Background: BDNF and Ca2+ mobilization is important for microglial function.
Results: We showed BDNF elevates intracellular Ca2+ through TRPC3 channels.
Conclusion: TRPC3 is important for BDNF suppression of microglial activation.
Significance: TRPC3 might be important for the treatment of psychiatric disorders.
Keywords: Brain-derived Neurotrophic Factor (BDNF), Calcium, Microglia, Nitric Oxide, Transient Receptor Potential Channels (TRP Channels)
Abstract
Microglia are immune cells that release factors, including proinflammatory cytokines, nitric oxide (NO), and neurotrophins, following activation after disturbance in the brain. Elevation of intracellular Ca2+ concentration ([Ca2+]i) is important for microglial functions such as the release of cytokines and NO from activated microglia. There is increasing evidence suggesting that pathophysiology of neuropsychiatric disorders is related to the inflammatory responses mediated by microglia. Brain-derived neurotrophic factor (BDNF) is a neurotrophin well known for its roles in the activation of microglia as well as in pathophysiology and/or treatment of neuropsychiatric disorders. In this study, we sought to examine the underlying mechanism of BDNF-induced sustained increase in [Ca2+]i in rodent microglial cells. We observed that canonical transient receptor potential 3 (TRPC3) channels contribute to the maintenance of BDNF-induced sustained intracellular Ca2+ elevation. Immunocytochemical technique and flow cytometry also revealed that BDNF rapidly up-regulated the surface expression of TRPC3 channels in rodent microglial cells. In addition, pretreatment with BDNF suppressed the production of NO induced by tumor necrosis factor α (TNFα), which was prevented by co-adiministration of a selective TRPC3 inhibitor. These suggest that BDNF induces sustained intracellular Ca2+ elevation through the up-regulation of surface TRPC3 channels and TRPC3 channels could be important for the BDNF-induced suppression of the NO production in activated microglia. We show that TRPC3 channels could also play important roles in microglial functions, which might be important for the regulation of inflammatory responses and may also be involved in the pathophysiology and/or the treatment of neuropsychiatric disorders.
Introduction
Microglia are immune cells that release proinflammatory cytokines, nitric oxide (NO), and neurotrophins, when they are activated in response to brain injury or immunological stimuli (1). There is increasing evidence suggesting that pathophysiology of neuropsychiatric disorders is related to inflammatory responses mediated by microglial cells (2, 3).
In the rodent brain, microglial cells secrete brain-derived neurotrophic factor (BDNF), and BDNF promotes the proliferation and survival of microglia themselves (4). In addition, pretreatment with BDNF suppressed the release of NO from murine microglial cells activated by IFN-γ (5). To date, BDNF is also well known for its involvement in the pathophysiology of neuropsychiatric disorders (4, 5).
Elevation of intracellular Ca2+ is important in activation of microglial cell functions, including proliferation, release of NO, and migration (1). We have reported previously that BDNF induces a sustained increase in intracellular Ca2+ concentration ([Ca2+]i) through the activation of the phospholipase C (PLC)2 pathway in rodent microglial cells (5). We also tested the effect of 2-aminoethoxydiphenyl borate or SKF-96365, both of which can inhibit canonical transient receptor potential (TRPC) channels (6, 7) and showed that sustained activation of TRPC channels occurred after a brief treatment with BDNF and contributed to the maintenance of BDNF-induced sustained intracellular Ca2+ elevation (5).
In this study, we examined whether TRPC3 channels contribute to the maintenance of BDNF-induced sustained intracellular Ca2+ elevation using the pyrazole compound 3 (Pyr3), a selective inhibitor of TRPC3 channels, which does not affect the activity of other TRPC channel members (8, 9), in rodent microglial cells. Although mRNAs of many TRPC channels, including TRPC3, are shown to be expressed in cultured rat microglia (10), this is the first report showing that TRPC3 channels could also play important roles in microglial functions.
EXPERIMENTAL PROCEDURES
Materials
The drugs used in the present study include Fura2-AM, 4,5-diaminofluorescein diacetate, U73122, and human recombinant TNFα (from Sigma) and polyclonal rabbit anti-TRPC3 channel antibody (ACC-016; Alomone Labs, Jerusalem, Israel). Recombinant IFN-γ and mouse GM-CSF were purchased from R&D Systems. Human recombinant BDNF (Sigma) was diluted with the standard external solution to obtain the final concentration (20 ng/ml; 0.73 nm), which is sufficient to promote the proliferation of microglial cells (4, 5). The final concentration of dimethyl sulfoxide was always <0.1%.
Microglial Cells
Primary microglial cells were prepared from the whole brain of 3-day postnatal Sprague-Dawley rats as described previously (5, 11, 12). Primary mixed cells were prepared from the whole brain of 3-day-postnatal Sprague-Dawley rats using a Cell Strainer (BD Biosciences). Primary rat microglial cells were selected after attachment to Aclar film (Nisshin EM) for 2 h in DMEM supplemented with 10% FBS (10% FBS/DMEM). Aclar films were gently washed with PBS and then transferred to fresh 10% FBS/DMEM, and the fresh microglia expanded for 1–2 days. The purity of isolated microglia was assessed by immunocytochemical staining for the microglial marker, Iba-1, and >99% of cells stained positively (13, 14). The 6-3 microglial cells were established from neonatal C57BL/6J (H-2b) mice as described previously (5, 11–14).
The 6-3 cells were cultured in Eagle's minimal essential medium supplemented with 0.3% NaHCO3, 2 mm glutamine, 0.2% glucose, 10 g/ml insulin, and 10% FCS. Cells were maintained at 37 °C in a 10% CO2, 90% air atmosphere. GM-CSF was supplemented into the culture medium, at a final concentration of 1 ng/ml, to maintain proliferation of the 6-3 cells. Culture medium was renewed twice per week.
The rat microglial cell line, highly aggressive proliferating immortalized (HAPI) cells (15), was kindly donated by Drs. N. P. Morales and F. Hyodo of Kyushu University. The cells were cultured in DMEM (low glucose; Invitrogen), 5% FBS (Hyclone), 4 mm glutamine (Invitrogen), 100 000 units/liter penicillin G, 100 mg/liter streptomycin (Mediatech), and maintained in 5% CO2 at 37 °C.
siRNA Transfection
To down-regulate TRPC3 channels, siRNA transfection was performed. The 6-3 microglial cells were cultured in growth medium without antibiotics in a 35-mm glass-based dish until 60–80% confluent. TRPC3-targeting siRNA or scrambled control siRNA (sc-42667 and sc-37007, 80 pmol/dish, respectively; Santa Cruz Biotechnology, Santa Cruz, CA) were transfected into 6-3 microglial cells using siRNA transfection reagent (sc-29528, 6 μl/dish; Santa Cruz Biotechnology) in siRNA transfection medium (sc-36868, 1 ml/dish; Santa Cruz Biotechnology) according to the manufacturer's instructions. Six hours after transfection, normal serum and antibiotics were added at final concentrations of 10 and 1%, respectively. The next day, the medium containing transfection mixtures was replaced with fresh growth medium. At 48 h, the transfected cells were used for intracellular Ca2+ imaging.
Intracellular Ca2+ Imaging
Intracellular Ca2+ imaging using Fura-2-AM was performed as reported previously (5, 16, 17). In brief, the experiments were performed in the external standard solution (150 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 10 mm glucose, and 10 mm HEPES, pH 7.4, with Tris-OH) at room temperature (27 °C). For Fura-2 excitation, the cells were illuminated with two alternating wavelengths, 340 and 380 nm using a computerized system for a rapid dual wavelength Xenon arc. The emitted light was recorded at 510 nm using a cooled CCD camera (Hamamatsu Photonics). The [Ca2+]i was calculated from the ratio (R) of fluorescence recorded at 340 and 380 nm excitation wavelengths for each pixel within a microglial cell boundary. All data presented were obtained from at least five dishes and three different cell preparations.
Immunocytochemistry
After microglial cells were fixed, indirect immunofluorescence was performed using the following antibodies: polyclonal rabbit anti-TRPC3 channel antibody (ACC-016; Alomone Labs, Jerusalem, Israel), which recognizes intracellular C terminus of mouse TRPC3 channel and mouse anti-CD45 monoclonal antibody. These specimens were incubated in primary antibodies, and FITC- or Texas red-conjugated secondary antibodies were used for detection. Fluorescent images were captured with a fluorescence microscope (Axio Scope A1, Carl Zeiss, Oberkochen, Germany).
Flow Cytometry
Flow cytometry was performed using a FACS Canto II (BD Biosciences) with FACS Diva software (BD Biosciences). Flow cytometry data were analyzed using FlowJo software (Tree Star, San Carios, CA). The HAPI microglial cells were harvested by non-enzymatic cell dissociation solution (Sigma) and cell lifter (Corning). The cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. After blocking, the cells were stained with anti-TRPC3 antibody (ACC-016). After washing, the cells were stained with Alexa Fluor 488-conjugated secondary antibody (Invitrogen). The fluorescence intensity of the cells was measured.
Intracellular NO Imaging
The microglial cells were loaded with 10 μm 4,5-diaminofluorescein diacetate (Sigma), a cell membrane-permeable dye that binds intracellular NO (18), for 20 min before the measurement. For DAF-2 excitation, the cells were illuminated with a wavelength, 490 nm, using a computerized system. The signal obtained at 490 nm was previously shown to be, among the excitation wavelengths, quantitatively largest and most representative of change in intracellular NO (19). The emitted light was collected at 510 nm using a cooled CCD camera. The intracellular DAF-2 fluorescence intensity (F) was recorded for each pixel within a cell boundary. The ratio (F/F0) of fluorescence intensity was estimated from the intensity of fluorescence recorded prior to stimulation (F0).
All data are expressed as the mean ± S.E., and statistical comparisons were made using an unpaired t test. Significance was established at a level of p < 0.05.
RESULTS
We have previously reported that BDNF induces sustained increase in intracellular Ca2+ in rodent microglial cells (Fig. 1A, inset) (5). The increase in intracellular Ca2+ was sustained for >40 min even after the washout of BDNF until the end of recording. We applied the Pyr3, a selective inhibitor of TRPC3 channels (8, 9), after the onset of BDNF-induced sustained intracellular Ca2+ elevation to investigate the involvement of TRPC3 channels in the maintenance of long lasting [Ca2+]i elevation. After the onset of BDNF-induced intracellular Ca2+ elevation, Pyr3 (0.3 μm) was applied and found to suppress the [Ca2+]i in the 6-3 (n = 35 cells; data not shown) and primary (n = 78 cells; Fig. 1A) microglial cells. As shown in Fig. 1B, application of Pyr3 suppressed BDNF-induced intracellular Ca2+ elevation in a dose-dependent manner with the IC50 value of 0.5 μm. We observed that 10 μm Pyr3 suppressed the [Ca2+]i to near basal levels in the 6-3 (n = 22) and primary (n = 21) microglial cells.
FIGURE 1.
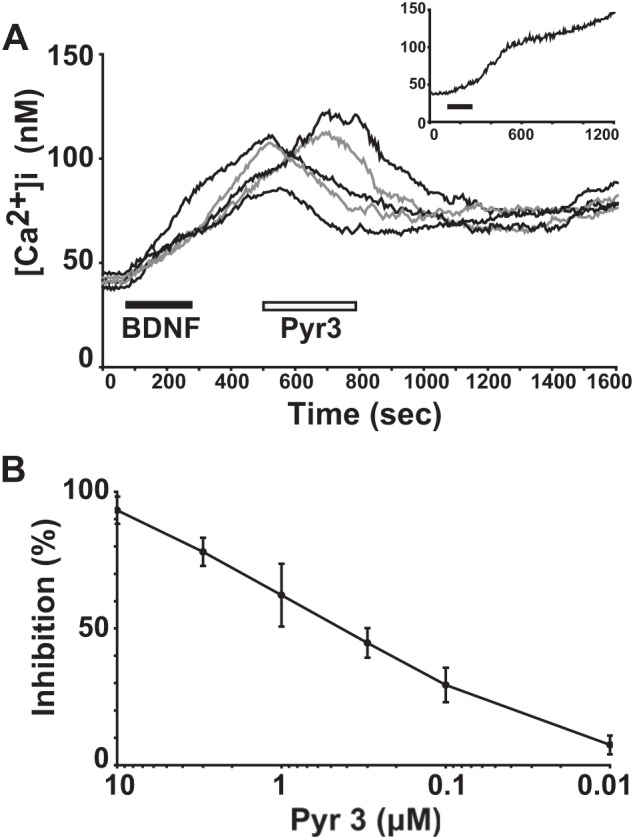
TRPC3 channels contribute to the maintenance of BDNF-induced sustained intracellular Ca2+ elevation in rodent microglial cells. A, five representative traces showing the effect of 0.3 μm Pyr3, a selective inhibitor of TRPC3 channels, after the onset of 20 ng/ml BDNF-induced sustained elevation of [Ca2+]i in primary rat microglial cells. Inset, the inset shows a brief (3 min) treatment of BDNF-induced sustained increase in [Ca2+]i in primary rat microglial cells. The average trace of five [Ca2+]i traces in response to BDNF is shown. B, the dose-response effect of different concentrations of Pyr3 on inhibition of the amplitude of [Ca2+]i increase obtained 15 min after BDNF treatment in primary rat microglial cells. Values are the mean ± S.E.
To confirm the involvement of TRPC3 channels in the BDNF-induced increase in [Ca2+]i, we down-regulated TRPC3 protein expression using siRNA. As expected, down-regulation of TRPC3 with siRNA suppressed the elevation of [Ca2+]i induced by BDNF (Fig. 2). These indicate that sustained activation of TRPC3 channels could occur after a brief application of BDNF and contribute to the maintenance of BDNF-induced sustained intracellular Ca2+ elevation in rodent microglial cells.
FIGURE 2.

Down-regulation of TRPC3 channels abolished the BDNF-induced elevation of intracellular Ca2+ in rodent microglial cells. The average traces showing the effect of BDNF (20 ng/ml) on intracellular Ca2+ mobilization in 6-3 microglial cells transfected with TRPC3-targeting siRNA (black line) and scrambled control siRNA (gray line). The average trace was determined from 10 representative traces of intracellular Ca2+ in each condition.
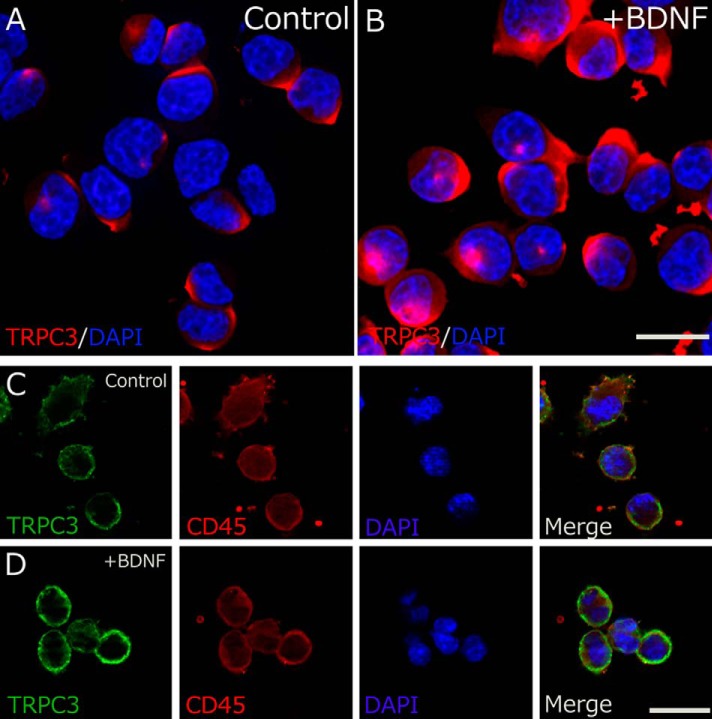
Next, we performed immunocytochemistry to examine the association between TRPC3 surface expression and BDNF in rodent microglia. RT-PCR analysis has shown previously that TRPC3 mRNA is expressed in cultured microglial cells derived from rats (10). We also confirmed the same results in primary microglial cells and 6-3 murine microglial cells (data not shown). Although only weak TRPC3 immunoreactivity was observed in somata of control HAPI microglial cells, a dramatic increase in TRPC3 expression was observed in BDNF-treated HAPI microglial cells (Fig. 3, A and B). Double immunostaining for TRPC3 and CD45 (cytoplasmic staining of immune cells) demonstrated that TRPC3 was strongly stained on the cell surface of HAPI microglial cells after the BDNF application, suggesting that BDNF rapidly up-regulated the surface expression of TRPC3 channels in rodent microglial cells (Fig. 3, C and D).
FIGURE 3.
BDNF up-regulates the surface expression of TRPC3 channels in rodent microglial cells. TRPC3 (red) is markedly up-regulated in the BDNF-treated (20 ng/ml, 10 min) HAPI cells (B) compared with control cells (A). C and D, two representative confocal images of HAPI microglial cells showing substantial staining of TRPC3 (green) and CD45 (red). The surface expression of TRPC3 is up-regulated in BDNF-treated HAPI cells (D) compared with control cells (C). The nuclei are stained with 4′,6-diamidino-2-phenylindole (blue). The scale bars indicate 20 μm.
To quantify the above-mentioned results, we next examine the effect of BDNF on surface expression of TRPC3 channels in HAPI microglial cells using flow cytometry. We observed that BDNF rapidly increased the relative expression of surface TRPC3 channels in HAPI microglial cells (n = 3; Fig. 4). Altogether, these indicate that BDNF induces sustained intracellular Ca2+ elevation possibly through the up-regulation of surface TRPC3 channels in rodent microglial cells.
FIGURE 4.
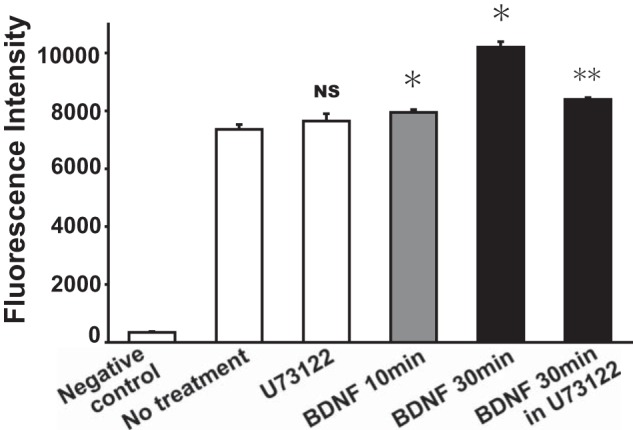
Quantification of the effect of BDNF on the surface expression of TRPC3 channels in rodent microglial cells. Flow cytomety showing that treatment of BDNF (20 ng/ml) rapidly increased the fluorescence intensity of surface expression of TRPC3 channels in HAPI microglial cells. In contrast, pretreatment of U73122 (5 μm), a membrane-permeable specific PLC inhibitor, significantly reduced the amplitude of BDNF-induced increase in the expression of surface TRPC3 channels in HAPI microglial cells. *, p < 0.05 versus no treatment; **, p < 0.01 versus BDNF (30 min). Negative control was obtained from secondary antibody alone. NS, nonsignificant.
We have previously shown that the activation of PLC is involved in the induction of BDNF-induced intracellular Ca2+ elevation in rodent microglial cells (5). In the next examination, we observed that pretreatment of U73122 (5 μm), a membrane-permeable specific PLC inhibitor, significantly reduced the amplitude of BDNF-induced increase in relative expression of surface TRPC3 channels in HAPI microglial cells (n = 3; Fig. 4). Thus, the activation of PLC could also be important for the up-regulation of surface TRPC3 channels induced by BDNF in rodent microglial cells.
We have previously reported that pretreatment with BDNF suppressed the release of NO from murine microglial cells activated by IFN-γ (5). In addition, pretreatment of BDNF suppressed the IFN-γ-induced elevation of [Ca2+]i, along with a rise in basal [Ca2+]i in rodent microglial cells (5). Thus, BDNF-induced elevation of basal levels of [Ca2+]i could regulate the microglial intracellular signal transduction to suppress the release of NO induced by IFN-γ (4, 5). We next tested whether TRPC3 channels could be important for the BDNF-induced suppression of NO production in rodent microglial cells.
In the present study, 50 units/ml IFN-γ induced sustained intracellular Ca2+ elevation in both 6-3 and primary microglial cells as reported previously (data not shown) (5). After the onset of IFN-γ-induced intracellular Ca2+ elevation, 3 μm Pyr3 was applied and found to suppress the [Ca2+]i to near basal levels in the 6-3 (n = 24; data not shown) and primary (n = 47; Fig. 5A) microglial cells. Thus, TRPC3 channels could also contribute to the maintenance of IFN-γ-induced sustained intracellular Ca2+ elevation in rodent microglial cells used in this study.
FIGURE 5.

TRPC3 channels are not involved in TNFα-induced sustained intracellular Ca2+ elevation in rodent microglial cells. A and B, an average trace showing the effect of the 3 μm Pyr3 after the onset of 50 units/ml IFN-γ-induced or 2 μg/ml TNFα-induced sustained elevation of [Ca2+]i in primary rat microglial cells. Each panel demonstrates the average trace determined from 10 representative traces of [Ca2+]i in each condition.
TNFα, one of the proinflammatory cytokines, was shown to induce a gradual increase in intracellular Ca2+ in cultured astrocytes at a concentration of 2 μg/ml (20). In the present study, 2 μg/ml TNFα rapidly increased [Ca2+]i in both 6-3 (n = 23; data not shown) and primary microglial cells (n = 41; data not shown). Once the intracellular Ca2+ level rose, it gradually increased without attenuation even after the washout of TNFα until the end of recording. Interestingly, 3 μm Pyr3 applied after the onset of TNFα-induced intracellular Ca2+ elevation did not affect [Ca2+]i in 6-3 (n = 21) and primary (n = 58; Fig. 5B) microglial cells. These suggest that TRPC3 channels could not be important for the maintenance of TNFα-induced sustained intracellular Ca2+ elevation in rodent microglial cells we used.
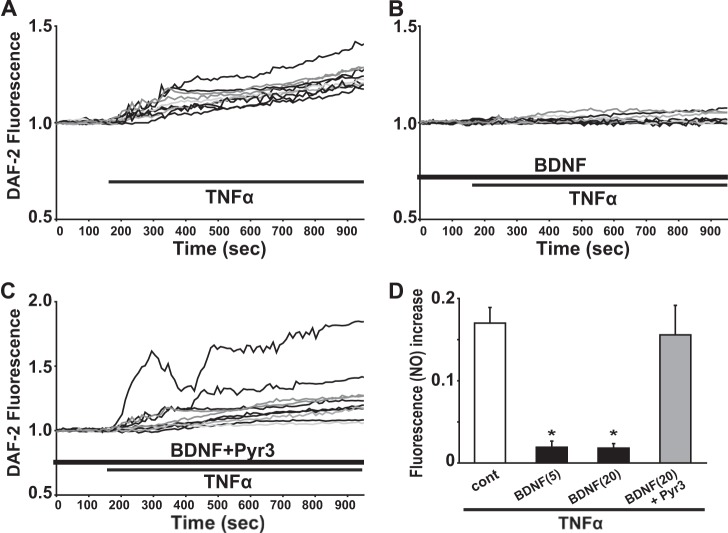
We next tested the effect of TNFα on intracellular NO mobilization, using DAF-2 imaging to detect endogenously produced NO in rodent microglia. An application of 2 μg/ml TNFα induced a gradual increase in DAF-2 fluorescence in both 6-3 (n = 101; Fig. 6A) and primary (n = 45; data not shown) microglial cells tested. The reaction between DAF-2 and NO is shown to be irreversible and the accumulated level of DAF-2 fluorescence reflects the total amount of intracellular NO production (18, 21). We observed that the increase in intracellular DAF-2 fluorescence was sustained for > 40 min even after the washout of TNFα until the end of recording. Additionally, in the presence of 50 μm l-N6-(1-iminoethyl)lysine, a membrane-permeable selective inhibitor of inducible nitric oxide synthase (22), TNFα failed to elevate the DAF-2 fluorescence in both 6-3 (n = 43) and primary (n = 11) microglial cells (data not shown).
FIGURE 6.
Pretreatment with BDNF suppressed the production of NO induced by TNFα in 6-3 microglial cells. A, 10 representative traces showing the treatment of 2 μg/ml TNFα induced the increase in the DAF-2 fluorescence in murine 6-3 microglial cells. B, 10 representative traces showing 24-h pretreatment with 20 ng/ml BDNF suppressed the TNFα-induced increase in the DAF-2 fluorescence in murine 6-3 microglial cells. C, 10 representative traces showing 24-h pretreatment with both 20 ng/ml BDNF and 0.2 μm Pyr3 did not suppress the TNFα-induced increase in the DAF-2 fluorescence in murine 6-3 microglial cells. D, bar graphs showing that pretreatment with BDNF suppressed the production of NO induced by TNFα treatment and TRPC3 channels could be important for the BDNF-induced suppression of the NO production in murine 6-3 microglial cells. BDNF (5) and BDNF (20) mean 5 ng/ml BDNF and 20 ng/ml BDNF for each.
We measured the effect of 24-h pretreatment with BDNF (20 ng/ml) on the production of intracellular NO induced by TNFα in rodent microglia. In 6-3 microglial cells that were pretreated with BDNF for 24 h, TNFα (2 μg/ml) also induced a gradual increase in the DAF-2 fluorescence (Fig. 6B). However, pretreatment of BDNF significantly reduced the amplitude of TNFα-induced increase in the DAF-2 fluorescence at 15 min after a treatment of TNFα in 6-3 microglial cells (0.171 ± 0.019, n = 101 in control; 0.019 ± 0.007, n = 27 in 5 ng/ml BDNF; 0.018 ± 0.006, n = 68 in 20 ng/ml BDNF; p < 0.001; Fig. 6D). In contrast, 24 h pretreatment of both BDNF (20 ng/ml) and Pyr3 (0.2 μm) did not reduce the amplitude of TNFα-induced increase in the DAF-2 fluorescence in 6-3 microglial cells (0.171 ± 0.019, n = 101 in control; 0.156 ± 0.036, n = 69 in BDNF + Pyr3; p = 0.37; Fig. 6, C and D). These suggest that pretreatment with BDNF suppressed the production of NO induced by TNFα. In addition, TRPC3 channels could be important for the BDNF-induced suppression of NO production in rodent microglial cells.
DISCUSSION
We found that TRPC3 channels mainly contributed to the maintenance of BDNF-induced sustained intracellular Ca2+ elevation in rodent microglial cells. In addition, we suggest that TRPC3 channels could be important for BDNF-induced suppression of NO production in rodent microglial cells activated by TNFα.
BDNF-induced elevation of basal levels of [Ca2+]i could regulate the microglial intracellular signal transduction to suppress the release of NO induced by IFN-γ (4, 5, 23). We herein showed that pretreatment with BDNF also suppressed the production of NO in murine microglial cells activated by TNFα, which was prevented by co-administration of Pyr3. We also found that pretreatment with both BDNF and Pyr3 did not elevate the basal [Ca2+]i in rodent microglial cells (data not shown). These suggest that BDNF-induced elevation of basal levels of [Ca2+]i mediated by TRPC3 channels could be important for the BDNF-induced suppression of NO production in rodent microglial cells.
We observed an application of Pyr3 did not suppress the elevation of [Ca2+]i induced by TNFα in rodent microglial cells. TRPM2 channels, a member of the melastatin subfamily of TRP channels, are shown to mediate the TNFα-induced intracellular [Ca2+]i oscillation (24), suggesting that TRPM2 channels might be involved in the TNFα-induced sustained [Ca2+]i increase in rodent microglial cells.
We have recently reported that pretreatment with antidepressants (13) or antipsychotics (14, 25) significantly inhibits the release of NO from activated microglia. In this study, we observed that pretreatment with BDNF significantly inhibited the production of NO in microglia activated by TNFα. TNFα plays a key role in the induction of sickness behaviors (26) and also in the development of depressive symptoms (27). Thus, this would suggest that BDNF might have an anti-inflammatory effect through the inhibition of microglial activation and could be useful for the treatment of neuropsychiatric disorders. We need to further examine the mechanism underlying the up-regulation of surface TRPC3 channels induced by BDNF in rodent microglial cells.
Acknowledgments
We thank Drs. Shigeki Kiyonaka and Yasuo Mori of Kyoto University for providing us with the pyrazole compound 3 and also thank Dr. Makoto Sawada of Nagoya University for providing us with the microglial cell line, 6-3.
This work was supported by research grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to Y. M., T. A. K., S. K., and A. M.).
- PLC
- phospholipase C
- TRPC3
- canonical transient receptor potential 3
- Pyr3
- pyrazole compound 3
- HAPI
- highly aggressive proliferating immortalized
- DAF
- 4,5-diaminofluorescein.
REFERENCES
- 1. Kettenmann H., Hanisch U. K., Noda M., Verkhratsky A. (2011) Physiology of microglia. Physiol. Rev. 91, 461–553 [DOI] [PubMed] [Google Scholar]
- 2. Monji A., Kato T. A., Mizoguchi Y., Horikawa H., Seki Y., Kasai M., Yamauchi Y., Yamada S., Kanba S. (2013) Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog. Neuropsychopharmacol. Biol. Psychiatry 42, 115–121 [DOI] [PubMed] [Google Scholar]
- 3. Kato T. A., Yamauchi Y., Horikawa H., Monji A., Mizoguchi Y., Seki Y., Hayakawa K., Utsumi H., Kanba S. (2013) Neurotransmitters, psychotropic drugs and microglia: clinical implications for psychiatry. Curr. Med. Chem. 20, 331–344 [DOI] [PubMed] [Google Scholar]
- 4. Mizoguchi Y., Monji A., Kato T. A., Horikawa H., Seki Y., Kasai M., Kanba S., Yamada S. (2011) Possible role of BDNF-induced microglial intracellular Ca2+ elevation in the pathophysiology of neuropsychiatric disorders. Mini Rev. Med. Chem. 11, 575–581 [DOI] [PubMed] [Google Scholar]
- 5. Mizoguchi Y., Monji A., Kato T., Seki Y., Gotoh L., Horikawa H., Suzuki S. O., Iwaki T., Yonaha M., Hashioka S., Kanba S. (2009) Brain-derived neurotrophic factor induces sustained elevation of intracellular Ca2+ in rodent microglia. J. Immunol. 183, 7778–7786 [DOI] [PubMed] [Google Scholar]
- 6. Amaral M. D., Pozzo-Miller L. (2007) TRPC3 channels are necessary for brain-derived neurotrophic factor to activate a nonselective cationic current and to induce dendritic spine formation. J. Neurosci. 27, 5179–5189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bootman M. D., Collins T. J., Mackenzie L., Roderick H. L., Berridge M. J., Peppiatt C. M. (2002) 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 16, 1145–1150 [DOI] [PubMed] [Google Scholar]
- 8. Kiyonaka S., Kato K., Nishida M., Mio K., Numaga T., Sawaguchi Y., Yoshida T., Wakamori M., Mori E., Numata T., Ishii M., Takemoto H., Ojida A., Watanabe K., Uemura A., Kurose H., Morii T., Kobayashi T., Sato Y., Sato C., Hamachi I., Mori Y. (2009) Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proc. Natl. Acad. Sci. U.S.A. 106, 5400–5405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim M. S., Lee K. P., Yang D., Shin D. M., Abramowitz J., Kiyonaka S., Birnbaumer L., Mori Y., Muallem S. (2011) Genetic and pharmacologic inhibition of the Ca2+ influx channel TRPC3 protects secretory epithelia from Ca2+-dependent toxicity. Gastroenterology 140, 2107–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ohana L., Newell E. W., Stanley E. F., Schlichter L. C. (2009) The Ca2+ release-activated Ca2+ current (I(CRAC)) mediates store-operated Ca2+ entry in rat microglia. Channels 3, 129–139 [DOI] [PubMed] [Google Scholar]
- 11. Kato T., Mizoguchi Y., Monji A., Horikawa H., Suzuki S. O., Seki Y., Iwaki T., Hashioka S., Kanba S. (2008) Inhibitory effects of aripiprazole on interferon-γ-induced microglial activation via intracellular Ca2+ regulation in vitro. J. Neurochem. 106, 815–825 [DOI] [PubMed] [Google Scholar]
- 12. Seki Y., Suzuki S. O., Masui K., Harada S., Nakamura S., Kanba S., Iwaki T. (2011) A simple and high-yield method for preparation of rat microglial cultures utilizing Aclar plastic film. Neuropathology 31, 215–222 [DOI] [PubMed] [Google Scholar]
- 13. Horikawa H., Kato T. A., Mizoguchi Y., Monji A., Seki Y., Ohkuri T., Gotoh L., Yonaha M., Ueda T., Hashioka S., Kanba S. (2010) Inhibitory effects of SSRIs on IFN-γ induced microglial activation through the regulation of intracellular calcium. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 1306–1316 [DOI] [PubMed] [Google Scholar]
- 14. Kato T. A., Monji A., Yasukawa K., Mizoguchi Y., Horikawa H., Seki Y., Hashioka S., Han Y. H., Kasai M., Sonoda N., Hirata E., Maeda Y., Inoguchi T., Utsumi H., Kanba S. (2011) Aripiprazole inhibits superoxide generation from phorbol-myristate-acetate (PMA)-stimulated microglia in vitro: implication for antioxidative psychotropic actions via microglia. Schizophr. Res. 129, 172–182 [DOI] [PubMed] [Google Scholar]
- 15. Cheepsunthorn P., Radov L., Menzies S., Reid J., Connor J. R. (2001) Characterization of a novel brain-derived microglial cell line isolated from neonatal rat brain. Glia 35, 53–62 [DOI] [PubMed] [Google Scholar]
- 16. Mizoguchi Y., Ishibashi H., Nabekura J. (2003) The action of BDNF on GABAA currents changes from potentiating to suppressing during maturation of rat hippocampal CA1 pyramidal neurons. J. Physiol. 548, 703–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mizoguchi Y., Kanematsu T., Hirata M., Nabekura J. (2003) A rapid increase in the total number of cell surface functional GABAA receptors induced by brain-derived neurotrophic factor in rat visual cortex. J. Biol. Chem. 278, 44097–44102 [DOI] [PubMed] [Google Scholar]
- 18. Kojima H., Nakatsubo N., Kikuchi K., Kawahara S., Kirino Y., Nagoshi H., Hirata Y., Nagano T. (1998) Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal. Chem. 70, 2446–2453 [DOI] [PubMed] [Google Scholar]
- 19. Patel V. H., Brack K. E., Coote J. H., Ng G. A. (2008) A novel method of measuring nitric-oxide-dependent fluorescence using 4,5-diaminofluorescein (DAF-2) in the isolated Langendorff-perfused rabbit heart. Pflugers Archiv. 456, 635–645 [DOI] [PubMed] [Google Scholar]
- 20. Köller H., Thiem K., Siebler M. (1996) Tumour necrosis factor-α increases intracellular Ca2+ and induces a depolarization in cultured astroglial cells. Brain 119, 2021–2027 [DOI] [PubMed] [Google Scholar]
- 21. Kalinchuk A. V., McCarley R. W., Porkka-Heiskanen T., Basheer R. (2010) Sleep deprivation triggers inducible nitric oxide-dependent nitric oxide production in wake-active basal forebrain neurons. J. Neurosci. 30, 13254–13264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moore W. M., Webber R. K., Jerome G. M., Tjoeng F. S., Misko T. P., Currie M. G. (1994) L-N6-(1-iminoethyl)lysine: a selective inhibitor of inducible nitric oxide synthase. J. Med. Chem. 37, 3886–3888 [DOI] [PubMed] [Google Scholar]
- 23. Hoffmann A., Kann O., Ohlemeyer C., Hanisch U. K., Kettenmann H. (2003) Elevation of basal intracellular calcium as a central element in the activation of brain macrophages (microglia): suppression of receptor-evoked calcium signaling and control of release function. J. Neurosci. 23, 4410–4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nazıroğlu M. (2011) TRPM2 cation channels, oxidative stress and neurological diseases: where are we now? Neurochem. Res. 36, 355–366 [DOI] [PubMed] [Google Scholar]
- 25. Kato T. A., Monji A., Mizoguchi Y., Hashioka S., Horikawa H., Seki Y., Kasai M., Utsumi H., Kanba S. (2011) Anti-inflammatory properties of antipsychotics via microglia modulations: are antipsychotics a “fire extinguisher” in the brain of schizophrenia? Mini. Rev. Med. Chem. 11, 565–574 [DOI] [PubMed] [Google Scholar]
- 26. Simen B. B., Duman C. H., Simen A. A., Duman R. S. (2006) TNFα signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol. Psychiatry. 59, 775–785 [DOI] [PubMed] [Google Scholar]
- 27. Dantzer R., O'Connor J. C., Freund G. G., Johnson R. W., Kelley K. W. (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]