal tubular cells confers resistance against oxidative injury and cisplatin toxicity. Nephrol. Dial. Transplant. 14, 1385–1390 [DOI] [PubMed] [Google Scholar]
- 17. Vidyasagar A., Reese S. R., Hafez O., Huang L. J., Swain W. F., Jacobson L. M., Torrealba J. R., Chammas P. E., Wilson N. A., Djamali A. (2013) Tubular expression of heat-shock protein 27 inhibits fibrogenesis in obstructive nephropathy. Kidney Int. 83, 84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bahta A. W., Farjo N., Farjo B., Philpott M. P. (2008) Premature senescence of balding dermal papilla cells in vitro is associated with p16(INK4a) expression. J. Invest. Dermatol. 128, 1088–1094 [DOI] [PubMed] [Google Scholar]
- 19. Thannickal V. J. (2012) Mechanisms of pulmonary fibrosis: role of activated myofibroblasts and NADPH oxidase. Fibrogenesis Tissue Repair 5, S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thannickal V. J. (2010) Aging, antagonistic pleiotropy and fibrotic disease. Int. J. Biochem. Cell Biol. 42, 1398–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thannickal V. J., Lee D. Y., White E. S., Cui Z., Larios J. M., Chacon R., Horowitz J. C., Day R. M., Thomas P. E. (2003) Myofibroblast differentiation by transforming growth factor-β1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J. Biol. Chem. 278, 12384–12389 [DOI] [PubMed] [Google Scholar]
- 22. Hecker L., Vittal R., Jones T., Jagirdar R., Luckhardt T. R., Horowitz J. C., Pennathur S., Martinez F. J., Thannickal V. J. (2009) NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 15, 1077–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bai G., Hock T. D., Logsdon N., Zhou Y., Thannickal V. J. (2014) A far-upstream AP-1/Smad binding box regulates human NOX4 promoter activation by transforming growth factor-β. Gene 540, 62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walczak H., Iwai K., Dikic I. (2012) Generation and physiological roles of linear ubiquitin chains. BMC Biol. 10, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martin-Garrido A., Brown D. I., Lyle A. N., Dikalova A., Seidel-Rogol B., Lassègue B., San Martín A., Griendling K. K. (2011) NADPH oxidase 4 mediates TGF-β-induced smooth muscle α-actin via p38MAPK and serum response factor. Free Radic. Biol. Med. 50, 354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xiao Q., Luo Z., Pepe A. E., Margariti A., Zeng L., Xu Q. (2009) Embryonic stem cell differentiation into smooth muscle cells is mediated by Nox4-produced H2O2. Am. J. Physiol. Cell Physiol. 296, C711–723 [DOI] [PubMed] [Google Scholar]
- 27. Lambeth J. D. (2004) NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4, 181–189 [DOI] [PubMed] [Google Scholar]
- 28. Senturk S., Mumcuoglu M., Gursoy-Yuzugullu O., Cingoz B., Akcali K. C., Ozturk M. (2010) Transforming growth factor-β induces senescence in hepatocellular carcinoma cells and inhibits tumor growth. Hepatology 52, 966–974 [DOI] [PubMed] [Google Scholar]
- 29. Weyemi U., Lagente-Chevallier O., Boufraqech M., Prenois F., Courtin F., Caillou B., Talbot M., Dardalhon M., Al Ghuzlan A., Bidart J. M., Schlumberger M., Dupuy C. (2012) ROS-generating NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene 31, 1117–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kodama R., Kato M., Furuta S., Ueno S., Zhang Y., Matsuno K., Yabe-Nishimura C., Tanaka E., Kamata T. (2013) ROS-generating oxidases Nox1 and Nox4 contribute to oncogenic Ras-induced premature senescence. Genes Cells 18, 32–41 [DOI] [PubMed] [Google Scholar]
- 31. Hecker L., Logsdon N. J., Kurundkar D., Kurundkar A., Bernard K., Hock T., Meldrum E., Sanders Y. Y., Thannickal V. J. (2014) Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci. Transl. Med. 6, 231–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang X., Hu G., Betts C., Harmon E. Y., Keller R. S., Van De Water L., Zhou J. (2011) Transforming growth factor-β1-induced transcript 1 protein, a novel marker for smooth muscle contractile phenotype, is regulated by serum response factor/myocardin protein. J. Biol. Chem. 286, 41589–41599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Srinivasan R., Forman S., Quinlan R. A., Ohanian J., Ohanian V. (2008) Regulation of contractility by Hsp27 and Hic-5 in rat mesenteric small arteries. Am. J. Physiol. Heart Circ. Physiol. 294, H961–969 [DOI] [PubMed] [Google Scholar]
- 34. Dabiri G., Tumbarello D. A., Turner C. E., Van de Water L. (2008) TGF-β1 slows the growth of pathogenic myofibroblasts through a mechanism requiring the focal adhesion protein, Hic-5. J. Invest. Dermatol. 128, 280–291 [DOI] [PubMed] [Google Scholar]
- 35. Dabiri G., Tumbarello D. A., Turner C. E., Van de Water L. (2008) Hic-5 promotes the hypertrophic scar myofibroblast phenotype by regulating the TGF-β1 autocrine loop. J. Invest. Dermatol. 128, 2518–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thannickal V. J. (2013) Mechanistic links between aging and lung fibrosis. Biogerontology 14, 609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ojha J., Masilamoni G., Dunlap D., Udoff R. A., Cashikar A. G. (2011) Sequestration of toxic oligomers by HspB1 as a cytoprotective mechanism. Mol. Cell. Biol. 31, 3146–3157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yerbury J. J., Gower D., Vanags L., Roberts K., Lee J. A., Ecroyd H. (2013) The small heat shock proteins αB-crystallin and Hsp27 suppress SOD1 aggregation in vitro. Cell Stress Chaperones 18, 251–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Du Y., Gu H. J., Gong Q. M., Yang F., Ling J. Q. (2009) HSP25 affects the proliferation and differentiation of rat dental follicle cells. Int. J. Oral Sci. 1, 72–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takahashi-Horiuchi Y., Sugiyama K., Sakashita H., Amano O. (2008) Expression of heat shock protein 27 with the transition from proliferation to differentiation of acinar precursor cell in regenerating submandibular gland of rats. Tohoku J. Exp. Med. 214, 221–230 [DOI] [PubMed] [Google Scholar]
- 41. Liu T., Warburton R. R., Guevara O. E., Hill N. S., Fanburg B. L., Gaestel M., Kayyali U. S. (2007) Lack of MK2 inhibits myofibroblast formation and exacerbates pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 37, 507–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Acunzo J., Katsogiannou M., Rocchi P. (2012) Small heat shock proteins HSP27 (HspB1), αB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. Int. J. Biochem. Cell Biol. 44, 1622–1631 [DOI] [PubMed] [Google Scholar]
- 43. Ito H., Kamei K., Iwamoto I., Inaguma Y., García-Mata R., Sztul E., Kato K. (2002) Inhibition of proteasomes induces accumulation, phosphorylation, and recruitment of HSP27 and αB-crystallin to aggresomes. J. Biochem. 131, 593–603 [DOI] [PubMed] [Google Scholar]
- 44. Hecker L., Jagirdar R., Jin T., Thannickal V. J. (2011) Reversible differentiation of myofibroblasts by MyoD. Exp. Cell Res. 317, 1914–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]