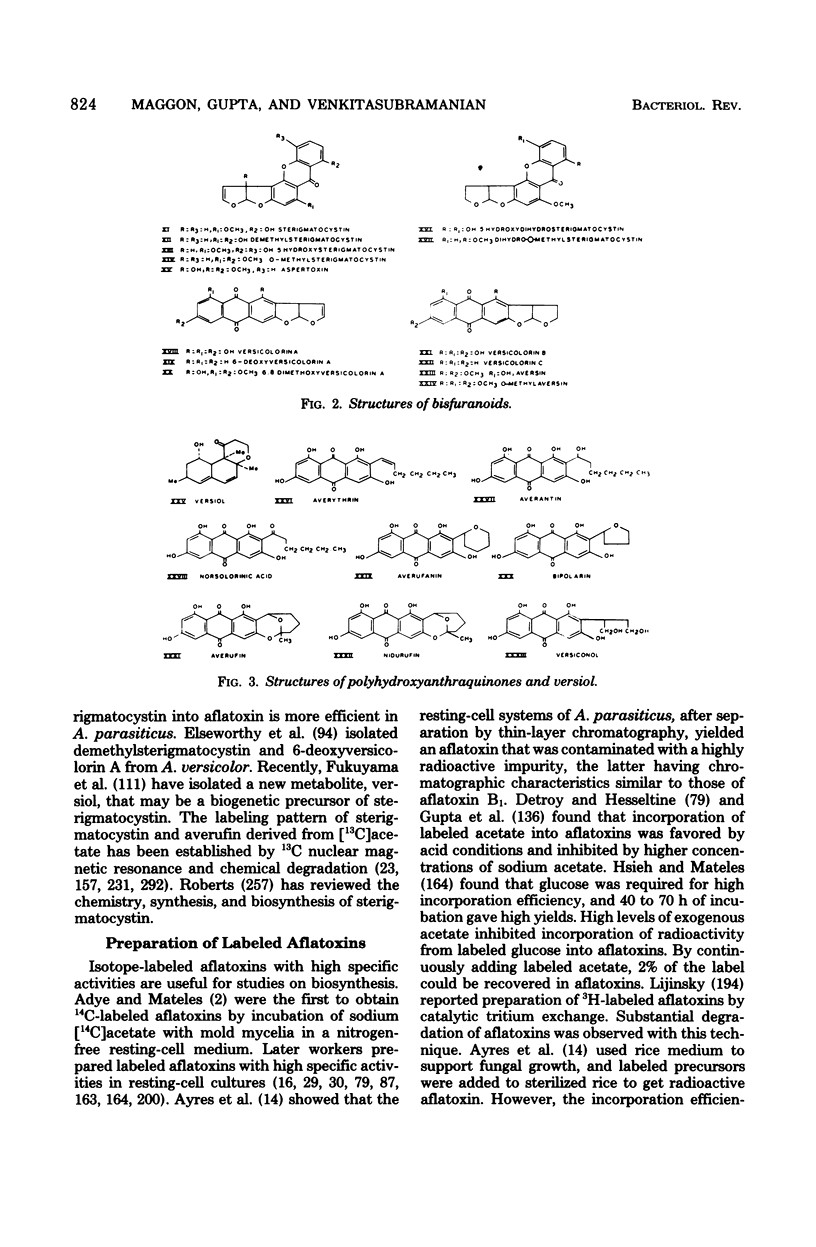
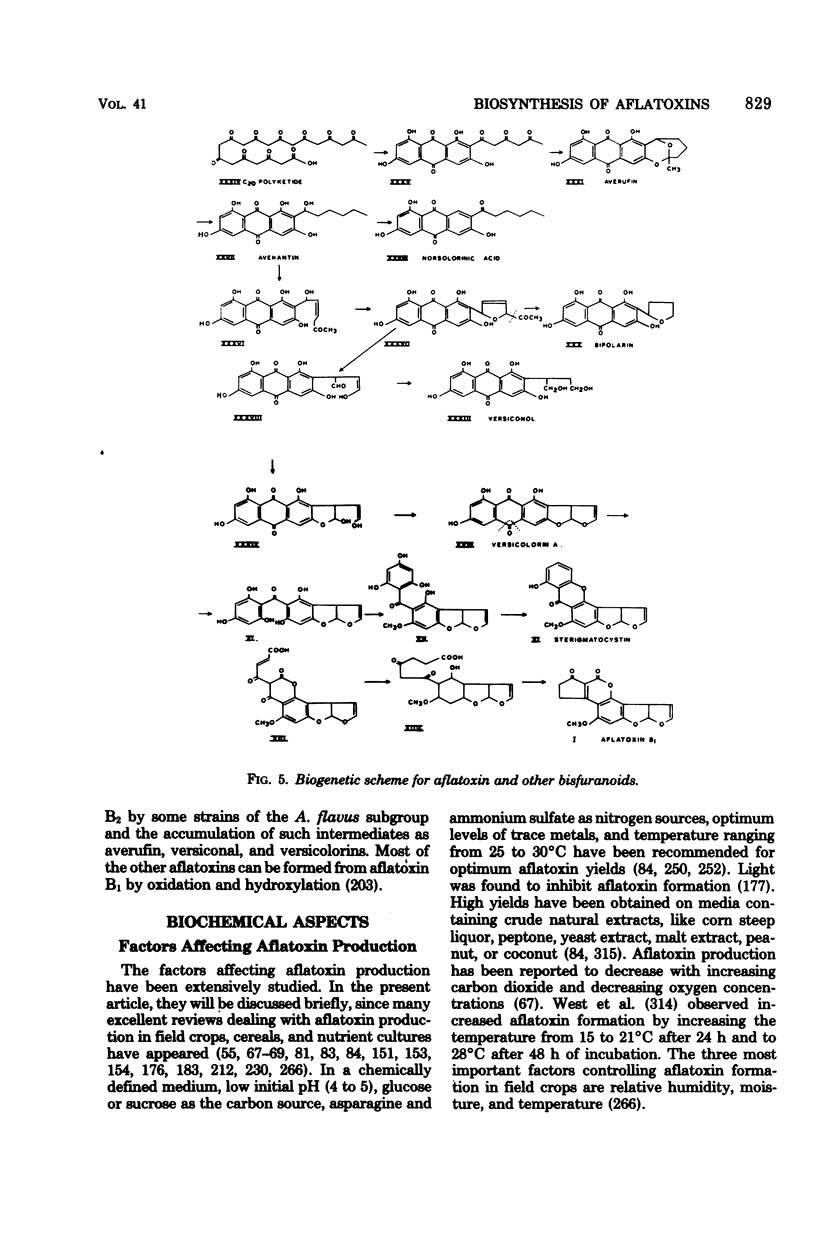
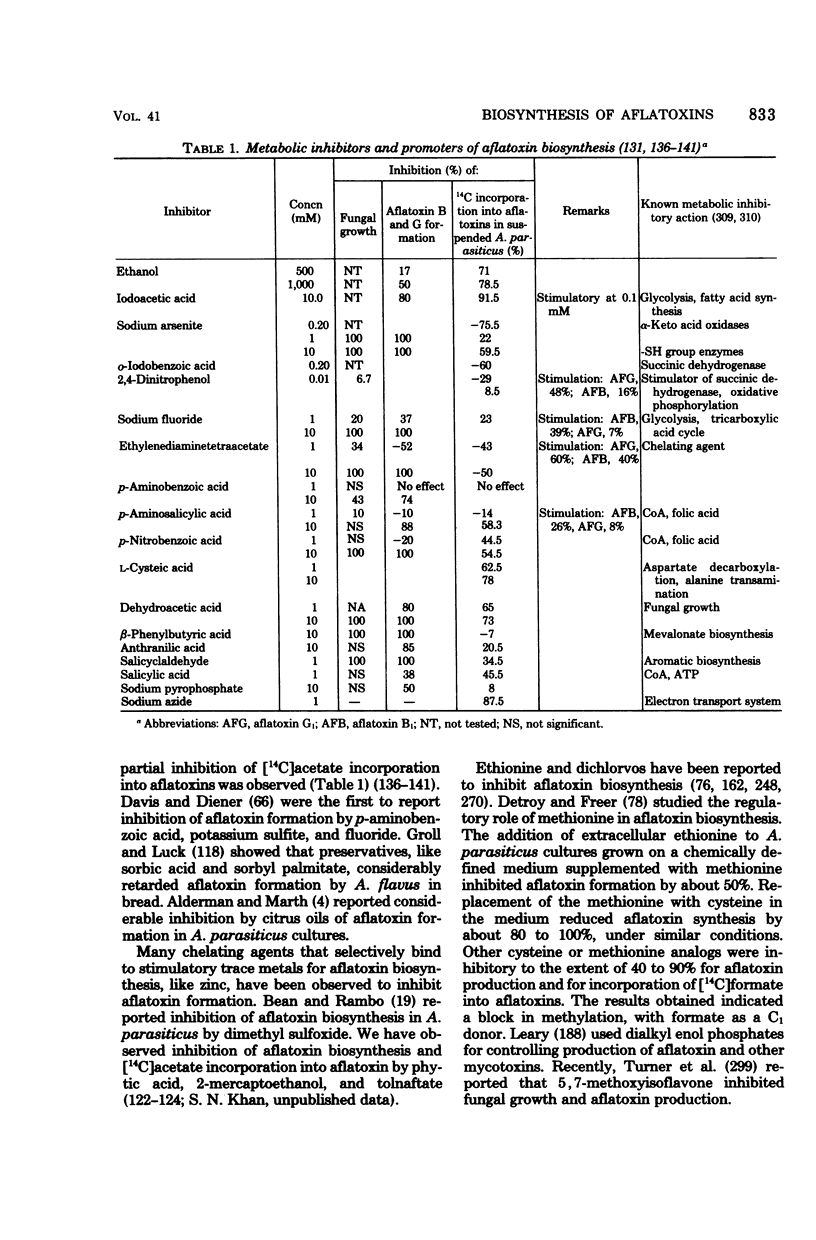
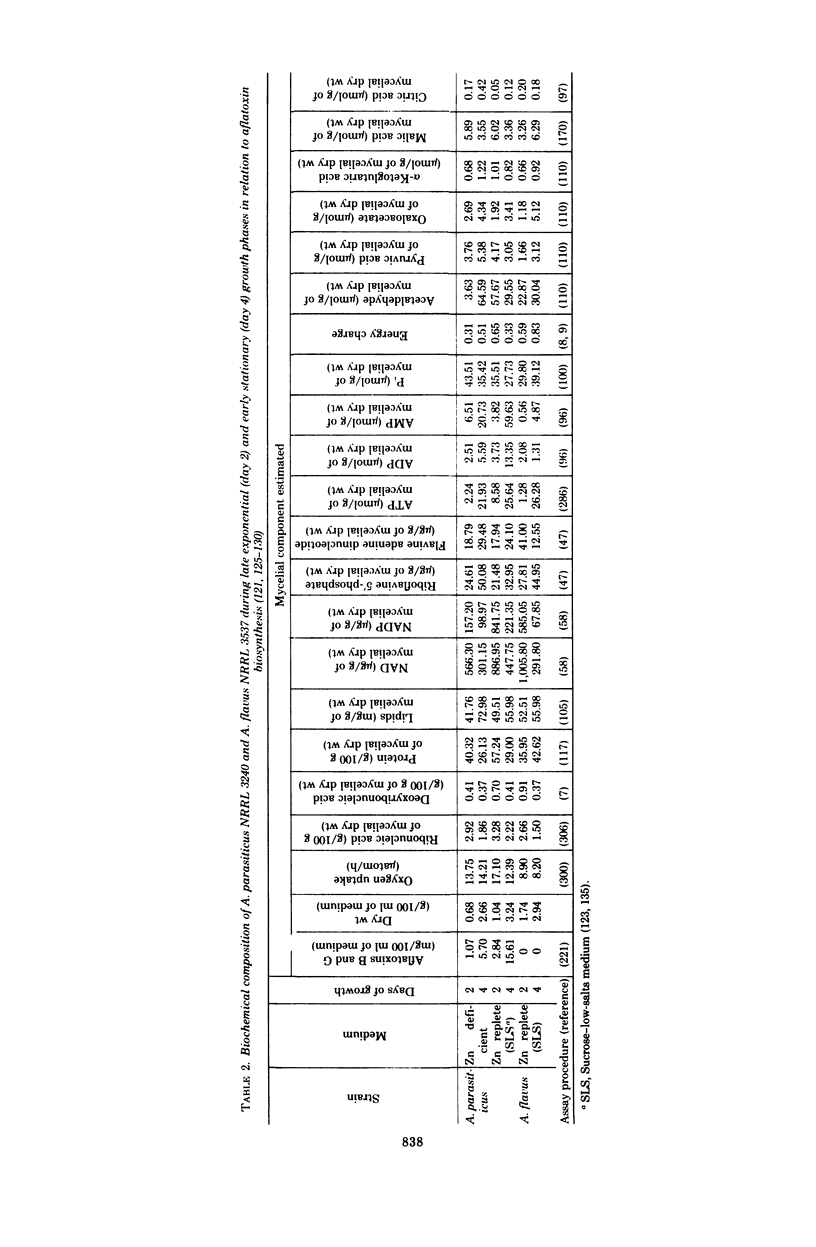
Full text
PDF




























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADYE J., MATELES R. I. INCORPORATION OF LABELLED COMPOUNDS INTO AFLATOXINS. Biochim Biophys Acta. 1964 May 11;86:418–420. doi: 10.1016/0304-4165(64)90077-7. [DOI] [PubMed] [Google Scholar]
- Abou-Sabé M., Burday M., Gentsch J. On the regulation of adenosine 3', 5'-monophosphate synthesis in bacteria. I. Effect of carbon source variation on cyclic AMP synthesis in Escherichia coli B/r. Biochim Biophys Acta. 1975 Apr 7;385(2):281–293. doi: 10.1016/0304-4165(75)90356-6. [DOI] [PubMed] [Google Scholar]
- Alderman G. G., Marth E. H. Inhibition of growth and aflatoxin production of Aspergillus parasiticus by citrus oils. Z Lebensm Unters Forsch. 1976 Apr 28;160(4):353–358. doi: 10.1007/BF01106324. [DOI] [PubMed] [Google Scholar]
- Applegate K. L., Chipley J. R. Daily variations in the production of aflatoxins by Aspergillus flavus NRRL-3145 following exposure to 60 Co irradiation. J Appl Bacteriol. 1974 Sep;37(3):359–372. doi: 10.1111/j.1365-2672.1974.tb00452.x. [DOI] [PubMed] [Google Scholar]
- Applegate K. L., Chipley J. R. Effects of 60Co gamma irradiation on aflatoxin B1 and B2 production by Aspergillus flavus. Mycologia. 1974 May-Jun;66(3):436–445. [PubMed] [Google Scholar]
- Auld D. S., Kawaguchi H., Livingston D. M., Vallee B. L. Reverse transcriptase from avian myeloblastosis virus: a zinc metalloenzyme. Biochem Biophys Res Commun. 1974 Apr 23;57(4):967–972. doi: 10.1016/0006-291x(74)90790-6. [DOI] [PubMed] [Google Scholar]
- Ayres J. L., Lee D. J., Sinnhuber R. O. Mycotoxins. Preparation of 14 C- and 3 H-labeled aflatoxins. J Assoc Off Anal Chem. 1971 Sep;54(5):1027–1031. [PubMed] [Google Scholar]
- BORROW A., BROWN S., JEFFERYS E. G., KESSELL R. H., LLOYD E. C., LLOYD P. B., ROTHWELL A., ROTHWELL B., SWAIT J. C. THE KINETICS OF METABOLISM OF GIBBERELLA FUJIKUROI IN STIRRED CULTURE. Can J Microbiol. 1964 Jun;10:407–444. doi: 10.1139/m64-054. [DOI] [PubMed] [Google Scholar]
- BRODIE J. D., WASSON G., PORTER J. W. ENZYME-BOUND INTERMEDIATES IN THE BIOSYNTHESIS OF MEVALONIC AND PALMITIC AICDS. J Biol Chem. 1964 May;239:1346–1356. [PubMed] [Google Scholar]
- Basappa S. C., Jayaraman A., Sreenivasamurthy V., Parpia H. A. Effect of B-group vitamins & ethyl alcohol on aflatoxin production by Aspergillus oryzae. Indian J Exp Biol. 1967 Oct;5(4):262–263. [PubMed] [Google Scholar]
- Bassir O., Adekunle A. A. Production of aflatoxin B 1 from defined natural cultures of Aspergillus flavus (Link). Mycopathol Mycol Appl. 1972 Mar 26;46(3):241–246. doi: 10.1007/BF02053412. [DOI] [PubMed] [Google Scholar]
- Bauer K. Zur Biosynthese der Penicilline: Bildung von 5-(2-Aminoadipyl)-cysteinyl-valin in Extration von Penicillium chryogenum. Z Naturforsch B. 1970 Oct;25(10):1125–1129. [PubMed] [Google Scholar]
- Bean G. A., Rambo G. W. Use of dimethyl sulfoxide to control aflatoxin production. Ann N Y Acad Sci. 1975 Jan 27;243:237–245. doi: 10.1111/j.1749-6632.1975.tb25362.x. [DOI] [PubMed] [Google Scholar]
- Bennett J. W., Lee L. S., Gaar G. G. Effect of acetone on production of aflatoxins and versicolorin pigments by resting cell cultures of Aspergillus parasiticus. Mycopathologia. 1976 Jun 4;58(1):9–12. doi: 10.1007/BF00493586. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Uptake and retention of metals by cell walls of Bacillus subtilis. J Bacteriol. 1976 Sep;127(3):1502–1518. doi: 10.1128/jb.127.3.1502-1518.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biollaz M., Büchi G., Milne G. The biogenesis of bisfuranoids in the genus Aspergillus. J Am Chem Soc. 1968 Aug 28;90(18):5019–5020. doi: 10.1021/ja01020a043. [DOI] [PubMed] [Google Scholar]
- Biollaz M., Büchi G., Milne G. The biosynthesis of the aflatoxins. J Am Chem Soc. 1970 Feb 25;92(4):1035–1043. doi: 10.1021/ja00707a050. [DOI] [PubMed] [Google Scholar]
- Borichewski R. M. Keto acids as growth-limiting factors in autotrophic growth of Thiobacillus thiooxidans. J Bacteriol. 1967 Feb;93(2):597–599. doi: 10.1128/jb.93.2.597-599.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost P. E., Demain A. L. Studies on the cell-free biosynthesis of beta-lactam antibiotics. Biochem J. 1977 Mar 15;162(3):681–687. doi: 10.1042/bj1620681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock D. J., Bloch K. Control of the synthesis of long-chain fatty acids and triacetic acid in E. coli. Biochem Biophys Res Commun. 1966 Jun 13;23(5):775–780. doi: 10.1016/0006-291x(66)90469-4. [DOI] [PubMed] [Google Scholar]
- Brunner R., Röhr M., Zinner M. Zur Biosynthese des Penicillins. Untersuchungen zur enzymatischen Aktivierung von Phenylessigsäure und Phenoxyessigsäure sowie zur Bildung von Penicillin aus 6-Amino-penicillansäure und aktivierter Seitenkettensäure durch Mycelhomogenate und zellfreie Extrakte von Penicillium chrysogenum. Hoppe Seylers Z Physiol Chem. 1968 Jan;349(1):95–103. doi: 10.1515/bchm2.1968.349.1.95. [DOI] [PubMed] [Google Scholar]
- Bryzgalova T. E., Orlova N. V. Obrazovanie organicheskikh kislot aktivnym shtammom Act. rimosus i neaktivnym mutantom v sviazi s biosintezom oksitetratsiklina. Antibiotiki. 1975 Jan;20(1):11–15. [PubMed] [Google Scholar]
- Campbell T. C., Hayes J. R. The role of aflatoxin metabolism in its toxic lesion. Toxicol Appl Pharmacol. 1976 Feb;35(2):199–222. doi: 10.1016/0041-008x(76)90282-9. [DOI] [PubMed] [Google Scholar]
- Campbell T. C., Stoloff L. Implication of mycotoxins for human health. J Agric Food Chem. 1974 Nov-Dec;22(6):1006–1015. doi: 10.1021/jf60196a016. [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvapil M. New aspects in the biological role of zinc: a stabilizer of macromolecules and biological membranes. Life Sci. 1973 Oct 16;13(8):1041–1049. doi: 10.1016/0024-3205(73)90372-x. [DOI] [PubMed] [Google Scholar]
- Ciegler A., Lillehoj E. B. Mycotoxins. Adv Appl Microbiol. 1968;10:155–219. doi: 10.1016/s0065-2164(08)70192-8. [DOI] [PubMed] [Google Scholar]
- Cocucci M. C., Rossi G. Biochemical and morphological aspects of zinc deficiency in Rhodotorula gracilis. Arch Mikrobiol. 1972;85(4):267–279. doi: 10.1007/BF00549265. [DOI] [PubMed] [Google Scholar]
- Coleman J. E. The role of Zn(II) in transcription by T7 RNA polymerase. Biochem Biophys Res Commun. 1974 Sep 23;60(2):641–648. doi: 10.1016/0006-291x(74)90289-7. [DOI] [PubMed] [Google Scholar]
- Curdová E., Kremen A., Vanek Z., Hostálek Z. Regulation and biosynthesis of secondary metabolites. XVIII. Adenylate level and chlorotetracycline production in Streptomyces aureofaciens. Folia Microbiol (Praha) 1976;21(6):481–487. doi: 10.1007/BF02876940. [DOI] [PubMed] [Google Scholar]
- Davis N. D., Diener U. L., Agnihotri V. P. Production of aflatoxins B1 and G1 in chemically defined medium. Mycopathol Mycol Appl. 1967 Apr 28;31(3):251–256. doi: 10.1007/BF02053422. [DOI] [PubMed] [Google Scholar]
- Davis N. D., Diener U. L., Eldridge D. W. Production of aflatoxins B1 and G1 by Aspergillus flavus in a semisynthetic medium. Appl Microbiol. 1966 May;14(3):378–380. doi: 10.1128/am.14.3.378-380.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. D., Diener U. L. Inhibition of Aflatoxin Synthesis by p-Aminobenzoic Acid, Potassium Sulfite, and Potassium Fluoride. Appl Microbiol. 1967 Nov;15(6):1517–1518. doi: 10.1128/am.15.6.1517-1518.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demain A. L. How do antibiotic-producing microorganisms avoid suicide? Ann N Y Acad Sci. 1974 May 10;235(0):601–612. doi: 10.1111/j.1749-6632.1974.tb43294.x. [DOI] [PubMed] [Google Scholar]
- Demain A. L. Mutation and the production of secondary metabolites. Adv Appl Microbiol. 1973;16:177–202. doi: 10.1016/s0065-2164(08)70027-3. [DOI] [PubMed] [Google Scholar]
- Demain A. L. Riboflavin oversynthesis. Annu Rev Microbiol. 1972;26:369–388. doi: 10.1146/annurev.mi.26.100172.002101. [DOI] [PubMed] [Google Scholar]
- Detroy R. W., Ciegler A. Aflatoxin biosynthesis in Aspergillus parasiticus: effect of methionine analogs. Can J Microbiol. 1971 May;17(5):569–574. doi: 10.1139/m71-094. [DOI] [PubMed] [Google Scholar]
- Detroy R. W., Ciegler A. Induction of yeastlike development in Aspergillus parasiticus. J Gen Microbiol. 1971 Mar;65(3):259–264. doi: 10.1099/00221287-65-3-259. [DOI] [PubMed] [Google Scholar]
- Detroy R. W., Freer S., Ciegler A. Aflatoxin and anthraquinone biosynthesis by nitrosoguanidine-derived mutants of Aspergillus parasiticus. Can J Microbiol. 1973 Nov;19(11):1373–1378. doi: 10.1139/m73-221. [DOI] [PubMed] [Google Scholar]
- Detroy R. W., Hesseltine C. W. Secondary biosynthesis of aflatoxin B in Aspergillus parasiticus. Can J Microbiol. 1970 Oct;16(10):959–963. doi: 10.1139/m70-164. [DOI] [PubMed] [Google Scholar]
- Diener U. L., Davis N. D. Aflatoxin production by isolates of Aspergillus flavus. Phytopathology. 1966 Dec;56(12):1390–1393. [PubMed] [Google Scholar]
- Dimroth P., Greull G., Seyffert R., Lynen F. 6-Methylsalicylic acid synthetase. Hoppe Seylers Z Physiol Chem. 1972 Feb;353(2):126–126. [PubMed] [Google Scholar]
- Dimroth P., Walter H., Lynen F. Biosynthese von 6-Methylsalicylsäure. Eur J Biochem. 1970 Mar 1;13(1):98–110. doi: 10.1111/j.1432-1033.1970.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Donkersloot J. A., Hsieh D. P., Mateles R. I. Incorporation of precursors into aflatoxin-B1. J Am Chem Soc. 1968 Aug 28;90(18):5020–5021. doi: 10.1021/ja01020a044. [DOI] [PubMed] [Google Scholar]
- Donkersloot J. A., Mateles R. I., Yang S. S. Isolation of averufin from a mutant of Aspergillus parasiticus impaired in aflatoxin biosynthesis. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1051–1055. doi: 10.1016/0006-291x(72)90939-4. [DOI] [PubMed] [Google Scholar]
- Drew S. W., Demain A. L. Methionine control of cephalosporin C formation. Biotechnol Bioeng. 1973 Jul;15(4):743–754. doi: 10.1002/bit.260150408. [DOI] [PubMed] [Google Scholar]
- ETTINGER R. H., GOLDBAUM L. R., SMITH L. H., Jr A simplified photometric method for the determination of citric acid in biological fluids. J Biol Chem. 1952 Dec;199(2):531–536. [PubMed] [Google Scholar]
- Eigener U. Adenine nucleotide pool variations in intact Nitrobacter winogradskyi cells. Arch Microbiol. 1975 Mar 10;102(3):233–240. doi: 10.1007/BF00428373. [DOI] [PubMed] [Google Scholar]
- Eigener U., Bock E. Study of the regulation of oxidation and CO2 assimilation in intact Nitrobacter winogradskyi cells. Arch Microbiol. 1975 Mar 10;102(3):241–246. doi: 10.1007/BF00428374. [DOI] [PubMed] [Google Scholar]
- Ellis J. J. An orange-yellow mutant of Aspergillus parasiticus produces aflatoxin. Mycologia. 1969 May-Jun;61(3):651–653. [PubMed] [Google Scholar]
- Enomoto M., Saito M. Carcinogens produced by fungi. Annu Rev Microbiol. 1972;26:279–312. doi: 10.1146/annurev.mi.26.100172.001431. [DOI] [PubMed] [Google Scholar]
- FITCH W. M., CHAIKOFF I. L. Extent and patterns of adaptation of enzyme activities in livers of normal rats fed diets high in glucose and fructose. J Biol Chem. 1960 Mar;235:554–557. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Failla M. L., Benedict C. D., Weinberg E. D. Accumulation and storage of Zn2+ by Candida utilis. J Gen Microbiol. 1976 May;94(1):23–36. doi: 10.1099/00221287-94-1-23. [DOI] [PubMed] [Google Scholar]
- Fishbein L., Falk H. L. Chromatography of mold metabolites. I. Aflatoxins, ochratoxins and related compounds. Chromatogr Rev. 1970 Jan;12(1):42–87. doi: 10.1016/0009-5907(70)80013-8. [DOI] [PubMed] [Google Scholar]
- Fitzell D. L., Hsieh D. P., Yao R. C., La Mar G. N. Biosynthesis of averufin from acetate by Aspergillus parasiticus. J Agric Food Chem. 1975 May-Jun;23(3):442–444. doi: 10.1021/jf60199a039. [DOI] [PubMed] [Google Scholar]
- Forrest W. W. Adenosine triphosphate pool during the growth cycle in Streptococcus faecalis. J Bacteriol. 1965 Oct;90(4):1013–1018. doi: 10.1128/jb.90.4.1013-1018.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest W. W., Walker D. J. The generation and utilization of energy during growth. Adv Microb Physiol. 1971;5:213–274. doi: 10.1016/s0065-2911(08)60408-7. [DOI] [PubMed] [Google Scholar]
- GESER G. [Studies on Aspergillus niger van Tiegh, on zinc uptake and activity changes of some enzymes in zinc deficiency]. Arch Mikrobiol. 1962;41:408–440. [PubMed] [Google Scholar]
- Gentry M. J., Smith D. K., Schnute S. F., Werber S. L., Weinberg E. D. Pseudomonas culture longevity: control by phosphate. Microbios. 1971 Dec;4(15):205–215. [PubMed] [Google Scholar]
- Goldblatt L. A. Chemistry and control of aflatoxin. Pure Appl Chem. 1970;21(3):331–353. doi: 10.1351/pac197021030331. [DOI] [PubMed] [Google Scholar]
- Goodwin B. C. Synchronization of Escherichia coli in a chemostat by periodic phosphate feeding. Eur J Biochem. 1969 Oct;10(3):511–514. doi: 10.1111/j.1432-1033.1969.tb00718.x. [DOI] [PubMed] [Google Scholar]
- Grove J. F. New metabolic products of Aspergillus flavus. I. Asperentin, its methyl ethers, and 5'-hydroxyasperentin. J Chem Soc Perkin 1. 1972;19:2400–2406. doi: 10.1039/p19720002400. [DOI] [PubMed] [Google Scholar]
- Grove J. F. New metabolic products of Aspergillus flavus. II. Asperflavin, anhydroasperflavin, and 5,7-dihydroxy-4-methylphthalide. J Chem Soc Perkin 1. 1972;19:2406–2411. doi: 10.1039/p19720002406. [DOI] [PubMed] [Google Scholar]
- Grove J. F. New metabolic products of Aspergillus flavus. IV. 4'-Hydroxyasperentin and 5'-hydroxyasperentin 8-methyl ether. J Chem Soc Perkin 1. 1973;22:2704–2706. doi: 10.1039/p19730002704. [DOI] [PubMed] [Google Scholar]
- Gupta S. K., Maggon K. K., Venkitasubramanian T. A. Effect of Zinc on tricarboxylic acid cycle intermediates and enzymes in relation to aflatoxin biosynthesis. J Gen Microbiol. 1977 Mar;99(1):43–48. doi: 10.1099/00221287-99-1-43. [DOI] [PubMed] [Google Scholar]
- Gupta S. K., Maggon K. K., Venkitasubramanian T. A. Effect of zinc on adenine nucleotide pools in relation to aflatoxin biosynthesis in Aspergillus parasiticus. Appl Environ Microbiol. 1976 Dec;32(6):753–756. doi: 10.1128/aem.32.6.753-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. K., Maggon K. K., Venkitasubramanian T. A. Inhibition of aflatoxin formation by 2-mercaptoethanol. Appl Environ Microbiol. 1976 Sep;32(3):324–326. doi: 10.1128/aem.32.3.324-326.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. K., Venkitasubramanian T. A. Production of aflatoxin on soybeans. Appl Microbiol. 1975 Jun;29(6):834–836. doi: 10.1128/am.29.6.834-836.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. K., Venkitasubramanian T. A. The effect of zinc and phytic acid on the incorporation of 1-14C-acetate into aflatoxin by resting mycelia of Aspergillus parasiticus. Z Lebensm Unters Forsch. 1975 Oct 31;159(2):107–111. doi: 10.1007/BF01135786. [DOI] [PubMed] [Google Scholar]
- Gupta S. R., Prasanna H. R., Viswanathan L., Venkitasubramanian T. A. Carboxylic acids as carbon sources for aflatoxin production. Experientia. 1974 Nov 15;30(11):1244–1246. doi: 10.1007/BF01945162. [DOI] [PubMed] [Google Scholar]
- Gupta S. R., Prasanna H. R., Viswanathan L., Venkitasubramanian T. A. Effect of some inhibitors on aflatoxin-production in a synthetic medium and on the incorporation of acetate-1- 14C into aflatoxins by resting mycelia of Aspergillus parasiticus. Bull Environ Contam Toxicol. 1976 Apr;15(4):447–453. doi: 10.1007/BF01685070. [DOI] [PubMed] [Google Scholar]
- Gupta S. R., Prasanna H. R., Viswanathan L., Venkitasubramanian T. A. Factors influencing the biosynthesis of aflatoxins. Indian J Biochem Biophys. 1975 Jun;12(2):179–181. [PubMed] [Google Scholar]
- Gupta S. R., Prasanna H. R., Viswanathan L., Venkitasubramanian T. A. Production of aflatoxins, and acetate(1-14C) incorporation, by Aspergillus parasiticus. J Gen Microbiol. 1974 Jan;80(1):31–36. doi: 10.1099/00221287-80-1-31. [DOI] [PubMed] [Google Scholar]
- Gupta S. R., Prasanna H. R., Viswanathan L., Venkitasubramanian T. A. Synthesis of aflatoxins by the non-growing mycelia of Aspergillus parasiticus and the effect of inhibitors. J Gen Microbiol. 1975 Dec;91(2):417–420. doi: 10.1099/00221287-91-2-417. [DOI] [PubMed] [Google Scholar]
- Gupta S. R., Prasanna H. R., Viswanathan L., Venkitasubramanian T. A. The effect of inorganic salts and some biologically important compounds on the incorporation of 1-14C acetate into aflatoxins by resting mycelia of Aspergillus parasiticus. Z Lebensm Unters Forsch. 1975 Feb 7;157(1):19–22. doi: 10.1007/BF01785723. [DOI] [PubMed] [Google Scholar]
- Gupta S. R., Prasanna H. R., Viswanathan L., Venkitasurbramanian T. A. Malonate as a precursor in the biosynthesis of aflatoxins. J Gen Microbiol. 1975 Jun;88(2):317–320. doi: 10.1099/00221287-88-2-317. [DOI] [PubMed] [Google Scholar]
- Gupta S. R., Viswanathan L., Venkitasubramanian T. A. A comparative study of the lipids of a toxigenic & a non-toxigenic strain of Aspergillus flavus. Indian J Biochem. 1970 Jun;7(2):108–111. [PubMed] [Google Scholar]
- Gupta S. R., Viswanathan L., Venkitasubramanian T. A. A comparative study of toxigenic and non-toxigenic strains of Aspergillus flavus. J Gen Microbiol. 1971 Feb;65(2):243–247. doi: 10.1099/00221287-65-2-243. [DOI] [PubMed] [Google Scholar]
- Gupta S. R., Viswanathan L., Venkitasubramanian T. A. Incorporation of 32P-orthophosphate into phospholipids by a toxigenic and a nontoxigenic strain of Aspergillus flavus. Mycopathol Mycol Appl. 1970 Dec 28;42(1):137–144. doi: 10.1007/BF02051834. [DOI] [PubMed] [Google Scholar]
- HOSTALEK Z. RELATIONSHIP BETWEEN THE CARBOHYDRATE METABOLISM OF STREPTOMYCES AUREOFACIENS AND THE BIOSYNTHESIS OF CHLORTETRACYCLINE. I. THE EFFECT OF INTERRUPTED AERATION, INORGANIC PHOSPHATE AND BENZYL THIOCYANATE ON CHLORTETRACYCLINE BIOSYNTHESIS. Folia Microbiol (Praha) 1964 Mar;18:78–88. doi: 10.1007/BF02868788. [DOI] [PubMed] [Google Scholar]
- Hacking A., Harrison J. Mycotoxins in animal feeds. Soc Appl Bacteriol Symp Ser. 1976;4:243–250. [PubMed] [Google Scholar]
- Harris C. M., Roberson J. S., Harris T. M. Biosynthesis of griseofulvin. J Am Chem Soc. 1976 Aug 18;98(17):5380–5386. doi: 10.1021/ja00433a053. [DOI] [PubMed] [Google Scholar]
- Hayes A. W., Davis N. D., Diener U. L. Effect of Aeration on Growth and Aflatoxin Production by Aspergillus flavus in Submerged Culture. Appl Microbiol. 1966 Nov;14(6):1019–1021. doi: 10.1128/am.14.6.1019-1021.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesseltine C. W. Mycotoxin research in India. Mycopathologia. 1976 Jul 16;58(3):157–163. doi: 10.1007/BF00496024. [DOI] [PubMed] [Google Scholar]
- Hesseltine C. W. Mycotoxins. Mycopathol Mycol Appl. 1969 Dec 29;39(3):371–383. doi: 10.1007/BF02052805. [DOI] [PubMed] [Google Scholar]
- Hesseltine C. W. Natural occurrence of mycotoxins in cereals. Mycopathol Mycol Appl. 1974 Aug 30;53(1):141–153. doi: 10.1007/BF02127204. [DOI] [PubMed] [Google Scholar]
- Hesseltine C. W., Shotwell O. L., Ellis J. J., Stubblefield R. D. Aflatoxin formation by Aspergillus flavus. Bacteriol Rev. 1966 Dec;30(4):795–805. doi: 10.1128/br.30.4.795-805.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesseltine C. W., Sorenson W. G., Smith M. Taxonomic studies of the aflatoxin-producing strains in the aspergillus flavus group. Mycologia. 1970 Jan-Feb;62(1):123–132. [PubMed] [Google Scholar]
- Holzer H. Some aspects of regulation of metabolism by ATP. Adv Enzyme Regul. 1970;8:85–97. doi: 10.1016/0065-2571(70)90010-5. [DOI] [PubMed] [Google Scholar]
- Hostálek Z., Tobek I., Bobyk M. A., Kulayev I. S. Role of ATP-glucokinase and polyphosphate glucokinase in Streptomyces aureofaciens. Folia Microbiol (Praha) 1976;21(2):131–138. doi: 10.1007/BF02876980. [DOI] [PubMed] [Google Scholar]
- Hsieh D. P. Inhibition of aflatoxin biosynthesis of dichlorvos. J Agric Food Chem. 1973 May-Jun;21(3):468–470. doi: 10.1021/jf60187a035. [DOI] [PubMed] [Google Scholar]
- Hsieh D. P., Lin M. T., Yao R. C. Conversion of sterigmatocystin to aflatoxin B 1 by Aspergillus parasiticus. Biochem Biophys Res Commun. 1973 Jun 8;52(3):992–997. doi: 10.1016/0006-291x(73)91035-8. [DOI] [PubMed] [Google Scholar]
- Hsieh D. P., Lin M. T., Yao R. C., Singh R. Biosynthesis of aflatoxin. Conversion of norsolorinic acid and other hypothetical intermediates into aflatoxin B1. J Agric Food Chem. 1976 Nov-Dec;24(6):1170–1174. doi: 10.1021/jf60208a018. [DOI] [PubMed] [Google Scholar]
- Hsieh D. P., Mateles R. I. Preparation of labeled aflatoxins with high specific activities. Appl Microbiol. 1971 Jul;22(1):79–83. doi: 10.1128/am.22.1.79-83.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh D. P., Mateles R. I. The relative contribution of acetate and glucose to aflatoxin biosynthesis. Biochim Biophys Acta. 1970 Jun;208(3):482–486. doi: 10.1016/0304-4165(70)90222-9. [DOI] [PubMed] [Google Scholar]
- Hsieh D. P., Yang S. L. Preparation of 14C-labeled sterigmatocystin in liquid media. Appl Microbiol. 1975 Jan;29(1):17–20. doi: 10.1128/am.29.1.17-20.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh D. P., Yao R. C., Fitzell D. L., Reece C. A. Letter: Origin of the bisfuran ring structure in aflatoxin biosynthesis. J Am Chem Soc. 1976 Feb 18;98(4):1020–1021. doi: 10.1021/ja00420a029. [DOI] [PubMed] [Google Scholar]
- Hutner S. H. Inorganic nutrition. Annu Rev Microbiol. 1972;26:313–346. doi: 10.1146/annurev.mi.26.100172.001525. [DOI] [PubMed] [Google Scholar]
- Jackson J. F., Atkinson M. R. The requirement for bivalent cations in formation of nicotinamide-adenine dinucleotide by nicotinamide mononucleotide adenylyltransferase of pig-liver nuclei. Biochem J. 1966 Oct;101(1):208–213. doi: 10.1042/bj1010208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janglová Z., Suchý J., Vanek Z. Regulation of biosynthesis of secondary metabolites. VII. Intracellular adenosine-5'-triphosphate concentration in Streptomyces aureofaciens. Folia Microbiol (Praha) 1969;14(3):208–210. doi: 10.1007/BF02872780. [DOI] [PubMed] [Google Scholar]
- Jarvis B. Mycotoxins in food. Soc Appl Bacteriol Symp Ser. 1976;4:251–267. [PubMed] [Google Scholar]
- Jechová V., Hostálek Z., Vanek Z. Regulation of biosynthesis of secondary metabolites. V. Decarboxylating malate dehydrogenase in Streptomyces aureofaciens. Folia Microbiol (Praha) 1969;14(2):128–134. doi: 10.1007/BF02892881. [DOI] [PubMed] [Google Scholar]
- Jemmali M., Poisson J., Guilbot A. Production d'aflatoxines dans les produits céréaliers. Influence de difféfenties conditions. Ann Nutr Aliment. 1969;23(2):151–166. [PubMed] [Google Scholar]
- Joffe A. Z., Lisker N. Effects of light, temperature, and pH value on aflatoxin production in vitro. Appl Microbiol. 1969 Sep;18(3):517–518. doi: 10.1128/am.18.3.517-518.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPRALEK F. The physiology of riboflavin production by Eremothecium ashbyi. J Gen Microbiol. 1962 Nov;29:403–419. doi: 10.1099/00221287-29-3-403. [DOI] [PubMed] [Google Scholar]
- Kalra V. K., Murti C. R., Brodie A. F. Resolution and reconstitution of the succinoxidase pathway of Mycobacterium phlei. Arch Biochem Biophys. 1971 Dec;147(2):734–743. doi: 10.1016/0003-9861(71)90433-4. [DOI] [PubMed] [Google Scholar]
- Krupinski V. M., Robbers J. E., Floss H. G. Physiological study of ergot: induction of alkaloid synthesis by tryptophan at the enzymatic level. J Bacteriol. 1976 Jan;125(1):158–165. doi: 10.1128/jb.125.1.158-165.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers K. E., Davis N. D., Diener U. L. Influence of atmospheric gases on aflatoxin production by Aspergillus flavus in peanuts. Phytopathology. 1967 Oct;57(10):1086–1090. [PubMed] [Google Scholar]
- Law A., Threlfall D. R., Whistance G. R. Isoprenoid quinone precursors of ubiquinone-10(X-H2) in Aspergillus flavus. Biochem J. 1970 May;117(4):799–800. doi: 10.1042/bj1170799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. S., Bennett J. W., Cucullu A. F., Ory R. L. Biosynthesis of aflatoxin B1. Conversion of versicolorin A to aflatoxin B1 by Aspergillus parasiticus. J Agric Food Chem. 1976 Nov-Dec;24(6):1167–1170. doi: 10.1021/jf60208a017. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Bennett J. W., Cucullu A. F., Stanley J. B. Synthesis of versicolorin A by a mutant strain of Aspergillus parasiticus deficient in aflatoxin production. J Agric Food Chem. 1975 Nov-Dec;23(6):1132–1134. doi: 10.1021/jf60202a011. [DOI] [PubMed] [Google Scholar]
- Lee L., Bennett J. W., Goldblatt L. A., Lundin R. E. Norsolorinic acid from a mutant strain of Aspergillus parasiticus. J Am Oil Chem Soc. 1971 Feb;48(2):93–94. doi: 10.1007/BF02635696. [DOI] [PubMed] [Google Scholar]
- Lillehoj E. B., Garcia W. J., Lambrow M. Aspergillus flavus infection and aflatoxin production in corn: influence of trace elements. Appl Microbiol. 1974 Nov;28(5):763–767. doi: 10.1128/am.28.5.763-767.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M. T., Hsieh D. P. Averufin in the biosynthesis of aflatoxin B. J Am Chem Soc. 1973 Mar 7;95(5):1668–1669. doi: 10.1021/ja00786a056. [DOI] [PubMed] [Google Scholar]
- Lin M. T., Hsieh D. P., Yao R. C., Donkersloot J. A. Conversion of averufin into aflatoxins by Aspergillus parasiticus. Biochemistry. 1973 Dec 4;12(25):5167–5171. doi: 10.1021/bi00749a023. [DOI] [PubMed] [Google Scholar]
- Lopes-Cardozo M., Vaartjes W. J., Van Den Bergh S. G. Regulation of pyruvate metabolism by the mitochondrial energy state: The effect of palmityl-coenzyme A. FEBS Lett. 1972 Dec 15;28(3):265–270. doi: 10.1016/0014-5793(72)80727-0. [DOI] [PubMed] [Google Scholar]
- MATELES R. I., ADYE J. C. PRODUCTION OF AFLATOXINS IN SUBMERGED CULTURE. Appl Microbiol. 1965 Mar;13:208–211. doi: 10.1128/am.13.2.208-211.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER P. A., HASH J. H., LINCKS M., BOHONOS N. BIOSYNTHESIS OF 5-HYDROXYTETRACYCLINE. Biochem Biophys Res Commun. 1965 Feb 3;18:325–331. doi: 10.1016/0006-291x(65)90707-2. [DOI] [PubMed] [Google Scholar]
- Maggon K. K., Venkitasubramanian T. A. Effect of groundnut on aflatoxin production. Indian J Exp Biol. 1973 Jul;11(4):356–357. [PubMed] [Google Scholar]
- Maggon K. K., Venkitasubramanian T. A. Metabolism of aflatoxins B1 and G1 by Aspergillus parasiticus. Experientia. 1973 Oct 15;29(10):1210–1211. doi: 10.1007/BF01935075. [DOI] [PubMed] [Google Scholar]
- Maggon K. K., Viswanathan L., Venkitasubramanian T. A., Mukerji K. G. Aflatoxin production by some Indian strains of Aspergillus flavus Link ex Fries. J Gen Microbiol. 1969 Nov;59(1):119–124. doi: 10.1099/00221287-59-1-119. [DOI] [PubMed] [Google Scholar]
- Marsh P. B., Simpson M. E., Trucksess M. W. Effects of trace metals on the production of aflatoxins by Aspergillus parasiticus. Appl Microbiol. 1975 Jul;30(1):52–57. doi: 10.1128/am.30.1.52-57.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehan V. K., Chohan J. S. Aflatoxin B 1 producing potential of isolates of Aspergillus flavus Link ex Fries from cotton, maize and wheat. Mycopathol Mycol Appl. 1973 Apr 30;49(4):263–274. doi: 10.1007/BF02050720. [DOI] [PubMed] [Google Scholar]
- Mehan V. K., Chohan J. S. Relative performance of selected toxigenic and non-toxigenic isolates of Aspergillus flavus Link ex Fries on different culture media. Indian J Exp Biol. 1973 May;11(3):191–193. [PubMed] [Google Scholar]
- Miović M. L., Gibson J. Nucleotide pools and adenylate energy charge in balanced and unbalanced growth of Chromatium. J Bacteriol. 1973 Apr;114(1):86–95. doi: 10.1128/jb.114.1.86-95.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Vogel G., Krippahl G., Lynen F. Patulin biosynthesis: the role of mixed-function oxidases in the hydroxylation of m-cresol. Eur J Biochem. 1974 Nov 15;49(2):443–455. doi: 10.1111/j.1432-1033.1974.tb03849.x. [DOI] [PubMed] [Google Scholar]
- Naik M., Modi V. V., Patel N. C. Studies on aflatoxin synthesis in Aspergillus flavus. Indian J Exp Biol. 1970 Oct;8(4):345–346. [PubMed] [Google Scholar]
- Newberne P. M. Mycotoxins: toxicity, carcinogenicity, and the influence of various nutritional conditions. Environ Health Perspect. 1974 Dec;9:1–32. doi: 10.1289/ehp.9-1475399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell B. L., Burpo C. E., Savage J. E. Evaluation of zinc availability in foodstuffs of plant and animal origin. J Nutr. 1972 May;102(5):653–660. doi: 10.1093/jn/102.5.653. [DOI] [PubMed] [Google Scholar]
- Pachler K. G., Steyn P. S., Vleggaar R., Wessels P. L. Carbon-13 nuclear magnetic resonance assignments and biosynthesis of aflatoxin B1 and sterigmatocystin. J Chem Soc Perkin 1. 1976;(11):1182–1189. doi: 10.1039/p19760001182. [DOI] [PubMed] [Google Scholar]
- Pai M. R., Bai N. J., Venkitasubramanian T. A. Production of aflatoxin m in a liquid medium. Appl Microbiol. 1975 Jun;29(6):850–851. doi: 10.1128/am.29.6.850-851.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa K. E. Mutant of Aspergillus flavus producing more aflatoxin B2 than B1. Appl Environ Microbiol. 1977 Jan;33(1):206–206. doi: 10.1128/aem.33.1.206-.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi A. F., Vallee B. L. Zinc metalloenzymes: characteristics and significance in biology and medicine. Am J Clin Nutr. 1969 Sep;22(9):1222–1239. doi: 10.1093/ajcn/22.9.1222. [DOI] [PubMed] [Google Scholar]
- Passeron S., Jiménez de Asua L., Carminatti H. Fructose 1,6-diphosphate, a reactivator of Cu++-inhibited pyruvate kinase from liver. Biochem Biophys Res Commun. 1967 Apr 7;27(1):33–38. doi: 10.1016/s0006-291x(67)80035-4. [DOI] [PubMed] [Google Scholar]
- Patterson D. S. Metabolism as a factor in determining the toxic action of the aflatoxins in different animal species. Food Cosmet Toxicol. 1973 Apr;11(2):287–294. doi: 10.1016/s0015-6264(73)80496-1. [DOI] [PubMed] [Google Scholar]
- Paulus H. Polymyxin synthetase:L-2,4-diaminobutyrate activating enzyme,. Methods Enzymol. 1975;43:579–584. doi: 10.1016/0076-6879(75)43120-2. [DOI] [PubMed] [Google Scholar]
- Pirt S. J., Righelato R. C. Effect of Growth Rate on the Synthesis of Penicillin by Penicillium chrysogenum in Batch and Chemostat Cultures. Appl Microbiol. 1967 Nov;15(6):1284–1290. doi: 10.1128/am.15.6.1284-1290.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj H. G., Shankaran R., Viswanathan L., Venkitasubramanian T. A. A comparative study of the incorporation of [1-14C] acetate into phospholipids by a toxigenic and a non-toxigenic strain of Aspergillus flavus. J Gen Microbiol. 1970 Jul;62(1):89–94. doi: 10.1099/00221287-62-1-89. [DOI] [PubMed] [Google Scholar]
- Raj H. G., Viswanathan L., Murthy H. S., Venkitasubramanian T. A. Biosynthesis of aflatoxins by cell-free preparations from Aspergillus flavus. Experientia. 1969 Nov 15;25(11):1141–1142. doi: 10.1007/BF01900235. [DOI] [PubMed] [Google Scholar]
- Rasmussen R. K., Klein H. P. Activation of fatty acid synthesis in cell-free extracts of Saccharomyces cerevisiae. J Bacteriol. 1968 Jan;95(1):157–161. doi: 10.1128/jb.95.1.157-161.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy T. V., Viswanathan L., Venkitasubramanian T. A. High aflatoxin production on a chemically defined medium. Appl Microbiol. 1971 Sep;22(3):393–396. doi: 10.1128/am.22.3.393-396.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehácek Z., Kozová J. Production of alkaloids and differentiation in a submerged culture of Claviceps purpurea (Fr.) Tul. Folia Microbiol (Praha) 1975;20(2):112–117. doi: 10.1007/BF02876766. [DOI] [PubMed] [Google Scholar]
- Rehácek Z., Sajdl P., Kozová J., Malik K. A., Ricicová A. Correlation of certain alterations in metabolic activity with alkaloid production by submerged Claviceps. Appl Microbiol. 1971 Dec;22(6):949–956. doi: 10.1128/am.22.6.949-956.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. C. Aflatoxins and sterigmatocystins. Fortschr Chem Org Naturst. 1974;31(0):119–151. doi: 10.1007/978-3-7091-7094-6_3. [DOI] [PubMed] [Google Scholar]
- Rodricks J. V., Lustig E., Campbell A. D., Stoloff L. Aspertoxin, a hydroxy derivative of O-methylsterigmatocystin from aflatoxin-producing cultures of Aspergillus flavus. Tetrahedron Lett. 1968 May;(25):2975–2978. doi: 10.1016/s0040-4039(00)89626-4. [DOI] [PubMed] [Google Scholar]
- Romano A. H., Kornberg H. L. Regulation of sugar utilization by Aspergillus nidulans. Biochim Biophys Acta. 1968 Jun 24;158(3):491–493. doi: 10.1016/0304-4165(68)90312-7. [DOI] [PubMed] [Google Scholar]
- Sanwal B. D. Allosteric controls of amphilbolic pathways in bacteria. Bacteriol Rev. 1970 Mar;34(1):20–39. doi: 10.1128/br.34.1.20-39.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlanderer G., Dellweg H. Cyclid AMP and catabolite repression in yeasts, In Schizosaccharomyces pombe glucose lowers both intracellular adenosine 3':5'-monophosphate levels and the activity of catabolite-sensitive enzymes. Eur J Biochem. 1974 Nov 1;49(1):305–316. doi: 10.1111/j.1432-1033.1974.tb03835.x. [DOI] [PubMed] [Google Scholar]
- Schoental R. Aflatoxins. Annu Rev Pharmacol. 1967;7:343–356. doi: 10.1146/annurev.pa.07.040167.002015. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Carlton W. W. Accumulation of only aflatoxin B 2 by a strain of Aspergillus flavus. Appl Microbiol. 1973 Jan;25(1):146–148. doi: 10.1128/am.25.1.146-148.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H. W., Cole R. J., Grigsby R. D., Hein H., Jr Inhibition of aflatoxin production and tentative identification of an aflatoxin intermediate "versiconal acetate" from treatment with dichlorvos. Appl Microbiol. 1974 Feb;27(2):394–399. doi: 10.1128/am.27.2.394-399.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H. W., Kelton W. H. Production of sterigmatocystin by some species of the genus Aspergillus and its toxicity to chicken embryos. Appl Microbiol. 1975 Oct;30(4):589–591. doi: 10.1128/am.30.4.589-591.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H. W., Verrett M. J. Production of aflatoxin by Aspergillus wentii Wehmer. Can J Microbiol. 1969 Aug;15(8):895–898. doi: 10.1139/m69-159. [DOI] [PubMed] [Google Scholar]
- Sekiguchi J., Gaucher G. M. Conidiogenesis and secondary metabolism in Penicillium urticae. Appl Environ Microbiol. 1977 Jan;33(1):147–158. doi: 10.1128/aem.33.1.147-158.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C. H., Marth E. H. Some cultural conditions that control biosynthesis of lipid and aflatoxin by Aspergillus parasiticus. Appl Microbiol. 1974 Mar;27(3):452–456. doi: 10.1128/am.27.3.452-456.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C. N., McCoy E., Marth E. H. Nitrification by aflatoxigenic strains of Aspergillus flavus and Aspergillus parasiticus. J Gen Microbiol. 1974 Oct;84(2):357–363. doi: 10.1099/00221287-84-2-357. [DOI] [PubMed] [Google Scholar]
- Singh R., Hsieh D. P. Aflatoxin biosynthetic pathway: elucidation by using blocked mutants of Aspergillus parasiticus. Arch Biochem Biophys. 1977 Jan 15;178(1):285–292. doi: 10.1016/0003-9861(77)90193-x. [DOI] [PubMed] [Google Scholar]
- Singh R., Hsieh D. P. Enzymatic conversion of sterigmatocystin into aflatoxin B1 by cell-free extracts of Aspergillus parasiticus. Appl Environ Microbiol. 1976 May;31(5):743–745. doi: 10.1128/aem.31.5.743-745.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. E., Ng W. S. Fluorometric determination of glycolytic intermediates and adenylates during sequential changes in replacement culture of Aspergillus niger. Can J Microbiol. 1972 Nov;18(11):1657–1664. doi: 10.1139/m72-257. [DOI] [PubMed] [Google Scholar]
- Stebbing N. Precursor pools and endogenous control of enzyme synthesis and activity in biosynthetic pathways. Bacteriol Rev. 1974 Mar;38(1):1–28. doi: 10.1128/br.38.1.1-28.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. Utilization of energy for growth and maintenance in continuous and batch cultures of microorganisms. A reevaluation of the method for the determination of ATP production by measuring molar growth yields. Biochim Biophys Acta. 1973 Feb 12;301(1):53–70. doi: 10.1016/0304-4173(73)90012-8. [DOI] [PubMed] [Google Scholar]
- TABER W. A., TERTZAKIAN G. SEQUENTIAL PRIMARY AND SECONDARY SHUNT METABOLISM IN PENICILLIUM CHRYSOGENUM. Appl Microbiol. 1965 Jul;13:590–594. doi: 10.1128/am.13.4.590-594.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber R. A., Schroeder H. W. Aflatoxin-producing potential of isolates of the Aspergillus flavus-oryzae group from peanuts (Arachis hypogaea). Appl Microbiol. 1967 Jan;15(1):140–144. doi: 10.1128/am.15.1.140-144.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M. Metal ions and ribosomal conformation. Biochim Biophys Acta. 1969 Nov 19;195(1):76–86. doi: 10.1016/0005-2787(69)90604-2. [DOI] [PubMed] [Google Scholar]
- Turner R. B., Lindsey D. L., Davis D. D., Bishop R. D. Isolation and identification of 5,7-dimethoxyisoflavone, an inhibitor of Aspergillus flavus from peanuts. Mycopathologia. 1975 Dec 8;57(1):39–40. doi: 10.1007/BF00431177. [DOI] [PubMed] [Google Scholar]
- VOLKIN E., COHN W. E. Estimation of nucleic acids. Methods Biochem Anal. 1954;1:287–305. doi: 10.1002/9780470110171.ch11. [DOI] [PubMed] [Google Scholar]
- Van Walbeek W., Scott P. M., Thatcher F. S. Mycotoxins from food-borne fungi. Can J Microbiol. 1968 Feb;14(2):131–137. doi: 10.1139/m68-022. [DOI] [PubMed] [Google Scholar]
- Vogel G., Lynen F. 6-Methylsalicylic acid synthetase. Methods Enzymol. 1975;43:520–530. doi: 10.1016/0076-6879(75)43114-7. [DOI] [PubMed] [Google Scholar]
- WINDER F. G., O'HARA C. Effects of iron deficiency and of zinc deficiency on the composition of Mycobacterium smegmatis. Biochem J. 1962 Jan;82:98–108. doi: 10.1042/bj0820098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. B. Enzymatic reactions involved in streptomycin biosynthesis and metabolism. Lloydia. 1971 Dec;34(4):363–371. [PubMed] [Google Scholar]
- Weinberg E. D. Secondary metabolism: raison d'être. Perspect Biol Med. 1971;14(4):565–577. doi: 10.1353/pbm.1971.0033. [DOI] [PubMed] [Google Scholar]
- West S., Wyatt R. D., Hamilton P. B. Improved yield of aflatoxin by incremental increases of temperature. Appl Microbiol. 1973 Jun;25(6):1018–1019. doi: 10.1128/am.25.6.1018-1019.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wogan G. N. Chemical nature and biological effects of the aflatoxins. Bacteriol Rev. 1966 Jun;30(2):460–470. doi: 10.1128/br.30.2.460-470.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wogan G. N. Mycotoxins. Annu Rev Pharmacol. 1975;15:437–451. doi: 10.1146/annurev.pa.15.040175.002253. [DOI] [PubMed] [Google Scholar]
- Wogan G. N., Pong R. S. Aflatoxins. Ann N Y Acad Sci. 1970 Oct 30;174(2):623–635. doi: 10.1111/j.1749-6632.1970.tb45587.x. [DOI] [PubMed] [Google Scholar]
- Wold W. S., Suzuki I. Regulation by zinc and adenosine 3',5'-cyclic monophosphate of growth and citric acid accumulation in Aspergillus niger. Can J Microbiol. 1976 Aug;22(8):1093–1101. doi: 10.1139/m76-160. [DOI] [PubMed] [Google Scholar]
- Wold W. S., Suzuki I. The citric acid fermentation by Aspergillus niger: regulation by zinc of growth and acidogenesis. Can J Microbiol. 1976 Aug;22(8):1083–1092. doi: 10.1139/m76-159. [DOI] [PubMed] [Google Scholar]
- Yao R. C., Hsieh D. P. Step of dichlorvos inhibition in the pathway of aflatoxin biosynthesis. Appl Microbiol. 1974 Jul;28(1):52–57. doi: 10.1128/am.28.1.52-57.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zicot M., Boland J. Sur certains paramètres hémodynamiques, continus et pulsatilis, des poumons isolés de lapin. C R Seances Soc Biol Fil. 1972;166(4):718–721. [PubMed] [Google Scholar]
- Zimmer T. L., Laland S. G. Gramicidine S synthetase. Methods Enzymol. 1975;43:567–579. doi: 10.1016/0076-6879(75)43119-6. [DOI] [PubMed] [Google Scholar]
- Zonneveld B. J. The effect of glucose and manganese on adenosine-3',5'-monophosphate levels during growth and differentiation of Aspergillus nidulans. Arch Microbiol. 1976 May 3;108(1):41–44. doi: 10.1007/BF00425091. [DOI] [PubMed] [Google Scholar]



