Background: We investigated both trafficking and function of Arrestin 1 (Arr1) in Drosophila.
Results: We show that Arr1 translocates to rhabdomeres to modulate deactivation of phosphorylated rhodopsin in adult photoreceptors but becomes internalized in pupal photoreceptors.
Conclusion: Arr1 plays distinct roles in pupal and adult photoreceptors.
Significance: Trafficking and function of arrestin may be regulated depending on the physiological context.
Keywords: Arrestin, Protein Translocation, Receptor Endocytosis, Rhodopsin, Trafficking, Visual Transduction
Abstract
Arrestin regulates many facets of G-protein coupled receptor signaling. In Drosophila, Arrestin 1 (Arr1) is expressed at a lower level than Arrestin 2 (Arr2), and the role of Arr1 in visual physiology is less understood. Here we generated transgenic flies expressing enhanced green fluorescent protein tagged Arr1 (Arr1-eGFP) and explored its trafficking in live photoreceptors. We show that Arr1-eGFP is localized in the cytoplasm and displays light-dependent translocation to the rhabdomere possibly by interacting with photoactivated rhodopsin 1 (Rh1*). In the adult, translocation of Arr1-eGFP occurs with slower kinetics when compared with that of Arr2-eGFP. This slower kinetic activity may be attributable to a reduced level of phosphorylated Rh1*. Indeed, a reduced level of phosphorylated Rh1* recruits a lower level of Arr1-eGFP to rhabdomeres. To investigate whether Arr1 is required for the deactivation of phosphorylated Rh1*, we show that in flies with reduced Arr1 prolonged depolarizing afterpotential can be triggered with fewer light pulses, indicating that inactivation of phosphorylated Rh1* is compromised when the Arr1 level is reduced. Consistently, Arr1 is no longer required for deactivation of Rh1 in flies expressing phosphorylation-deficient Rh1. Previously it was reported that Arr1 displays light-dependent internalization. Unexpectedly, in adult photoreceptors we failed to observe endocytosis of Arr1-eGFP. In contrast, we show that in pupal photoreceptors Arr1-eGFP becomes internalized and sequestered in vesicles within the cytoplasm. Taken together, we propose that Arr1 plays distinct roles during development and adulthood. Arr1 orchestrates the recycling of phosphorylated Rh1* in pupae whereas it regulates the deactivation in adult.
Introduction
Members of the arrestin family have emerged as key regulatory proteins of diverse signaling processes mediated by many transmembrane receptors including G-protein coupled receptors (1), Frizzled (2), transforming growth factor β receptors (3), and receptor protein-tyrosine kinases (4). Vertebrate arrestin 1 was initially identified as a regulatory protein for rhodopsin in vertebrate photoreceptors (5, 6). In the visual signaling process, activated rhodopsin activates a heterotrimeric G-protein that triggers a cascade of biochemical reactions leading to a change in the photoreceptor membrane potential. Subsequently, activated rhodopsin becomes phosphorylated by rhodopsin kinase, and arrestin binding to the phosphorylated rhodopsin renders rhodopsin unable to activate the downstream G-protein leading to the termination of the visual response (7).
Drosophila photoreceptors express two distinct visual arrestins: Arr1 2 (8, 9) and Arr2 (10, 11). These two arrestins share 46% sequence identities and are of different sizes; Arr1 contains 364 amino acids, missing the extended C terminus found on Arr2, whereas Arr2 consists of 401 amino acids (8). Moreover, Arr1 is considered a “minor” arrestin and is expressed 85% less than Arr2 (12). Biochemically, Arr1 recognizes activated rhodopsin that is also phosphorylated (14), yet Arr2 binds only to activated rhodopsin (13). We wanted to determine why photoreceptors require two arrestins. It is likely that each arrestin participates in distinct electrophysiological and cellular functions in photoreceptors, contributed by the unique molecular and biochemical properties of each arrestin.
Indeed, Arr1 and Arr2 participate in distinct visual electrophysiology. Arr2 is involved in the fast deactivation of activated rhodopsin (12). Arr1 appears not critical for the deactivation of the visual response, as the arr1 mutant alone displays no overt electrophysiological defects by patch clamp analysis (12). However, the absence of Arr1 in the arr1;arr2 double mutant greatly enhances slow recovery when compared with the arr2 mutant alone. It was reported that Arr1 undergoes light-dependent endocytosis and is required for the internalization of the phosphorylated rhodopsin (14). Arr1 may be critical in modulating the functional rhodopsin levels of the membrane as well as in promoting the re-sensitization of the internalized rhodopsin.
In this report we explored the function and trafficking of Arr1 in photoreceptors. We generated transgenic flies expressing enhanced green fluorescence protein (eGFP)-tagged Arr1 in R1–6 photoreceptors and demonstrated that cytoplasmic Arr1-eGFP redistributes to the rhabdomere by associating with phosphorylated Rh1*. Interestingly, the light-dependent translocation of Arr1-eGFP occurs at much slower kinetics when compared with that of Arr2-eGFP. Moreover, Arr1-eGFP undergoes the light-dependent endocytosis only in pupal but not in adult photoreceptors. To investigate whether Arr1 plays a role in the visual electrophysiology, we performed electroretinogram recordings (ERG) using a light stimulation paradigm that generates phosphorylated Rh1*. We show that prolonged depolarizing afterpotential, which is caused by the continued activity of Rh1*, can be easily triggered in arr11. Furthermore, Arr1 is no longer needed for deactivation in transgenic flies expressing phosphorylation-deficient Rh1. These findings support the notion that Arr1 is required for the timely deactivation of phosphorylated rhodopsin in adult photoreceptors.
EXPERIMENTAL PROCEDURES
Recombinant DNA and Molecular Biology
Arr1 cDNA was obtained by polymerase chain reaction (PCR) using a retinal cDNA library as the template. A full-length Arr1 cDNA with engineered EcoRI (at 5′) and XhoI (at 3′) restriction enzyme sites was subcloned into pCR2.1 (Invitrogen) using the TA cloning strategy. The nucleotide sequences were confirmed by automatic DNA sequencing. A modified Arr1 cDNA lacking a stop codon was obtained by PCR and subcloned into pEGFP (Clontech, Mountain View, CA) for expressing an Arr1 fusion protein with the eGFP inserted at its C terminus. The nucleotide sequences for Arr1-eGFP were then subcloned into the YC4 transgenic vector that contains the Drosophila Rh1 promoter (15) responsible for directing the transgenic expression of Arr1-eGFP in R1–6 photoreceptors.
P-element-mediated Germ Line Transformation
A recombinant DNA construct containing the nucleotide sequence of Arr1-eGFP and a transposase plasmid (“wings-clipped”) were injected into yw embryos (The Rainbow Transgenics Inc, Camarillo, CA). Multiple transgenic lines were selected based on the rescue of the yellow phenotype in the wings. Flies with the transgene integrated into the second or the third chromosome were selected for further analysis. The expression of Arr1-eGFP was investigated by Western blotting and by fluorescence microscopy.
Generation of Anti-Arr1 Antibodies
Polyclonal antibodies were obtained by immunizing rabbits with the bacterially expressed full-length Arr1 protein (AnaSpec, Fremont, CA). Antibodies were characterized by Western blotting.
Quantitative Western Blotting
Fly heads were isolated and extracted using 2× Laemmli sample buffer. Solubilized proteins were size-fractionated by SDS/PAGE (10%) and transferred onto a nitrocellulose filter. After incubation with the desired primary antibodies, filters were incubated with fluorophore-conjugated secondary antibodies (e.g. Alexa Fluor 680 goat anti-rabbit IgG, Invitrogen). The fluorescent signal of the secondary antibodies was detected and quantified by the Odyssey Infrared Imaging system (LI-COR, Lincoln, NE). Polyclonal antibodies against GFP (Vanderbilt Antibody and Protein Resource), Arr1, INAD (inactivation no afterpotential D), and TRP (transient receptor potential) were used in the Western blot analysis.
Fluorescence Microscopy
Adult flies (2–5 days old) were anesthetized by CO2, immobilized in clay placed in a 50-mm Petri dish, and imaged. Pupae (>p13 or >80% pupal development) (16) were selected from vials and positioned in clay placed in a Petri dish for imaging after removing the puparium. The preparation was examined using an upright Olympus AX70 microscope equipped with 10× lens (for detecting deep pseudopupil (dpp)) or 40× water immersion lens (LUMPLFL 40×). The images were acquired using IPLab image acquisition software (BioVision Technologies, Exton, PA) and the Retiga camera from QImaging (Surrey, BC, Canada). Multiple images (n ≥ 3) were taken for each fly.
Fly Handling for Microscopy
Adult flies or pupae were sorted and manipulated under a dissecting microscope with a light source of 600 lux for <1 min for adult or 3 min for pupae. During imaging the compound eyes were subjected to blue light (1300 lux) from the fluorescent microscope, and images were taken after 4 min when translocation of Arr1-eGFP reached a steady state. For light-dependent translocation studies, 2–5-day-old flies were kept in the dark overnight and manipulated under ambient room light (300 lux) for <1 min. For light-dependent endocytosis studies, pupae were manipulated under ambient room light (300 lux) for <3 min. After light treatment, pupal eyes were dissected under a stereo microscope (600 lux) in <3 min to obtain dissociated photoreceptors. Light intensity was measured using a handhold light meter.
Fluorescent Image Analysis
All image manipulation was performed under the guideline of Rossner and Yamada (17). Fluorescent images included in the figures are similar in appearance as the raw images. The fluorescence intensity of Arr1-eGFP or Arr2-eGFP in the rhabdomere or the cytosol was analyzed using ImageJ (National Institutes of Health). The average fluorescence in a given rhabdomere was obtained by highlighting the area using the circle tool after background subtraction. The average cytosol fluorescence was obtained using similar strategies. Three independent measurements were performed for each image to obtain an average. For light-dependent translocation, the fluorescence intensity of dpp was calculated and plotted.
Dissociation of Photoreceptors from Pupal Eyes
Pupae (>p13 or >80% pupal development) were removed under room light (300 lux). The pupae case was cut open to expose the compound eyes, which were dissected and transferred onto a drop of 1× PBS in a slide. Ommatidia were gently dissociated using insect needles under a stereo microscope (600 lux). The dissection and dissociation were completed in <2 min. The preparation was immediately imaged using the Olympus AX70 microscope.
Electroretinogram Recordings
ERG recordings were performed on 2–3-day-old flies reared in a 12-h light/12-h dark cycle. All flies used were white-eyed in the genetic background of either w− or cn,bw. Flies were immobilized with non-drying modeling clay, and glass electrodes were filled with 0.7% NaCl solution. Blue light stimulation (10 lux) was generated by a fiber optic light source (Oriel, Stratford, CT) with a blue light filter, and signals were amplified by means of Axopatch 200B (Molecular Devices, Sunnyvale, CA). ERG data were digitalized using AxonScope 9.0 (Molecular Devices). The following light stimulation protocol was employed for evoking prolonged depolarizing afterpotential (PDA). Flies were first dark-adapted for 3 min and given 2 pulses of the orange light (35 lux) to ensure the complete photoconversion of metarhodopsin to rhodopsin. Subsequently, flies were stimulated continuously with a 10-s blue light every 20 s (10 s on with 10 s off) until photoreceptors achieved a steady-state depolarization, which indicates that PDA is triggered. Each fly of a given phenotype was tested three times, and the number of blue light pulses required to generate PDA were obtained. All recordings were made at 25 °C.
Statistical Analysis
Data are represented as the mean ± S.E. from at least three independent experiments (n ≥ 3) unless otherwise noted in the figures legends. Comparisons between control and experimental conditions were performed using a two-tailed Student's t test. The differences were considered significant when p < 0.05.
Drosophila Stocks
The ninaEP332 mutants were obtained from the Bloomington Stock Center. arr11, arr21, and arr23 stocks were provided by Dr. C. Zuker (Columbia University), and transgenic flies expressing modified Rh1 (Rh1CT S>A; ninaE and Rh1Δ356; ninaE) were from Dr. S. Britt (University of Colorado Health Science Center). Standard crosses were made to introduce Arr1-eGFP into various genetic backgrounds and to generate double mutants.
RESULTS
Characterization of Transgenic Lines Expressing Arr1-eGFP
Arr1 associates with phosphorylated Rh1* to orchestrate its endocytosis. To explore the trafficking of Arr1 in live photoreceptors, we generated transgenic flies expressing modified Arr1 with an eGFP tag incorporated at its C terminus. The expression of this Arr1-eGFP chimera is directed by the Rh1 promoter (18) that restricts its expression to R1–6 photoreceptors of the compound eye.
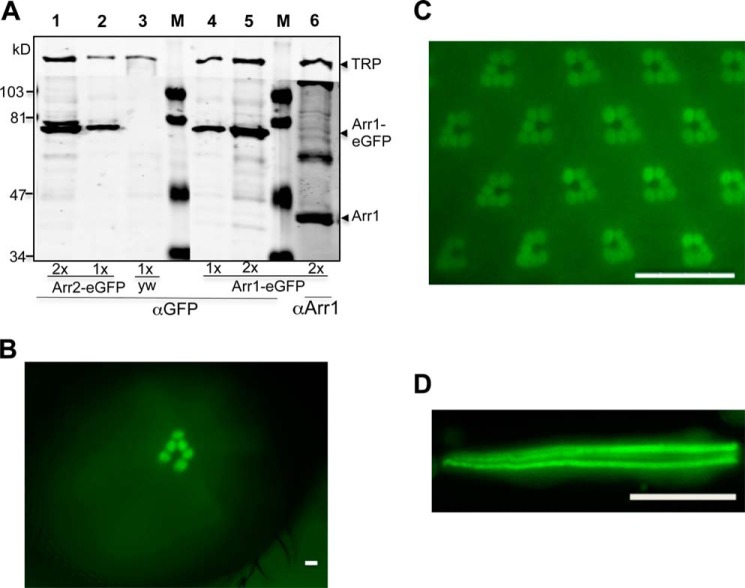
We characterized multiple Arr1-eGFP transgenic lines by Western blotting using anti-GFP antibodies. We detected a polypeptide in fly head extracts of ∼70 kDa in size (Fig. 1A, lanes 4 and 5), which corresponds to the predicted size of Arr1-eGFP. Consistently, this 70-kDa species is absent in non-transgenic wild-type flies (Fig. 1A, lane 3). The steady-state level of Arr1-eGFP was comparable to that of Arr2-eGFP in transgenic flies that we previously generated (Fig. 1A, lanes 1 and 2). Notably, the expression of Arr1-eGFP is relatively low, and its steady-state level corresponds to <5% of endogenous Arr1 (Fig. 1A, lane 6). The low level of Arr1-eGFP is not sufficient to rescue the retinal degeneration phenotype of arr11 (not shown). Here Arr1-eGFP serves as a fluorescent marker for investigating the dynamic redistribution of Arr1 in live photoreceptors.
FIGURE 1.
Characterization of transgenic flies expressing Arr1-eGFP in R1–6 photoreceptors. A, Western blot analysis. Shown is a representative Western blot comparing the levels of Arr1-eGFP and Arr2-eGFP as well as the relative levels of Arr1-eGFP and endogenous Arr1 in transgenic flies. Lanes 1 and 2 contain head extracts from Arr2-eGFP expressing flies, whereas lane 3 was from the parental strain yw. Lanes 4–6 contain head extracts from Arr1-eGFP-expressing flies. Double amounts of extracts were loaded in lanes 1, 5, and 6. The estimated size for Arr1-eGFP is about 71 kDa, and that of Arr2-eGFP, about 75 kDa. Arr1 corresponds to a polypeptide of 44 kDa, whereas TRP (transient receptor potential) is a polypeptide of 150 kDa, which was used as a loading control. Protein size markers (M) are indicated on the left. B, enrichment of Arr1-GFP in the rhabdomeres can be detected as fluorescent “deep pseudopupil” in adult eyes. C, subcellular localization of Arr1-eGFP in wild-type photoreceptors under blue light illumination. D, Arr1-eGFP is highly concentrated in rhabdomeres but also present in the cytoplasm of isolated photoreceptors. Scale bar, 20 μm.
We characterized the expression of Arr1-eGFP by fluorescence microscopy; we show that Arr1-eGFP is expressed in the compound eye of adult flies (Fig. 1, B and C). Indeed, a fluorescent version of dpp that represents Arr1-eGFP binding to phosphorylated Rh1* in the rhabdomere (Fig. 1B) can be readily detected. By water immersion fluorescence microscopy, we found that Arr1-eGFP is concentrated in the rhabdomeres but also present in the cytoplasm of photoreceptors (Fig. 1C). Similarly, Arr1-eGFP is enriched in rhabdomeres of isolated ommatidia prepared from newly eclosed flies (Fig. 1D).
Light-dependent Translocation of Arr1-eGFP Occurs with Slower Kinetics Than Arr2-eGFP
Visual arrestin interacts with photoactivated rhodopsin and displays light-dependent translocation by trafficking from the cytoplasm to membranes where rhodopsin is localized. For insights into regulation controlling the interaction between Arr1 and Rh1 rhodopsin, we explored the real-time kinetics of the interaction between Arr1-eGFP and Rh1* in live photoreceptors.
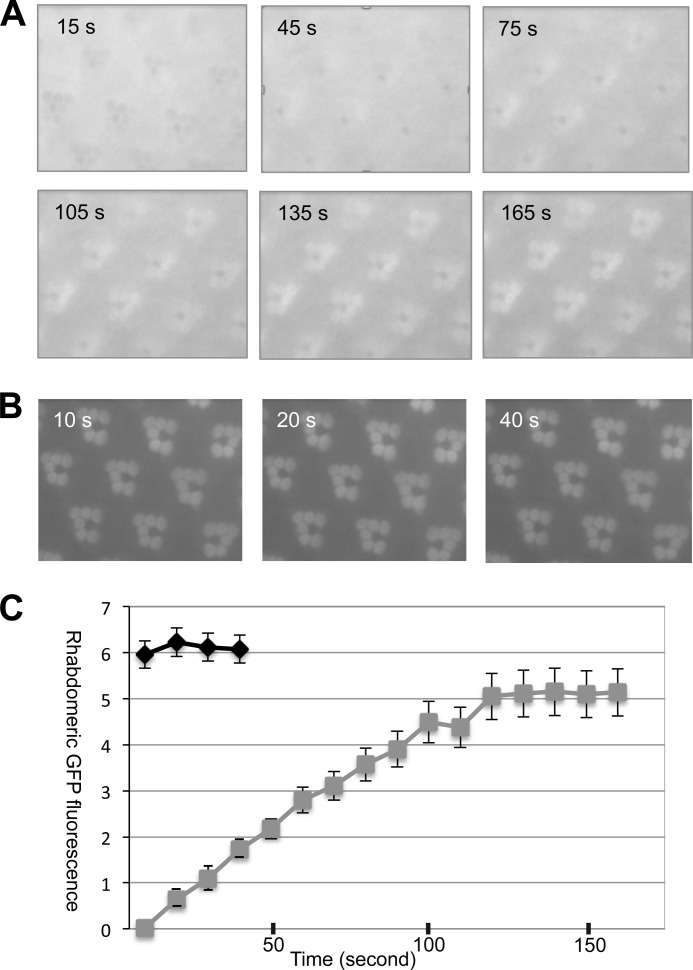
We immobilized 2–5-day-old flies and captured time-lapse fluorescence images during continuous blue light exposure for 4 min. Representative images taken at the indicated times are shown in Fig. 2A. Notably, Arr1-eGFP was initially localized in the cytoplasm of R1–6 photoreceptors. The absorption of the blue light by Rh1 possibly caused the rhabdomeres in this image to appear as darkened circles. As light stimulation continued, Arr1-eGFP became recruited to the rhabdomere, and subsequently its fluorescence “masked” the dark rhabdomeres (Fig. 2A). The light-dependent recruitment of Arr1-eGFP also can be observed by the appearance of the “fluorescent dpp.” Based on the changes of the fluorescence intensity in the dpp, we calculated the kinetics of translocation (Fig. 2C). Translocation of Arr1-eGFP reached a steady state by 150 ± 15 s (n = 3) (Fig. 2C). Under a similar condition, Arr2-eGFP displayed a more robust translocation, which achieved a steady state by 10 s (Fig. 2C). The light-dependent translocation of Arr1-eGFP is at least 15-fold slower than that of Arr2-eGFP. A lower level of phosphorylated Rh1* and/or a reduced rate of interaction may cause the slow translocation kinetics of Arr1-eGFP.
FIGURE 2.
Kinetics of the light-dependent translocation of Arr1-eGFP and Arr2-eGFP in adult retinas. A, subcellular localization of Arr1-eGFP during continued blue light illumination. Selected time-lapse images are shown (see supplemental Movie 1). Arr1-eGFP initially was in the cytosol but began to migrate to rhabdomeres at ∼45 s after the blue light was switched on. Translocation reached a steady state after around 165 s. B, light-dependent redistribution of Arr2-eGFP. The light-dependent translocation of Arr2-eGFP occurred much faster than that of Arr1-eGFP. C, a comparison of translocation kinetics of Arr2-eGFP (black) and Arr1-eGFP (gray) in wild-type background. Data are represented as the mean ± S.E. (n = 3).
Probing the Potential Interaction between Arr1-eGFP and Rh1* in Adult Photoreceptors
We speculated that the slow translocation of Arr1-eGFP is partly due to a reduced rate of interaction with phosphorylated Rh1* and/or a low level of phosphorylated Rh1*. These two causes can also hamper the recruitment of Arr1-eGFP to the rhabdomeres. Thus we quantified the steady-state level of Arr1-eGFP in the rhabdomeres after at least 4 min of light exposure. First, we estimated the intensity of the Arr1-eGFP fluorescence in the rhabdomeres and cytoplasm of photoreceptors, respectively. We calculated the difference in intensity between rhabdomeres and cytoplasm to obtain “enrichment in the rhabdomere,” which is divided by the intensity of the cytoplasm to calculate the rhabdomere enrichment index (REI).
In wild-type flies, Arr1-eGFP displayed an REI of 0.22 ± 0.02 (n = 5). In ninaEP332 flies, Arr1-eGFP displayed a much lower REI (Fig. 3D). ninaEP332 contains a point mutation in the Rh1 gene (19) leading to a greatly reduced Rh1 level. This reduced Rh1 level leads to greatly reduced recruitment of Arr1-eGFP to the rhabdomeres. Consistent with the notion that phosphorylation of Rh1* is required for Arr1 binding, we observed that the rhabdomeric enrichment of Arr1-eGFP is drastically reduced in flies expressing phosphorylation-deficient Rh1 (Fig. 3, B and D). These modified Rh1s that either miss the C-terminal sequence (Δ356) or lack the putative phosphorylation sites (S>A) (14, 23) appear to be expressed at a level similar to that of the endogenous Rh1 in wild-type flies based on the intensity of dpp.
FIGURE 3.
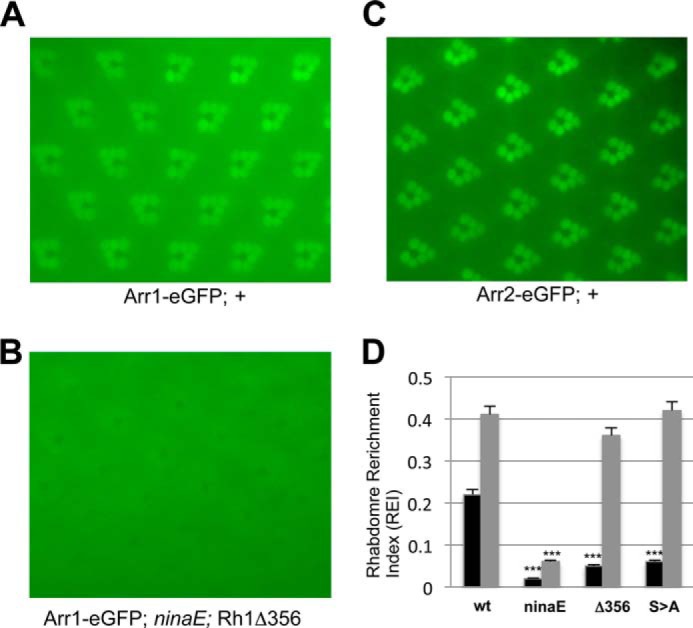
Interaction between Arr1-eGFP and Rh1 in live photoreceptors. Subcellular localization of Arr1-eGFP in flies expressing wild type (A) and Rh1Δ356 (B). C, subcellular distribution of Arr2-eGFP in wild-type photoreceptors. D, a comparison of the REI between Arr2-eGFP (gray) or Arr1-eGFP (black) in wild-type (wt) or mutant Rh1 backgrounds. The genotype for S>A is Rh1CT S>A; ninaE7. Data are represented as the mean ± S.E. (n = 5). ***, p < 0.001 when compared with the value obtained in the wild-type background. All images were taken after at least 4 min of blue light exposure.
As a comparison, we calculated the REI for Arr2-eGFP (20). Arr2-eGFP (Fig. 3C) is expressed at a level similar to that of Arr1-eGFP (Fig. 1A). We obtained an REI of 0.41 ± 0.01 (n = 5) for Arr2-eGFP in the wild-type background, which is similar to that in flies expressing either the C-terminal truncation or the phosphorylation-deficient Rh1 (Fig. 3D). Although Arr2 recognizes a distinct pool of activated but non-phosphorylated Rh1, it may not directly compete with Arr1. Consistently, we found that the REI for Arr1-eGFP is relatively unchanged in the null allele of arr23 background (not shown).
PDA Can Be Evoked Using Less Light in arr11 Photoreceptors
Whether Arr1 is critical for the termination of the visual response remains debatable. The slower kinetics of the interaction between Arr1 and phosphorylated Rh1 suggest against a role of Arr1 in the fast deactivation of Rh1 in visual signaling. Previous electrophysiological analysis using patch clamp recording indicates that Arr1 is involved in deactivation only in the arr23 genetic background. This specificity of deactivation occurs because removal of Arr1 exacerbates the deactivation defects of arr23 mutants (12), whereas arr12 mutants exhibit no defects. We speculate that Arr1 interacts only with phosphorylated Rh1* and may be required under conditions that accumulate phosphorylated Rh1*.
To investigate whether Arr1 participates in the deactivation of Rh1*, we performed ERG recordings of adult eyes. ERG is the extracellular recording measuring the light-induced changes of membrane potential. ERG is also instrumental for uncovering the stoichiometric relationship between Rh1 and Arr2 in the visual response. When Arr2 becomes limiting to inactivate Rh1*, excess Rh1* leads to persistent depolarization or PDA. Thus it is possible that PDA could be triggered when Arr1 becomes limiting to inactivate phosphorylated Rh1*.
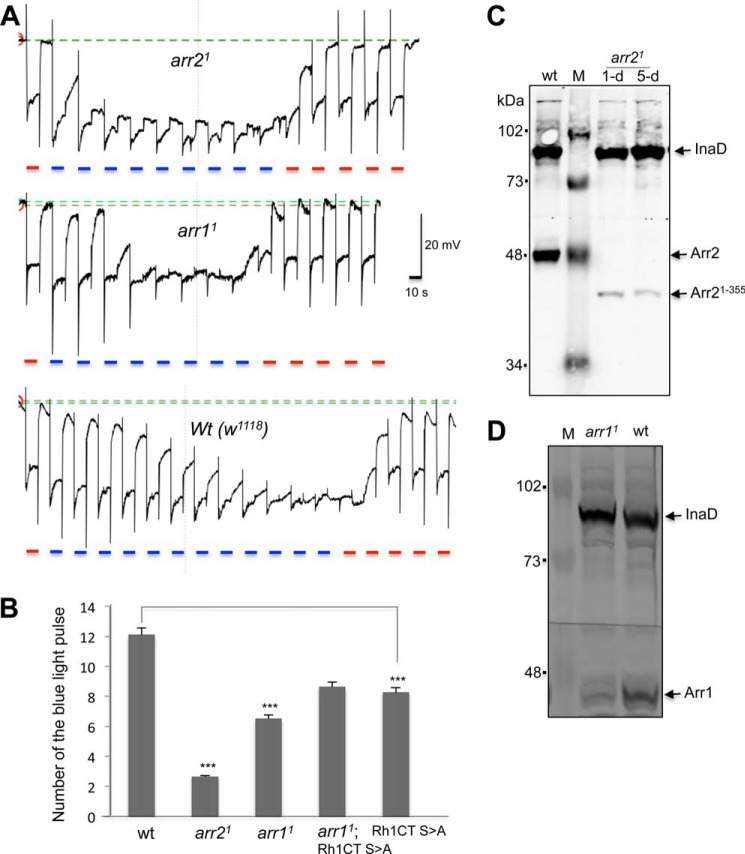
To promote the accumulation of phosphorylated Rh1*, we chose a light stimulation protocol by exposing eyes to repetitive pulses of 10-s blue light (λ480 nm). Moreover, we employed a blue light of moderate intensity that allowed us to distinguish the amount of light required for initiating PDA among wild-type and arrestin mutants. As shown in Fig. 4A, wild-type flies that have a full complement of both Arr1 and Arr2 require 12.1 ± 0.3 (n = 6) pulses of the blue light to trigger PDA. In contrast, arr21 mutants that express 5.3% of the truncated Arr2 (Fig. 4C) require 2.6 ± 0.2 pulses (n = 4) to evoke PDA (Fig. 4, A and B). Notably, arr11 mutants are also prone to PDA and require 6.5 ± 0.2 pulses (n = 6) (Fig. 4, A and B). arr11 is a hypomorphic allele expressing about 10–15% of Arr1 (12) (Fig. 4D). Based on our findings, we conclude that Arr1 is critical for the deactivation of phosphorylated Rh1* under the experimental light condition.
FIGURE 4.
The arr11 photoreceptors require fewer light pulses to initiate PDA. A, representative ERG recordings of arr21 (top panel), arr11 (middle), and wild-type (wt) (bottom) flies by repetitive blue light pulses for generating PDA. PDA were reversed by orange light stimulation. Light pulses (blue or orange) are indicated below. B, a comparison of the number of blue light pulses needed for generating PDA in various mutants. Data are represented as the mean ± S.E. from several experiments as indicated under “Results.” ***, p < .0001, compared with wild type. The p value equals 0.23 when arr11;Rh1CT S>A was compared with Rh1CT S>A. C, Western blotting analysis of arr21. Head extracts from 1-day-old and 3-day-old arr21 flies were analyzed. arr21 expresses a truncated Arr2. Anti-InaD was used as a control. D, Western blotting shows arr11 contains a reduced level of Arr1, compared with wild type. M, mass markers.
Removal of Arr1 Fails to Modify PDA in Phosphorylation-deficient Rh1 Flies
We further explored the relationship between phosphorylated Rh1* and Arr1 by ERG. We reasoned if Arr1 is involved in the deactivation of the phosphorylated Rh1*, expression of phosphorylation-deficient Rh1 would eliminate the requirement of Arr1 for the deactivation. To test this hypothesis, we generated double mutants (arr11; Rh1CT S>A; ninaE) by introducing arr11 into transgenic flies expressing the modified Rh1 in which the putative phosphorylation sites were modified (14). We compared the ERG phenotype of the double mutant with that of single mutants expressing modified Rh1 (Rh1CT S>A; ninaE). Significantly, we found that in both single (8.3 ± 0.1, n = 4) and double mutants (8.6 ± 0.2, n = 4) PDA can be triggered using similar numbers of the blue light pulses (Fig. 4B), strongly indicating that the PDA phenotype of flies expressing phosphorylation-deficient Rh1 is similar with or without the presence of Arr1. Thus Arr1 is not required for the deactivation of the phosphorylation-deficient Rh1*. Notably, flies expressing modified Rh1 (8.3 ± 0.1, n = 4) require fewer light pulses to initiate PDA when compared with wild type (12.1 ± 0.3, n = 6). This may be partly due to a different ratio between arrestins and modified Rh1 in the mutants. Taken together, we conclude that Arr1 is required for the fast deactivation of phosphorylated Rh1* to terminate the visual response.
Light-dependent Internalization of Arr1-eGFP Occurs Only in Pupal Photoreceptors
Previously, Satoh and Ready (14) reported that Arr1 plays a role in the light-dependent internalization of Rh1* by indirect immunofluorescence studies. These authors showed that upon light stimulation Arr1 first translocates to rhabdomeres then undergoes internalization and finally becomes enriched in multivesicular bodies of photoreceptors.
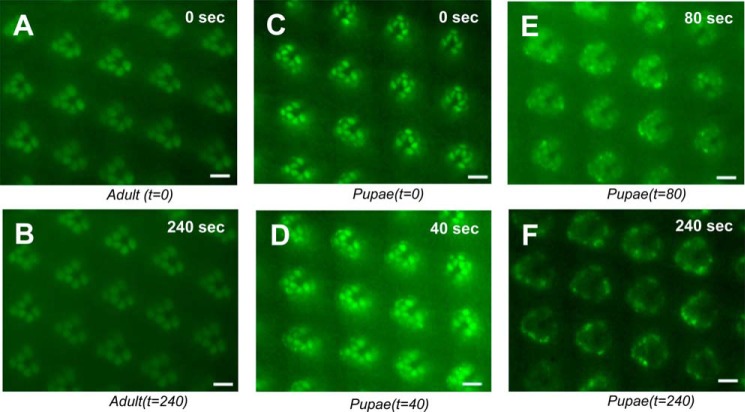
We investigated the real-time kinetics of Arr1 endocytosis in Arr1-eGFP expressing transgenic flies (Fig. 5). Unexpectedly, we show that in adult photoreceptors Arr1-eGFP is not internalized but remains associated with the rhabdomeres after light stimulation (Fig. 5, A and B). To investigate whether internalization of Arr1-eGFP occurs in pupal photoreceptors, we isolated and imaged late-stage pupae (>p13, or >80% pupal development) in which visual signaling proteins including Rh1 and Arr1 are expressed. We show that Arr1-eGFP first translocates to rhabdomeres upon blue light stimulation (Fig. 5C). Subsequently, Arr1-eGFP appears internalized and is sequestered in vesicles in the adjacent cytoplasm (Fig. 5D). Notably, the intensity of Arr1-eGFP in the rhabdomeres gradually decreases (Fig. 5E). After blue light exposure for 4 min, the intensity of Arr1-eGFP in the rhabdomeres is greatly reduced, and the remaining Arr1-eGFP is present near the base of the rhabdomeres (Fig. 5F). This dynamic redistribution may reflect the trafficking of Arr1-eGFP from the rhabdomere toward the base where endocytosis takes place.
FIGURE 5.
Arr1-eGFP undergoes light-dependent endocytosis in pupal but not in adult photoreceptors. In adult photoreceptors, blue light triggers redistribution of Arr1-eGFP to the rhabdomeres (A). After an additional 4 min of blue light exposure, Arr1-eGFP remains associated with rhabdomeres (B). In pupal eyes, rhabdomere-localized Arr1-eGFP (C) quickly internalized as vesicles emerge in the adjacent cytoplasm (D). Upon continued blue light illumination, Arr1-eGFP in the rhabdomeres was greatly reduced (E), and Arr1-GFP could be detected only near the base of rhabdomeres after 4 min of light exposure (F). See supplemental Movie 2 for the light-dependent endocytosis in pupae. Scale bar, 5 μm.
Significantly, the light-dependent internalization of Arr1-eGFP is observed only in pupal photoreceptors at and after 80% development. In the more mature pupal photoreceptors from pupae that are ready to emerge as adults, however, internalization is not detected. These results demonstrate that Arr1 undergoes light-dependent endocytosis in a restricted period during the development of pupal photoreceptors.
Endocytosis Results in Arr1-eGFP Containing Cytosolic Vesicles in Dissociated Photoreceptors
To further explore the light-dependent internalization of Arr1-eGFP, we investigated its subcellular localization. After the light treatment, pupal heads were isolated and placed in a drop of 1× PBS on a slide. After removing the pupal cuticle, the compound eyes were gently dissected to obtain dissociated photoreceptors. By fluorescence microscopy, we show that Arr1-eGFP is found in vesicles within the cytoplasm of photoreceptors (Fig. 6, A and B). Additionally, Arr1-eGFP also can be detected in rhabdomeres as well as in the cytoplasm (Fig. 6, A and B). Without light treatment, we failed to observe Arr1-eGFP-containing vesicles; instead, Arr1-eGFP remains in rhabdomeres and the cytoplasm of pupal photoreceptors (Fig. 6C). In dissociated adult photoreceptors, Arr1-eGFP is enriched in the rhabdomeres with (Fig. 6, D and E) or without (Fig. 6F) light treatment. Taken together, we conclude that the light-dependent internalization of Arr1-eGFP takes place only in pupal but not in adult photoreceptors. Internalization of Arr1-eGFP results in its sequestration in numerous cytosolic vesicles.
FIGURE 6.
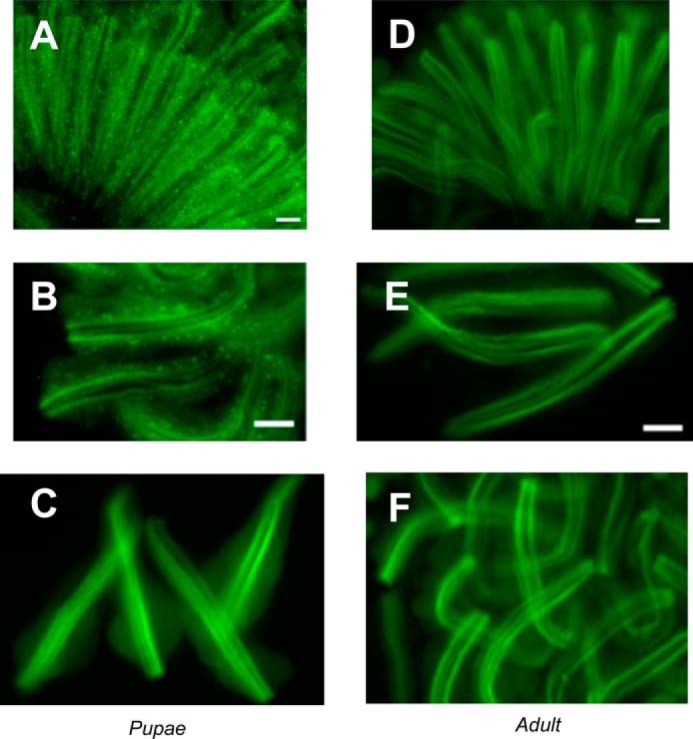
Endocytosis of Arr1-eGFP is characterized by the presence of cytosolic vesicles in dissociated photoreceptors. Dissociated photoreceptors isolated from pupae or adult that were pretreated either with or without blue light for 4 min were imaged. Blue light treated pupal photoreceptors contain numerous cytosolic vesicles enriched with Arr1-eGFP (A and B). Without blue light, Arr1-eGFP was not sequestered in vesicles but was localized in rhabdomeres and cytoplasm of pupal photoreceptors (C). In isolated adult photoreceptors, Arr1-eGFP was preferentially localized in rhabdomeres with (D and E) or without prior blue light treatment (F). Scale bar, 10 μm.
DISCUSSION
Drosophila photoreceptors express Arr1 and Arr2, each with distinct biochemical properties (10, 13, 14, 21) and molecular characteristics (12, 14). Although Arr2 is more abundant and plays a major role in the fast deactivation of the visual response (12), the role of Arr1 is less understood. Previous immunolocalization studies showed that Arr1 displays light-dependent endocytosis and may promote the recycling of phosphorylated Rh1* (14). To further our understanding into the function of Arr1 in photoreceptors, we explored the trafficking of Arr1 in live photoreceptors by generating transgenic flies expressing an eGFP-tagged Arr1.
In adult photoreceptors, Arr1-eGFP displays light-dependent translocation to the rhabdomeres. Consistent with previous reports, Arr1-eGFP appears to recognize Rh1* that is also phosphorylated. Interestingly, translocation of Arr1-eGFP is much slower than that of Arr2-eGFP, which may be attributable partly to a low level of phosphorylated Rh1*. Phosphorylation of Rh1* is regulated by the rhodopsin kinase that belongs to the family of G-protein-coupled receptor kinases. In Drosophila, there are two putative G-protein-coupled receptor kinases, Gprk1 and Gprk2 (22). Gprk1 may be the critical rhodopsin kinase involved in the phosphorylation of Rh1* in adult photoreceptors (23).
Despite the slow kinetics of the interaction between phosphorylated Rh1* and Arr1, Arr1 is specifically required for the deactivation of phosphorylated Rh1* in adult eyes and may explain our observed phenotypes. Consequently, when the level of phosphorylated Rh1* is greater than that of Arr1, PDA can be triggered. The requirement of Arr1 to inactivate phosphorylated Rh1* is also supported by the previous observation in retinal degeneration C (rdgC) flies. Vinós et al. (24) reported that rdgC flies lacking rhodopsin phosphatase requires less light to initiate PDA. We speculate that PDA is easily triggered in rdgC flies because the loss of phosphatase elevates phosphorylated Rh1* above Arr1 levels. Taken together, we propose that Arr1 is critically involved during intense light conditions in which a substantial amount of Rh1* becomes phosphorylated.
We found differences between the endocytosis of Arr1 in adult versus pupal live photoreceptors. We failed to observe the light-dependent internalization of Arr1-eGFP in adult photoreceptors. This finding along with our electrophysiological result indicates that in adult photoreceptors Arr1 is mainly involved in fast deactivation of the visual signaling. In pupal photoreceptors, we observed the light-regulated internalization of Arr1-eGFP. Internalization of Arr1-eGFP after the intense light treatment is further supported by the abundance of vesicles in the cytoplasm of dissociated photoreceptors. Based on this result, we speculate that in pupal photoreceptors Arr1 is involved in recycling of phosphorylated Rh1*. Thus the activity of phosphorylated Rh1* can be modulated by Arr1 via distinct mechanisms depending on the stages of photoreceptor development. In pupal photoreceptors, Arr1 binds to phosphorylated Rh1* and orchestrates the endocytosis of the phosphorylated Rh1*-Arr1 complex. The fate of internalized Rh1* remains to be explored, and it may be recycled or degraded. Importantly, in adult photoreceptors Arr1 is not involved in the internalization of phosphorylated Rh1*.
How does Arr1 transition from the endocytosis competent state to the incompetent state? This transition takes place around eclosion and may be regulated post-translationally. For instance, Arr1 undergoes light-dependent phosphorylation in adult photoreceptors (25). Phosphorylation of Arr1 may reduce its interaction with the endocytic machinery in a way similar to phosphorylation of β-arrestin 1 by extracellular signal-regulated kinases (26). It is also possible that Rh1* may be differentially phosphorylated in photoreceptors similar to the phosphorylation of β2-adrenergic receptor by either G-protein coupled receptor kinase 2 or 5/6 (27), leading to a different trafficking pattern in Arr1.
We hypothesize that adult photoreceptors efficiently recycle phosphorylated Rh1* to maintain optimal visual sensitivity. For example, phosphorylated Rh1* may be readily dephosphorylated in rhabdomeres by RdgC (24, 28, 29), the critical rhodopsin phosphatase, to promote fast recycling of Rh1 in adult eyes. This hypothesis also predicts a low steady-state level of phosphorylated Rh1*, which may be responsible for the slow translocation of Arr1-eGFP we observed in adult eyes.
Here we explored the functions and trafficking of Arr1 in Drosophila photoreceptors. We show that Arr1 appears to interact with phosphorylated Rh1* to control its cellular fates and signaling. Specifically we demonstrate that cytoplasmic Arr1 displays light-dependent redistribution in transgenic flies expressing Arr1-eGFP possibly by associating with phosphorylated Rh1* in the rhabdomeres. Arr1 interaction occurs with a slower kinetics compared with the interaction between Arr2 and Rh1*. After translocation, Arr1-eGFP remains in the rhabdomeres but is not internalized in adult photoreceptors. In contrast, Arr1-eGFP becomes internalized in pupal photoreceptors. These different trafficking itineraries thus reflect the distinct role of Arr1 in photoreceptor function.
In the adult, Arr1 is required for the visual response to promote fast deactivation of phosphorylated Rh1*. When the level of phosphorylated Rh1* exceeds that of Arr1, PDA can be evoked. Consistently, Arr1 is not required in flies expressing phosphorylation-deficient Rh1. In pupae, Arr1 appears to associate with phosphorylated Rh1* and becomes internalized. Arr1 may orchestrate the recycling of rhodopsin in pupal eyes in which the accumulation of rhodopsin is required for the elongation of rhabdomeres.
Supplementary Material
Acknowledgment
We thank William Haberstroh for technical help.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 EY019519 (to B.-H.S.) and P30 EY08126 (to Vanderbilt University).

This article contains supplemental Movies 1 and 2.
- Arr1
- arrestin 1
- Arr2
- arrestin 2
- dpp
- deep pseudopupil
- eGFP
- enhanced green fluorescent protein
- ninaE
- neither inactivation nor afterpotential E
- PDA
- prolonged depolarizing afterpotential
- rdgC
- retinal degeneration C
- Rh1
- rhodopsin 1
- ERG
- electroretinogram
- REI
- rhabdomere enrichment index.
REFERENCES
- 1. Lefkowitz R. J., Shenoy S. K. (2005) Transduction of receptor signals by β-arrestins. Science 308, 512–517 [DOI] [PubMed] [Google Scholar]
- 2. Chen W., ten Berge D., Brown J., Ahn S., Hu L. A., Miller W. E., Caron M. G., Barak L. S., Nusse R., Lefkowitz R. J. (2003) Dishevelled 2 recruits β-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science 301, 1391–1394 [DOI] [PubMed] [Google Scholar]
- 3. Chen W., Kirkbride K. C., How T., Nelson C. D., Mo J., Frederick J. P., Wang X. F., Lefkowitz R. J., Blobe G. C. (2003) β-Arrestin 2 mediates endocytosis of type III TGF-β receptor and down-regulation of its signaling. Science 301, 1394–1397 [DOI] [PubMed] [Google Scholar]
- 4. Noma T., Lemaire A., Naga Prasad S. V., Barki-Harrington L., Tilley D. G., Chen J., Le Corvoisier P., Violin J. D., Wei H., Lefkowitz R. J., Rockman H. A. (2007) β-Arrestin-mediated β1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J. Clin. Invest. 117, 2445–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilden U., Wüst E., Weyand I., Kühn H. (1986) Rapid affinity purification of retinal arrestin (48 kDa protein) via its light-dependent binding to phosphorylated rhodopsin. FEBS Lett. 207, 292–295 [DOI] [PubMed] [Google Scholar]
- 6. Xu J., Dodd R. L., Makino C. L., Simon M. I., Baylor D. A., Chen J. (1997) Prolonged photoresponses in transgenic mouse rods lacking arrestin. Nature 389, 505–509 [DOI] [PubMed] [Google Scholar]
- 7. Kühn H., Wilden U. (1987) Deactivation of photoactivated rhodopsin by rhodopsin-kinase and arrestin. J. Recept. Res. 7, 283–298 [DOI] [PubMed] [Google Scholar]
- 8. Smith D. P., Shieh B. H., Zuker C. S. (1990) Isolation and structure of an arrestin gene from Drosophila. Proc. Natl. Acad. Sci. U.S.A. 87, 1003–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hyde D. R., Mecklenburg K. L., Pollock J. A., Vihtelic T. S., Benzer S. (1990) Twenty Drosophila visual system cDNA clones: one is a homolog of human arrestin. Proc. Natl. Acad. Sci. U.S.A. 87, 1008–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. LeVine H., 3rd, Smith D. P., Whitney M., Malicki D. M., Dolph P. J., Smith G. F., Burkhart W., Zuker C. S. (1990) Isolation of a novel visual-system-specific arrestin: an in vivo substrate for light-dependent phosphorylation. Mech. Dev. 33, 19–25 [DOI] [PubMed] [Google Scholar]
- 11. Yamada T., Takeuchi Y., Komori N., Kobayashi H., Sakai Y., Hotta Y., Matsumoto H. (1990) A 49-kilodalton phosphoprotein in the Drosophila photoreceptor is an arrestin homolog. Science 248, 483–486 [DOI] [PubMed] [Google Scholar]
- 12. Dolph P. J., Ranganathan R., Colley N. J., Hardy R. W., Socolich M., Zuker C. S. (1993) Arrestin function in inactivation of G protein-coupled receptor rhodopsin in vivo. Science 260, 1910–1916 [DOI] [PubMed] [Google Scholar]
- 13. Kiselev A., Socolich M., Vinós J., Hardy R. W., Zuker C. S., Ranganathan R. (2000) A molecular pathway for light-dependent photoreceptor apoptosis in Drosophila. Neuron 28, 139–152 [DOI] [PubMed] [Google Scholar]
- 14. Satoh A. K., Ready D. F. (2005) Arrestin1 mediates light-dependent rhodopsin endocytosis and cell survival. Curr. Biol. 15, 1722–1733 [DOI] [PubMed] [Google Scholar]
- 15. Mishra P., Socolich M., Wall M. A., Graves J., Wang Z., Ranganathan R. (2007) Dynamic scaffolding in a G protein-coupled signaling system. Cell 131, 80–92 [DOI] [PubMed] [Google Scholar]
- 16. Bainbridge S. P., Bownes M. (1981) Staging the metamorphosis of Drosophila melanogaster. J. Embryol. Exp. Morphol. 66, 57–80 [PubMed] [Google Scholar]
- 17. Rossner M., Yamada K. M. (2004) What's in a picture? The temptation of image manipulation. J. Cell Biol. 166, 11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mismer D., Rubin G. M. (1987) Analysis of the promoter of the ninaE opsin gene in Drosophila melanogaster. Genetics 116, 565–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Tousa J. E., Baehr W., Martin R. L., Hirsh J., Pak W. L., Applebury M. L. (1985) The Drosophila ninaE gene encodes an opsin. Cell 40, 839–850 [DOI] [PubMed] [Google Scholar]
- 20. Kristaponyte I., Hong Y., Lu H., Shieh B. H. (2012) Role of rhodopsin and arrestin phosphorylation in retinal degeneration of Drosophila. J. Neurosci. 32, 10758–10766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsumoto H., Kurien B. T., Takagi Y., Kahn E. S., Kinumi T., Komori N., Yamada T., Hayashi F., Isono K., Pak W. L. (1994) Phosrestin I undergoes the earliest light-induced phosphorylation by a calcium/calmodulin-dependent protein kinase in Drosophila photoreceptors. Neuron 12, 997–1010 [DOI] [PubMed] [Google Scholar]
- 22. Cassill J. A., Whitney M., Joazeiro C. A., Becker A., Zuker C. S. (1991) Isolation of Drosophila genes encoding G protein-coupled receptor kinases. Proc. Natl. Acad. Sci. U.S.A. 88, 11067–11070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee S. J., Xu H., Montell C. (2004) Rhodopsin kinase activity modulates the amplitude of the visual response in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 101, 11874–11879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vinós J., Jalink K., Hardy R. W., Britt S. G., Zuker C. S. (1997) A G protein-coupled receptor phosphatase required for rhodopsin function. Science 277, 687–690 [DOI] [PubMed] [Google Scholar]
- 25. Matsumoto H., Yamada T. (1991) Phosrestins I and II: arrestin homologs which undergo differential light-induced phosphorylation in the Drosophila photoreceptor in vivo. Biochem. Biophys. Res. Commun. 177, 1306–1312 [DOI] [PubMed] [Google Scholar]
- 26. Lin F. T., Miller W. E., Luttrell L. M., Lefkowitz R. J. (1999) Feedback regulation of β-arrestin1 function by extracellular signal-regulated kinases. J. Biol. Chem. 274, 15971–15974 [DOI] [PubMed] [Google Scholar]
- 27. Shenoy S. K., Drake M. T., Nelson C. D., Houtz D. A., Xiao K., Madabushi S., Reiter E., Premont R. T., Lichtarge O., Lefkowitz R. J. (2006) β-Arrestin-dependent, G protein-independent ERK1/2 activation by the β2 adrenergic receptor. J. Biol. Chem. 281, 1261–1273 [DOI] [PubMed] [Google Scholar]
- 28. Byk T., Bar-Yaacov M., Doza Y. N., Minke B., Selinger Z. (1993) Regulatory arrestin cycle secures the fidelity and maintenance of the fly photoreceptor cell. Proc. Natl. Acad. Sci. U.S.A. 90, 1907–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Steele F. R., Washburn T., Rieger R., O'Tousa J. E. (1992) Drosophila retinal degeneration C (rdgC) encodes a novel serine/threonine protein phosphatase. Cell 69, 669–676 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.