Abstract
Conflicts of interests: the authors declare no potential conflict of interests.
Background
Polychlorinated biphenyls (PCBs) have been found to be associated with diabetes in some, but not all, studies performed so far. The aim of this study was to assess the association between PCB serum levels and glycaemia and diabetes in people living in Brescia, a highly industrialised PCB-polluted town in Northern Italy.
Design and Methods
527 subjects were enrolled in a cross-sectional population-based study: they were interviewed face-to-face in 2003 and also provided a blood sample under fasting conditions. The concentration of 24 PCB congeners was determined using gas-chromatography (GC/MS). Subsequently, all subjects were included in a follow-up (cohort) study. According to the Local Health Authority health-care database, subjects were considered to be diabetic if they had diabetes at interview time (prevalent cases) or during a 7-year follow-up (incident cases).
Results
A total of 53 subjects (10.0%) were diabetics: 28 had diabetes at enrolment and other 25 developed the disease subsequently. Diabetes frequency increased according to the serum concentrations of total PCBs and single PCB congeners, but no association was found when estimates were adjusted for education, body mass index, age and gender by logistic regression analysis. Accordingly, glycaemia increased with PCB serum levels, but no association was observed when multiple regression analysis, including confounding factors, was performed.
Conclusions
This study does not support the hypothesis that PCB environmental exposure is strictly associated with diabetes or glycaemia.
Key words: polychlorinated biphenyls, diabetes, glycaemia.
Significance for public health
The aim of this research was to assess the association between polychlorinated biphenyls (PCBs) serum levels, glycaemia and diabetes, with a cross-sectional population-based study and a subsequent cohort study with a 7 year follow-up. An association between diabetes and concentration of PCBs serum levels was found by crude analysis, but it disappeared when adjusting for education, body mass index, age and gender as confounders.
The results of this study, which was carried on people living in a highly PCB-polluted area, provide information on the possible intervention of Public Health to contrast onset of diseases related to PCB exposure and put forward some useful suggestions for an environmental policy.
Introduction
Polychlorinated biphenyls (PCBs) are defined as persistent organic pollutants (POPs) and toxic chemicals, which persist in the environment for long periods of time and biomagnify as they move up through the food chain.1 PCBs are mixtures of up to 209 different compounds (congeners), depending on the number and position of chlorine atoms attached to the biphenyl molecule. PCBs were widely used in the past, especially in electrical, heat transfer and hydraulic equipment, and in plasticizers, paints, plastic and rubber products, but their production was stopped in many countries in response to the negative impact that these compounds had on environmental and human health.1 PCBs, especially the 12 PCB congeners that have dioxin-like activity, have been linked to several and adverse health effects on human and animals, such as: cancer,1 damage to the nervous system, reproductive disorders and disruption of the endocrine and immune systems.2 Recently, it has been suggested that POPs stored in body fat may contribute to the onset of diabetes or insulin resistance, in addition to obesity, unhealthy diet and physical inactivity.3 PCBs, especially PCB 153, and PCDD/Fs were found to be associated with diabetes in various studies carried out in the 2000s.4-9 However, no clear pathogenic mechanism has been identified and the association of dioxins and PCBs with diabetes hasn’t been found in other studies.10-14 So, up to date, no conclusion can be drawn at present and more research is required.3 In Brescia, a highly industrialised town in Northern Italy with about 200,000 inhabitants, a chemical factory produced PCBs from 1930 to 1984, and these compounds polluted the soil of nearby agricultural areas via irrigation channels, entered the food chain and thus accumulated in human beings.15,16 Other POPs, including polychlorinated dibenzo-para-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs), have also been found in the environment, animal-derived food (meat, eggs, milk and dairy products) and human sera, with a high correlation with PCB levels.17,18 The aim of this study was to assess the association between PCB serum levels and glycaemia and diabetes in people living in this town.
Design and methods
Study population
In the first phase of this cross-sectional and cohort study (2003-2004), we performed a survey on a random sample of adult residents in Brescia (Italy). Here, there was an important factory which produced organochlorine compounds, including PCBs, from the 1930s to 1984. PCBs were released into drainage water, which was discharged in irrigation channels and then accumulated in the soil of a nearby agricultural area, entering the food chain. Therefore, PCB polluted food, mainly animal products (chicken, eggs, milk and cheese), was consumed by several individuals living in the town.15,16 Four different areas and their residents were identified according to soil PCB concentrations. The stratified sampling design was based on separate frames for subjects resident in each of these 4 areas, separately for sex and divided into six age groups, from 20 to 79 years. An invitation letter was sent to 1200 subjects: 308 of them did not respond the letter and could not be found by telephone. Among the 892 contacted people, 579 (64.8%) agreed to participate and gave a blood sample for laboratory determination; 527 of them were face-to-face interviewed by a trained nurse using a structured questionnaire, including questions on demographic variables, weight and height, residential and occupational history, smoking habit, alcohol intake, and presence of past and current diseases.
Body mass index (BMI) was computed by dividing the weight in kilograms by the square of the height in meters. We indentified 3 categories of BMI according to the cut-off points proposed by the WHO:19 normal weight (BMI<25 kg/m2), overweight (25≤BMI<30 kg/m2) and obese (BMI≤30 kg/m2).
Subjects were questioned about the presence of diabetes and current use of insulin or oral hypoglycaemic agents. They were considered to have diabetes (prevalent cases) if they reported a history of type I or type II diabetes and/or if they were currently using insulin or oral hypoglycaemic agents. In Italy, citizens are registered in the General Registry Health database of the National Health Service (NHS) with a unique identification code. All subjects affected by chronic diseases can benefit free of charge analyses, drugs, physicians’ visits, and other services provided by the NHS. All these data are made available to each Local Health Authority. Therefore, in order to detect the occurrence of diabetes, we merged our database with Brescia Local Health Authority (LHA) health-care one, including both administrative and health data of all the subjects living in the area. A complete description and validity considerations on this database are given elsewhere.20 In the second phase of the study, all subjects enrolled in the survey were included in a cohort study. During a 7-year follow-up, from 1st January 2004 to 31st December 2011, all the new cases of diabetes, which occurred in the cohort, were retrieved using the LHA database (incident cases).
The Ethics Committee of the LHA approved the project and each participant provided informed consent.
Blood sampling and storage
A 20-mL blood sample was collected from each subject. Fasting plasma glucose was determined using the glucose-oxidase method. Serum lipid profile, including total cholesterol and triglycerides, was evaluated using enzymatic methods.
The serum was separated by centrifugation at 3500 rpm (5 min) and stored at –20°C until the chemical analyses were performed.
Polychlorinated biphenyls analyses
We determined the serum concentration of 24 PCB congeners - 28, 31, 52, 77, 81, 101, 105, 114, 118, 123, 126, 128, 138, 153, 156, 157, 167, 169, 170, 180, 189, 194, 206 and 209 - according to Ballschmiter and Zell’s study.21
PCBs were measured using a Hewlett-Packard 6890N gas chromatograph coupled with an MSD HP 5973. Analytical procedure, limit of quantification and method accuracy assessment are reported elsewhere.15 PCB analysis was performed at the Laboratory of Occupational Hygiene and Toxicology, Brescia University, Italy.
For the 12 dioxin-like congeners (DL-PCBs) 77, 81, 126, 169, 105, 114, 118, 123, 156, 157, 167 and 189, the total toxic equivalency (TEQ) was computed using toxic equivalency factor (TEF) defined by Van den Berg’s study.22 A minimum concentration of half the quantification limit (0.05 ng/mL) was attributed to subjects with no detection for PCB congeners that were detectable in at least 30% of subjects. A null value was attributed to congeners with detectable values in less than 30% of subjects, according to common suggestions.15 Total PCB concentration was calculated by summing up values of PCB congeners.
Since PCBs are transported in the lipidic fraction of the blood, their concentration depends on the amount of serum lipids. The ratio of PCB concentrations to total lipid levels was therefore computed (lipidadjusted PCB concentration) and expressed as ng/g lipid, the standard practice in studies investigating PCB serum levels. We calculated the total lipid concentration from cholesterol and triglyceride levels, using the formula suggested by Philips’ work:23 total serum lipids (g/dL) = 2.27 × serum cholesterol (g/dL) + triglycerides (g/dL) + 0.623.
Statistical analysis
The distribution of total PCB serum levels was examined using common statistical techniques for exploratory analysis. Due to the asymmetrical, non-normal distribution of total PCBs and of each PCB congener, the mean, median, range and 95th centile of the distributions are shown. PCB congeners were classified on the basis of their different functional groups, according to the classification proposed by various authors as:24-26 i) congeners with immunotoxic activities (PCB 138, 153 and 180); ii) low-chlorinated PCBs with pseudo-oestrogen activity (PCB 28, 52 and 153); iii) high-chlorinated PCBs with anti-oestrogenic activities (PCB 170, 180 and 194); iv) PCBs that can induce phenobarbital (PCB 101, 153, 180 and 194). The proportions of subjects with diabetes (prevalent and incident cases separately and all together) were compared according to the total PCB distribution tertiles, specific PCB congeners and PCB functional groups and TEQ DL- PCBs. Logistic regression analysis was also performed to evaluate the association between diabetes, PCB serum levels (distribution tertiles), adjusting for education, age, gender and BMI, and the corresponding odds ratios (ORs) were computed. Glycaemia levels according to the total PCB distribution tertiles, PCB 153 and PCB functional groups were compared using the analysis of variance on log-transformed data. Multiple regression of log-glycaemia as the dependent variable was also performed, with PCBs levels, education, gender, age and BMI as independent variables. All the statistical tests were two-sided with a 0.05 P-value to refute the null hypothesis. Statistical analysis was performed using STATA software for personal computer (Stata Statistical Software release 10, 2003; Stata Corporation, College Station, Texas, USA).
Results
A total of 527 subjects were enrolled. Table 1 shows the distribution of participants according to education, age, gender and BMI: 48.5% of subjects were males, mean age was 50.6 years, mean BMI was 24.8 kg/m2, and 56% had less than 8 years of schooling. According to BMI categories, 291 subjects (55.2%) had normal weight, 163 (30.9%) were overweight and 73 (13.9%) were obese.
Table 1.
Age, gender, body mass index and education of subjects enrolled in the study.
| Males N. (%) | Females N. (%) | Total N. (%) | |
|---|---|---|---|
| All subjects | 256 (48.5) | 271 (51.5) | 527 (100) |
| Age (years) | |||
| 20-39 | 72 (28.2) | 81 (29.9) | 153 (29.0) |
| 40-59 | 89 (34.7) | 105 (38.7) | 194 (36.8) |
| 60-79 | 95 (37.1) | 85 (31.4) | 180 (34.2) |
| Mean ± SD age (years) | 51.7±16.4 | 50.3±16.0 | 50.6±6.2 |
| Mean ± SD BMI (kg/m2) | 25.3±3.8 | 24.3±5.0 | 24.8±4.5 |
| Education (years) ≤5 |
59 (23.0) | 79 (29.2) | 138 (26.2) |
| 6-8 | 70 (27.4) | 79 (29.2) | 149 (28.3) |
| >8 | 127 (49.6) | 113 (41.6) | 240 (45.5) |
SD, standard deviation; BMI, body mass index.
Table 2 shows descriptive statistics of the lipid-adjusted serum levels of total PCBs, PCB congeners and PCB functional groups. The median serum concentration of total PCBs was 713.7 ng/g lipid and the range was 55.4-34377.8 ng/g lipid. PCB 153 and PCB 180 contributed most to total PCB level: the medians of PCB 153 and 180 were 207.9 (range 4.7-5618.1) ng/g lipid and 233.9 (range 9.1-11296.4) ng/g lipid, respectively. The PCB congeners set out in the table were over the detection limit in all subjects. No data are shown for congeners that were undetectable in all or almost all samples, i.e. PCB 28 (0% of detectable values), PCB 52 (0%), PCB 101 (1.5%) and PCB 126 (0%). We found no differences between males and females in serum levels of total and single PCB congeners. In the cross sectional study, 28 subjects were found to have diabetes (prevalence: 5.3%; 95% CI: 3.3-7.2). All the 527 subjects, who were already included in the cross-sectional study, were also enrolled in the cohort study. After 7 years of follow-up, 32 (6.0%) of them were dead and 9 (1.7%) were not found in the LHA database (lost at follow-up). Other 25 subjects of the cohort developed diabetes (incident cases) among those who did not have the disease at the enrolment (cumulative incidence: 5.0%; 95% CI: 3.1-6.9). Overall, 53 (10.0%) subjects were found to have diabetes according to the initial interview (n=28) or during the follow-up (n=25). For all the subjects, who reported diabetes at the interview, the presence of that disease was confirmed via the LHA database.
Table 2.
Lipid-adjusted polychlorinated biphenyls (PCB) congener, total PCB, PCB functional group and total toxic equivalency dioxin like PCBs serum levels (ng/g lipid).
| PCB congener (ng/g lipid) | Mean | Median | 95th percentile | Range |
|---|---|---|---|---|
| Total PCBs | 1309.3 | 713.7 | 3520.9 | 55.4-34377.8 |
| 138 | 187.5 | 118.2 | 481.9 | 4.7-4192.9 |
| 153 | 331.6 | 207.9 | 883.3 | 4.7-5618.1 |
| 170 | 113.0 | 64.4 | 332.3 | 5.6-2737.6 |
| 180 | 423.7 | 233.9 | 1032.5 | 9.1-11296.4 |
| 194 | 114.2 | 41.0 | 352.4 | 4.1-4216.3 |
| Immunotoxic PCBs (PCB138+153+180) | 942.9 | 565.7 | 2459.9 | 37.4-18732.4 |
| Pseudo-oestrogen PCBs (PCB28+52+153) | 331.6 | 207.9 | 883.3 | 4.7-5618.1 |
| High chlorinated anti-oestrogenic PCBs (PCB170+180+194) | 651.0 | 342.4 | 1668.5 | 27.4-18122.5 |
| Phenobarbital inducer PCBs (PCB101+153 +180+194) | 869.8 | 499.9 | 2233.8 | 37.4-20402.1 |
| TEQ DL-PCBs | 0.13 | 0.05 | 0.43 | 0.03-4.3 |
PBC, polychlorinated biphenyls congener; TEQ, total toxic equivalency.
Prevalent and incident cases of diabetes were considered all together for the comparison with non-diabetics subjects (controls). The diabetes cases were older than controls, with a median age of 62.4 and 51.3 years, respectively (P<0.001). PCBs concentration increased with the age in both diabetes cases and subjects without diabetes (P<0.01): in the whole sample, PCB serum levels were higher in subjects aged 62.4 years and older (median: 1229.5 ng/g lipid) compared with younger subjects (median: 554.8 ng/g lipid) (P<0.001).
The median serum levels of total PCBs, according to the BMI, were, with respect to normal weight subjects (628.8 ng/g lipid), higher in overweight subjects (867.2 ng/g lipid, P<0.001), but not significantly different in obese subjects (684.6 ng/g lipid; P=0.1). Diabetes prevalence increased with age and BMI, with a maximum of 20.5% among subjects aged 60 years and over, and 29.4% among the 68 obese subjects (BMI of 30 and over), more than six times the proportion in normal-weight individuals (29.4% vs 4.1%). Only one (5%) of those obese subjects had total PCB levels higher than the median of the distribution. When comparing prevalent and incident diabetes cases, we found no differences in serum levels of total PCBs between them: mean serum levels of PCBs were 1360.7 and 1166.9 ng/g lipid in prevalent and incident cases, respectively (P=0.18).
The odds ratios for diabetes according to tertiles of serum total PCB concentration, adjusted for education, BMI, age and gender, were: for prevalence of diabetes: OR=0.5 (0.1-2.2) for both 2nd and 3rd vs 1st tertiles; for cumulative incidence of diabetes: OR=2.3 (0.6-9.6) for 2nd and 0.9 (0.2-5.0) for 3rd vs 1st tertiles, respectively. There was no association between diabetes and total PCBs, PCB 153, 4 PCB functional groups and TEQ DL-PCBs concentration both in prevalent cases and in incident cases. According to those findings, we decided to analyse the association between diabetes and PCBs concentration combining all diabetes cases together to increase the power of the study.
Table 3 shows the overall cases of diabetes (prevalent and incident cases together) and the odds ratios (ORs) for diabetes according to distribution tertiles for the serum concentration of lipid-adjusted total PCBs, PCB 153, PCB functional groups and TEQ DL-PCBs. The proportion of diabetes cases increased with the concentration of total PCBs, single PCB congeners and PCB functional group. However, diabetes did not show a statistically significant association with PCB when adjusting for education, BMI, age and gender by logistic regression analysis. The differences between the crude and adjusted OR estimates were entirely due to age, which therefore was a confounder of the association whereas gender, BMI and education were not. Diabetes was associated with obesity (BMI≤30), but not overweight (24.9≤BMI≤30), the OR for obesity being 7.2 (3.4-16.2), and none of these associations was confounded or modified by PCB serum levels. No differences were found when restricting the analysis to the 25 incident diabetes cases only. No association was found between diabetes and serum concentration of lipid-adjusted PCBs according to concentration tertiles when analysing subjects with normal weight, overweight and obesity separately. No difference was found in the OR estimates for PCB tertiles when dichotomizing subjects in those under and over 62.4 years of age (median age of diabetics): the ORs for the 3rd vs 1st tertile were 0.8 (95% CI:0.2-3.8) and 0.3 (95% CI:0.1-1.5), respectively. A high dispersion of glycaemia according to PCB lipid-adjusted values was observed (Figure 1), with a weak linear correlation (Spearman’s r=0.2). However, multiple regression analysis, with logarithm of glycaemia as the dependent variable, showed no association with log-transformed total PCB concentration when including age, BMI, education and gender. Table 4 shows the geometric mean of glycaemia according to tertiles of lipid-adjusted total PCBs, PCB 153 and four functional groups in 474 subjects without diabetes. Statistically significant associations between logarithm of glycaemia and tertiles of PCB lipid-adjusted levels were found for total PCBs, PCB153 and four PCB functional groups using one-way ANOVA on log-transformed glycaemia data, which were not confirmed when adjusting for education, BMI, age and gender by multi-way ANOVA. The associations between PCB serum levels and diabetes and glycaemia were also analysed in men and women separately, providing similar results to those observed in total subjects.
Table 3.
Association between diabetes and tertiles of serum concentration of lipid-adjusted total polychlorinated biphenyls (PCBs), PCB 153, four PCB functional groups and total toxic equivalency dioxin like PCBs.
| Lipid-adjusted PCB concentration (ng/g lipid) | Presence of diabetes No. of subjects/total | Prevalence (95% CI) | Crude odds ratio (95% CI) | Adjusted odds ratio* (95% CI) |
|---|---|---|---|---|
| Total | 53/527 | 10.0 (7.4-12.5) | ||
| Total PCBs | ||||
| ≤433.7 | 8/173 | 4.6 (1.4-7.7) | 1 | 1 |
| 433.7-996.7 | 22/174 | 12.6 (7.6-17.5) | 2.9 (1.3-6.9) | 1.1 (0.4-3.0) |
| >996.7 | 23/180 | 12.8 (7.9-17.6) | 3.0 (1.3-6.9) | 0.7 (0.2-2.0) |
| PCB 153 | ||||
| ≤135.8 | 8/173 | 4.6 (1.4-7.7) | 1 | 1 |
| 135.8-282.0 | 19/174 | 10.9 (6.2-15.5) | 2.5 (1.0-5.9) | 1.1 (0.4-2.8) |
| >282.0 | 26/180 | 14.4 (9.2-19.5) | 3.4 (1.5-7.9) | 0.9 (0.3-2.4) |
| Immunotoxic PCBs (PCB138+153+180) | ||||
| ≤374.4 | 8/172 | 4.6 (1.4-7.7) | 1 | 1 |
| 374.4-752.2 | 21/175 | 12.0 (7.1-16.8) | 2.8 (1.2-6.5) | 0.9 (0.3-2.6) |
| >752.2 | 24/180 | 13.3 (8.3-18.2) | 3.1 (1.3-7.2) | 0.7 (0.2-2.1) |
| Pseudo-oestrogen PCBs (PCB28+ 52+ 153) | ||||
| ≤135.8 | 8/173 | 4.6 (1.4-7.7) | 1 | 1 |
| 135.8-282.0 | 19/174 | 10.9 (6.2-15.5) | 2.5 (1.0-5.9) | 1.0 (0.4-2.8) |
| >282.0 | 26/180 | 14.4 (9.2-19.5) | 3.4 (1.5-7.9) | 0.9 (0.3-2.4) |
| High chlorinated anti-oestrogenic PCBs (PCB170+180+194) | ||||
| ≤203.4 | 9/173 | 5.2 (1.8-8.5) | 1 | 1 |
| 2.03.4-488.9 | 24/174 | 13.8 (8.6-18.9) | 2.9 (1.3-6.4) | 1.0 (0.4-2.8) |
| >488.9 | 20/180 | 11.1 (6.5-15.6) | 2.2 (1.0-5.1) | 0.6 (0.2-1.8) |
| Phenobarbital inducer PCBs (PCB101+153+180+194) | ||||
| ≤309.2 | 8/173 | 4.6 (1.4-7.7) | 1 | 1 |
| 309.2-669.9 | 22/174 | 12.6 (7.6-17.5) | 2.9 (1.3-6.9) | 1.0 (0.4-2.7) |
| >669.9 | 23/180 | 12.8 (7.9-17.6) | 3.0 (1.3-6.9) | 0.7 (0.2-2.2) |
| TEQ DL-PCBs | ||||
| ≤0.03 | 12/228 | 5.2 (2.3-8.0) | 1 | 1 |
| 0.03-0.09 | 15/98 | 15.3 (8.0-22.4) | 3.2 (1.4-7.2) | 1.2 (0.5-3.2) |
| >0.09 | 26/201 | 12.9 (8.2-17.5) | 2.6 (1.3-5.4) | 0.8 (0.3-1.9) |
*Logistic regression models included age, gender, education and body mass index. CI, confidence interval; PBC, polychlorinated biphenyls; TEQ, total toxic equivalency.
Figure 1.
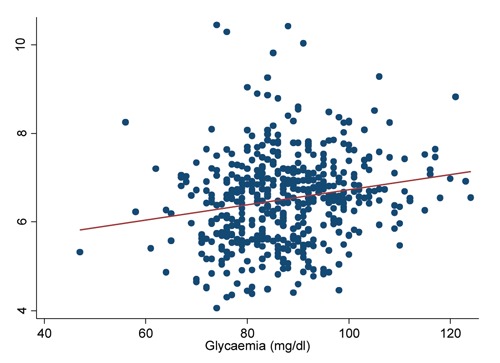
Distribution of glycaemia values according to log-transformed, lipid-adjusted, total polychlorinated biphenyls serum levels.
Table 4.
Geometric mean of glycaemia according to tertiles of lipid-adjusted polychlorinated biphenyls in 474 subjects without diabetes.
| Tertiles of lipid-adjusted PCB concentration (ng/g lipid) | Geometric mean of glycaemia | One-way ANOVA on log-transformed data P values | Multi-way ANOVA* on log-transformed data P values |
|---|---|---|---|
| Total | 86.7 | ||
| Total PCBs | |||
| ≤433.7 | 84.2 | 0.0009 | 0.17 |
| 433.7-996.7 | 88.8 | ||
| >996.7 | 88.0 | ||
| PCB 153 | |||
| ≤135.8 | 84.3 | 0.001 | 0.75 |
| 135.8-282.0 | 88.0 | ||
| >282.0 | 88.8 | ||
| Immunotoxic PCBs (PCB138+153+180) | |||
| ≤374.4 | 84.3 | 0.001 | 0.80 |
| 374.4-752.2 | 87.9 | ||
| >752.2 | 88.8 | ||
| Pseudo-oestrogen PCBs (PCB28+52+153) | |||
| ≤135.8 | 84.3 | 0.001 | 0.75 |
| 135.8-282.0 | 88.0 | ||
| >282.0 | 88.8 | ||
| High chlorinated anti-oestrogenic PCBs (PCB170+180+194) | |||
| >203.4 | 84.2 | 0.009 | 0.18 |
| 2.03.4-488.9 | 88.9 | ||
| >488.9 | 87.2 | ||
| Phenobarbital inducer PCBs (PCB101+153+180+194) | |||
| ≤309.2 | 84.5 | 0.004 | 0.43 |
| 309.2-669.9 | 88.2 | ||
| >669.9 | 88.3 | ||
| TEQ DL-PCB | |||
| ≤0.03 | 85.1 | 0.01 | 0.14 |
| 0.03-0.09 | 86.8 | ||
| >0.09 | 89.3 | ||
*Fitted model included age, gender, education and body mass index. PBC, polychlorinated biphenyls; TEQ, total toxic equivalency
Discussion
In spite of a high exposure of the study population to PCBs and possibly PCDD/Fs for about half a century, we found no relationship between total PCB serum levels and diabetes. An association of diabetes with the concentration of lipid-adjusted total PCBs, PCB 153, PCB functional group and TEQ DL-PCB serum levels was found by crude analysis, but it disappeared when adjusting for age.
Accordingly, glycaemia increased with serum levels of total PCB, PCB 153, and immunotoxic, high-chlorinated and phenobarbital-inducing PCBs, but again no association was found when multiple regression analysis, including confounders, was performed. ANOVA models using PCB distribution tertiles confirmed these findings.
Several cross-sectional studies and cohort studies have evaluated the association between serum concentration of PCBs and diabetes, glycaemia or insulin resistance so far. Some of them have been conducted among people with high environmental exposure to PCBs or high intake of contaminated food,27,28 but the majority of them included subjects with low serum concentrations of PCBs.4,6,7,9,29-31 Many, 4,6-9,27,28,30-35 but not all,5,10-14,36 studies found a statistically significant association between PCB concentration and diabetes, glycaemia or insulin resistance. Two of them found a positive association among women, but not among men,37,38 and one found a positive association for some PCB congeners but not sum of PCBs.29 One study found PCB effects only at low doses, suggesting a non-traditional dose-response relationship of POPs with diabetes,30,32 although opposite results (associations only at the highest levels of PCB contaminations and dose-effect relationship) were reported by many others.6,8,27,29,34 These findings are substantially in agreement with those reported in a recent National Toxicology Program Workshop Review, although it did not include the studies published in 2011-12.39 Overall, these discrepancies does not seem to depend on study area, study design, PCB serum levels, age and gender of investigated people, and may be due to differences in exposure, in terms of quantity and quality of PCBs investigated, and to not having taking account of confounding factors and interaction.
Various mechanisms to measure diabetogenic effect of dioxin and PCB have been suggested.3,40,41 These chemicals exert their effects by binding to the aryl-hydrocarbon receptor, which could promote diabetogenesis by antagonism of peroxisome proliferation-activated receptors.41 Some authors, however, suggest that the effect of POPs may be due to an interaction with the classic risk factors for diabetes, more than to a direct causal relationship. Indeed, the NHANES survey conducted on the U.S. general population found that obese people had a higher frequency of insulin resistance than normal weight individuals, but that the association between obesity and insulin resistance was present only in people with high POP levels.8,42 Other authors have suggested that PCB may act through increased inflammatory responses or like steroid hormones,43 which may play a role in the development of diabetes.44 However, an independent substantial role of POPs in causing diabetes at the commonly found environmental levels seems questionable to us, as the present worldwide diabetes epidemic has been related universally to an increase in obesity, high calorie and high glycaemic load diets and physical inactivity, and various trials have clearly shown that changes in diet and exercise can prevent diabetes effectively, although they obviously cannot remove POPs from the body, as noted by others.3
Serious environmental contamination of a PCB mixture was found in the Brescia area, with a prevalence of hexa/hepta-chlorinated congeners and a minor component of lower chlorinated congeners and dioxin-like congeners. PCDDs and PCDFs, but not TCDD, were also found, although the DL-PCBs contributed to the overall toxicity equivalent (TEQ) substantially, sometimes outweighing the contribution of PCDD/Fs.17,18
This study has various strengths. First, it is a population-based study, aiming to investigate PCB serum levels and the possible effects of this exposure in a random sample of the general adult population living in the town. Second, all the people included in the study were living in an industrialised town that has been highly polluted by PCBs, PCDDs and PCDFs for many years. The high levels of PCBs still found in the soil in some areas of the town (up to 8 mg/kg) suggest a persistent exposure to PCBs for the whole population, long enough to allow an effect on glycaemia metabolism or diabetes to be detected. In fact, we found that some of the people studied had very high serum PCB levels, with total PCB lipid-adjusted values ranging up to 34377.8 ng/g lipid, 13 times higher than the reference value for the adult population living in highly industrialized areas in Italy (2650 ng/g lipid) and similar to those found in other PCB-polluted areas,45 such as Anniston (USA) and Michalovce (Slovakia).27,28 We also examined 24 PCB congeners, including the most commonly implicated as endocrine disruptors, and we collected information on serum lipid values and body mass index in order to check for possible confounders.
Furthermore, the presence of diabetes was confirmed in all cases using the LHA database, including clinical data of high validity for each subject.
This study has some limitations as well. First of all, the relatively small number of subjects with diabetes, which is not unexpected since a random sample of the general adult population was included in the study, most of whom were healthy. In our samples the diabetes percentage was 12.9% in males and 7.3% in females, in agreement with national data (10% in males and 7% in females).46
In conclusion, this cross-sectional and cohort study in a highly PCB-polluted area does not support the hypothesis that environmental exposure to PCBs and other POPs is associated with diabetes or glycaemia.
Acknowledgements
we are grateful to the nurses who performed the interviews and to the Local Health Authority technical personnel who collaborated in the study. The Ethics Committee of the Brescia Local Health Authority approved the project.
References
- 1.Faroon OM, Keith S, Jones D, De Rosa C. Carcinogenic effects of polychlorinated biphenyls. Toxicol Ind Health 2001;17:41-62 [DOI] [PubMed] [Google Scholar]
- 2.Faroon OM, Keith S, Jones D, De Rosa C. Effects of polychlorinated biphenyls on development and reproduction. Toxicol Ind Health 2001;17:63-93 [DOI] [PubMed] [Google Scholar]
- 3.Carpenter DO. Environmental contaminants as risk factors for developing diabetes. Environ Health 2008;23:59-74 [DOI] [PubMed] [Google Scholar]
- 4.Longnecker MP, Klebanoff MA, Brock JW, et al. Polychlorinated biphenyl serum levels in pregnant subjects with diabetes. Diabetes Care 2001;24:1099-101 [DOI] [PubMed] [Google Scholar]
- 5.Rignell-Hydbom A, Rylander L, Hagmar L. Exposure to persistent organochlorine pollutants and type 2 diabetes mellitus. Hum Exp Toxicol 2007;26:447-52 [DOI] [PubMed] [Google Scholar]
- 6.Fierens S, Mairesse H, Heilier JF, et al. Dioxin/polychlorinated biphenyl body burden, diabetes and endometriosis: findings in a population-based study in Belgium. Biomarkers 2003;8:529-34 [DOI] [PubMed] [Google Scholar]
- 7.Rylander L, Rignell-Hydbom A, Hagmar L. A cross-sectional study of the association between persistent organochlorine pollutants and diabetes. Environ Health 2005;4:28-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DH, Lee IK, Song K, et al. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999-2002. Diabetes Care 2006;29:1638-44 [DOI] [PubMed] [Google Scholar]
- 9.Everett CJ, Frithsen IL, Diaz VA, et al. Association of a polychlorinated dibenzo-p-dioxin, a polychlorinated biphenyl, and DDT with diabetes in the 1999-2002 National Health and Nutrition Examination Survey. Environ Res 2007;103:413-8 [DOI] [PubMed] [Google Scholar]
- 10.Airaksinen R, Rantakokko P, Eriksson JG, et al. Association between type 2 diabetes and exposure to persistent organic pollutants. Diabetes Care 2011;34:1972-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turyk M, Anderson HA, Knobeloch L, et al. Prevalence of diabetes and body burdens of polychlorinated biphenyls, polybrominateddiphenyl ethers, and p,p’-diphenyldichloroethene in Great Lakes sport fish consumers. Chemosphere 2009;75:674-9 [DOI] [PubMed] [Google Scholar]
- 12.Turyk M, Anderson H, Knobeloch L, et al. Organochlorine exposure and incidence of diabetes in a cohort of Great Lakes sport fish consumers. Environ Health Perspect 2009;117:1076-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Codru N, Schymura MJ, Negoita S, et al. Diabetes in relation to serum levels of polychlorinated biphenyls and chlorinated pesticides in adult Native Americans. Environ Health Perspect 2007;115:1442-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glynn AW, Granath F, Aune M, et al. Organochlorines in Swedish women: determinants of serum concentrations. Environ Health Perspect 2003;111:349-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apostoli P, Magoni M, Bergonzi R, et al. Assessment of reference values for polychlorinated biphenyl concentration in human blood. Chemosphere 2005;61:413-21 [DOI] [PubMed] [Google Scholar]
- 16.Donato F, Magoni M, Bergonzi R, et al. Exposure to polychlorinated biphenyls in residents near a chemical factory in Italy: the food chain as main source of contamination. Chemosphere 2006;64:1562-72 [DOI] [PubMed] [Google Scholar]
- 17.Turrio-Baldassarri L, Abate V, Alivernini S, et al. A study on PCB, PCDD/PCDF industrial contamination in a mixed urban-agricultural area significantly affecting the food chain and the human exposure. part I: soil and feed. Chemosphere 2007;67:1822-30 [DOI] [PubMed] [Google Scholar]
- 18.Turrio-Baldassarri L, Abate V, Battistelli CL, et al. PCDD/F and PCB in human serum of differently exposed population groups of an Italian city. Chemosphere 2008;73:S228-34 [DOI] [PubMed] [Google Scholar]
- 19.WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. 1995. Technical Report Series No. 854. Available from: http://whqlibdoc.who.int/trs/WHO_ TRS_854.pdf [PubMed]
- 20.Lonati F, Scarcella C, Indelicato A, et al. [Brescia Local Health Authority Population Database: a method based on current data for monitoring chronic diseases and management]. Epidemiol Prev 2008; 32:137-144 [Article in Italian]. [PubMed] [Google Scholar]
- 21.Ballschmiter K, Zell M. Analysis of polychlorinated biphenyls (PCB) by glass capillary gas chromatography. Fresenius Z Anal Chem 1980;302:20-31 [Google Scholar]
- 22.Van den Berg M, Birnbaum LS, Denison M, et al. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci 2006;93:223-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips DL, Pirkle JL, Burse VW, et al. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol 1989;18:495-500 [DOI] [PubMed] [Google Scholar]
- 24.Hansen, LG. Stepping backward to improve assessment of PCB congener toxicities. Environ Health Perspect 1998;106:171-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolff MS, Camann D, Gammon M, et al. Proposed PCB congener groupings for epidemiological studies. Environ Health Perspect 1997;105:13-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cocco P, Brennan P, Ibba A, et al. Plasma polychlorobiphenyl and organochlorine pesticide level and risk of major lymphoma subtypes. Occup Environ Med 2008;65:132-40 [DOI] [PubMed] [Google Scholar]
- 27.Ukropec J, Radikova Z, Huckova M, et al. High prevalence of prediabetes and diabetes in a population exposed to high levels of an organochlorine cocktail. Diabetologia 2010;53:899-906 [DOI] [PubMed] [Google Scholar]
- 28.Silverstone AE, Rosenbaum PF, Weinstock RS, et al. Polychlorinated Biphenyl (PCB) exposure and diabetes: results from the Anniston Community Health Survey. Environ Health Perspect 2012;120:727-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee DH, Lee IK, Jin SH, et al. Association between serum concentrations of persistent organic pollutants and insulin resistance among nondiabetic adults: results from the National Health and Nutrition Examination Survey 1999-2002. Diabetes Care 2007;30:622-8 [DOI] [PubMed] [Google Scholar]
- 30.Lee DH, Steffes MW, Sjödin A, et al. Low dose of some persistent organic pollutants predicts type 2 diabetes: a nested case-control study. Environ Health Perspect 2010;118:1235-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philibert A, Schwartz H, Mergler D. An exploratory study of diabetes in a First Nation community with respect to serum concentrations of p,p’-DDE and PCBs and fish consumption. Int J Environ Res Public Health 2009;6:3179-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grandjean P, Henriksen JE, Choi AL, et al. Marine food pollutants as a risk factor for hypoinsulinemia and type 2 diabetes. Epidemiology 2011;22:410-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Persky V, Piorkowski J, Turyk M, et al. Associations of polychlorinated biphenyl exposure and endogenous hormones with diabetes in post-menopausal women previously employed at a capacitor manufacturing plant. Environ Res 2011;111:817-24 [DOI] [PubMed] [Google Scholar]
- 34.Uemura H, Arisawa K, Hiyoshi M, et al. Associations of environmental exposure to dioxins with prevalent diabetes among general inhabitants in Japan. Environ Res 2008;108:63-8 [DOI] [PubMed] [Google Scholar]
- 35.Lee DH, Steffes MW, Sjödin A, et al. Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS One 2011;6:e15977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jørgensen ME, Borch-Johnsen K, Bjerregaard P. A cross-sectional study of the association between persistent organic pollutants and glucose intolerance among Greenland Inuit. Diabetologia 2008;51:1416-22 [DOI] [PubMed] [Google Scholar]
- 37.Vasiliu O, Cameron L, Gardiner J, et al. Polybrominated biphenyls, polychlorinated biphenyls, body weight, and incidence of adult-onset diabetes mellitus. Epidemiology 2006;17:352-9 [DOI] [PubMed] [Google Scholar]
- 38.Wang SL, Tsai PC, Yang CY, et al. Increased risk of diabetes and polychlorinated biphenyls and dioxins: a 24-year follow-up study of the Yucheng cohort. Diabetes Care 2008;31:1574-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect 2012;120:779-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujiyoshi PT, Michalek JE, Matsumura F. Molecular epidemiologic evidence for diabetogenic effects of dioxin exposure in U.S. Air force veterans of the Vietnam war. Environ Health Perspect 2006;114:1677-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remillard RB, Bunce NJ. Linking dioxins to diabetes: epidemiology and biologic plausibility. Environ Health Perspect 2002;110:853-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dirinck E, Jorens PG, Covaci A, et al. Obesity and persistent organic pollutants: possible obesogenic effect of organochlorine pesticides and polychlorinated biphenyls. Obesity 2011;19:709-14 [DOI] [PubMed] [Google Scholar]
- 43.Hennig B, Meerarani P, Slim R, et al. Proinflammatory properties of coplanar PCBs: in vitro and in vivo evidence. Toxicol Appl Pharmacol 2002;181:174-83 [DOI] [PubMed] [Google Scholar]
- 44.Swedenborg E, Pongratz I. AhR and ARNT modulate ER signaling. Toxicology 2010;268:132-8 [DOI] [PubMed] [Google Scholar]
- 45.SIVR Società Italiana Valori di Riferimento. Available from: http://www.valoridiriferimento.com/lista.htm Accessed on: June 25, 2012.
- 46.Il progetto cuore, Istituto Superiore di Sanità [in Italian]. Available from: http://www.cuore.iss.it/fattori/glicemia.asp Accessed on: June 25, 2012.


