Background: Loz1 represses gene expression when zinc is in excess.
Results: A double zinc finger domain and accessory domain preceding zinc finger 1 are required for zinc-dependent repression.
Conclusion: The zinc-sensing domain in Loz1 coincides with the DNA binding domain.
Significance: Data presented here provide new insights into mechanisms of zinc sensing.
Keywords: Metal Homeostasis, Transcription Repressor, Yeast Genetics, Zinc, Zinc Finger, Metallosensor
Abstract
The Loz1 transcription factor from Schizosaccharomyces pombe plays an essential role in zinc homeostasis by repressing target gene expression in zinc-replete cells. To determine how Loz1 function is regulated by zinc, we employed a genetic screen to isolate mutants with impaired zinc-dependent gene expression and analyzed Loz1 protein truncations to map a minimal zinc-responsive domain. In the screen, we isolated 36 new loz1 alleles. 27 of these alleles contained mutations resulting in the truncation of the Loz1 protein. The remaining nine alleles contained point mutations leading to an amino acid substitution within a C-terminal double zinc finger domain. Further analysis of two of these substitutions revealed that they disrupted Loz1 DNA activity in vitro. By analyzing Loz1 protein truncations, we found that the last 96 amino acids of Loz1 was the smallest region that was able to confer partial zinc-dependent repression in vivo. This 96-amino acid region contains the double zinc finger domain and an accessory domain that enhances DNA binding. These results were further supported by the findings that MtfA, a transcription factor from Aspergillus nidulans that contains a related double zinc finger, is unable to complement loz1Δ, whereas a chimera of MtfA containing the Loz1 accessory domain is able to complement loz1Δ. Together, our studies indicate that the double zinc finger domain and adjacent accessory domain preceding zinc finger 1 are necessary for DNA binding and zinc-dependent repression.
Introduction
Zinc is a metal nutrient that is essential for all life. At the cellular level, zinc is a cofactor in over 300 enzymes, including alcohol dehydrogenases, RNA polymerases, and alkaline phosphatases (1). Several structural motifs, including the zinc finger and RING finger, are also stabilized by zinc ions (2). These types of domains are commonly found in regulatory factors and often play a role in mediating interaction with DNA, RNA, or proteins. In addition to its role in protein structure/function, zinc is an important signaling molecule in some cells (3).
Although zinc has many important biological roles, in excess, zinc can be toxic to cell growth. As a consequence, all organisms rely on mechanisms to maintain optimal intracellular levels of zinc. In eukaryotes, zinc-responsive transcription factors play a primary role in maintaining zinc homeostasis by controlling the expression of genes necessary for zinc transport and/or zinc storage (4). Transcription factors that regulate gene expression in response to zinc levels include Zap1 from Saccharomyces cerevisiae, MTF-1 in mammals and fish, Loz1 in Schizosaccharomyces pombe, and bZip19 and bZip23 in Arabidopsis thaliana (5–8).
The majority of what is known about how eukaryotic cells sense zinc comes from studies of the two transcriptional activators, Zap1 and MTF-1 (4). Zap1 is active in zinc-limited cells, whereas MTF-1 is active in zinc-replete cells. Both factors contain multiple zinc-responsive domains. Zap1 contains two transactivation domains that are independently regulated by zinc (9, 10). Zinc also inhibits Zap1 DNA binding activity (11). In mammals, zinc regulates MTF-1 DNA binding activity, cellular localization, and transactivation domain function (8). The presence of these multiple zinc-responsive domains ensures that the activity of each factor can be precisely controlled by changes in cellular zinc status.
In S. pombe, Loz1 represses gene expression under conditions of zinc excess. Loz1 target genes include zrt1, adh4, and the adh1AS transcript, which encode a zinc uptake transporter, a mitochondrial alcohol dehydrogenase, and an antisense transcript that inhibits the expression of adh1 (alcohol dehydrogenase 1), respectively (6, 12). Loz1 also negatively regulates the expression of its own gene (6). Although most studies to date have focused on the mechanisms by which zinc-responsive transcriptional activators sense zinc, in this study, we investigated how Loz1 is regulated by zinc. We found that a minimal domain containing two C2H2-type zinc finger domains and a neighboring accessory domain is sufficient for DNA binding and zinc-dependent repression.
EXPERIMENTAL PROCEDURES
Yeast Strains and Growth Conditions
All strains created in this study are derivatives of the wild-type strain JW81 (h− ade6-M210 leu1-32 ura4-D18) (13), adh1Δ strain ABY83 (h+ ade6-M210 leu1-32 ura4-D18 adh1Δ::kanMX6), or loz1Δ strain ABY540 (h− ade6-M210 leu1-32 ura4-D18 loz1Δ::kanMX6) (6). Strains were created by integrating linearized plasmid constructs using standard transformation procedures. S. pombe strains were grown in YES medium or in zinc-limited Edinburgh minimal medium (ZL-EMM)2 with or without the indicated zinc supplement (12). For all experiments with ZL-EMM, cells were pregrown to exponential phase in YES medium. Cells were washed twice in ZL-EMM, diluted to a final A600 of 0.5 and grown for a further 14–16 h in ZL-EMM with or without zinc.
Plasmid Construction
All plasmids used in this study were linearized with NruI, BsiWI, or NcoI before integration at the leu1 or ade6 locus in the yeast strains, JW81, ABY83, or ABY540 (6). The fusion of the zhf1 promoter to the lacZ reporter gene was generated by PCR-amplifying an ∼1-kb fragment of the zhf1 promoter. PCR primers contained EagI and BamHI restriction sites to facilitate cloning into similar sites in the vector JK-lacZ (12). The construction of the adh4-lacZ and loz1-lacZ reporters has been described previously (6, 12). The reporter pTN-zrt1-lacZ is a derivative of the plasmid pTN215 (NBRP ID FYP484), which was obtained from the National BioResource Project, Japan. pTN-zrt1-lacZ was generated by introducing the lacZ gene into the PstI/ApaI sites of pTN215 to generate pTN-lacZ. The zrt1 promoter was then introduced as a SacII/PstI fragment to generate pTN-zrt1-lacZ. pZ-loz1GFP is a derivative of pL-loz1GFP (6) in which a KpnI/EcoRI loz1 promoter fragment was replaced with a PCR-amplified 1-kb zhf1 promoter fragment. The S489F and M513I mutations were introduced into pTH-Loz1 plasmid using QuikChange mutagenesis (Agilent Technologies). The pTH-Loz1 ZF fusion was generated by amplifying the C terminus of Loz1 with primers containing BamHI and XhoI sites. The resulting PCR product was digested with BamHI/XhoI and was cloned into similar sites in the vector Pet32a (EMD Millipore). Constructs expressing pL-Loz1GFP truncations were generated by PCR-amplifying the respective region of the loz1 ORF. PCR primers contained EcoRI and BamHI restriction sites to facilitate cloning into EcoRI/BamHI-digested pL-loz1GFP. Constructs expressing Loz1-GFP truncations from the zhf1 promoter were generated by replacing the loz1 promoter with a KpnI/EcoRI zhf1 promoter fragment released from pZ-loz1GFP. pZ-MtfAGFP was generated by using overlapping PCR to amplify the mtfA ORF from an Aspergillus nidulans genomic DNA template. Primers contained EcoRI/BamHI sites to allow replacement of the loz1 ORF with MtfA in the vector pZ-loz1GFP. Chimeras of Loz1 and MtfA were generated by a similar strategy using overlapping PCR and pZ-MtfAGFP and pL-Loz1GFP plasmid templates. All plasmid constructs were confirmed by sequence analysis.
RNA Blot, Immunoblot Analysis, and β-Galactosidase Assays
For RNA blot analysis, total RNA was purified using hot acidic phenol, and total RNA was separated on formaldehyde gels by standard procedures. All single-stranded 32P-labeled RNA probes were generated from a PCR template using a MAXISCRIPT T7 kit (Ambion) according to the manufacturer's instructions. PCR primers used for probe generation have been described previously (6, 12). Total protein extracts were prepared from yeast as described previously (6). Immunoblots were incubated with the primary antibodies anti-GFP (G1544, Sigma) and anti-Act1 (ab3280-500, Abcam) and secondary antibodies IRDye800CW-conjugated anti-mouse IgG (LI-COR) and IRDye680-conjugated anti-rabbit IgG (LI-COR). Signal intensities were analyzed using the Odyssey infrared image system (LI-COR). β-Galactosidase assays were performed as described previously (12).
Recombinant Protein Purification and EMSAs
TH-Loz1 truncations and mutated derivatives were expressed in Escherichia coli and were purified using Ni2+-nitrilotriacetic acid Superflow (Qiagen) columns as described previously (6). Double-stranded DNA probes for the EMSAs were created by end labeling with [γ-32P]dATP (PerkinElmer Life Sciences) using T4 polynucleotide kinase. Binding reactions were performed in buffer containing a final concentration of 50 μg/ml BSA (New England Biolabs), 1 μg of poly(dI-dC) (Sigma), 200 ng of the indicated protein, and 40 μmol of labeled probe. Competition studies were performed by incubating protein with the indicated unlabeled probe for 20 min at room temperature before the addition of the labeled probe. Reactions were incubated for an additional 10 min before protein·DNA complexes were resolved on 6% (w/v) acrylamide Tris borate-EDTA gels. Gels were dried and subjected to phosphorimaging analysis.
RESULTS
Loz1 Function Is Regulated by Zinc at a Post-translational Level
In previous studies, we found that Loz1 represses its own expression in zinc-replete cells (6). Because the levels of Loz1 within a cell could potentially influence target gene regulation, we initially investigated how autoregulation of loz1 levels contributed to the overall zinc-dependent regulation of Loz1 target genes. To determine the extent to which autoregulation affected gene expression, constructs were generated in which a Loz1-GFP fusion protein was expressed from its own promoter (pL-loz1GFP) or the zhf1 promoter (pZ-loz1GFP). The zhf1 promoter was used because previous studies suggested that zhf1 expression is not regulated by zinc (12, 14, 15). These results were also confirmed by measuring β-galactosidase activity in wild-type cells expressing a zhf1-lacZ reporter gene and control zinc-regulated adh4-lacZ and loz1-lacZ reporters (Fig. 1A). Following growth in ZL-EMM with or without a zinc supplement, no major changes in β-galactosidase activity were observed in wild-type cells expressing the zhf1-lacZ reporter gene, consistent with zinc not affecting expression from the zhf1 promoter. To determine the extent to which loz1 expression level influences target gene expression, pL-loz1GFP, pZ-loz1GFP, or the empty vector was introduced into loz1Δ cells, and zinc-dependent changes in gene expression were examined by RNA blot analysis (Fig. 1B). Expression of pL-loz1GFP or pZ-loz1GFP led to the strong repression of adh4 and zrt1 expression in zinc-replete cells. Interestingly, the introduction of pL-loz1GFP or pZ-loz1GFP resulted in slightly lower levels of adh4 expression under zinc-limiting conditions relative to the adh4 transcript levels observed in loz1Δ. However, when related constructs lacking the GFP tag were expressed in loz1Δ cells, adh4 expression was fully induced under zinc-limiting conditions (data not shown). Thus, the slight increase in repression observed at the adh4 locus in the presence of pL-loz1GFP and pZ-loz1GFP is probably a result of the introduction of the C-terminal GFP tag. Despite this slight overcompensation, zinc-dependent changes in gene expression were similar whether loz1GFP was expressed from its own promoter or at a constant level from the zhf1 promoter. These results indicate that although Loz1 regulates its own expression, other post-transcriptional mechanisms must play a major role in the regulation of Loz1 function by zinc.
FIGURE 1.
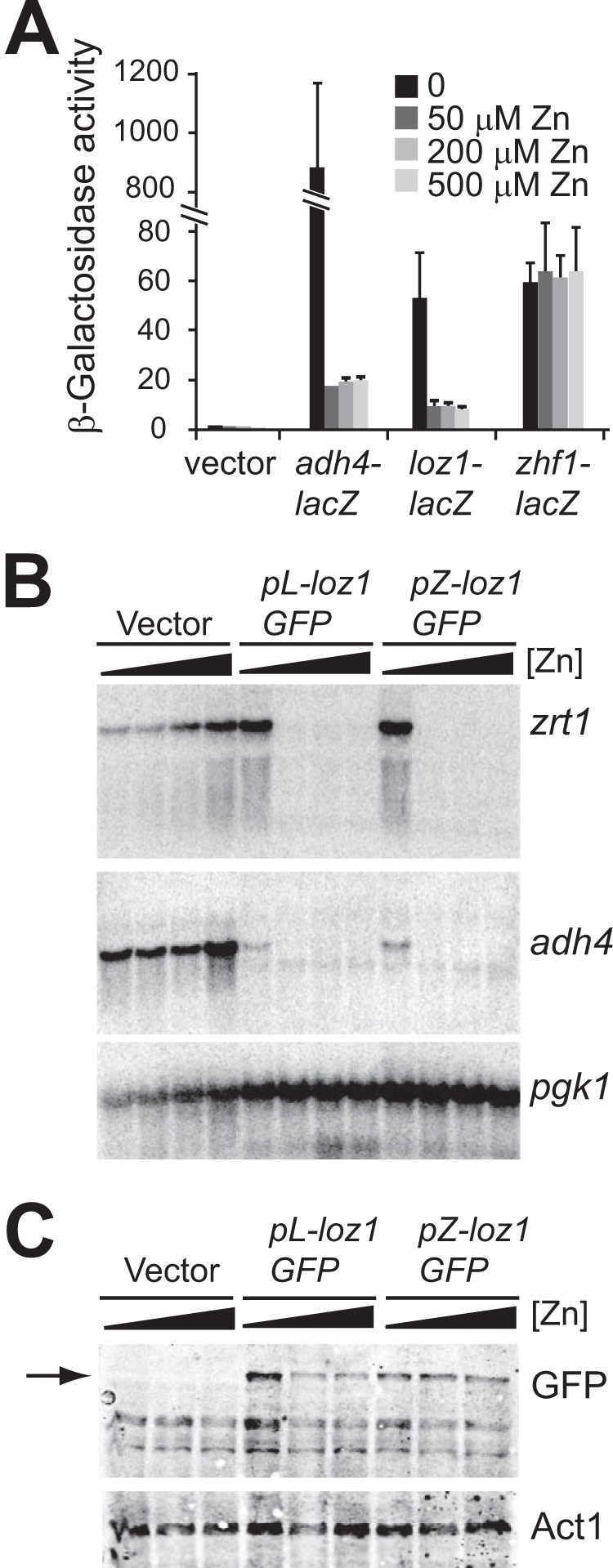
Loz1 is regulated at a post-translational level by zinc. A, wild-type cells containing the empty vector or the integrated adh4-lacZ, loz1-lacZ, or zhf1-lacZ reporter constructs were grown in ZL-EMM supplemented with the indicated amount of zinc before β-galactosidase activity was measured. B, loz1Δ cells containing the empty vector, pL-loz1GFP, or pZ-loz1GFP were grown in ZL-EMM supplemented with 0, 50, 200, or 500 μm zinc. Total RNA was extracted and subjected to RNA blot analysis. Blots were probed for zrt1, adh4, and the loading control pgk1 (phosphoglycerate kinase 1). C, immunoblot analysis of crude protein extracts prepared from loz1Δ cells containing the indicated plasmids. Cells were grown in ZL-EMM supplemented with 0, 50, or 200 μm zinc. Immunoblots were probed with antibodies raised against GFP or the loading control actin (Act1). An arrow indicates the band that is specific to Loz1-GFP. Shown are the means from three independent experiments, and the error bars indicate ± S.D.
Zinc could directly or indirectly alter Loz1 function by affecting mRNA stability, protein translation, protein stability, cellular localization, DNA binding activity, and/or a repressor function. To determine whether zinc affected Loz1 protein levels, loz1Δ cells expressing pZ-loz1GFP were grown in ZL-EMM supplemented with 0–200 μm zinc, and crude protein extracts were prepared for immunoblot analysis (Fig. 1C). As controls, crude protein extracts were also prepared from loz1Δ cells expressing the empty vector or pL-loz1GFP. When immunoblots were probed for GFP, a single band was detected that was specific to cells expressing either of the Loz1GFP fusions. Consistent with loz1 autoregulating its own expression, an ∼2-fold reduction in Loz1GFP levels was observed in zinc-replete cells expressing pL-loz1GFP. However, when loz1GFP was expressed from the zhf1 promoter, Loz1GFP accumulated at a constant level. The zhf1-driven Loz1GFP was also localized to the nucleus under both zinc-limiting and zinc-replete conditions (data not shown). Thus, when expressed at a constant level, Loz1 protein accumulates in the nucleus under both zinc-limiting and zinc-replete conditions; however, Loz1 only represses target gene expression when zinc levels are high. Together, these results suggest that zinc directly or indirectly regulates Loz1 function at a post-translational level.
Isolation of Mutations That Disrupt Loz1 Function
To understand how Loz1 is regulated by zinc, we used a genetic approach to isolate amino acid residues that were critical for Loz1 function. In our previous study, we isolated an adh1Δ strain containing a spontaneous partial loss of function mutation in loz1 (designated loz1-1) (6). Under nutrient-rich conditions, the loz1-1 allele conferred a growth advantage to adh1Δ cells. It also resulted in adh1Δ cells surviving in the presence of the respiration inhibitor antimycin A. Based on these observations, we screened for further antimycin A-resistant adh1Δ colonies. We hypothesized that these colonies might also contain spontaneous mutations that directly or indirectly affected Loz1 function.
To identify antimycin A-resistant adh1Δ strains, 1 × 107 adh1Δ cells were plated onto YES medium. Cells were grown for 3 days at 31 °C and then were transferred to YES plates containing antimycin A. After 2–5 days of growth, ∼5–8 colonies were detected per 1 × 107 cells. Similar results were obtained when adh1Δ cells were transferred onto plates supplemented with antimycin A and 500 μm zinc or with antimycin A and a 50 μm concentration of the zinc ion chelator EDTA (Fig. 2A) (data not shown). During these studies, we also noted that when adh1Δ cells are plated onto YES medium, a few colonies grew faster than others. When one of these faster growing colonies was isolated and transferred to antimycin A plates, it was also able to survive.
FIGURE 2.
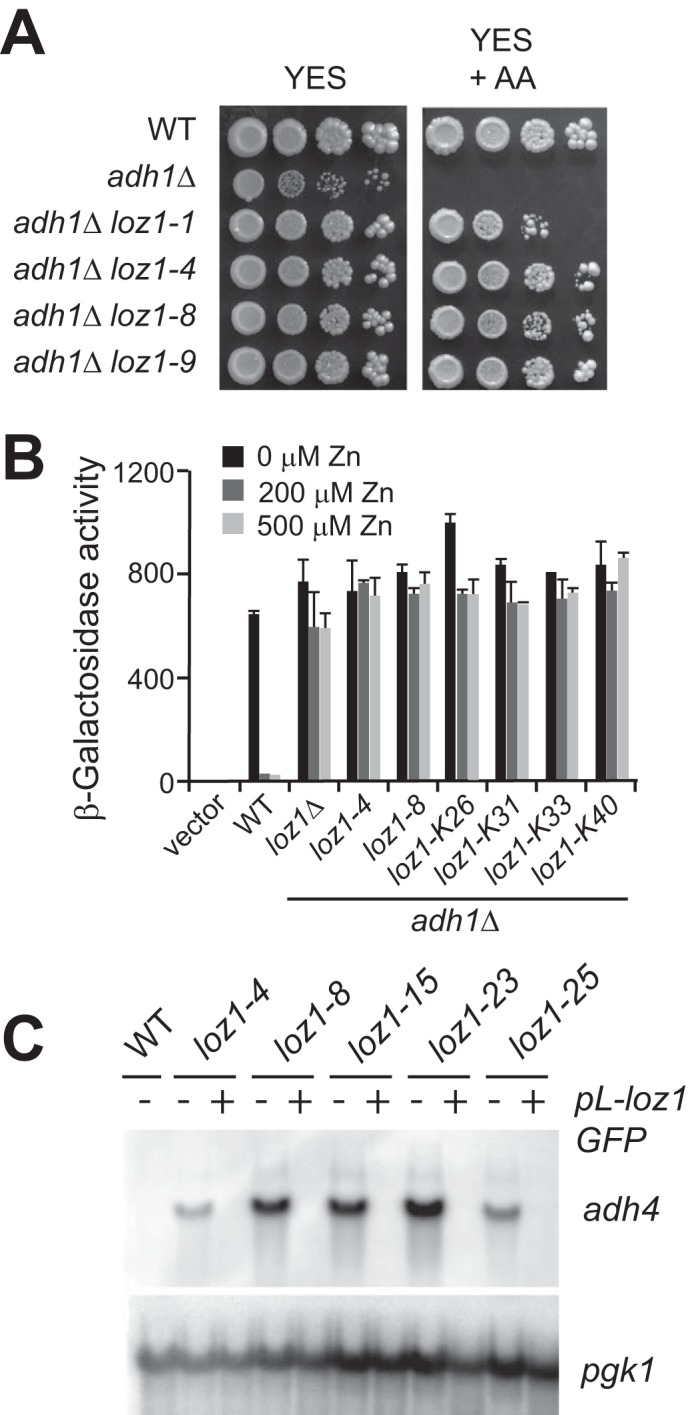
Abnormal zinc homeostasis in antimycin A-resistant adh1Δ cells. A, wild-type, adh1Δ, adh1Δ loz1-1, adh1Δ loz1-4, adh1Δ loz1-8, and adh1Δ loz1-9 cells were grown overnight in YES medium before cells were spotted in 10-fold serial dilutions onto YES medium without or with (+AA) a 10 μg/μl antimycin A supplement. Plates were incubated for 3 days at 31 °C before photography. B, wild-type cells expressing the empty vector (Vector) or adh4-lacZ reporter (WT) or adh1Δ cells containing the indicated loz1 allele and the adh4-lacZ reporter were grown in ZL-EMM with or without a 200 or 500 μm zinc supplement. Cells were harvested, and β-galactosidase activity was measured using standard procedures. C, wild-type cells or haploid strains containing the indicated loz1 alleles or these strains with the plasmid pL-loz1GFP were grown overnight in YES medium. Total RNA was extracted and subjected to RNA blot analysis. Error bars, S.E.
To determine if zinc homeostasis was impaired in any of the antimycin A-resistant adh1Δ cells, adh4 transcript levels were examined by RNA blot analysis in 36 independently isolated colonies. In adh1Δ cells grown in nutrient-rich YES medium, adh4 gene expression is repressed. However, when the 36 antimycin A-resistant strains were grown under similar conditions, adh4 transcripts accumulated to high levels, suggesting that each of these isolates contained a mutation(s) that resulted in adh4 expression under nutrient-rich conditions (data not shown). Because Loz1 is required for the transcriptional repression of adh4 in zinc-replete cells, we also tested whether the increased adh4 expression observed in the antimycin A resistant strains was typically dependent upon the adh4 promoter. For this, an adh4-lacZ reporter gene was introduced into six of the antimycin A-resistant colonies, and β-galactosidase activity was measured following growth in ZL-EMM with or without a 200 or 500 μm zinc supplement (Fig. 2C). As controls, adh4-lacZ reporter activity was also examined in wild-type and adh1Δ loz1Δ cells grown under similar conditions. We were unable to measure adh4-lacZ activity in adh1Δ cells because this strain has a severe growth defect in ZL-EMM with or without zinc (data not shown). Growth in ZL-EMM with a 200 or 500 μm zinc supplement led to an ∼30-fold decrease in β-galactosidase in wild-type cells. Although a small zinc-dependent change in β-galactosidase was observed in the presence of the loz1-K26 allele, for the most part, there was no major change in β-galactosidase activity in any of the antimycin A-resistant colonies. These results are consistent with the mutation(s) in these strains impairing gene repression. Further genetic backcrosses to wild type cells revealed that the impaired zinc-responsive gene expression observed in the antimycin A-resistant colonies was a result of a single site mutation in the genome (data not shown). In addition, introduction of pL-loz1GFP into five backcrossed isolates fully restored zinc-dependent repression of adh4 (Fig. 2C). Because these results were consistent with at least five of the strains containing a mutation within the loz1 ORF, DNA was isolated from each antimycin A-resistant adh1Δ colony, and the loz1 ORF was sequenced. All 36 colonies contained a mutation in the loz1 ORF (Tables 1 and 2). Of these, 18 were nucleotide insertions, deletions, or duplications resulting in a frameshift; 9 contained point mutations leading to the introduction of a premature stop codon; and 9 contained point mutations that resulted in a change to an amino acid residue within the double zinc finger domain (Fig. 3).
TABLE 1.
loz1 alleles containing a point mutation
| Allele | DNA mutation | Amino acid substitution | Isolation condition |
|---|---|---|---|
| loz1-1 | C1528G | R510G | Spontaneous mutation identified via transformationa |
| loz1-4 | T1430C | F477S | 10 μg/ml antimycin A |
| loz1-5 | G865T | G289Stop | 10 μg/ml antimycin A |
| loz1-8 | G1418A | C473Y | 10 μg/ml antimycin A |
| loz1-15 | T1404G | Y468Stop | 10 μg/ml antimycin A |
| loz1-K5 | T1408C | C470R | Spontaneous mutation in YES medium |
| loz1-23 | T1417G | C473G | 5 μg/ml antimycin A |
| loz1-25 | C1466T | S489F | 5 μg/ml antimycin A |
| loz1-K25 | G298T | E100Stop | 10 μg/ml antimycin A |
| loz1-K26 | C1558G | H520D | 10 μg/ml antimycin A |
| loz1-K27 | C931T | Q311Stop | 10 μg/ml antimycin A |
| loz1-K29 | C952T | Q318Stop | 10 μg/ml antimycin A + 500 μm zinc |
| loz1-K30 | C874T | Q292Stop | 10 μg/ml antimycin A + 500 μm zinc |
| loz1-K31 | G1436T | R479M | 10 μg/ml antimycin A |
| loz1-K33 | G1539A | M513I | 10 μg/ml antimycin A + 500 μm zinc |
| loz1-K34 | C889T | Q297Stop | 10 μg/ml antimycin A + 500 μm zinc |
| loz1-K35 | C1327T | R443Stop | 10 μg/ml antimycin A + 500 μm zinc |
| loz1-K36 | T666G | Y222Stop | 10 μg/ml antimycin A + 500 μm zinc |
| loz1-K40 | C1456T | H486Y | 10 μg/ml antimycin A |
a The original loz1-1 allele reported in Ref. 6.
TABLE 2.
Other loz1 alleles
| Type of mutation | Allele | Mutation | Summary | Isolation condition |
|---|---|---|---|---|
| Insertion | loz1-9 | nt 428TC | 2-bp insertion | 10 μg/ml antimycin A |
| loz1-12 | nt 486T | 1-bp insertion | 10 μg/ml antimycin A | |
| loz1-K18 | nt 1404A | 1-bp insertion | 10 μg/ml antimycin A | |
| loz1-K20 | nt 529A | 1-bp insertion | 10 μg/ml antimycin A | |
| loz1-K22 | nt 796A | 1-bp insertion | 10 μg/ml antimycin A | |
| loz1-K23 | nt 1283T | 1-bp insertion | 10 μg/ml antimycin A | |
| loz1-K24 | nt 636T | 1-bp insertion | 10 μg/ml antimycin A | |
| loz1-K32 | nt 349C | 1-bp insertion | 10 μg/ml antimycin A + 500 μm zinc | |
| loz1-K37 | nt 486T | 1-bp insertion | 10 μg/ml antimycin A + 500 μm zinc | |
| loz1-K38 | nt 486T | 1-bp insertion | 10 μg/ml antimycin A | |
| Deletion | loz1-K13 | nt 1484 | 1-bp deletion | 10 μg/ml antimycin A |
| loz1-K17 | nt 515 | 1-bp deletion | 10 μg/ml antimycin A | |
| loz1-K19 | nt 1193 | 1-bp deletion | 10 μg/ml antimycin A | |
| loz1-K28 | nt 609 | 1-bp deletion | 10 μg/ml antimycin A + 50 μm EDTA | |
| loz1-K39 | nt 1341–1342 | 2-bp deletion | 10 μg/ml antimycin A | |
| Duplication | loz1-K14 | Duplication of nt 681–709 | 29-bp duplication | 10 μg/ml antimycin A |
| loz1-K16 | Duplication of nt 681–709 | 29-bp duplication | 10 μg/ml antimycin A | |
| loz1-K21 | Duplication of nt 870–880 | 11-bp duplication | 10 μg/ml antimycin A |
FIGURE 3.
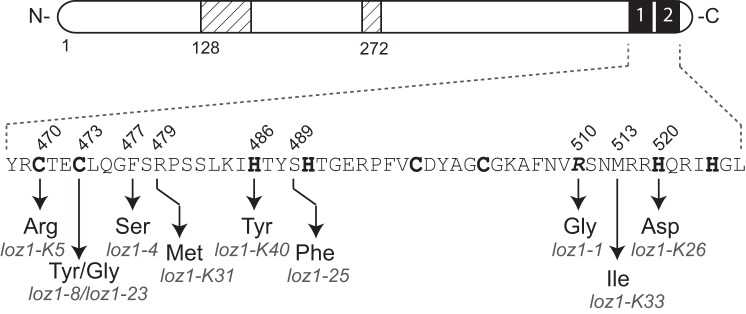
A schematic diagram illustrating amino acid substitutions present in different Loz1 alleles. Cysteine and histidine residues predicted to coordinate zinc are shown in boldface type, and the original loz1-1 mutation is shown in boldface and italic type.
Mutations in the Loz1 Zinc Finger Domain Affect DNA Binding Function
Previously, we had found that a C470G substitution in zinc finger 1 prevented Loz1 from binding to a GNNGATC cis-acting element in vitro, suggesting that the Loz1 double zinc finger domain was necessary for DNA binding (6). In further support of the Loz1 zinc finger domains having a direct role in DNA binding, all of the newly isolated alleles primarily targeted amino acid residues predicted to be involved in zinc ion coordination (C470R, C473Y, C473G, H486Y, and H520D), the formation of the zinc finger hydrophobic core (F477S), or making site-specific interactions with DNA (R479M). Two exceptions were the loz1-25 allele (S489F) and the loz1-K33 allele (M513I), each containing amino acid substitutions that do not target amino acids known to be critical for zinc finger formation or interactions with DNA. To determine whether these amino acid substitutions affected DNA binding function, recombinant proteins containing amino acids 427–522 of Loz1 fused to a Trx-His tag (TH-Loz1) with or without S489F or M513I substitutions were purified from E. coli using nickel-nitrilotriacetic acid affinity chromatography (Fig. 4B). The tag alone and TH-loz1 C470G were also purified as controls. The ability of each protein to bind to a radiolabeled double-stranded oligonucleotide containing the predicted Loz1 binding site (GNNGATC) was then examined using electrophoretic mobility shift assays (EMSAs) (Fig. 4, A and C). As expected, a DNA·protein complex was detected when the TH-Loz1 fusion was incubated with the wild-type oligonucleotide. Formation of this complex was inhibited by incubation with excess levels of a non-radiolabeled wild-type oligonucleotide but not a non-radiolabeled mutant oligonucleotide. When similar levels of TH-Loz1 recombinant proteins containing the C470G, S489F, or M513I substitutions were incubated with the wild-type oligonucleotide, no complex was detected in the presence of the C470G and S489F substitutions. A weak DNA·protein complex was observed in the presence of the M513I substitution, suggesting that this mutation significantly impaired but did not entirely disrupt DNA binding activity. Thus, consistent with the zinc finger domain being critical for Loz1 function, all of the amino acid substitutions identified in the screen impaired or disrupted DNA binding or targeted amino acids predicted to be critical for zinc finger formation.
FIGURE 4.
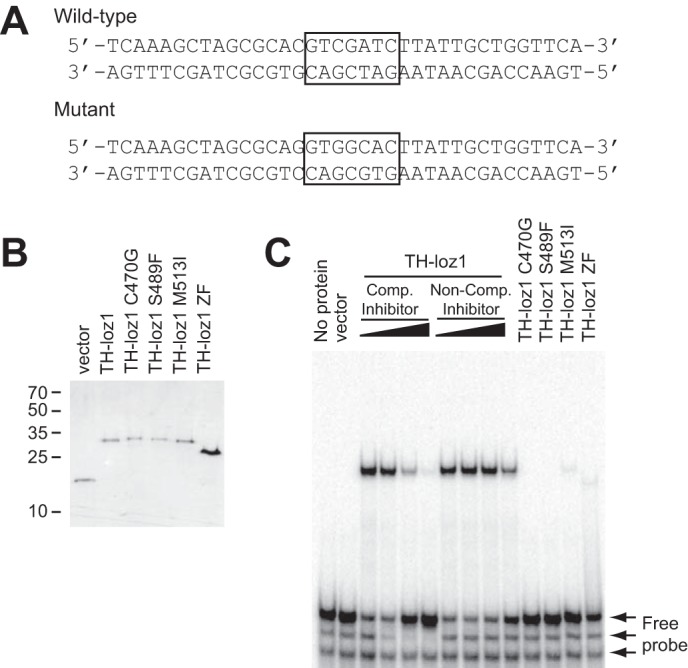
S489F and M513I substitutions affect Loz1 DNA binding in vitro. A, DNA sequence of the wild-type and mutated oligonucleotide probes. The loz1 binding site is boxed. B, SDS-PAGE analysis of the purified Trx-histidine tag (vector) and recombinant proteins TH-loz1, TH-loz1 C470G, TH-loz1 S489F, TH-loz1 M513I, or TH-loz1 ZF. Proteins were visualized by staining with Coomassie Blue. The sizes of the molecular mass markers are shown in kDa on the left. C, representative EMSA using 40 μmol of 32P-labeled double-stranded oligonucleotide and 200 ng of the indicated purified recombinant protein. For competition studies, 0, 50×, 200×, or 500× of the WT unlabeled oligonucleotide (Comp Inhibitor), or mutant oligonucleotide (Non-Comp Inhibitor) was added to the reactions.
A Minimal Region Required for Zinc Responsiveness Maps to the DNA Binding Domain
Because the genetic screen did not identify any amino acid substitutions outside of the DNA binding domain and Loz1 contains a number of other conserved regions, a series of Loz1-GFP truncations were generated to determine which region(s) of Loz1 was required for zinc-responsive regulation (Fig. 5A). Each truncation was expressed from its native promoter in loz1Δ cells, and immunoblot analysis and fluorescence microscopy were performed to confirm that the fusion protein was produced and was localized to the nucleus (Fig. 5B) (data not shown). For unknown reasons, the smaller truncations (Fig. 5B, constructs #3–5) accumulated to higher levels than those of the full-length Loz1GFP under zinc-limited conditions (construct #1). With the exception of construct 2, all fusion proteins were found within the nucleus. A heterologous nuclear localization signal from the SV40 large T antigen was therefore added to construct 2 to ensure its nuclear localization. Although the nuclear localization signal containing construct was able to partially rescue loz1Δ phenotypes (see below), we were not able to detect it by immunoblot analysis (Fig. 5B, construct #2), suggesting that it may accumulate to lower levels or be less stable than the other truncations.
FIGURE 5.
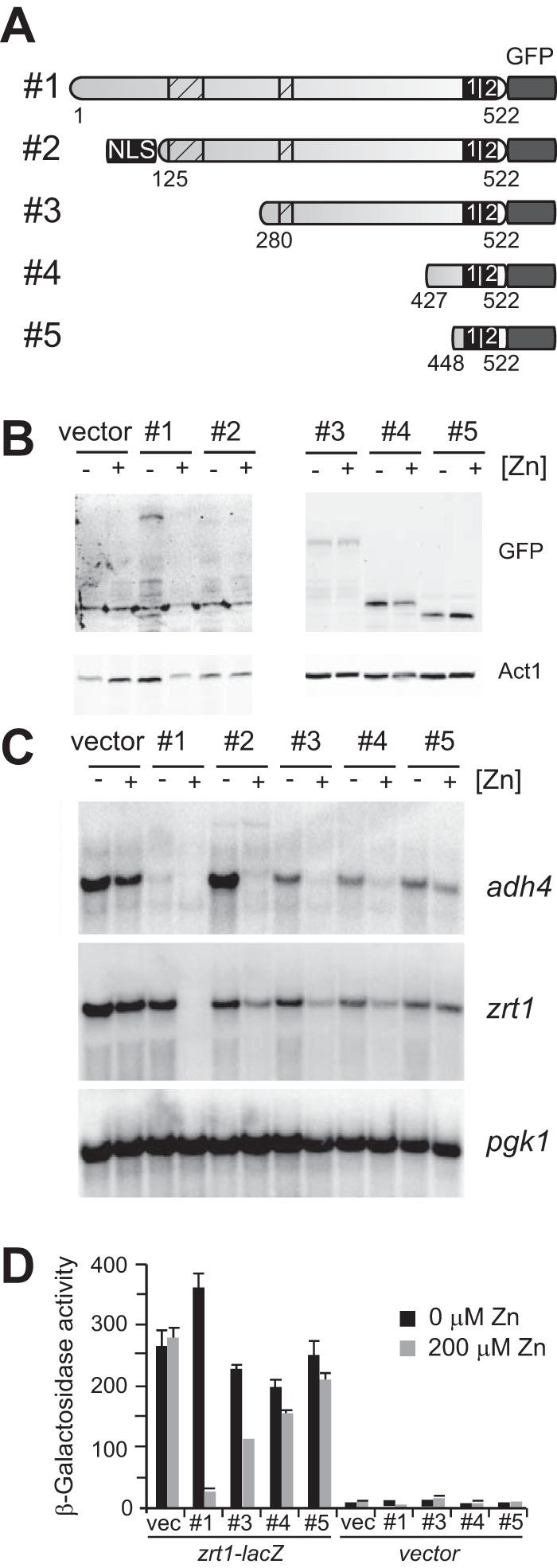
Mapping a minimal zinc-responsive domain. A, schematic diagram of pL-Loz1GFP truncations. Shown are a C-terminal GFP tag, the double zinc finger domain (numbered black boxes), regions conserved in Loz1 homologs from other Schizosaccharomyces sp. (striped boxes), and introduced nuclear localization signal. The amino acids included in each truncation are also indicated. B and C, loz1Δ cells expressing the constructs shown in A were grown in ZL-EMM with or without a 200 μm zinc supplement. Cells were harvested, and crude protein extracts were prepared for immunoblot analysis (B), or total RNA was extracted for RNA blot analysis (C). D, loz1Δ cells co-expressing the constructs shown in A and reporter gene pTN-zrt1-lacZ (zrt1-lacZ) or empty vector pTN-lacZ (vector) were grown as described in B before β-galactosidase activity was measured by standard procedures. Error bars, S.E.
To determine whether any of the truncations were functional, the ability of each fusion protein to repress target gene expression was examined by RNA blot analysis (Fig. 5C). Although the smallest truncation (construct #5) was not able to confer repression, introduction of constructs 2–4 resulted in the strong repression of adh4 expression in zinc-replete cells and a more modest repression of zrt1 expression in zinc-replete cells. To determine the extent to which the smaller truncation affected zrt1 expression, a zrt1-lacZ reporter was co-expressed with the full-length Loz1GFP and the Loz1GFP truncation 3–5. When these cells were grown in ZL-EMM with or without a zinc supplement, only the full-length Loz1GFP resulted in the strong repression of β-galactosidase activity in zinc-replete cells (Fig. 5D). However, minor zinc-dependent changes in β-galactosidase activity were observed in cells expressing constructs 3 and 4, consistent with the mild zinc-dependent regulation observed in the RNA blot analysis.
Because the above truncations were expressed from the loz1 promoter and were therefore subject to negative autoregulation, depending on their activity, similar experiments were performed using zhf1-driven truncations (Fig. 6, A–C). This analysis indicated that when expressed at a constant level, construct 4 was able to mediate partial zinc-responsive regulation of adh4 and zrt1 gene expression. Thus, when expressed at a constant level, the minimal region of Loz1 containing the last 96 amino acids is sufficient to confer at least some zinc-dependent regulation. However, full repression of target gene expression requires additional N-terminal sequences.
FIGURE 6.
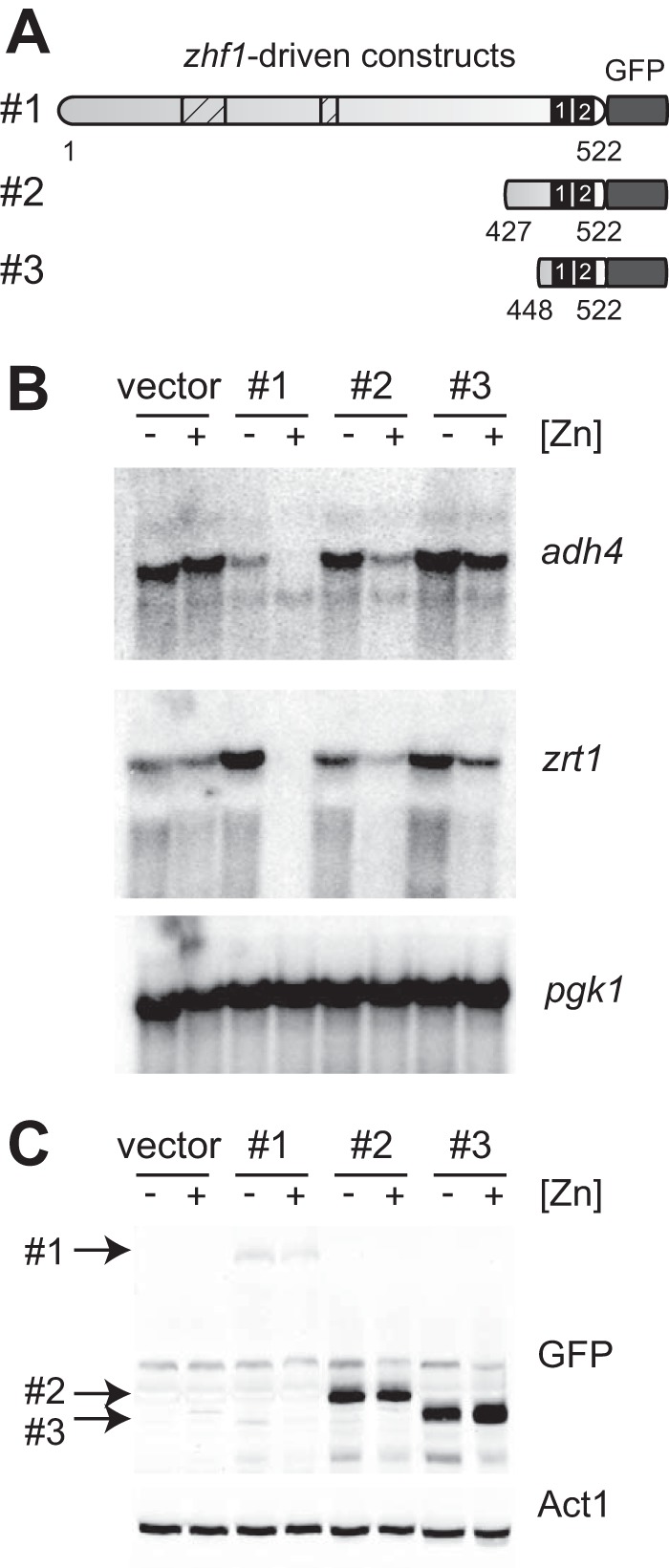
A minimal domain containing the last 96 amino acids of Loz1 is sufficient to confer zinc-dependent repression of adh4. A, schematic diagram of pZ-loz1GFP truncations. See the legend to Fig. 5A for details. B and C, loz1Δ cells expressing the constructs shown in A were grown in ZL-EMM with or without a 200 μm zinc supplement. Cells were harvested, and total RNA was extracted and subjected to RNA blot analysis (B), or crude protein extracts prepared for immunoblot analysis (C).
An N-terminal Zinc Finger Accessory Domain Enhances DNA Binding
The 96-amino acid region that can confer partial zinc-dependent repression contains both C2H2 zinc fingers and an additional 40 amino acid residues adjacent to zinc finger 1. In other transcription factors that bind to DNA via zinc finger domains, an accessory domain that is adjacent to the first or last zinc finger can be critical for DNA recognition and/or high affinity binding (16–19). Because the minimal zinc-responsive domain included a 40-amino acid region that was not part of the double zinc finger domain, we tested whether additional N-terminal sequences that were adjacent to zinc finger 1 were required for DNA binding in vitro. For this, a recombinant protein containing amino acids 455–522 of Loz1 fused to a Trx-His tag (TH-Loz1 ZF) was purified from E. coli. The ability of this protein to bind to a GNNGATC element was then examined by EMSA (Fig. 4C, TH-Loz1 ZF). In EMSAs, a DNA·protein complex was detected in the presence of TH-Loz1 ZF and the double-stranded oligonucleotide containing the Loz1 binding site. However, the levels of this complex were significantly lower than those of the TH-Loz1·DNA complex. Similar results were also obtained using a recombinant protein that contained amino acids 448–552 of Loz1 (data not shown). Thus, Loz1 amino acids 427–552 are sufficient for high affinity DNA binding in vitro and can confer partial zinc-dependent repression in vivo. However, in the absence of the adjacent 40-amino acid “accessory domain,” DNA binding affinity is severely reduced in vitro, and zinc-dependent regulation is lost in vivo. Together, these results suggest that the N-terminal accessory domain is necessary for high affinity DNA binding.
The partial zinc-dependent repression observed in cells expressing amino acids 427–522 of Loz1 (Fig. 5A, construct #4) could result from N-terminal sequences being necessary for gene repression (e.g. these sequences might be important for the recruitment of co-repressors or chromatin-remodeling proteins), or they might result from the N-terminal region of Loz1 being necessary for full zinc responsiveness. To determine whether the Loz1 C-terminal region was sufficient for zinc-dependent repression, we took advantage of the differences in sequence conservation between Loz1 and MtfA (master transcription factor A) from A. nidulans. MtfA is a transcription factor that plays a primary role in regulating secondary metabolism and morphogenesis (20). MtfA contains a double zinc finger domain that shares significant sequence similarities to the Loz1 zinc fingers (Fig. 7A). However, outside of this domain (including the 40-amino acid accessory domain), there is no sequence conservation. Because the sequence conservation between Loz1 and MtfA was limited to the zinc fingers, we tested whether expression of mtfA-GFP from the zhf1 promoter would complement loz1Δ (Fig. 7, B and C). Despite the strong homology in the zinc finger domain, only a small zinc-dependent decrease in adh4 or zrt1 gene expression was observed in the loz1Δ cells expressing mtfA-GFP. However, when chimeras of MtfA containing the Loz1 zinc finger inclusive of the 40-amino acid accessory domain (construct 3) or in which the MtfA “accessory domain” was replaced with the Loz1 accessory domain (construct 4) were expressed in loz1Δ cells, both zrt1 and adh4 gene expression were robustly regulated by zinc. The derepression of adh4 and zrt1 expression in zinc-limited cells was not a result of altered protein stability or cellular localization, because all fusion proteins were stable (Fig. 7D) and nucleus-localized (data not shown). Together, these results are consistent with the Loz1 zinc finger domains and adjacent accessory domain having a dual role in DNA binding and zinc-dependent repression in vivo.
FIGURE 7.
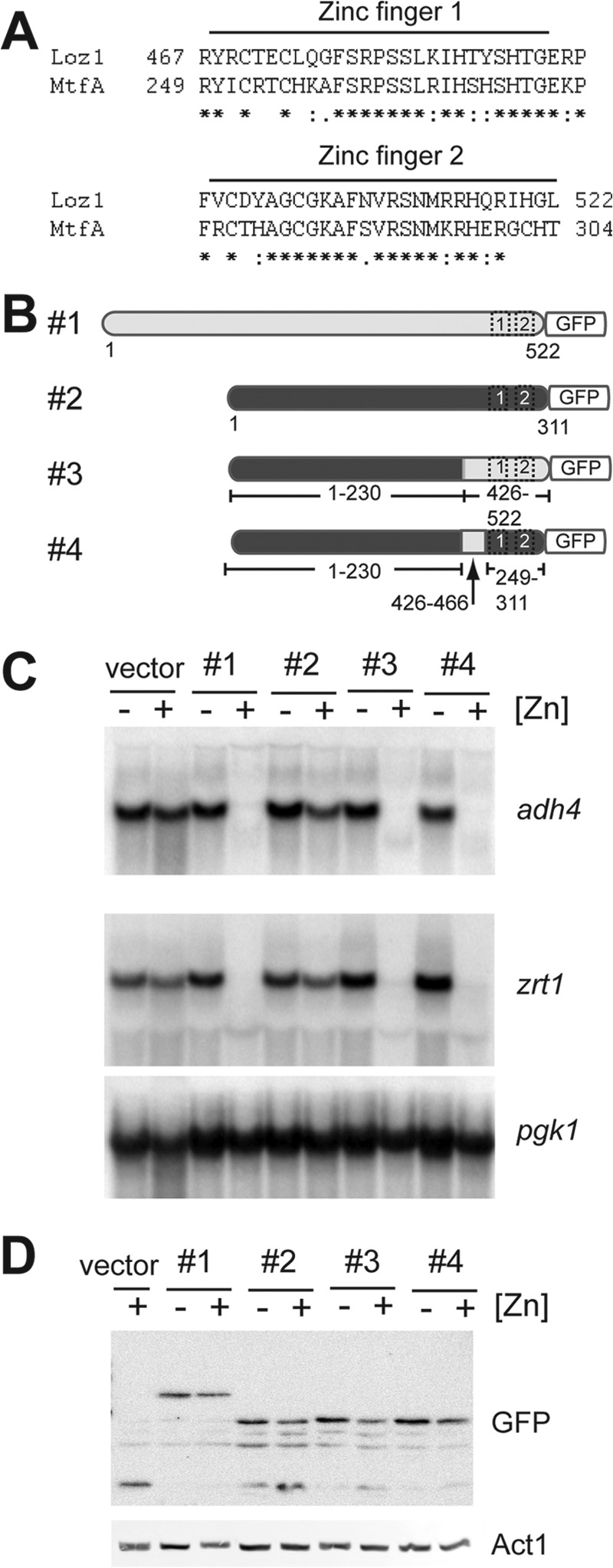
pZ-MtfAGFP is regulated by zinc in loz1Δ cells. A, an alignment of the double zinc finger domains from Loz1 and MtfA. B, schematic diagram of Loz1/MtfA chimeras. Loz1 sequences are shown in light gray, and MtfA sequences are shown in dark gray. Numbers represent amino acid number in the respective proteins. C and D, loz1Δ cells expressing the constructs shown in B were grown in ZL-EMM with or without a 200 μm zinc supplement. Cells were harvested, and total RNA was extracted for RNA blot analysis (C), or crude protein extracts were prepared for immunoblot analysis (D).
DISCUSSION
In our previous work, we isolated a partial loss of function mutation in loz1, which conferred a growth advantage to adh1Δ cells and enabled their survival on plates containing the respiration inhibitor antimycin A. In this study, we isolated 36 additional adh1Δ colonies that were able to grow in the presence of antimycin A. Further characterization of these colonies revealed that they all contained a mutation leading to the loss of Loz1 function. These results strongly support our previous observations that disruption of Loz1 function is advantageous to the growth of adh1Δ cells and highlight a novel approach to identify mutations that impair Loz1 function.
All of the newly identified loz1 alleles resulted in the premature truncation of the Loz1 protein or an amino acid substitution within the Loz1 double zinc finger domain. Of the alleles leading to an amino acid substitution, most altered residues involved in zinc ion coordination or the formation of the hydrophobic core. One exception was the loz1-K31 allele, which led to an R479M substitution at position −1 of the α helix in zinc finger 1. In C2H2-type zinc finger domains, side chains from amino acid residues at positions −1, 2, 3, and 6 of the α helix frequently make hydrogen bond contacts with DNA (2). Thus, the isolation of an allele encoding an R479M substitution is consistent with the Loz1 zinc finger domains being necessary for site-specific DNA binding. An unexpected mutation was S489F in zinc finger 1. This residue is not predicted to coordinate zinc or interact with DNA. However, it is adjacent to a histidine residue that is critical for zinc ion coordination. Thus, the introduction of a bulky, hydrophobic residue at this position could interfere with zinc ion binding. Another unanticipated mutation was an M513I substitution at position 4 of the α helix in zinc finger 2. This mutation disrupted Loz1 function in vivo (Fig. 2B) and impaired DNA binding function in vitro (Fig. 4C). Although the side chains of Met-513 are not predicted to make contacts with DNA, methionine residues have a higher helix-forming propensity than isoleucines (21). Thus, this substitution may have destabilized the α helix in zinc finger 2, preventing Loz1 from effectively interacting with DNA. Because this residue is highly conserved in other Loz1 homologs, it is also possible that this residue has another yet to be identified function. Thus, the adh1Δ screen revealed a number of novel amino acid substitutions that interfere with Loz1 activity and further emphasized that the double zinc finger domain is critical for Loz1 function.
To gain additional insight into how Loz1 senses zinc, N-terminal truncations and chimeric proteins were generated to map a minimal domain necessary for zinc responsiveness. These studies revealed that 1) an accessory domain that is adjacent to zinc finger 1 is necessary for high affinity DNA binding and zinc-dependent repression, 2) a minimal region containing the last 96 amino acids of Loz1 is sufficient to mediate partial zinc-dependent regulation of adh4 and zrt1, and 3) a chimera of MtfA and the Loz1 accessory domain is able to complement loz1Δ. Because MtfA contains no regions of homology with Loz1 outside of the double zinc finger domain, this latter result is consistent with the double zinc finger domain and adjacent accessory domain being necessary for zinc-dependent repression. Based on the above observations, we propose that the last 96 amino acids of Loz1 play a dual role in DNA binding and in zinc sensing. Because truncations or mutations (e.g. M513I) that reduce DNA binding affinity in vitro result in the loss of zinc-dependent repression in vivo, our current working model is that changes in intracellular zinc levels affect Loz1 DNA binding activity and that high affinity DNA binding is critical for repression in vivo. In Zap1 and MTF-1, zinc finger domains can have regulatory functions and act as intracellular sensors of zinc (22). Because the two zinc finger domains in Loz1 are necessary for zinc-dependent repression, they may play a central role in zinc sensing. For example, if one or both of the zinc finger domains were only occupied by zinc when it is in excess, this would result in Loz1 binding to DNA and acting as a repressor in zinc-replete cells. Alternately, the accessory domain contains four histidine residues. Because these residues could potentially coordinate zinc, this domain might also bind zinc in a regulatory capacity. For example, zinc binding to the accessory domain might result in a protein conformation that is able to bind to DNA with a high affinity. To test whether DNA binding is regulated by zinc in vivo, we have used ChIP to investigate whether Loz1 is bound to target gene promoters in zinc-replete cells. However, in these studies, we find that Loz1 is a relatively sticky protein in that it readily associates with chromatin purified from target and non-target gene promoters (data not shown). Our future studies will therefore use alternative strategies to address whether zinc affects DNA binding function in vivo.
Another aspect of Loz1 function our studies highlighted is that a minimal domain containing the last 96 amino acids of Loz1 is able to mediate strong repression of the adh4 promoter but only weak repression of zrt1 expression. In ongoing studies, we have found that the smaller truncations are also only able to modestly regulate the loz1 promoter (data not shown), suggesting that that the N-terminal region of Loz1 is necessary for robust regulation of zrt1 and loz1. Studies of the adh4 promoter have found that it is complex, in that it contains multiple binding sites for other transcriptional activators and repressors (6, 15). In addition, multiple Loz1 DNA response elements are located downstream of the transcriptional start site. Differences in the affinity and positions of DNA response elements can affect the regulatory action of a transcription factor. For example, Zap1 binds to two high affinity zinc-responsive elements in the ZRT2 promoter and activates gene expression in zinc-limited cells (23). However, when zinc is extremely limited, Zap1 binds to a third low affinity zinc-responsive element that is located downstream of the ZRT2 transcriptional start site. Binding at this site restricts the progression of RNA polymerase II, inhibiting gene expression (23, 24). Because the adh4 promoter contains multiple Loz1 binding sites that are located downstream of the transcriptional start site, binding of the smaller Loz1 proteins to these downstream sites may allow them to be effective repressors of adh4 expression in zinc-replete cells.
So far, a number of different domains have been implicated in zinc sensing in eukaryotes. These include C2H2-type zinc fingers in Zap1, MTF-1, and now Loz1 and a cysteine/histidine-rich domain in AD1 from Zap1 (4, 22). Deletion of a cysteine-rich metallothionein-like domain from the copper-responsive regulator Crr1 in Chlamydomonas also leads to increased expression of genes required for zinc uptake and the hyperaccumulation of zinc, suggesting that this may be another type of zinc-sensing domain (25, 26). Despite a growing number of eukaryotic zinc-responsive factors, it is largely unknown whether these factors sense changes in zinc levels through zinc ion binding or if they are indirectly regulated by zinc (e.g. through a zinc-dependent post-translational modification). Thus, future studies with Loz1 will help to provide important knowledge concerning zinc homeostasis and zinc sensing.
Acknowledgments
We thank Kaila Dafforn for help with strain generation and Dr. R. Michael Townsend for critical reading of the manuscript. We also thank Dr. Stephen Osmani for providing A. nidulans genomic DNA.
Footnotes
- ZL-EMM
- zinc-limited Edinburgh minimal medium
- nt
- nucleotide(s).
REFERENCES
- 1. Andreini C., Bertini I. (2012) A bioinformatics view of zinc enzymes. J. Inorg. Biochem. 111, 150–156 [DOI] [PubMed] [Google Scholar]
- 2. Klug A. (2010) The discovery of zinc fingers and their applications in gene regulation and genome manipulation. Annu. Rev. Biochem. 79, 213–231 [DOI] [PubMed] [Google Scholar]
- 3. Fukada T., Yamasaki S., Nishida K., Murakami M., Hirano T. (2011) Zinc homeostasis and signaling in health and diseases: zinc signaling. J. Biol. Inorg. Chem. 16, 1123–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ehrensberger K. M., Bird A. J. (2011) Hammering out details: regulating metal levels in eukaryotes. Trends Biochem. Sci. 36, 524–531 [DOI] [PubMed] [Google Scholar]
- 5. Eide D. J. (2009) Homeostatic and adaptive responses to zinc deficiency in Saccharomyces cerevisiae. J. Biol. Chem. 284, 18565–18569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corkins M. E., May M., Ehrensberger K. M., Hu Y. M., Liu Y. H., Bloor S. D., Jenkins B., Runge K. W., Bird A. J. (2013) Zinc finger protein Loz1 is required for zinc-responsive regulation of gene expression in fission yeast. Proc. Natl. Acad. Sci. U.S.A. 110, 15371–15376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Assunção A. G., Herrero E., Lin Y. F., Huettel B., Talukdar S., Smaczniak C., Immink R. G., van Eldik M., Fiers M., Schat H., Aarts M. G. (2010) Arabidopsis thaliana transcription factors bZIP19 and bZIP23 regulate the adaptation to zinc deficiency. Proc. Natl. Acad. Sci. U.S.A. 107, 10296–10301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Günther V., Lindert U., Schaffner W. (2012) The taste of heavy metals: gene regulation by MTF-1. Biochim. Biophys. Acta 1823, 1416–1425 [DOI] [PubMed] [Google Scholar]
- 9. Frey A. G., Eide D. J. (2011) Roles of two activation domains in Zap1 in the response to zinc deficiency in Saccharomyces cerevisiae. J. Biol. Chem. 286, 6844–6854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frey A. G., Eide D. J. (2012) Zinc-responsive coactivator recruitment by the yeast Zap1 transcription factor. Microbiologyopen 1, 105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frey A. G., Bird A. J., Evans-Galea M. V., Blankman E., Winge D. R., Eide D. J. (2011) Zinc-regulated DNA binding of the yeast Zap1 zinc-responsive activator. PLoS One 6, e22535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ehrensberger K. M., Mason C., Corkins M. E., Anderson C., Dutrow N., Cairns B. R., Dalley B., Milash B., Bird A. J. (2013) Zinc-dependent regulation of the adh1 antisense transcript in fission yeast. J. Biol. Chem. 288, 759–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu J. Q., Kuhn J. R., Kovar D. R., Pollard T. D. (2003) Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev. Cell 5, 723–734 [DOI] [PubMed] [Google Scholar]
- 14. Clemens S., Bloss T., Vess C., Neumann D., Nies D. H., Zur Nieden U. (2002) A transporter in the endoplasmic reticulum of Schizosaccharomyces pombe cells mediates zinc storage and differentially affects transition metal tolerance. J. Biol. Chem. 277, 18215–18221 [DOI] [PubMed] [Google Scholar]
- 15. Dainty S. J., Kennedy C. A., Watt S., Bähler J., Whitehall S. K. (2008) Response of Schizosaccharomyces pombe to zinc deficiency. Eukaryot. Cell 7, 454–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bowers P. M., Schaufler L. E., Klevit R. E. (1999) A folding transition and novel zinc finger accessory domain in the transcription factor ADR1. Nat. Struct. Biol. 6, 478–485 [DOI] [PubMed] [Google Scholar]
- 17. Kamashev D. E., Balandina A. V., Karpov V. L. (2000) Tramtrack protein-DNA interactions. A cross-linking study. J. Biol. Chem. 275, 36056–36061 [DOI] [PubMed] [Google Scholar]
- 18. Dutnall R. N., Neuhaus D., Rhodes D. (1996) The solution structure of the first zinc finger domain of SWI5: a novel structural extension to a common fold. Structure 4, 599–611 [DOI] [PubMed] [Google Scholar]
- 19. Shimizu M., Murase A., Hara M., Shindo H., Mitchell A. P. (2001) A C-terminal segment with properties of α-helix is essential for DNA binding and in vivo function of zinc finger protein Rme1p. J. Biol. Chem. 276, 37680–37685 [DOI] [PubMed] [Google Scholar]
- 20. Ramamoorthy V., Dhingra S., Kincaid A., Shantappa S., Feng X., Calvo A. M. (2013) The putative C2H2 transcription factor MtfA is a novel regulator of secondary metabolism and morphogenesis in Aspergillus nidulans. PLoS One 8, e74122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pace C. N., Scholtz J. M. (1998) A helix propensity scale based on experimental studies of peptides and proteins. Biophys. J. 75, 422–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choi S., Bird A. J. (2014) Zinc'ing sensibly: controlling zinc homeostasis at the transcriptional level. Metallomics 10.1039/C4MT00064A [DOI] [PubMed] [Google Scholar]
- 23. Bird A. J., Blankman E., Stillman D. J., Eide D. J., Winge D. R. (2004) The Zap1 transcriptional activator also acts as a repressor by binding downstream of the TATA box in ZRT2. EMBO J. 23, 1123–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carey L. B., van Dijk D., Sloot P. M., Kaandorp J. A., Segal E. (2013) Promoter sequence determines the relationship between expression level and noise. PLoS Biol. 11, e1001528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Herbig A., Bird A. J., Swierczek S., McCall K., Mooney M., Wu C. Y., Winge D. R., Eide D. J. (2005) Zap1 activation domain 1 and its role in controlling gene expression in response to cellular zinc status. Mol. Microbiol. 57, 834–846 [DOI] [PubMed] [Google Scholar]
- 26. Sommer F., Kropat J., Malasarn D., Grossoehme N. E., Chen X., Giedroc D. P., Merchant S. S. (2010) The CRR1 nutritional copper sensor in Chlamydomonas contains two distinct metal-responsive domains. Plant Cell 22, 4098–4113 [DOI] [PMC free article] [PubMed] [Google Scholar]


