Abstract
Background
An estimated 90% of HIV-infected people are likely to develop oral lesions in the course of HIV infection. Oro-pharyngeal candidiasis (OPC), an early marker for HIV-infection, can be diagnosed during an oral examination (OE). Primary healthcare (PHC) providers in Kenya are neither trained nor sufficiently equipped to perform this simple, cheap and non-invasive examination. The PHC system in Kenya offers an opportunity to integrate early recognition and management of oral lesions into general health care. This study aims to estimate the effect of a multifaceted intervention for PHC providers in training them to perform an OE. Specifically, our primary objective is to establish whether the intervention is effective in increasing: i) the frequency of early detection of HIV-related oral lesions; and ii) referral rates for HIV-testing.
Design and methods
The study has been designed in two parts: a retrospective clinical data record study and a prospective cohort study with pre-post control group design, carried out in 2 administrative divisions in Nairobi East district. The intervention group will receive one day of training on recognition of HIV-related oral lesions and other common oral conditions. Reminder sessions will be held at individual health facilities. Routine tally sheets will be used to record all patients with HIV-related oral lesions, dental caries and periodontal disease. A convenience sample of all the PHC in a division will be used. It will not be possible to blind investigators or assessors. Expected impact of the study for Public Health. Early recognition and treatment of HIV infection influences long-term survival rates and will reduce healthcare expenditure.
Acknowledgments
The project is funded by the Netherlands organisation for international cooperation in higher education (NUFFIC). We would like to thank all participating health facilities and health care workers for their willingness to take part in this study. LNK also thanks the Kenya Ministry of Public Health and Sanitation for permission to carry out this study. We also thank Mr. J Mulder from Radboud University Nijmegen Medical Centre, Department of Methodology, Information Management and Statistics, Nijmegen, The Netherlands for statistical advice.
Funding
this study is funded by a research grant from The Netherlands Organization for International Cooperation in Higher Education (NUFFIC, Grant nr: C&B-NFP-PHD.10/110), The Hague, The Netherlands.
Trial registration
Netherlands Trial Register NTR2627 (date registered 22nd November 2010).
Ethics approval
Kenyatta National Hospital/University of Nairobi Ethics and Research Committee (approval number KNH-ERC/A/474), and The Ministry of Public Health and Sanitation (Ref. N. MPHS/IB/1/14 Vol. III).
Key words: AIDS/HIV, detection, mucosal lesion
Significance for public health
Several oral mucosal lesions, especially oro-pharyngeal candidiasis (OPC), are markers of suspected HIV infection. In the course of their disease, over 90% of HIV-infected people are likely to suffer from these lesions. The appearance of oral mucosal lesions and particularly, of OPC in HIV- infected patients on highly active anti-retroviral therapy (HAART), may indicate virological failure. Continuation of anti-retroviral treatment in the presence of ongoing viral replication leads to increasing resistance to HAART, thereby decreasing options for second-line treatment. Primary healthcare providers (PHC), i.e. nurses and clinical officers, being the main providers of health services for the underprivileged, should be able to recognise OPC. PHC should be trained in performing an oral examination. Early detection by PHC of OPC in both patient groups, followed by appropriate management, might increase survival, improve quality of life and eventually reduce national health care costs.
Introduction
Early detection of HIV infection, allowing immediate management, influences long-term survival rates. Several oral lesions, especially oro-pharyngeal candidiasis (OPC), are early markers of suspected Human Immunodeficiency Virus (HIV) infection. Referral of suspected HIV patients for a blood test when their immunity is only moderately compromised might improve their quality of life. In many developing countries, however, early detection of oral symptoms of HIV-related lesions is inadequate.1 In the course of their disease, over 90% of HIV-infected people are likely to suffer from oral lesions. These cause pain and discomfort leading to reduced food intake and, consequently, to weight loss.2 Most HIV medications require adequate food intake in order to minimise side effects and optimise absorption. Inadequate food intake may lead to non-adherence to drugs and may even result in drug resistance and development of OPC.3, 4
Identification and management of HIV-related oral lesions requires skilled clinicians. In Kenya, the main health service providers at primary health care (PHC) facilities are clinical officers (COs) and nurses. They are trained to perform a general medical examination, are allowed to make a diagnosis and, consequently, to treat patients or refer them for specialised care. They also give general care to HIV patients, but their knowledge and skills in detecting, recognising, diagnosing and managing oral HIV-related lesions are usually inadequate. Moreover, they are neither familiar with nor trained in performing an oral examination (OE).5 They even show some resistance towards performing OE (LNK, personal data collected during interviews with PHC providers, 2010). Furthermore, owing to stigmatisation, low self-esteem and lack of information on available care, HIV patients may possibly not demand oral health service provision during consultations. Integration of oral health care into the current health care system requires additional education of PHC providers, enabling them to perform OE in order to manage common oral conditions and to identify oral lesions that can be referred early to higher levels of care.6,7 In addition, the appearance of oral lesions, and particularly of OPC, in HIV-infected patients on highly active anti-retroviral therapy (HAART) may indicate virological failure.8 Continuation of anti-retroviral treatment in the presence of ongoing viral replication leads to increasing resistance to HAART, thereby reducing the options for second-line treatment.9, 10 The resistant virus may also be transmitted through sexual contact, thus limiting the effects of first-line treatment in other people. In poor resource settings such as those found in Kenya, where virological tests may not always be available, routine OE of patients on HAART may be a simple and practical tool for the early identification and subsequent management of HAART-failure.
Study aims
The primary objective of this study is to estimate the effectiveness of educating PHC providers to perform an OE, and compare it with that of care usually provided by PHC providers not familiar with carrying out an OE. The specific intention is to establish whether the training is effective in increasing: i) the frequency of detection of suspected HIV-related oral lesions; ii) rates of referral for HIV testing; and iii) the accuracy of the prescribed HIV tests. The secondary objective is to estimate the effects of the intervention training session on secondary outcomes, i.e. improvement of PHC providers’: i) knowledge; ii) recognition; and iii) management of (HIV-related) oral lesions. This will be carried out using questionnaires and clinical data. In addition, recognition and registration of easily visible signs of periodontal disease, dental caries and malocclusions, which are recorded in the routine tally sheet, will be assessed.
Design and Methods
Study design
The study is made up of two parts: a retrospective clinical data record study and a prospective cohort study using a pre-post control group design (Figure 1) in 2 administrative divisions. All health facilities (HF) within a division will be fully involved. To minimise contamination between intervention and control cohorts, all HFs within one administrative division will be clustered.
Figure 1.
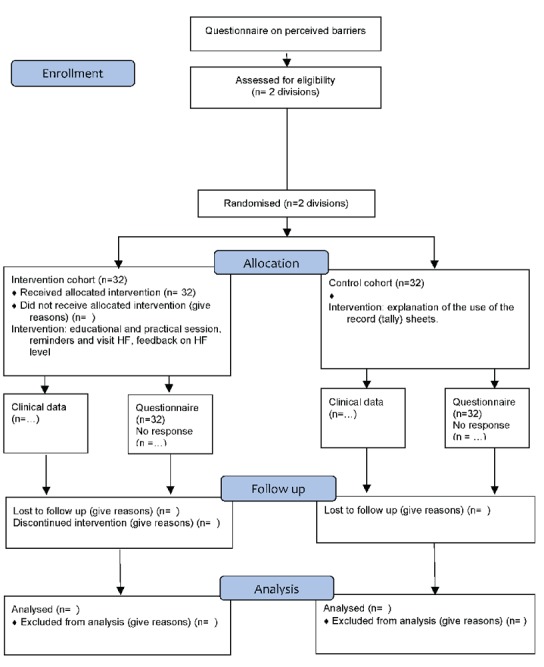
Design timeframe and flow of the participants through all stages of the study (according to CONSORT 2010 Flow Diagram).
Group composition
The convenience sample will consist of all healthcare workers in a division. All healthcare workers will be invited to complete the questionnaire and, for the intervention cohort, to participate in the scheduled sessions. All COs and nurses (n=32) from HFs (n=4) in Njiru division, Nairobi East district, (Dandora 1, Dandora 2, Njiru and Ruai) will be clustered into cohort 1. All health workers (n=32) in Makadara Division (Kaloleni, Lungalunga, Remand and Police Band) will be clustered into cohort 2. The two divisions are geographically far apart and the distance between them will prevent any interaction during the study. Staff numbers, workloads at health centres, and personal and background characteristics are all comparable. Randomization Software (EASY RA1 Easy Randomizer® Version 4.1, State University of Michigan, USA) will be used in selecting Njiru Division as the intervention cohort. Consequently, Makadara Division will be designated as control cohort.
Blinding
It will not be possible to blind investigators or participants in the intervention cohort. Outcome assessors, extracting data from patient registers at HF level, can also not be blinded. The study biostatistician will be blinded for group allocation for first analysis at HF level, but can not be blinded for division analysis.
Implementation protocol
Control cohort
Participants in the control division will be supplied with regular tally sheets and spatulas, and will be asked to continue their usual routine care.
Intervention cohort
Prior to the intervention, semi-structured interviews will be conducted with PHC providers in the whole Nairobi East District, in order to identify any barriers to performing an OE (Koyio, 2010). The findings of these interviews will be used to tailor the training programme to the local context.11 PHC providers will receive a one-day training intervention consisting of a combination of didactic lectures and small group discussions led by experienced dentists, and practical sessions. Lectures are intended to improve: i) knowledge and recognition of common oral HIV-related lesions; and ii) detection of easily visible common oral diseases; e.g. distinct dental decay (tooth cavities) and severe periodontal disease (gum disorders, calculus). In addition, practical skills in diagnosis and management of HIV-related oral lesions, in line with the Kenya training curricula, will be offered.12 These modules included hands-on training in performing an OE and examination of 10 selected subjects with various HIV-related oral lesions. Reminder sessions, i.e. personal visits and regular telephone calls from LNK, will be provided after one and two months. The personal visits will include supportive supervision and power point presentations, again showing the appearance and the clinical importance of oral lesions. In addition, small group discussions will be held to elicit feedback about participants’ first experiences and perceived barriers to performing OE. Written informed consent will be obtained from all participants.
Materials
Routine record (tally) sheets for recording general examinations will enable PHC providers in both cohorts to record patients with: a) HIV-related oral lesions (to be referred for HIV testing) and their test results; b) dental decay; c) periodontal (gum) disorders. The importance of record keeping will be emphasized and health facility managers will be asked to request that their staff use these sheets for recording diagnosed conditions, including suspected oral HIV-related oral lesions. After training, the intervention cohort will receive torches, washable cotton mouth masks and spatulas, enabling them to perform effective OEs.
Data collection
Clinical baseline data for the year preceding the implementation will be collected for intervention and control divisions, to determine how the two cohorts compare on clinic demographics and health service delivery regarding HIV testing services. A written pre-test questionnaire will be used to gain personal background data and to assess current knowledge and skills in both groups. As part of the training programme, all participants of the intervention cohort will be asked to clinically examine 10 selected HIV patients (8 with typical oral HIV-related oral lesions and 2 with no lesions), allowing their current clinical performance to be assessed. Diagnosis will be registered for every individual participant on a marked registration form. On the training day, all participants will have their knowledge immediately assessed. The written questionnaire will be administered under supervision to the control cohort on the same day at their place of work. Immediately after the intervention session, participants will be asked to complete a questionnaire asking the same knowledge questions as those in the pre-test form, as well as a training evaluation form. Before and during the study, in both divisions, all patients leaving the health facility will be interviewed and a structured form will be used to assess their reasons for the visit and to discover whether an OE has been performed by a PHC worker. For early (+3 months) and midterm (+9 months) effect assessment of the intervention and reminder sessions, the questionnaire will be re-administered to both cohorts. In the intervention cohort, the same photographs as those presented at the training session will have to be assessed to detect any change in knowledge. Possible barrier factors confronting PHC providers in the intervention cohort in performing routine OE will also be assessed, using a structured questionnaire. Case registrations in the HF register and tally sheets will be used to assess frequencies of people with HIV-related oral lesions, distinct dental decay, and severe periodontal disease, malocclusion, and HIV-related oral lesions.
Statistical analysis
The primary outcome measures related to the effect of the training intervention on increasing the frequency of early detection of HIV-related oral lesions, and tally sheets will be used to collect referral rates for the prescription of HIV testing at an HF level. Completed questionnaires collected from individual PHC providers working within an HF will be used to assess secondary outcomes related to changes in knowledge and recognition of common oral pathology. For the intervention cohort, baseline data will be compared with data from 1st, 2nd and 3rd post-training assessments, and with recorded data from the control cohort. All analyses will be carried out using the SAS Version 9.2 statistical program, SAS Institute, Cary, NC, USA. The χ2 tests will be used for analysis. Logistic regression will be used to simultaneously test the effect of background variables. The Jackknife procedure will be used to estimate the standard error (variance) to compensate for the dependences of data inherent in clustering of PHC workers per unit.13 Table 1 summarises the categories of data to be analysed in both cohorts for pre-training and post-training assessments.
Table 1.
Categories of planned data collection for evaluation of the training programme for PHC providers in Nairobi East district in the test and control cohort.
| Time frame | Data collection for analysis | |
|---|---|---|
| Intervention cohort | Control cohort | |
| Pre-training (-9 months) (-3 months) |
Baseline records data, clinical records data, and patient interviews. | Baseline records data, clinical records data, and patient interviews. |
| Training 1st assessment (0 months) 2nd assessment (+3 months) 3rd assessment (+9 months) |
Baseline (pre-training) assessment. 1st post training assessment immediately after the training. 2nd post training assessment, clinical records data, patient interviews. 3rd post training assessment, clinical records data, patient interviews. |
Baseline assessment. No action. 2nd assessment, clinical records data, patient interviews. 3rd assessment, clinical records data, patient interviews. |
Main outcome benefits
The intervention session is intended to increase the competences and practical skills of PHC providers in early identification and management of HIV-related oral lesions and other common oral health problems in their routine practice. As a result, the number of patients being identified early during consultations as having oral HIV-suspected lesions and being referred for HIV testing will increase. This will allow for earlier treatment in the course of HIV infection, enhancement of the patients’ quality of life, and a reduction in national healthcare costs. In addition, referral to other oral health care specialists will increase.
Patient safety
Patient confidentiality will be respected throughout the study. Information collected will be for research purposes only and results will only be shared anonymously. All patient data will be kept under lock and key. Data in electronic devices will be controlled and password-protected. Only members of the study team will have access to the files.
Ethical and legal aspects
The study protocol has been approved by the Kenyatta National Hospital/University of Nairobi Ethics and Research Committee (approval number KNH-ERC/A/474). This training programme is in line with the above-mentioned Kenya National training curriculum and guidelines. The Ministry of Public Health and Sanitation has given written approval for the training of the PHC providers, for collaboration with the National AIDS/STI Control Programme (NASCOP) in developing Information Education and Communication (IEC) and for training materials that will be used during the study (Ref. No. MPHS/IB/1/14 Vol. III).
Discussion
Clinical performance (e.g. OE by PHC providers during general consultations) is, among other things, influenced by: attitudes (accepting the importance of OE); subjective norms (opinion of others, e.g. whether supervisors are in favour of OE); and perceived behaviour control (competence and equipment). During the focus group discussions with PHC providers in Nairobi East district, some possible barriers that might influence this study became evident. PHC providers, for example, expressed fears that: i) they might get infected when performing an OE; ii) they might make a wrong diagnosis; iii) patients might reject OE if an oral problem was not a presenting complaint. Infection patterns will be explained, mouth masks will be provided, and an information and skills training programme will be implemented to diminish these barriers.
An OE will be an additional tool for provider-initiated HIV testing and counselling. In addition, patients with severe dental decay and gum disease can be referred for oral health care. Furthermore, identification of OPC in HAART patients will alert the clinicians to possible antiretroviral drug failure and prompt them to counsel patients on adherence, to minimise further resistance. This training programme for PHC providers will inform policymakers on integration of oral health care into PHC settings for all patients. After a positive effect has been proven, the training will be integrated into the existing NASCOP programmes and be implemented in the PHC settings. This will make it possible for the government to supply oral health care at minimal cost and in a sustainable way. Moreover, early detection and treatment of HIV-infected patients will significantly reduce the total costs of health care. The total number of days off work caused by HIV-related illness or complications will decrease while survival rates will rise.
Patient interviews might eventually influence clinical performance of PHC in both cohorts, as they will know that they are being tested. As data will be compared with pre-training data, an estimation of this effect on the control cohort can be made. Further data analysis might provide insights into how far these interviews have had an influence on the frequency of HIV testing.
References
- 1.Ranganathan K, Hemalatha R.Oral lesions in HIV infection in developing countries: an overview. Adv Dent Res 2006,19:63-8 [DOI] [PubMed] [Google Scholar]
- 2.Anabwani G, Navarro P.Nutrition HIV/AIDS in sub-Saharan. Africa: an overview. Nutrition 2005;21:96-9 [DOI] [PubMed] [Google Scholar]
- 3.Scrimshaw NS, SanGiovanni JP.Synergism of nutrition, infection and immunity: an overview. Am J Clin Nutr 1997;66:464S-77S [DOI] [PubMed] [Google Scholar]
- 4.Petersen PE.Global policy for improvement of oral health in the 21st century – implications to oral health research of World Health Assembly 2007, World Health Organization. Community Dent Oral Epidemiol 2009;37:1-8 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Primary Health Care now more than ever. Available from: http://www.who.int/whr/2008/whr08_en.pdf [Google Scholar]
- 6.Coulter ID, Marcus M, Freed JR, et al. Use of dental care by HIV-infected medical patients. J Dent Res 2000;79:1356-61 [DOI] [PubMed] [Google Scholar]
- 7.Andersson K, Nordenram G, Wårdh I, Berglund B.The district nurse’s perceptions of elderly patients’ oral health: a qualitative interview study. Acta Odontol Scand 2007;65:177-82 [DOI] [PubMed] [Google Scholar]
- 8.Perrin L, Telenti A.HIV Treatment failure: testing for HIV resistance in clinical practice. Science 1998;280:1871. [DOI] [PubMed] [Google Scholar]
- 9.Boden D, Hurley A, Zhang L, et al. HIV-1 Drug resistance in newly infected individuals. JAMA 1999;282:1135-41 [DOI] [PubMed] [Google Scholar]
- 10.Yerly S, Kaiser L, Race E, et al. Transmission of antiretroviral-drugresistant HIV-1 variants. Lancet 1999;354:729-33 [DOI] [PubMed] [Google Scholar]
- 11.Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ministry of Health. National Aids & STD Control Programme NASCOP (2005). Available from: http://www.womenchildrenhiv.org/wchiv?page=gtp-02-01. [Google Scholar]
- 13.Efron B.Bootstrap methods: another look at the jackknife. Ann Stat 1979;7:1-26 [Google Scholar]


