Background: Interleukin-1 signaling in fibroblasts is mediated through focal adhesions, which are enriched with tyrosine kinases and phosphatases.
Results: The focal adhesion targeting domain of FAK interacts with protein-tyrosine phosphatase-α (PTPα) to restrict interleukin-induced Ca2+ release, ERK activation, and MMP-9 expression.
Conclusion: Signaling through focal adhesions requires FAK and PTPα.
Significance: FAK and PTPα cooperate to restrict IL-1 signaling.
Keywords: Cell Adhesion, Fibroblast, Matrix Metalloproteinase (MMP), Phosphatase, Signaling, Tyrosine-protein Kinase (Tyrosine Kinase)
Abstract
Interleukin-1 (IL-1) signaling in fibroblasts is mediated through focal adhesions, organelles that are enriched with adaptor and cytoskeletal proteins that regulate signal transduction. We examined interactions of the focal adhesion kinase (FAK) with protein-tyrosine phosphatase-α (PTP-α) in IL-1 signaling. In wild type and FAK knock-out mouse embryonic fibroblasts, we found by immunoblotting, immunoprecipitation, immunostaining, and gene silencing that FAK is required for IL-1-mediated sequestration of PTPα to focal adhesions. Immunoprecipitation and pulldown assays of purified proteins demonstrated a direct interaction between FAK and PTPα, which was dependent on the FAT domain of FAK and by an intact membrane-proximal phosphatase domain of PTPα. Recruitment of PTPα to focal adhesions, IL-1-induced Ca2+ release from the endoplasmic reticulum, ERK activation, and IL-6, MMP-3, and MMP-9 expression were all blocked in FAK knock-out fibroblasts. These processes were restored in FAK knock-out cells transfected with wild type FAK, FAT domain, and FRNK. Our data indicate that IL-1-induced signaling through focal adhesions involves interactions between the FAT domain of FAK and PTPα.
Introduction
Interleukin-1 (IL-1) is a family of potent, pro-inflammatory cytokines that can induce multiple signaling cascades (1), which contribute to inflammatory tissue injury (2). IL-1 stimulates the expression of the early response genes c-fos and c-jun as well as multiple cytokines such as interleukin-6 (IL-6) (3) and inflammatory factors that drive extracellular matrix degradation (4) via the up-regulation of various proteases, including members of the matrix metalloproteinase (MMP)2 family such as MMP-9 in connective tissue cells (5). Enhanced expression of IL-6 (3), MMP-3 (6), and MMP-9 by IL-1 involves the activation of several interdependent signaling cascades that include the mitogen-activated protein kinase (MAPK) subfamily members, extracellular-signal regulated kinase (ERK), p38 MAPK, and stress-activated c-Jun N-terminal kinase (7). Although earlier studies have suggested a potential regulatory role of integrin receptors and the focal adhesion kinase (FAK) in MMP-9 expression (8), the mechanisms that coordinate the activation of these MAPK members in IL-6, MMP-3, and MMP-9 expression downstream of the IL-1 receptor are not well defined.
Focal adhesions (FAs) are mechanical linkages that connect the actin cytoskeleton to the extracellular matrix and serve as signaling platforms that concentrate numerous signaling and scaffolding proteins at regions of integrin receptor binding and clustering (9, 10). Spatial co-localization of interacting signaling molecules provides a pivotal regulatory locus for signal transduction. In adherent cells such as fibroblasts, the formation of FAs is required for IL-1-induced signaling cascades leading to tyrosine phosphorylation and activation of the focal adhesion kinase (11), Ca2+ release from the endoplasmic reticulum (ER) (12, 13), and activation of ERK (14, 15), which ultimately result in regulating the expression of MMPs (6, 16).
FAK is a ubiquitously expressed, nonreceptor protein-tyrosine kinase that associates with integrin receptors and plays an integral role in integrin-mediated signaling (17, 18). The N terminus of FAK contains the major autophosphorylation site, Tyr397, which binds the Src homology 2 domains of phospholipase Cγ (19), Shc (17), and Src family kinases (e.g. Src and Fyn) (20). Following binding, activated Src phosphorylates the C-terminal Tyr925 residue on FAK, which consequently recruits Grb2 (21). Phospholipase Cγ catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate generating a secondary messenger, inositol 1,4,5-trisphosphate, which mobilizes Ca2+, whereas Shc and Grb2 serve as adaptors for signal transduction by the ERK pathway. These processes indicate that FAK may be important in coupling components of Ca2+ and ERK signaling downstream of the IL-1 receptor by regulating tyrosine phosphorylation status of other signaling molecules and by interacting with other adaptor proteins.
The dynamic and reversible nature of tyrosine phosphorylation of focal adhesion proteins indicates an important role for protein-tyrosine kinases and phosphatases (PTPs) in focal adhesion-dependent signaling (22). Indeed, there are numerous PTPs in FAs. In particular, PTPα plays a prominent role in the regulation of FA remodeling and in cell adhesion and signaling (23). PTPα is a receptor-like PTP that activates Src family kinases (e.g. Src and Fyn) via dephosphorylation of an inhibitory C-terminal tyrosine residue (24–29). Through its interactions with Src, we showed that PTPα controls IL-1-induced phosphorylation of the inositol 1,4,5-trisphosphate receptor1 (IP3R1) and consequently Ca2+ release (30). Furthermore, PTPα regulates formation and maturation of FAs in response to mechanical force, reinforces connections between integrins and the cytoskeleton, and modulates cytoskeletal reorganization in response to integrin ligation (28, 29). In the absence of PTPα, fibroblasts show reduced spreading, increased numbers of abnormal FAs, decreased tyrosine phosphorylation of focal adhesion kinase and p130Cas, as well as attenuated ERK activation during adhesion and spreading (26).
Our previous studies have partially characterized the roles of PTPα in focal adhesion-dependent IL-1 signaling (6, 23, 30); however, it is not known whether PTPα and FAK collaborate to regulate IL-1 signaling through FAs. Furthermore, we do not understand how PTPα is spatially sequestered to FAs and whether it interacts with FAK to enable adhesion-restricted IL-1 signaling. Consequently, we examined the functional importance of FAK and three of its domains (band4.1-ezrin-radixin-moesin (FERM) homology domain, kinase domain, and focal adhesion-targeting (FAT) domain) in influencing the interactions between FAK and PTPα, as well as the impact on IL-1-induced signaling that leads to Ca2+ release, ERK activation, and IL-6, MMP-3, and MMP-9 expression.
EXPERIMENTAL PROCEDURES
Materials
Fibronectin, poly-l-lysine, bovine serum albumin (BSA), puromycin, doxycycline, and mouse monoclonal antibody to paxillin were purchased from Sigma. Rabbit polyclonal antibodies to FAK and PTPα were purchased from Millipore. Mouse monoclonal antibody to FAK and rabbit polyclonal antibodies to phospho-FAK (Tyr397) were obtained from Invitrogen. Rabbit monoclonal antibody to ERK1/2, mouse monoclonal antibody to phospho-ERK1/2 Thr202/Tyr204, and rabbit polyclonal antibodies to phospho-PTPα (Tyr789) were purchased from Cell Signaling (Beverly, MA). Recombinant human IL-1β and the Quantikine mouse MMP-3, MMP-9, and IL-6 ELISA immunoassays were purchased from R&D Systems (Minneapolis, MN). FuGENE HD transfection reagent was from Promega (Madison, WI). DharmaFECT 1 transfection reagent was purchased from Thermo Fisher Scientific (Lafayette, CO). Glutathione-Sepharose 4B, GSTrap 4B, and thrombin protease were obtained from GE Healthcare.
Cell Culture
FAK wild type (FAK+/+) and knock-out (FAK−/−) mouse embryonic fibroblasts (MEFs) were obtained from the American Type Cell Collection (ATCC, Manassas, VA). PTPα wild type (PTPα+/+) and knock-out (PTPα−/−) MEFs were isolated from PTPα+/+ and PTPα−/− embryos (E14) and were immortalized using a replication-defective, recombinant murine retrovirus expressing simian virus 40 large T-antigen (from D.D. Belsham, Department of Physiology, University of Toronto, Toronto, Ontario, Canada) following a previously described method (31). All MEFs were maintained in Dulbecco's Modified Eagle's Medium (DMEM) with 10% fetal bovine serum (FBS). Genetically modified NIH3T3 fibroblasts under control of a doxycycline-sensitive repressor (from David Shalloway, Department of Molecular Biology and Genetics, Cornell University, Ithaca, NY) that express hemagglutinin-tagged wild type PTPα (NIH3T3PTPα), PTPα C433S/C723S mutant (NIH3T3CCSS; essential cysteine in catalytic site of both phosphatase domains mutated to serine) and PTPαY789F mutant (NIH3T3Y789F) were grown in DMEM containing 5% FBS and 5 ng/ml doxycycline. About 14–16 h prior to experiments doxycycline was removed, to allow expression of recombinant PTPα. During routine propagation, all cells were also supplemented with 0.17% (w/v) penicillin V, 0.01 g/ml amphotericin B and 0.1% gentamycin sulfate, maintained in a 37 °C humidified with 5% CO2 and passaged with 0.01% trypsin (Invitrogen).
FAK Constructs and Transfection
pEGFP-FAK, pEGFP-FAK Y397F, pEGFP-FAK L1035S and pEGFP-FRNK constructs were provided by J. Thomas Parsons (Department of Microbiology and Cancer Center, University of Virginia, Charlottesville, VA). Transfections were performed with FuGENE® HD (Promega, Madison, WI) according to the manufacturer's instructions. The siRNA transfections were conducted with DharmaFECT 1 (Thermo Fisher Scientific, Boulder, CO) according to the manufacturer's protocol.
FAK Expression Constructs
The cDNA templates of chicken wild type FAK, FAK Y397F, FAK L1035S, and FRNK (from J. Thomas Parsons) were amplified using corresponding primers that were designed according to the GenBankTM number provided on Table 1 and with Phusion® HF DNA polymerase (New England Biolabs, Mississauga, Ontario, Canada), although PCR of FERM (amino acids 1–415), kinase (amino acids 416–676), and FAT (amino acids 908–1053) domains (32) of FAK (from J. Thomas Parsons) were carried out using Pfu DNA polymerase (Thermo Scientific Canada, Ontario, Canada). The resulting PCR products were either digested with BamHI and XhoI, as highlighted in the primer sequences, and cloned (T4 DNA ligase, New England Biolabs) into pGEX-4T-2 digested with the same enzymes, or ligated into BamHI- and XhoI-linearized pGEX-4T-2 directly using In-fusion® HD enzyme premix (Clontech) after gel purification. The sequences of the inserts were confirmed (ACGT Corp., Toronto, Ontario, Canada), and constructs were transformed into BL21 (DE3) Escherichia coli (Agilent, Mississauga, Ontario, Canada) for protein expression and purification.
TABLE 1.
Primer sequences used in the generation of GST fusion constructs
Fwd is forward, and Rev is reverse.
| Template | GenBankTM no. | Forward and reverse primer sequence (5′ to 3′) |
|---|---|---|
| FAK wild type | M86656.1 | Fwd GGTTCCGCGTGGATCCATGGCAGCAGCTTACCTTGATCCA |
| Rev GATGCGGCCGCTCGAGCTATTAGTGGGGCCTGGACTGGCT | ||
| FAK-Y397F | M86656.1 | Fwd GGTTCCGCGTGGATCCATGGCAGCAGCTTACCTTGATCCA |
| Rev GATGCGGCCGCTCGAGCTATTAGTGGGGCCTGGACTGGCT | ||
| FAK-L1035S | M86656.1 | Fwd GCGCGGATCCATGGCAGCAGCTTACCTTGATCCAAACTTG |
| Rev GCGC[underln]CTCGAGACTATTAGTGGGGCCTGGACTGGCTGATCA | ||
| FRNK | L07415.1 | Fwd GCGCGGATCCATGGAATCCAGGCGACAAGTCACAGTATCC |
| Rev GCGC[underln]CTCGAGACTATTAGTGGGGCCTGGACTGGCTGATCA | ||
| FERM | M86656.1 | Fwd GGTTCCGCGTGGATCCATGGCAGCAGCTTACCTTG |
| Rev GATGCGGCCGCTCGAGTCATTAATAATCTCTGGTTGAT | ||
| Kinase | M86656.1 | Fwd GGTTCCGCGTGGATCCGAAATTCAAAGGGAGAGAATT |
| Rev GATGCGGCCGCTCGAGTCATTAGAGTTGTGCTTTAAG | ||
| FAT | M86656.1 | Fwd GGTTCCGCGTGGATCCCAGGAAATCAGCCCTCCT |
| Rev GATGCGGCCGCTCGAGTCATTAGTGGGGCCTGGA |
Isolation of FAs
Focal adhesion complexes were isolated from cells incubated with collagen- or BSA-coated magnetite beads after specific incubation time periods as described (30). In brief, cell-bead complexes were scraped into ice-cold cytoskeleton extraction buffer (CSKB: 0.5% Triton X-100, 50 mm NaCl, 300 mm sucrose, 3 mm MgCl2, 20 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 mm phenylmethylsulfonyl fluoride, 10 mm PIPES, pH 6.8), and after washing the beads three times in CSKB using magnetic separation to remove nonspecific proteins, the remaining bead-associated focal adhesion proteins were eluted in Laemmli sample buffer by boiling for 10 min.
Subcellular Fractionation
Examination of ER fraction proteins was performed as described (33). Briefly, cells were resuspended in an isotonic buffer (10 mm Tris, pH 7.6, 100 mm CaCl2, 200 mm sucrose) and disrupted by Dounce homogenization using 20 strokes. The homogenate was centrifuged at 800 × g for 10 min. The supernatant was further centrifuged for 10 min at 8,000 × g, and the resulting supernatant was again centrifuged for 1.5 h at 28,000 × g. The resulting pellet constituted the microsomal ER-enriched fraction. The authenticity of this putative ER fraction was confirmed by immunoblotting for the ER-specific protein calnexin.
Immunoblotting
Equal amounts of protein as determined by the Bradford assay (Bio-Rad) were resolved by SDS-PAGE and transferred onto nitrocellulose membranes. Immunoblotting was performed by blocking membranes with 5% milk or 2% BSA in Tris-buffered saline solution (TBS) for 1 h and then probing with primary antibodies overnight at 4 °C in TBS with 0.1% Tween 20 and 5% milk or 2% BSA. The membranes were then incubated with HRP-conjugated secondary antibodies for 1 h in TBS with 0.1% Tween 20. All steps were done at room temperature, unless indicated otherwise. Labeled proteins were visualized by chemiluminescence using ECL reagent (Amersham Biosciences) according to the manufacturer's instructions.
Immunoprecipitation and Pulldown Assay
Cells were lysed in 1% Tris/NaCl/Triton immunoprecipitation buffer (20 mm Tris, pH 7.5, 1% Triton X-100, 0.1% SDS, 150 mm NaCl) containing 1 mm phenylmethylsulfonyl fluoride, 1 mm NaVO3, 10 μg/ml leupeptin, and 10 μg/ml aprotinin. Equal amounts of protein from cleared extracts were immunoprecipitated with Dynabeads (Invitrogen) following the manufacturer's immunoprecipitation protocol or employed in pulldown assays using glutathione-Sepharose bead (GST)-bound bacterially expressed fusion proteins and subsequently immunoblotted.
Total Internal Reflection Microscopy
MEFs were plated in 35-mm glass bottom micro-well dishes (MatTek) previously coated with fibronectin (10 μg/ml) and were serum-starved for 10–12 h at 37 °C before stimulation with IL-1 (20 ng/ml for 30 min). At room temperature, cells were then fixed (3.7% paraformaldehyde in PBS for 10 min), blocked (0.2% BSA in PBS for 20 min), and permeabilized (0.2% Triton X-100 in PBS for 15 min). Antibodies were diluted in PBS with 1% BSA. Immunofluorescence staining was performed with mouse anti-paxillin antibody (1:100), rabbit anti-FAK antibody (1:60), or rabbit anti-PTPα antibody (1:60) for 1 h at 37 °C. Dishes were washed with PBS, incubated with FITC- or TRITC-conjugated secondary antibodies for 1 h, and washed. The cells were viewed by total internal reflection fluorescence microscopy (Leica, LAS AF, Heidelberg, Germany).
Measurement of Ca2+ in ER Stores
For estimating [Ca2+]ER, cells were incubated with mag-fura-2/AM (4 μm) for 150 min at 37 °C in culture medium and measured by ratio fluorimetry as described (13). The nominally Ca2+-free buffer consisted of a bicarbonate-free medium containing 150 mm NaCl, 5 mm KCl, 10 mm d-glucose,1 mm MgSO4, 1 mm Na2HPO4, and 20 mm HEPES, pH 7.4, with an osmolarity of 291 mosm. For experiments requiring external Ca2+, 2 mm CaCl2 was added to the buffer; for experiments requiring chelation of external Ca2+, 1 mm EGTA was added. Visual inspection of mag-fura-2-loaded cells showed fluorescent labeling of intracellular organelles. Mag fura-2 ratios were obtained with a C·Imaging System (Compix, Inc., Cranberry, PA) using excitation wavelengths of 340 and 380 nm and an emission wavelength of 520 nm.
Quantitative Real Time PCR
After IL-1 stimulation in low serum (1% FBS) DMEM, total RNA was extracted from FAK MEFs using an RNeasy® mini kit (Qiagen, Mississauga, Ontario, Canada) according to the manufacturer's instructions. Total RNA samples (1 μg; as determined with a NanoDrop 1000 spectrophotometer) were reverse-transcribed using the iScriptTM cDNA synthesis kit (Bio-Rad) according to the manufacturer's instructions. Quantitative real time PCR was performed on a Bio-Rad CFX96 real time PCR system using SsoFastTMEvaGreen® Supermix (Bio-Rad) following the product protocol with validated mouse MMP-9 primers (forward, 5′-GACATAGACGGCATCCAGTATC-3′; reverse, 5′-GTGGGAGGTATAGTGGGA CA-3′) and mouse GAPDH primers (forward, 5′-AATGGTGAAGGTCGGTGTG-3′; reverse, 5′-GTGGAGTCATACTGGAACATGTAG-3′). Each PCR was done in duplicate, with triplicates of each set of assay. The relative expression of the MMP-9 gene to the housekeeping gene GAPDH was calculated using the 2−ΔΔCT method.
Enzyme-linked Immunosorbent Assay
Subconfluent cells (75–85%) were incubated for 3 h in low serum (1% FBS) DMEM prior to IL-1 treatment. Following IL-1 stimulation, supernatants were collected, concentrated ∼10× using Ultracel-3K centrifugal filters (Millipore), and stored at −20 °C until use. Levels of MMP-3, MMP-9, or IL-6 in culture supernatants were measured with a mouse MMP-3, MMP-9, or IL-6 ELISA kit, respectively (R&D Systems).
Experimental Design and Analysis
Data shown are representative of independent experiments conducted in triplicate. For numerical data, means ± S.E. were calculated. When appropriate, Student's t test was used to compare two groups. For three or more groups, analysis of variance was used. Statistical significance was set at p < 0.05.
RESULTS
Accumulation of PTPα in FAs Requires FAK
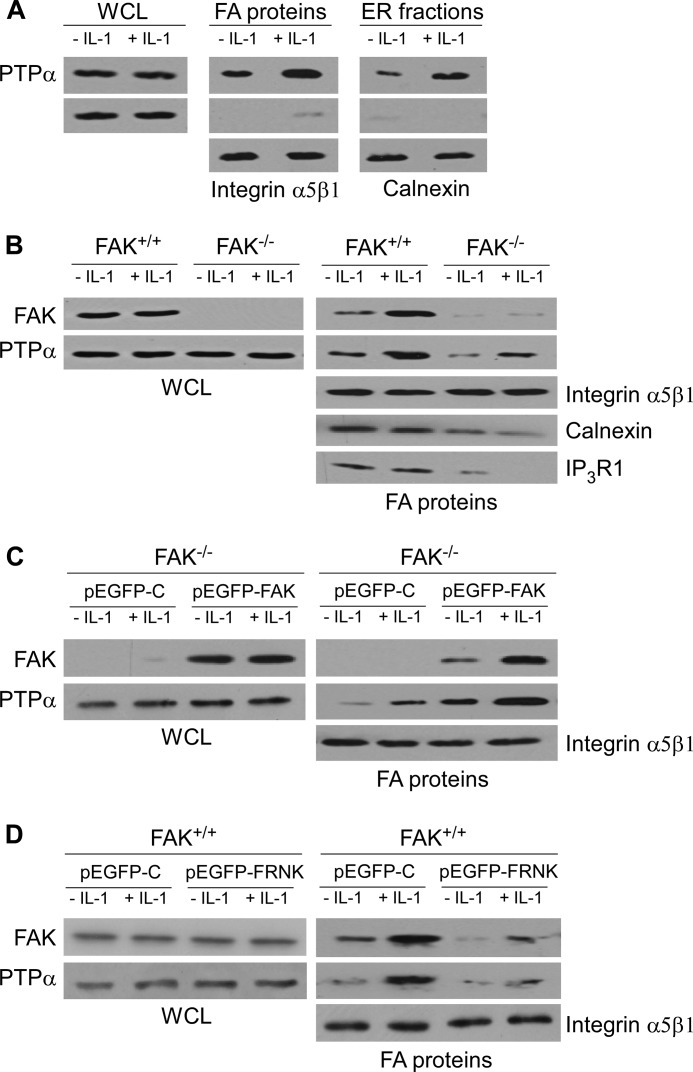
Focal adhesions are enriched with tyrosine phosphatases that may regulate signaling through FAs (34). PTPα is a critical adaptor in mediating functional links between FAs and the ER, a process that enables IL-1-induced Ca2+ signaling and MMP-3 expression (6, 30). To assess the fibroblasts used in the current experiments and to confirm key phenotypic features, we determined whether IL-1 affects the association of PTPα with FA- and ER-associated proteins in FAK+/+ MEFs. After IL-1 treatment, the relative abundance of PTPα in focal adhesion preparations (which also contain plasma membrane constituents) and in ER fractions was increased by IL-1 treatment (Fig. 1A). By contrast, the relative abundance of the loading control proteins GAPDH (in whole cell lysates), α5β1 integrin (in FA fractions), and calnexin (in ER fractions) was unchanged by IL-1 treatment.
FIGURE 1.
IL-1 induces recruitment of PTPα to FA and ER, and FAK is required for IL-1-induced PTPα accumulation in FAs. A, whole cell lysates (WCL), FA, and ER fractions were isolated as described under “Experimental Procedures” from FAK+/+ MEFs treated with (+) or without (−) IL-1 at 20 ng/ml for 30 min. Equivalent amounts of whole cell lysates, FA, and ER fractions were immunoblotted for PTPα, GAPDH, α5β1 integrin, and calnexin; the latter three were used as loading controls for WCL, FA, and ER fractions, respectively. B, WCL and FA proteins prepared from FAK+/+ and FAK−/− MEFs treated with vehicle (−) or with (+) IL-1 (20 ng/ml) for 30 min were immunoblotted for FAK and PTPα. FA proteins were also immunoblotted for α5β1 integrin, calnexin, and IP3R1. Same experiment as in B was done with C, FAK−/− MEFs transfected with pEGFP-control (pEGFP-C) and FAK wild type (pEGFP-FAK), as well as with D, FAK+/+ MEFs were transfected with pEGFP-control (pEGFP-C) and FAK-related non-kinase (pEGFP-FRNK).
We next determined whether the recruitment of PTPα to FAs in response to IL-1 was dependent on FAK. The relative abundance of FAK and PTPα in whole cell lysates or focal adhesion preparations was examined by immunoblotting lysates prepared from FAK wild type (FAK+/+) and FAK knock-out (FAK−/−) MEFs. IL-1 treatment enhanced the accumulation of PTPα and FAK in the FAs of FAK+/+ cells but had a much smaller effect on the abundance of PTPα in the FAs of FAK−/− MEFs (Fig. 1B). We also found that IL-1 promoted the recruitment of FAK and PTPα to focal adhesion fractions prepared from FAK−/− MEFs that were transfected with wild type FAK (pEGFP-FAK) (Fig. 1C). The IL-1-enhanced accumulation of FAK and PTPα in focal adhesion fractions was strongly reduced in FAK+/+ MEFs that had been transfected with FRNK (FAK-related non-kinase), which strongly localizes to FAs but acts as a dominant negative inhibitor of FAK (Fig. 1D). Notably, the amount of calnexin and IP3R1 in the FAs of FAK−/− MEFs were also much lower than FAK+/+ MEFs (Fig. 1B). Taken together, these data indicate that IL-1 signaling mediates associations of PTPα with FAs and with the ER that are dependent on FAK.
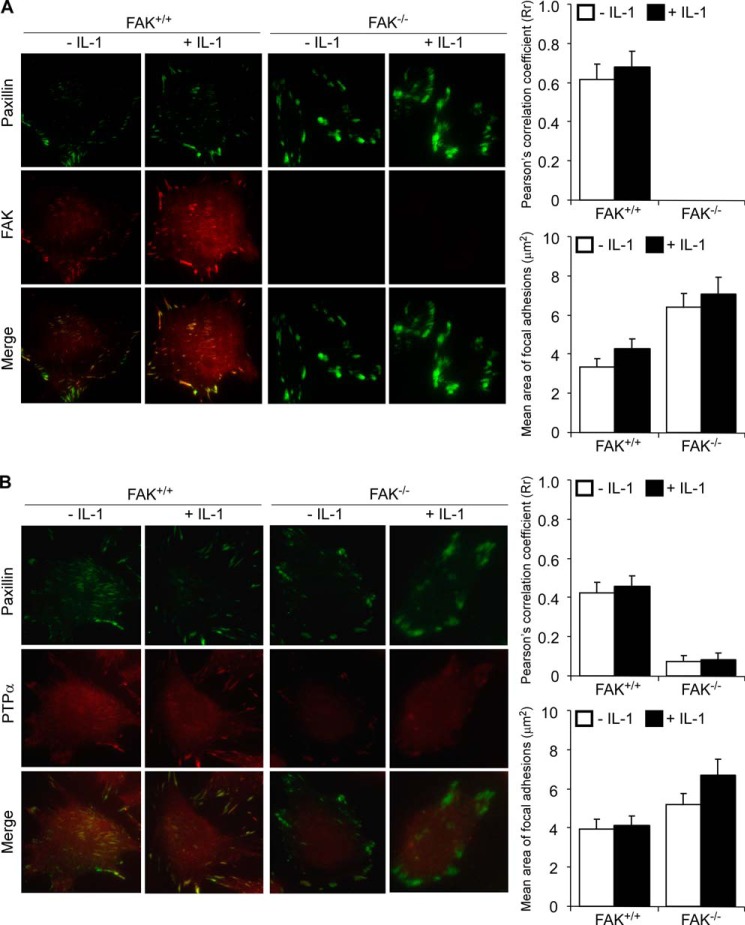
We examined the spatial association of FAK and PTPα in FAs using total internal reflection fluorescence microscopy of FAK+/+ and FAK−/− MEFs. Cells were treated with vehicle or with IL-1 for 30 min and co-immunostained for paxillin and FAK (Fig. 2A) or for paxillin and PTPα (Fig. 2B). The extent of co-localization of paxillin with FAK and the co-localization of paxillin with PTPα was quantified by calculating Pearson's correlation coefficient (35). These data showed that PTPα strongly co-localized with paxillin in FAK+/+ MEFs but not in FAK−/− MEFs (p < 0.01). IL-1 treatment did not significantly change the extent of co-localization of PTPα with paxillin or with FAK in FAK+/+ MEFs. These results indicate that FAK is required for accumulation of PTPα in FAs. Notably, the areas of FAs were much larger in FAK−/− than FAK+/+ MEFs, consistent with previous reports indicating that FAK null fibroblasts have defective FA turnover mechanisms that result in highly adherent, large and stable FAs (36). Similarly, PTPα−/− MEFs exhibit large, super-mature FAs (23), indicating the importance of both of these proteins in FA remodeling.
FIGURE 2.
FAK is required for sequestration of PTPα to FAs. FAK+/+ and FAK−/− MEFs treated with vehicle (−) or with IL-1 (+) at 20 ng/ml for 30 min were immunostained for paxillin and FAK (A) or paxillin and PTPα (B) and then imaged by total internal reflection fluorescence microscopy. Histograms of Pearson's correlation coefficient (Rr) obtained using ImageJ and mean area of FAs in the cell body estimated using MetaMorph software (Molecular Devices, Sunnyvale, CA) are given on the side panels for each set. Values from 20 to 30 cells in three independent experiments are expressed as mean ± S.D.
FAK Is Required for IL-1-stimulated PTPα-Tyr789 Phosphorylation
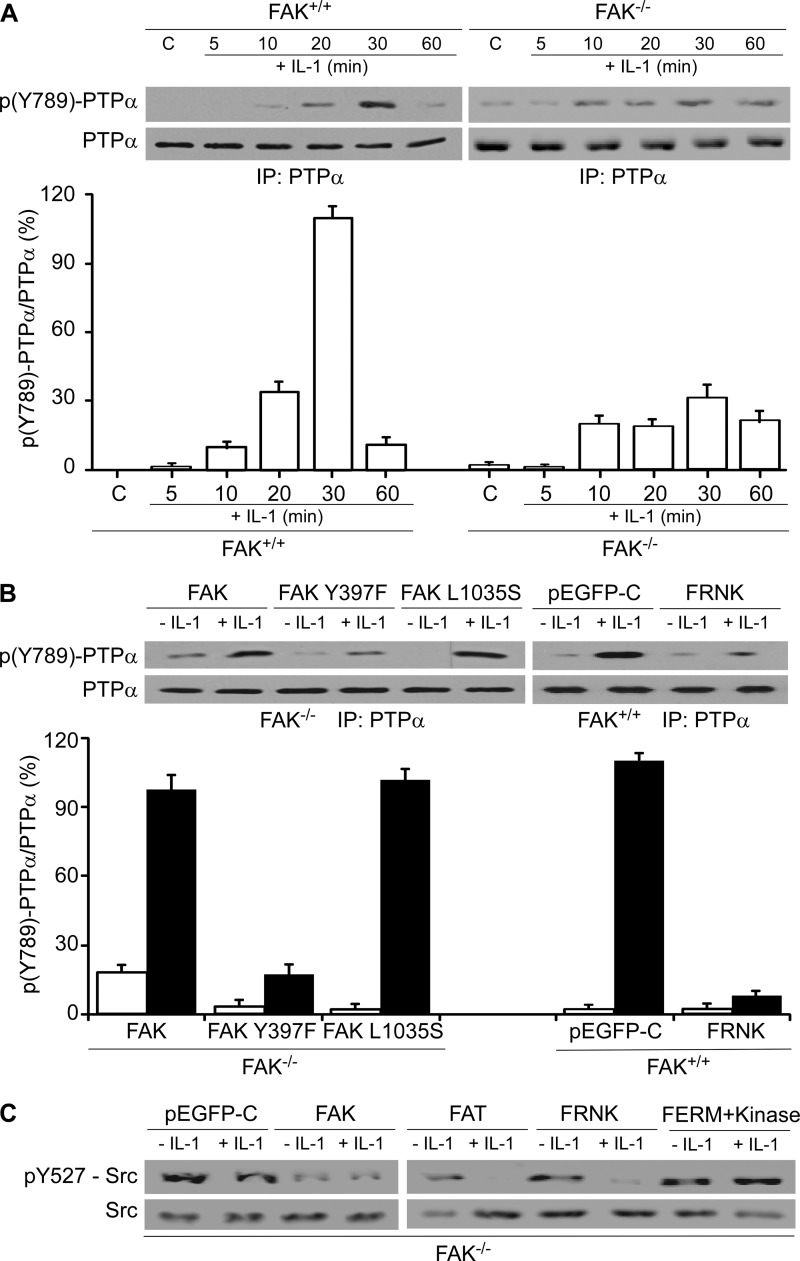
Because the phosphorylation of Tyr789 of PTPα regulates the localization of PTPα to FAs as well as integrin-induced cell spreading (29), we examined whether FAK is required for IL-1-induced phosphorylation of PTPα at Tyr789. FAK+/+ and FAK−/− MEFs were treated with vehicle or with IL-1, and PTPα immunoprecipitates were immunoblotted for PTPα-Tyr(P)789. FAK−/− MEFs exhibited more prolonged and 4-fold lower PTPα-Tyr789 phosphorylation compared with FAK+/+ MEFs after IL-1 stimulation (Fig. 3A, 30 min, p < 0.001). Transfection of FAK−/− MEFs with wild type FAK restored IL-1-induced PTPα-Tyr789 phosphorylation by 87% at 30 min (Fig. 3, A and B, p < 0.001). Although transfection of FAK−/− MEFs with a FAK-Y397F mutant did not significantly increase PTPα-Tyr789 phosphorylation in response to IL-1, expression of the FAK-L1035S mutant restored PTPα-Tyr789 phosphorylation levels comparable with that of wild type FAK. These data indicate that the Tyr397 autophosphorylation site of FAK may play a role in PTPα-Tyr789 phosphorylation (Fig. 3B). Furthermore, we found that in FAK+/+ MEFs transfected with FRNK, which strongly localizes to FAs and acts as a dominant negative inhibitor of endogenous FAK; the level of PTPα-Tyr789 phosphorylation in response to IL-1 was reduced by 91% (Fig. 3B, p < 0.001), thus underlining the importance of functional FAK in IL-1-induced PTPα-Tyr789 phosphorylation.
FIGURE 3.
FAK modulates IL-1-induced phosphorylation state of PTPα. A, PTPα immunoprecipitates (IP) from whole cell lysates of FAK+/+ and FAK−/− MEFs that had been treated with vehicle (C) or with IL-1 (20 ng/ml) for the indicated times were immunoblotted for Tyr(P)789-PTPα and PTPα. B, as above, PTPα immunoprecipitate was performed using FAK−/− MEFs, which were transfected with pEGFP-FAK (FAK), pEGFP-FAKY397F (FAK Y397F), or pEGFP-FAK L1035S (FAK L1035S); FAK+/+ MEFs were transfected with pEGFP-control (pEGFP-C) or pEGFP-FRNK (FRNK). Quantification by densitometry of immunoblots are shown as histograms of mean ratios of densities ± S.E. of Tyr(P)789-PTPα (pY527-PTPα) to PTPα (n = 3 replicates from three independent experiments). C, FAK−/− MEFs transfected with pEGFP-control (pEGFP-C), pEGFP-FAK (FAK), pEGFP-FAT (FAT), pEGFP-FRNK (FRNK), and pEGFP-FERM + kinase (FERM+Kinase) were treated with (+) or without (−) IL-1 at 20 ng/ml for 30 min. Equivalent amounts of whole cell lysates were immunoblotted for Tyr(P)527-Src and Src.
Src family kinase activation can be estimated from the phosphotyrosine levels of the inhibitory Tyr527 in Src, a target site for dephosphorylation by PTPα (37, 38). We examined the impact of FAK on IL-1-induced Src-Tyr527 phosphorylation. FAK−/− MEFs that were transfected with wild type FAK showed very low levels of phosphorylation of Tyr527 compared with control cells (Fig. 3C). Transfection of FAK−/− MEFs with the FAT domain or FRNK (C-terminal domain encompassing the FAT domain) strongly reduced Src-Tyr527 phosphorylation after IL-1 stimulation compared with transfection of cells with the FAK FERM + kinase domains, indicating that the FAT domain of FAK may interact with PTPα to enable Src activation.
PTPα Is Required for IL-1-stimulated FAK-Tyr397 Phosphorylation
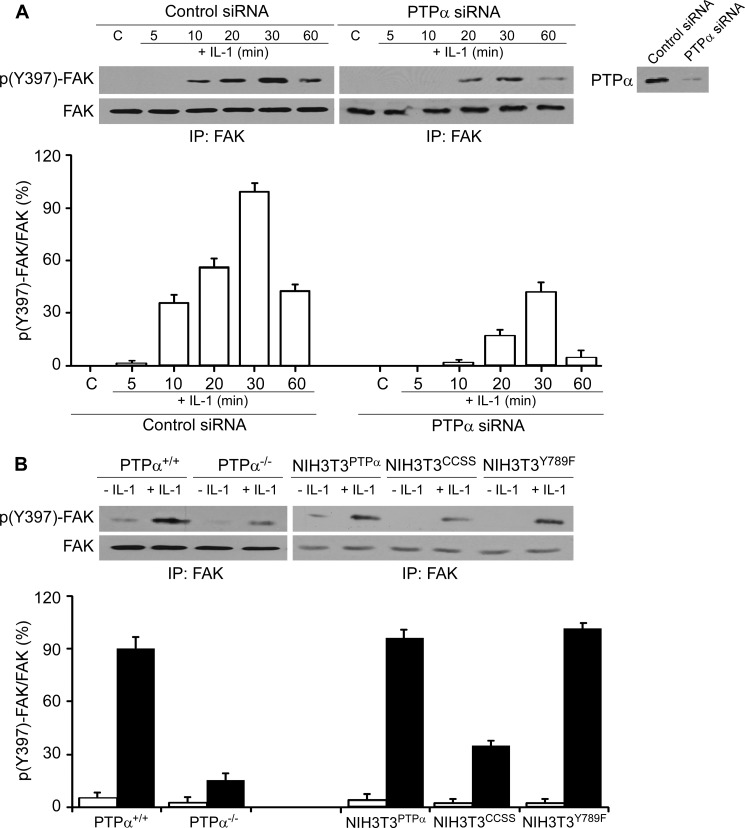
We determined whether autophosphorylation of FAK, which mediates its interaction with signaling molecules such as Src family kinases, is affected by PTPα. We examined IL-1-induced FAK-Tyr397 phosphorylation in cells with or without PTPα. Compared with cells transfected with control siRNA, knockdown of PTPα reduced by 55% IL-1-induced FAK-Tyr397 phosphorylation (30 min after IL-1 treatment; Fig. 4A, p < 0.01). Furthermore, there was a 75% reduction of IL-1-induced FAK-Tyr397 phosphorylation in PTPα−/− null cells compared with PTPα wild type cells 30 min after IL-1 stimulation (Fig. 4B, left panel, p < 0.001).
FIGURE 4.
PTPα modulates IL-1-induced phosphorylation state of FAK. A, FAK immunoprecipitates (IP) were obtained from PTPα+/+ MEFs transfected with control (C) or PTPα siRNA for 48 h and then treated with vehicle or with IL-1 (20 ng/ml) for the indicated times. FAK immunoprecipitates were immunoblotted for Tyr(P)397-FAK (p(Y)397-FAK) and FAK. Inset panel on right: lysates were immunoblotted for PTPα to confirm the efficacy of knockdown of PTPα. B, experiment described in A was repeated using PTPα+/+, PTPα−/−, NIH3T3PTPα, NIH3T3CCSS, or NIH3T3Y789F cells. Data in the histograms are means ± S.E. of the percentage ratios of Tyr(P)397-FAK to FAK (n = 3 replicates from four independent experiments).
We examined whether the phosphorylation of FAK was dependent on the presence of catalytically active PTPα. In cells that expressed phosphatase-inactive PTPα (i.e. NIH3T3 cells overexpressing a catalytically inert PTPα mutant C433S/C723S, NIH3T3CCSS), IL-1-induced FAK-Tyr397 phosphorylation was 62% lower than NIH3T3 fibroblasts expressing wild type PTPα (NIH3T3PTPα) (Fig. 4B, right panel, p < 0.01). In cells expressing the Y789F mutant (NIH3T3Y789F), FAK phosphorylation of Tyr397 in response to IL-1 was comparable with wild type cells. These results indicate that the catalytic activity but not phosphorylation of Tyr789 of PTPα is important for IL-1-induced FAK-Tyr397 phosphorylation.
Associations between FAK and PTPα
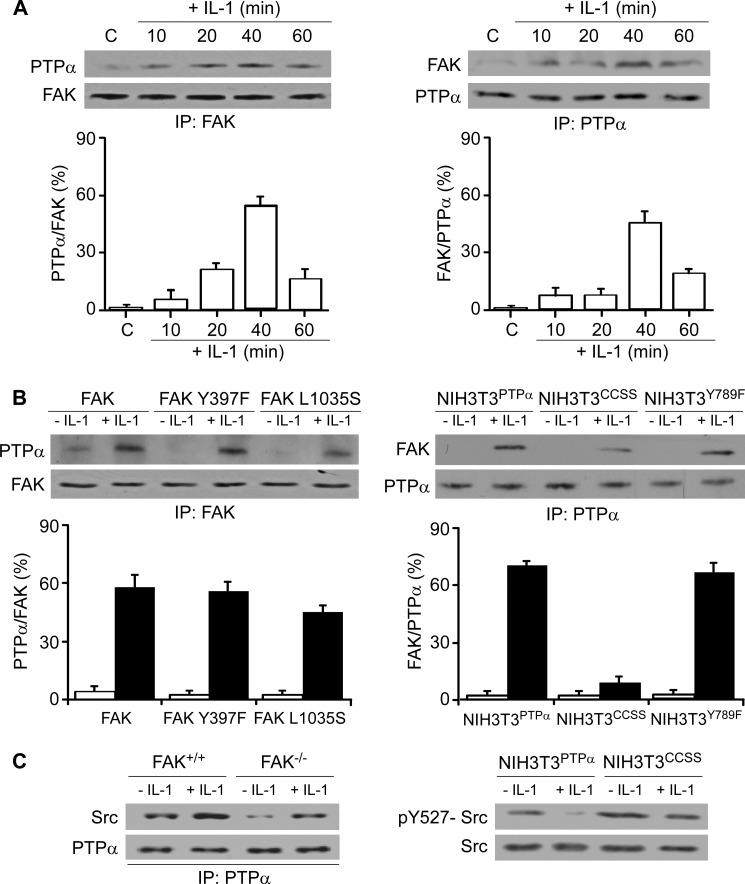
FAK and PTPα are both critical for focal adhesion-dependent cell adhesion through integrins (23, 28, 29, 39). To understand how PTPα is spatially sequestered to FAs and how it interacts with FAK to enable adhesion-restricted IL-1 signaling, we verified whether PTPα associates with FAK in response to IL-1. Examination of FAK immunoprecipitates that were immunoblotted for PTPα, or PTPα immunoprecipitates that were immunoblotted for FAK, showed a time-dependent enhancement of the association of FAK with PTPα that peaked ∼40 min after IL-1 stimulation (Fig. 5A).
FIGURE 5.
FAK associates with PTPα. A, FAK (left panel) and PTPα (right panel) immunoprecipitates (IP) were prepared from whole cell lysates of FAK+/+ MEFs that had been treated with vehicle (C) or with IL-1 (20 ng/ml) for the indicated times and immunoblotted for PTPα and FAK. B, left panel, above experiment was done with FAK−/− cells transfected with pEGFP-FAK (FAK), pEGFP-FAK Y397F (FAK Y397F), or pEGFP-FAK L1035S (FAK L1035S). Right panel, same procedure as above was carried out with NIH3T3PTPα, NIH3T3CCSS, and NIH3T3Y789F. Ratios of PTPα to FAK or FAK to PTPα were quantified by densitometry. Data are the percentage mean ratios of densities ± S.E. of four replicates from three separate experiments. C, left panel, PTPα immunoprecipitates were prepared from whole cell lysates of IL-1 treated (+) and untreated (−) FAK+/+ and FAK−/− MEFs and then immunoblotted for Src and PTPα. Right panel, NIH3T3PTPα and NIH3T3CCSS were treated with (+) or without (−) IL-1 at 20 ng/ml for 30 min. Whole cell lysates were immunoblotted for Tyr(P)527-Src (pY527-Src) and Src.
The phosphorylation of FAK at Tyr397 is critical for its kinase activity (40). In addition, interaction of FAK with paxillin is impaired by a point mutation in the FAT domain (Leu1035 to Ser), which disrupts paxillin binding. Accordingly, we examined the effect of expressing FAK Y397F and FAK L1035S mutants on the association of FAK with PTPα. FAK−/− MEFs were transfected with pEGFP-FAK (FAK wild type), pEGFP-FAK Y397F (FAK Y397F), or pEGFP-FAK L1035S (FAK L1035S). FAK immunoprecipitates were prepared from whole cell lysates of vehicle or IL-1-treated cells and immunoblotted for PTPα and FAK (Fig. 5B, left panel). Expression of the Y397F and L1035S mutants did not affect IL-1-induced association between FAK and PTPα, indicating that the Tyr397 and Leu1035 sites do not directly affect the association of FAK with PTPα.
We also examined the requirements for the catalytic and adaptor functions of PTPα to enable associations between FAK and PTPα with the use of cells expressing the C433S/C723S or Y789F mutants, respectively. NIH3T3 fibroblasts expressing wild type PTPα (designated as NIH3T3PTPα), catalytically inert PTPα (C433S/C723S; designated as NIH3T3CCSS), and PTPα Y789F mutant (designated as NIH3T3Y789F) were treated with vehicle or IL-1. PTPα immunoprecipitates were prepared from whole cell lysates and immunoblotted for FAK and PTPα. The association between FAK and PTPα was dependent on the integrity of the catalytic domain of PTPα because in NIH3T3 fibroblasts expressing the catalytically defective PTPα (C433S/C723S mutant), there was ∼85% reduction of FAK that co-precipitated with PTPα even after IL-1 treatment (Fig. 5B, right panel, p < 0.001). The expression of the Y789F mutant did not affect the association between FAK and PTPα. We suggest that the PTPα-FAK interaction may precede interactions with other proteins like paxillin and Grb2 and phosphorylation events associated with these proteins, which in turn affect the catalytic activity of FAK and PTPα.
We assessed whether FAK expression affects the association of PTPα with Src in cells treated with IL-1. FAK+/+ and FAK−/− MEFs were treated with IL-1 or vehicle. PTPα immunoprecipitates were prepared from whole cell lysates and immunoblotted for Src. We found that PTPα associates with Src after IL-1. However, this association was partly dependent on the presence of FAK because in FAK-null cells, the IL-1-induced association of PTPα with Src was decreased (Fig. 5C, left panel). Furthermore, we found that after IL-1 stimulation, Src-Tyr527 phosphorylation was abolished in NIH3T3 fibroblasts that expressed wild type PTPα (NIH3T3PTPα) compared with NIH3T3 fibroblasts expressing the catalytically defective PTPα (C433S/C723S mutant) (Fig. 5C, right panel), indicating that an intact catalytic phosphatase domain of PTPα is essential for Src-Tyr527 dephosphorylation and thus activation. Collectively, the association of FAK with PTPα appears to be important for the association of PTPα with Src and Src-Tyr527 dephosphorylation that is required for Src activation.
Interactions of FAK with PTPα
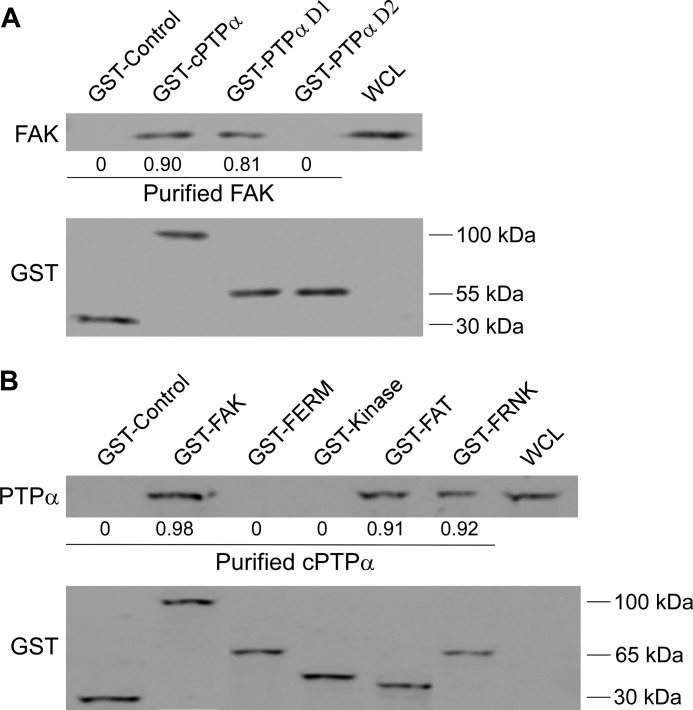
Direct associations between FAK and PTPα were examined with pulldown assays using purified GST-tagged PTPα proteins. These bacterially expressed mutant proteins included either the entire cytosolic domain (GST-cPTPα) or the catalytic domain D1 alone (GST-PTPα D1) or the D2 alone (GST-PTPα D2). Bead-bound proteins were incubated in a cell-free system with bacterially expressed FAK. GST-associated proteins and FAK were detected by immunoblotting (Fig. 6A). These data showed that purified FAK directly interacted with the whole cytosolic domain of PTPα as well as the D1 catalytic domain but not with the catalytic domain D2.
FIGURE 6.
Direct interaction between FAK and PTPα. A, to show direct interactions, GST-control, GST-cPTPα, GST-PTPα D1, and GST-PTPα D2 were incubated with purified FAK by removal of GST tag by enzymatic (thrombin) cleavage from GST-FAK bacterial fusion protein. Bead-associated proteins were analyzed by immunoblotting for FAK and GST. The numbers below the blots represent the ratios of FAK to GST levels. WCL, whole cell lysate. B, same procedure as above was performed with GST-control, GST-FAK, GST-FERM, GST-kinase, GST-FAT, and GST-FRNK by incubation with purified cPTPα by removal of GST tag using enzymatic (thrombin) cleavage from GST-cPTPα bacterial fusion protein. Bead-associated proteins were analyzed by immunoblotting for PTPα and GST. The numbers below the blots represent the ratios of PTPα to GST levels.
FAK exhibits a domain structure consisting of a FERM, kinase, and focal adhesion-targeting (FAT) domain (40). We assessed which of these FAK domains binds to PTPα. Wild type FAK, FERM, kinase, and FAT domains of FAK bound to GST were purified and incubated in a cell-free system with bacterially expressed cPTPα. GST-associated proteins were immunoblotted for PTPα. Equal protein loading was verified by immunoblotting for GST (Fig. 6B). These data showed that purified cPTPα interacted directly with wild type FAK as well as the FAT domain of FAK and FRNK, indicating that interactions between FAK and PTPα specifically require an intact FAT domain.
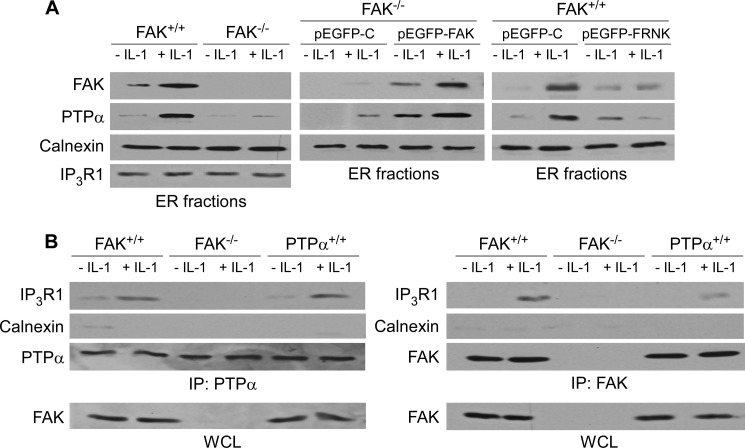
FAK Is Required for IL-1-induced PTPα Accumulation in ER
For many complex regulatory systems, including IL-1, anchorage of signaling proteins to specific organellar membranes is required for effective signal transduction (41). We showed that PTPα co-localizes with ER-associated proteins (e.g. calnexin, IP3R1) in FAs, and this is further enhanced by IL-1 (30). In the current experiments, we observed that IL-1 treatment enhanced accumulation of PTPα in ER fractions of FAK+/+ cells and in FAK−/− cells expressing wild type FAK (pEGFP-FAK) but not in FAK−/− MEFs (transfected with or without pEGFP-control (pEGFP-C)) (Fig. 7A). Furthermore, the ability of IL-1 to enhance the accumulation of FAK and PTPα in ER fractions was dramatically reduced in FAK+/+ cells transfected with FRNK (an endogenous inhibitor of FAK), indicating that FA localization of FAK is required for IL-1-induced PTPα accumulation in ER fractions.
FIGURE 7.
FAK is required for PTPα recruitment to ER. A, left panel, ER fractions from FAK+/+ and FAK−/− MEFs treated with vehicle (−) or with (+) IL-1 (20 ng/ml) for 30 min were immunoblotted for FAK, PTPα, calnexin, and IP3R1. Middle panel, above experiment was repeated with FAK−/− MEFs transfected with pEGFP-control (pEGFP-C) and pEGFP-FAK, and right panel, in FAK+/+ MEFs transfected with pEGFP-control (pEGFP-C) and pEGFP-FRNK. B, PTPα or FAK was immunopurified from whole cell lysates (WCL) of FAK+/+, FAK−/−, and PTPα+/+ MEFs that had been stimulated with vehicle (−) or with (+) IL-1. PTPα immunoprecipitates (IP) and whole cell lysates (left panel) were immunoblotted forIP3R1, calnexin, and PTPα. FAK immunoprecipitates and whole cell lysates (right panel) were immunoblotted for IP3R1, calnexin, and FAK.
Immunoblot analysis of PTPα or FAK from FAK+/+ or PTPα+/+ MEFs showed that IL-1 treatment enhanced the amount of IP3R1 that co-precipitated with PTPα and with FAK in whole cell lysates prepared from these cells. There was very little IP3R1 that co-precipitated with PTPα in FAK−/− cells (Fig. 7B). Collectively, these results indicate that FAK binds to PTPα, thereby facilitating IL-1-induced enrichment of PTPα in FAs and in proximal ER membranes and interaction with IP3R1.
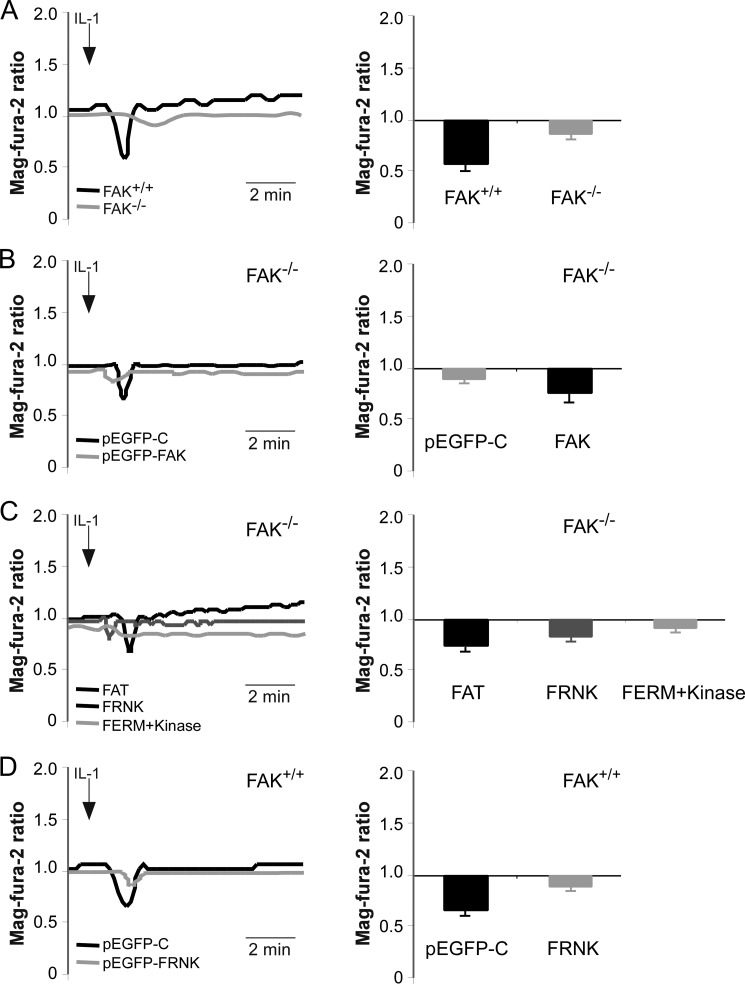
Requirement for FAK in IL-1-induced Ca2+ Signaling
IL-1 triggers FA-dependent Ca2+ release from the ER (12, 13). The experiments described above (Fig. 7B) demonstrated that IL-1 treatment enhanced co-precipitation of IP3R1 with FAK and PTPα. Because previous studies showed that FAK is critical in regulating cell adhesion, contractility, migration, and signaling (42), we determined whether FAK regulates Ca2+ signaling in response to IL-1. In FAK+/+ MEFs, IL-1 treatment resulted in a transient release of Ca2+ from the ER (Fig. 8A). By contrast, the amplitude of IL-1-induced ER Ca2+ signals was reduced by >5-fold (p < 0.01) in FAK−/− MEFs. This defect could be reversed by transfecting FAK null cells with wild type FAK (Fig. 8B). Notably, there was a >3- and 2-fold higher response (p < 0.01) in FAK−/− MEFs transfected with FAT domain or FRNK compared with FAK null cells (Fig. 8C). However, there was no change in FERM + kinase domain transfections. Similarly, FAK wild type (FAK+/+) cells expressing FRNK (Fig. 8D) exhibited a >3.5-fold (p < 0.01) lower ER Ca2+ release compared with cells expressing the pEGFP-control (pEGFP-C). Taken together, these data indicate that the FAT domain of FAK is important for IL-1-induced Ca2+ release from the ER.
FIGURE 8.
FAK is required for IL-1-induced Ca2+ release from the ER. Mag-fura-2 ratios were measured in mag-fura-2-loaded cells after treatment with IL-1 (20 ng/ml). A, FAK+/+ and FAK−/− MEFs; B, FAK−/− MEFs transfected with pEGFP-control (pEGFP-C) and FAK wild type (FAK); C, FAK−/− MEFs transfected with pEGFP-FAT (FAT), pEGFP-FRNK (FRNK), and pEGFP-FERM + kinase (FERM+Kinase); D, FAK+/+ MEFs transfected with pEGFP-control (pEGFP-C) and pEGFP-FRNK (FRNK) are depicted in histograms as mean ± S.E. of mag-fura-2 ratios from three replicates obtained from 4 to 5 independent experiments.
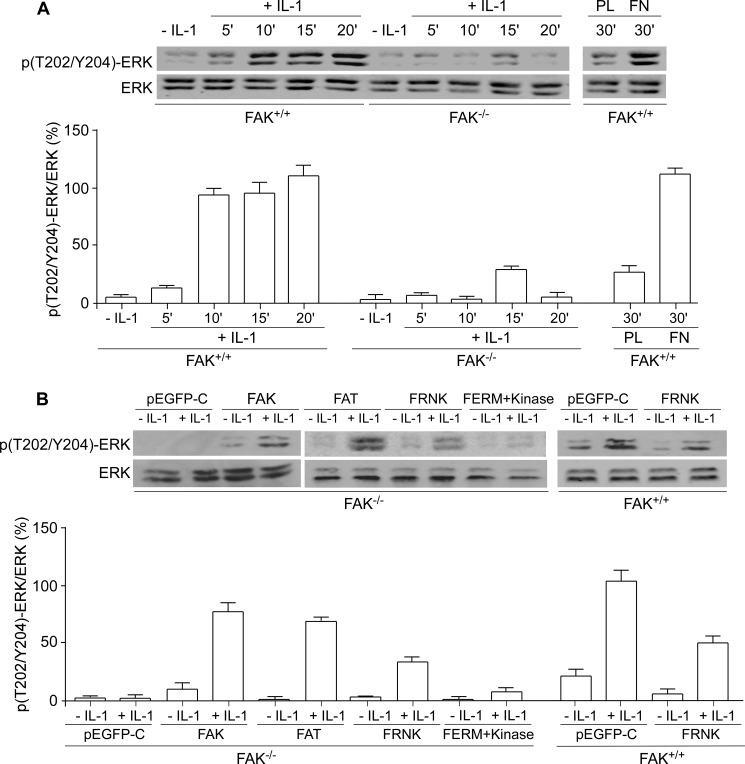
FAK Restricts IL-1-induced ERK Phosphorylation
In many signaling systems, Ca2+ release from the ER is required for ERK activation (43). Furthermore, FAK mediates activation of the ERK pathway in response to integrin ligation (17). Accordingly, we examined the role of FAK in IL-1-induced ERK activation in FAK+/+ and FAK−/− MEFs (Fig. 9A). FAK+/+ cells displayed a time-dependent increase in ERK phosphorylation in response to IL-1 treatment; by contrast, IL-1 did not induce ERK phosphorylation in FAK−/− MEFs As a control, when cells were plated on poly-l-lysine to prevent the formation of FAs, IL-1-induced ERK activation was not detectable in FAK+/+ MEFs (Fig. 9A). These data indicate that formation of FAs, along with the expression of FAK, is crucial for IL-1-induced ERK activation.
FIGURE 9.
IL-1-induced ERK phosphorylation is FAK-dependent. A, whole cell lysates from FAK+/+ and FAK−/− MEFs plated on fibronectin (FN) or poly-l-lysine (PL) and treated with vehicle or with IL-1 (20 ng/ml) for indicated time periods were immunoblotted for Thr(P)202/Tyr204-ERK (p(T202/Y204)-ERK) and ERK. B, above experiment was carried out using FAK−/− MEFs transfected with pEGFP-control (pEGFP-C), FAK wild type (FAK), pEGFP-FAT (FAT), pEGFP-FRNK (FRNK), and pEGFP-FERM + kinase (FERM+Kinase) as well as FAK+/+ MEFs transfected with pEGFP-control (pEGFP-C) and pEGFP-FRNK (FRNK). Ratios of Thr(P)202/Tyr204-ERK to ERK were quantified by densitometry and shown as the percentage mean ratios of densities ± S.E. of four replicates from three separate experiments.
To determine the role of FAK domains in ERK activation, we studied the effects of the FAT domain, FRNK, and the FERM + kinase domains on IL-1-induced signaling using transfected FAK−/− and FAK+/+ MEFs. In FAK−/− MEFs transfected with wild type FAK, the FAT domain, or FRNK, IL-1-induced ERK phosphorylation was increased. When FAK−/− MEFs were transfected with pEGFP-control or the FERM + kinase domains, there was markedly reduced IL-1-induced ERK phosphorylation. Similarly, in FAK+/+ cells transfected with pEGF-FRNK, IL-1-induced ERK phosphorylation was reduced (Fig. 9B). These data indicate that the FAT domain of FAK is required for IL-1 signaling to ERK.
FAK Regulates IL-1-induced IL-6, MMP-3, and MMP-9 Expression
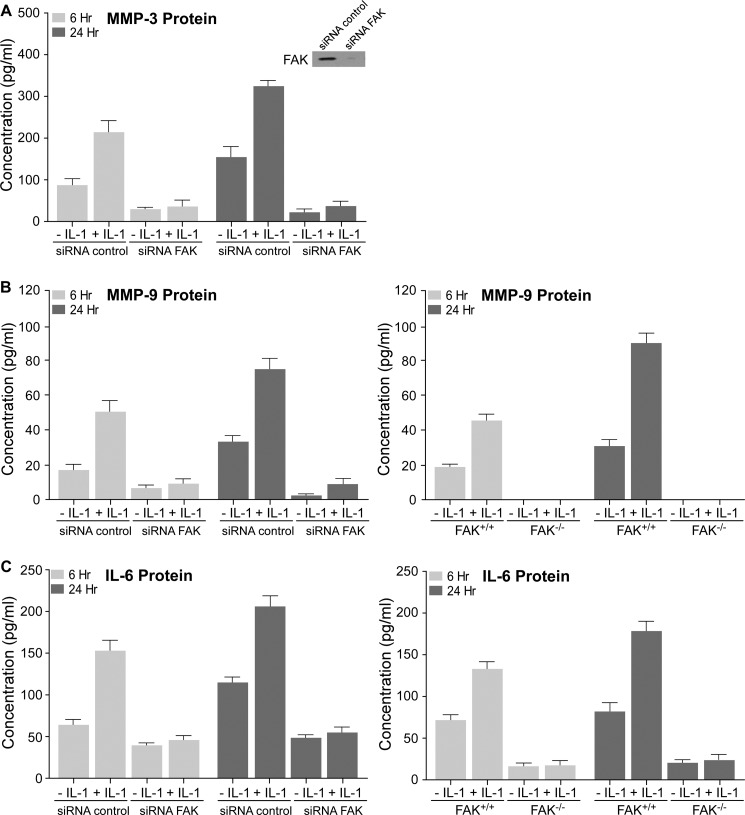
IL-1 signaling leads to the expression of the early response gene c-fos and several cytokines and inflammatory factors that drive extracellular matrix remodeling via MMPs (4). Currently, it is not known whether FAK mediates IL-1-induced expression of matrix-destructive genes. Hence, we examined the impact of FAK on IL-1-induced IL-6, MMP-3, and MMP-9 release into conditioned media of NIH3T3 fibroblasts. The cells were transfected with control siRNA or siRNA FAK and treated with IL-1 for 6 or 24 h. From ELISA analyses, FAK knockdown cells exhibited markedly reduced expression of MMP-3 in response to IL-1 at 6 and 24 h compared with control siRNA (Fig. 10A, p < 0.01). IL-1 also enhanced the secretion of MMP-9 and IL-6 at 6 and 24 h by NIH3T3 fibroblasts transfected with control siRNA and in FAK+/+ MEFs (p < 0.01) but not in cells transfected with siRNA FAK or in FAK−/− MEFs (Fig. 10, B and C).
FIGURE 10.
FAK regulates IL-1-induced MMP-3, MMP-9, and IL-6 protein expression. A, NIH3T3 fibroblasts were transfected with siRNA control or siRNA FAK for 48 h, and then treated with vehicle (−) or with IL-1 (+) (20 ng/ml) for 6 and 24 h. Culture medium was concentrated 5–10× and MMP-3protein were quantified by ELISA (see “Experimental Procedures”). Lysates of siRNA control- and siRNA FAK-transfected cells were immunoblotted for FAK to confirm the knockdown of FAK. B, left panel, MMP-9 protein of above NIH3T3 cells in concentrated conditioned media was quantified by ELISA. Right panel, same experiment was repeated with FAK+/+ and FAK−/− MEFs. C, left panel, IL-6 protein of above NIH3T3 cells in concentrated conditioned media was quantified by ELISA. Right panel, same experiment was repeated with FAK+/+ and FAK−/− MEFs. Values are mean ± S.E. from n = three independent experiments.
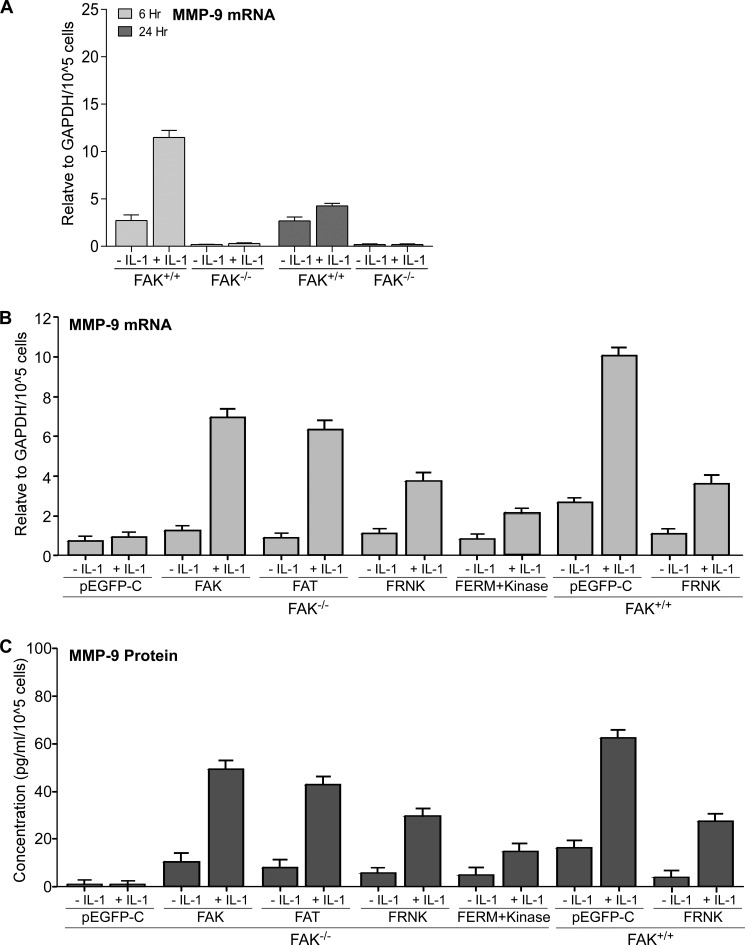
Notably, FAK has been implicated in matrix degradation leading to tumor cell metastasis (8) As MMP-9 is important for extracellular matrix remodeling and its expression is regulated by IL-1 (5, 7), we assessed the role of FAK in mediating MMP-9 mRNA expression after IL-1 treatment of FAK+/+ and FAK−/− MEFs (Fig. 11A). IL-1 enhanced production of MMP-9 mRNA at 6 and 24 h after IL-1 treatment in FAK+/+ MEFs (p < 0.01) but not in FAK−/− MEFs. We then determined which domain of FAK was involved in IL-1-induced MMP-9 expression at transcriptional and translational levels. Expression of wild type FAK, FAT domain, and FRNK in FAK−/− cells strongly increased mRNA and protein levels of MMP-9 in response to IL-1 compared with the FAK−/− cells transfected with control plasmid (pEGFP-C) (Fig. 11, B and C, p < 0.01). However, expression of MMP-9 was slightly increased in cells transfected with the FERM + kinase domains. When FAK+/+ cells were transfected with pEGF-FRNK, IL-1-induced MMP-9 expression was reduced >2-fold compared with FAK+/+ cells (Fig. 11, B and C, p < 0.01). These data indicate that the FAT domain is essential for IL-1-induced MMP-9 expression.
FIGURE 11.
FAK is required for IL-1-induced MMP-9 expression. A, FAK+/+ and FAK−/− MEFs were treated with vehicle or with IL-1 (20 ng/ml) for 6 and 24 h. Total RNA was extracted, and MMP-9 mRNA levels were quantified by qRT-PCR analysis. B, MMP-9 mRNA levels were quantified in FAK−/− MEFs transfected with pEGFP-control (pEGFP-C), FAK wild type (FAK), pEGFP-FAT (FAT), pEGFP-FRNK (FRNK), and pEGFP-FERM + kinase (FERM+Kinase) as well as FAK+/+ MEFs transfected with pEGFP-control (pEGFP-C) and pEGFP-FRNK (FRNK) that were treated with vehicle or with IL-1 (20 ng/ml) for 6 h, respectively. C, transfected cells in B were treated with vehicle or with IL-1 (20 ng/ml) for 24 h, and MMP-9 protein in concentrated conditioned media was quantified by ELISA (see “Experimental Procedures”). Values are mean ± S.E. from n = 3 independent experiments.
DISCUSSION
Our principal finding is that IL-1-induced signaling in fibroblasts involves association of FAK with PTPα. The interactions between FAK and PTPα require the FAT domain of FAK and the membrane-proximal domain of PTPα. As shown here, FAK is critical for IL-1-induced Ca2+ release from the ER and ERK activation and IL-6, MMP-3, and MMP-9 expression. Notably, in FAK-deficient fibroblasts, the structure and composition of FAs is altered (36), underscoring the importance of FAK in enabling IL-1 signaling through FAs.
FAs are signaling platforms enriched with molecules such as tyrosine kinases and phosphatases that regulate signaling through FAs (10, 41). Our results show that PTPα and FAK were recruited into FAs in response to IL-1, which is consistent with our previous observations indicating that IL-1 increases the size and maturity of FAs (44). Indeed, we found fewer but larger FAs in cultured fibroblasts genetically deficient in FAK, which is in agreement with earlier data indicating that FAK is involved in the formation and turnover of these adhesive structures (36). Likewise, defective FA remodeling in PTPα−/− cells leads to the formation of “super-mature” adhesions in these cells (23). Hence, FAK and PTPα are critical for maintaining FA dynamics and the propagation of downstream signaling events.
Our data show that the role of FAK extends beyond IL-1 signaling through FAs because we also observed that association of the ER-associated protein calnexin and IP3R1 with FAs was substantially reduced in FAK−/− cells compared with wild type cells. These findings suggest that the local FA environment was changed by the absence of FAK, resulting in loss of functional connections between the ER and FAs. Conceivably, the proximity of FAs with the ER is important for IL-1-induced signaling that involves ER-Ca2+ release and ERK activation (43).
Signaling platforms created by the spatial aggregation of interacting signaling molecules regulate the type and amplitude of signal transduction (34). The signaling events occurring immediately downstream of IL-1 that lead to the activation and interactions of FAK or PTPα are not defined. PTPα may provide a scaffolding function to promote PTPα-mediated catalysis of Src family kinase activation by dephosphorylating the inhibitory C-terminal Tyr527 residue of Src (45, 46), which subsequently enables autophosphorylation of FAK at Tyr397. Furthermore, FAK is also an adaptor protein in many signaling processes, due to its ability to bind proteins such as Src family kinases (47). These previous findings are consistent with our data that PTPα and its catalytic domains are important for IL-1-induced phosphorylation of FAK on Tyr397. In addition, our data indicate that FAK and its Tyr397 autophosphorylation site are necessary for IL-1-induced phosphorylation of PTPα on Tyr789. These findings suggest that PTPα phosphorylation may occur after the formation of the PTPα- Src family kinase-FAK complex.
PTPα is a receptor-like PTP (48, 49) that possesses an extracellular domain, a transmembrane domain, and a cytosolic domain containing two catalytic subdomains. Our data demonstrate that the accumulation of PTPα in FAs induced by IL-1 was strongly reduced in FAK−/− MEFs. These findings suggest that the enrichment of FAK in FAs may enable the recruitment of PTPα to FAs. We found that the FAT domain of FAK enhances interactions with PTPα, which may provide essential focal adhesion-dependent anchorage motifs for PTPα that are important for IL-1 signaling through FAs. Notably, the phosphatase domains of PTPα are essential for phosphatase activity (6, 23) and, as shown here, are important binding sites for FAK.
For many complex signaling systems, including IL-1, anchorage of signaling proteins to specific organellar membranes is required for effective signal transduction (34). In the context of signaling platforms in FAs, we found that PTPα and FAK are closely associated with the ER. We also found that the ER-associated proteins calnexin and IP3R1 co-localized with FAs in FAK+/+ MEFs and that FAK is required for IL-1-induced PTPα accumulation in the ER. As FAK binds to PTPα, these proteins may help to approximate FAs with the ER, thereby facilitating Ca2+ release and downstream signaling to ERK activation and MMP expression.
Previous studies have shown that in substrate-dependent cells, FAs are essential for IL-1-induced Ca2+ release and ERK activation (11–15) and that PTPα is a critical adaptor in mediating functional links between FAs and the ER to enable IL-1-induced Ca2+ signaling leading to ERK activation (6). This study demonstrates that only cells expressing intact FAK or FAT domain could respond to IL-1 stimulation by exhibiting Ca2+ release from the ER, ERK phosphorylation, and IL-6, MMP-3, and MMP-9 expression.
Collectively these data indicate that FAK is required for the recruitment of specific proteins, such as PTPα to FAs, possibly as a result of the catalytic and/or regulatory activities that reside in these structural domains. These domains in turn may modulate IL-1 signaling that is transduced through FAs (34). The novelty of our report is that the FAT domain of FAK provides a docking site for PTPs like PTPα, which then provide appropriate localization of other signaling proteins to FAs. These proteins in turn regulate downstream FA-restricted IL-1 signaling leading to IL-6, MMP-3, and MMP-9 expression.
This work was supported in part by National Institutes of Health Grant HL090669 from NHLBI (to G. P. D.). This work was also supported by Canadian Institute of Health Research Operating Grant MOP-384254 (to C. A. M.) and by an operating grant from the National Institutes of Health/NHLBI.
- MMP
- matrix metalloproteinase
- FA
- focal adhesion
- FAK
- focal adhesion kinase
- PTP-α
- protein-tyrosine phosphatase-α
- ER
- endoplasmic reticulum
- IP3R1
- inositol 1,4,5-trisphosphate receptor 1
- FERM
- band4.1-ezrin-radixin-moesin
- FAT
- focal adhesion targeting
- FRNK
- FAK-related non-kinase
- MEF
- mouse embryonic fibroblast
- TRITC
- tetramethylrhodamine isothiocyanate.
REFERENCES
- 1. Smith D. E., Renshaw B. R., Ketchem R. R., Kubin M., Garka K. E., Sims J. E. (2000) Four new members expand the interleukin-1 superfamily. J. Biol. Chem. 275, 1169–1175 [DOI] [PubMed] [Google Scholar]
- 2. Dinarello C. A. (2003) Setting the cytokine trap for autoimmunity. Nat. Med. 9, 20–22 [DOI] [PubMed] [Google Scholar]
- 3. Isshiki H., Akira S., Tanabe O., Nakajima T., Shimamoto T., Hirano T., Kishimoto T. (1990) Constitutive and interleukin-1 (IL-1)-inducible factors interact with the IL-1-responsive element in the IL-6 gene. Mol. Cell. Biol. 10, 2757–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldring S. R. (2003) Pathogenesis of bone and cartilage destruction in rheumatoid arthritis. Rheumatology 42, Suppl. 2, 11–16 [DOI] [PubMed] [Google Scholar]
- 5. Ruhul Amin A. R., Senga T., Oo M. L., Thant A. A., Hamaguchi M. (2003) Secretion of matrix metalloproteinase-9 by the proinflammatory cytokine, IL-1β: a role for the dual signalling pathways, Akt and Erk. Genes Cells 8, 515–523 [DOI] [PubMed] [Google Scholar]
- 6. Wang Q., Rajshankar D., Laschinger C., Talior-Volodarsky I., Wang Y., Downey G. P., McCulloch C. A. (2010) Importance of protein-tyrosine phosphatase-α catalytic domains for interactions with SHP-2 and interleukin-1-induced matrix metalloproteinase-3 expression. J. Biol. Chem. 285, 22308–22317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liang K. C., Lee C. W., Lin W. N., Lin C. C., Wu C. B., Luo S. F., Yang C. M. (2007) Interleukin-1β induces MMP-9 expression via p42/p44 MAPK, p38 MAPK, JNK, and nuclear factor-κB signaling pathways in human tracheal smooth muscle cells. J. Cell Physiol. 211, 759–770 [DOI] [PubMed] [Google Scholar]
- 8. Shibata K., Kikkawa F., Nawa A., Thant A. A., Naruse K., Mizutani S., Hamaguchi M. (1998) Both focal adhesion kinase and c-Ras are required for the enhanced matrix metalloproteinase 9 secretion by fibronectin in ovarian cancer cells. Cancer Res. 58, 900–903 [PubMed] [Google Scholar]
- 9. Wehrle-Haller B., Imhof B. (2002) The inner lives of focal adhesions. Trends Cell Biol. 12, 382–389 [DOI] [PubMed] [Google Scholar]
- 10. Burridge K., Chrzanowska-Wodnicka M. (1996) Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 12, 463–518 [DOI] [PubMed] [Google Scholar]
- 11. Arora P. D., Ma J., Min W., Cruz T., McCulloch C. A. (1995) Interleukin-1-induced calcium flux in human fibroblasts is mediated through focal adhesions. J. Biol. Chem. 270, 6042–6049 [DOI] [PubMed] [Google Scholar]
- 12. Luo L., Cruz T., McCulloch C. (1997) Interleukin 1-induced calcium signalling in chondrocytes requires focal adhesions. Biochem. J. 324, 653–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Q., Downey G. P., Choi C., Kapus A., McCulloch C. A. (2003) IL-1 induced release of Ca2+ from internal stores is dependent on cell-matrix interactions and regulates ERK activation. FASEB J. 17, 1898–1900 [DOI] [PubMed] [Google Scholar]
- 14. Lo Y. Y., Luo L., McCulloch C. A., Cruz T. F. (1998) Requirements of focal adhesions and calcium fluxes for interleukin-1-induced ERK kinase activation and c-fos expression in fibroblasts. J. Biol. Chem. 273, 7059–7065 [DOI] [PubMed] [Google Scholar]
- 15. MacGillivray M. K., Cruz T. F., McCulloch C. A. (2000) The recruitment of the interleukin-1 (IL-1) receptor-associated kinase (IRAK) into focal adhesion complexes is required for IL-1β-induced ERK activation. J. Biol. Chem. 275, 23509–23515 [DOI] [PubMed] [Google Scholar]
- 16. Reunanen N., Westermarck J., Häkkinen L., Holmström T. H., Elo I., Eriksson J. E., Kähäri V. M. (1998) Enhancement of fibroblast collagenase (matrix metalloproteinase-1) gene expression by ceramide is mediated by extracellular signal-regulated and stress-activated protein kinase pathways. J. Biol. Chem. 273, 5137–5145 [DOI] [PubMed] [Google Scholar]
- 17. Schlaepfer D. D., Hunter T. (1997) Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J. Biol. Chem. 272, 13189–13195 [DOI] [PubMed] [Google Scholar]
- 18. Hanks S. K., Calalb M. B., Harper M. C., Patel S. K. (1992) Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc. Natl. Acad. Sci. U.S.A. 89, 8487–8491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng C., Xing Z., Bian Z. C., Guo C., Akbay A., Warner L., Guan J. L. (1998) Differential regulation of Pyk2 and focal adhesion kinase (FAK). The C-terminal domain of FAK confers response to cell adhesion. J. Biol. Chem. 273, 2384–2389 [DOI] [PubMed] [Google Scholar]
- 20. Schaller M. D., Hildebrand J. D., Shannon J. D., Fox J. W., Vines R. R., Parsons J. T. (1994) Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol. Cell. Biol. 14, 1680–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schlaepfer D. D., Hunter T. (1996) Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol. Cell. Biol. 16, 5623–5633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Panetti T. S. (2002) Tyrosine phosphorylation of paxillin, FAK, and p130CAS: effects on cell spreading and migration. Front. Biosci. 7, d143–d150 [DOI] [PubMed] [Google Scholar]
- 23. Herrera Abreu M. T., Penton P. C., Kwok V., Vachon E., Shalloway D., Vidali L., Lee W., McCulloch C. A., Downey G. P. (2008) Tyrosine phosphatase PTPα regulates focal adhesion remodeling through Rac1 activation. Am. J. Physiol. Cell Physiol. 294, C931–C944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harder K. W., Moller N. P., Peacock J. W., Jirik F. R. (1998) Protein-tyrosine phosphatase α regulates Src family kinases and alters cell-substratum adhesion. J. Biol. Chem. 273, 31890–31900 [DOI] [PubMed] [Google Scholar]
- 25. Ponniah S., Wang D. Z., Lim K. L., Pallen C. J. (1999) Targeted disruption of the tyrosine phosphatase PTPα leads to constitutive downregulation of the kinases Src and Fyn. Curr. Biol. 9, 535–538 [DOI] [PubMed] [Google Scholar]
- 26. Su J., Muranjan M., Sap J. (1999) Receptor protein-tyrosine phosphatase α activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr. Biol. 9, 505–511 [DOI] [PubMed] [Google Scholar]
- 27. Lammers R., Lerch M. M., Ullrich A. (2000) The carboxyl-terminal tyrosine residue of protein-tyrosine phosphatase α mediates association with focal adhesion plaques. J. Biol. Chem. 275, 3391–3396 [DOI] [PubMed] [Google Scholar]
- 28. von Wichert G., Jiang G., Kostic A., De Vos K., Sap J., Sheetz M. P. (2003) RPTP-α acts as a transducer of mechanical force on αv/β3-integrin-cytoskeleton linkages. J. Cell Biol. 161, 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen M., Chen S. C., Pallen C. J. (2006) Integrin-induced tyrosine phosphorylation of protein-tyrosine phosphatase-α is required for cytoskeletal reorganization and cell migration. J. Biol. Chem. 281, 11972–11980 [DOI] [PubMed] [Google Scholar]
- 30. Wang Q., Rajshankar D., Branch D. R., Siminovitch K. A., Herrera Abreu M. T., Downey G. P., McCulloch C. A. (2009) Protein-tyrosine phosphatase-α and Src functionally link focal adhesions to the endoplasmic reticulum to mediate interleukin-1-induced Ca2+ signaling. J. Biol. Chem. 284, 20763–20772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brown M., McCormack M., Zinn K. G., Farrell M. P., Bikel I., Livingston D. M. (1986) A recombinant murine retrovirus for simian virus 40 large T cDNA transforms mouse fibroblasts to anchorage-independent growth. J. Virol. 60, 290–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Golubovskaya V. M., Cance W. G. (2007) Focal adhesion kinase and p53 signaling in cancer cells. Int. Rev. Cytol. 263, 103–153 [DOI] [PubMed] [Google Scholar]
- 33. Oakes S. A., Scorrano L., Opferman J. T., Bassik M. C., Nishino M., Pozzan T., Korsmeyer S. J. (2005) Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc. Natl. Acad. Sci. U.S.A. 102, 105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCulloch C. A., Downey G. P., El-Gabalawy H. (2006) Signalling platforms that modulate the inflammatory response: new targets for drug development. Nat. Rev. Drug Discov. 5, 864–876 [DOI] [PubMed] [Google Scholar]
- 35. Bolte S., Cordelières F. P. (2006) A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 [DOI] [PubMed] [Google Scholar]
- 36. Ilić D., Furuta Y., Kanazawa S., Takeda N., Sobue K., Nakatsuji N., Nomura S., Fujimoto J., Okada M., Yamamoto T. (1995) Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377, 539–544 [DOI] [PubMed] [Google Scholar]
- 37. Zheng X. M., Wang Y., Pallen C. J. (1992) Cell transformation and activation of pp60c-src by overexpression of a protein-tyrosine phosphatase. Nature 359, 336–339 [DOI] [PubMed] [Google Scholar]
- 38. den Hertog J., Pals C. E., Peppelenbosch M. P., Tertoolen L. G., de Laat S. W., Kruijer W. (1993) Receptor protein-tyrosine phosphatase α activates pp60c-src and is involved in neuronal differentiation. EMBO J. 12, 3789–3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zeng L., Si X., Yu W. P., Le H. T., Ng K. P., Teng R. M., Ryan K., Wang D. Z., Ponniah S., Pallen C. J. (2003) PTPα regulates integrin-stimulated FAK autophosphorylation and cytoskeletal rearrangement in cell spreading and migration. J. Cell Biol. 160, 137–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arold S. T. (2011) How focal adhesion kinase achieves regulation by linking ligand binding, localization and action. Curr. Opin. Struct. Biol. 21, 808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aplin A. E., Juliano R. L. (1999) Integrin and cytoskeletal regulation of growth factor signaling to the MAP kinase pathway. J. Cell Sci. 112, 695–706 [DOI] [PubMed] [Google Scholar]
- 42. Schaller M. D. (2010) Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J. Cell Sci. 123, 1007–1013 [DOI] [PubMed] [Google Scholar]
- 43. Cullen P. J. (2006) Decoding complex Ca2+ signals through the modulation of Ras signaling. Curr. Opin. Cell Biol. 18, 157–161 [DOI] [PubMed] [Google Scholar]
- 44. Herrera Abreu M. T., Wang Q., Vachon E., Suzuki T., Chow C. W., Wang Y., Hong O., Villar J., McCulloch C. A., Downey G. P. (2006) Tyrosine phosphatase SHP-2 regulates IL-1 signaling in fibroblasts through focal adhesions. J. Cell. Physiol. 207, 132–143 [DOI] [PubMed] [Google Scholar]
- 45. Arias-Salgado E. G., Lizano S., Sarkar S., Brugge J. S., Ginsberg M. H., Shattil S. J. (2003) Src kinase activation by direct interaction with the integrin β cytoplasmic domain. Proc. Natl. Acad. Sci. U.S.A. 100, 13298–13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schaller M. D., Hildebrand J. D., Parsons J. T. (1999) Complex formation with focal adhesion kinase: A mechanism to regulate activity and subcellular localization of Src kinases. Mol. Biol. Cell 10, 3489–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mitra S. K., Schlaepfer D. D. (2006) Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 18, 516–523 [DOI] [PubMed] [Google Scholar]
- 48. Tonks N. K. (2005) Redox redux: revisiting PTPs and the control of cell signaling. Cell 121, 667–670 [DOI] [PubMed] [Google Scholar]
- 49. Alonso A., Sasin J., Bottini N., Friedberg I., Friedberg I., Osterman A., Godzik A., Hunter T., Dixon J., Mustelin T. (2004) Protein-tyrosine phosphatases in the human genome. Cell 117, 699–711 [DOI] [PubMed] [Google Scholar]