Abstract
Background
Human papillomavirus (HPV) infection is the most common sexually transmitted infection and high-risk HPV types are a necessary cause for the development of cervical cancer. The present study investigated the HPV-type specific prevalence in 650 women, aged 15-76 years, with cytological abnormalities and the association between HPV infection and cervical disease in a subset of 160 women for whom cytological results for Pap-Test were available, during the period 2008-2011 in Cagliari (Southern Italy).
Design and Methods
HPV-DNA extraction was performed by lysis and digestion with proteinase K and it was typed by using the INNOLiPA HPV Genotyping Assay.
Results
Overall the HPV prevalence was 52.6%; high-risk genotypes were found in 68.9% of women and multiple-type infection in 36.1% of HPV-positive women. The commonest types were HPV-52 (23.4%), HPV-53 (15.7%), HPV-16 (15.4%) and HPV-6 (12.4%). Among the women with cytological diagnosis, any-type of HPV DNA was found in 49.4% of the samples and out of these 93.7% were high-risk genotypes. Genotype HPV 53 was the commonest type among women affected by ASCUS lesions (21.4%), genotype 52 in positive L-SIL cases (22.5%), genotype 16 H-SIL (27.3%).
Conclusions
This study confirmed the high prevalence of HPV infection and high-risk genotypes among women with cervical abnormalities while, unlike previously published data, genotype HPV-52 was the most common type in our series. These data may contribute to increase the knowledge of HPV epidemiology and designing adequate vaccination strategies.
Significance for public health.
Human papillomavirus (HPV) is the most common sexually-transmitted agent, which can cause cervical lesions and cancer in females. Efforts to reduce the burden of cervical cancer with cytology screening in the last years have had limited success. HPV infection and disease imposes a substantial burden of direct costs on the Italian National Health Service that have never been fully quantified. Monitoring HPV prevalence could represent a tool to follow the evolution of the infection in the vaccination and post-vaccination era, to understand the impact of HPV types in cervical diseases in Italy. Our survey shows an high frequency of infections sustained by HPV 52. Given the recent implementation of a widespread immunization program with vaccines not containing HPV 52, it has been relevant to prove the high prevalence of this HPV genotype from the beginning of the vaccination campaign, to avoid ascribing to the vaccination program a possible selection effect and the importance of non-vaccine HPV types in the burden of cervical disease, in order to assess the opportunity to realize new vaccine including other types.
Key words: HPV epidemiology, cervical abnormalities, HPV prevalence
Introduction
Genital Human Papillomavirus (HPV) infection is the most frequent sexually transmitted viral infection all over the world; it is responsible for clinical forms including both asymptomatic and benign form or malignant lesions of the cutaneous/mucosal genital tract.1-3 The association of HPV infection with cervical cancer is widely documented. The majority of cervical cancers are the consequence of persistent infection by high risk HPV genotypes.4-6 Cervical cancer is the second most frequent cancer in women worldwide, with 500.000 cases and 250.000 deaths yearly: its frequency is high both in developing and industrialized areas.7 HPV is associated with other types of cancers like anal, vaginal, vulva, penis and oropharingeal cancer causing further 0.7% of all cancers in women and men; overall, HPV is thus responsible for 5.2% of cancers worldwide.8-10
At present, more than 140 HPV types have been identified, of which about 40 infect the genital tract. Fifteen of these have been classified as high-risk types (HR-HPV) according to the International Agency for research on Cancer (IARC)11 and 12 are considered as low-risk types as they are responsible for ano-genital warts and Recurrent Respiratory Papillomatosis (RRP). The high risk types HPV-16 and HPV-18 are responsible for approximately 70% of cervical cancers;12-14 worldwide HPV-16 is present in 45% and HPV-18 in 16% of cervical lesions. Genotypes 31 and 45 account for another 10%.13
Italian investigations about HPV prevalence in healthy women and in women with cytological abnormalities reported HPV-16 as the most frequent genotype. However, consistent local differences of other HR genotypes distribution were observed posing the question about a different impact of HPV vaccination according to the local prevalent genotypes.15-21 Immunity induced by vaccination to HPV is type-specific. However, if we consider the phylogenetic tree including the different HPV types, we realize that a certain degree of cross-protection is possible, due to the high homology of some viral types with vaccine ones.22,23
The present study reports the frequency of HPV genotypes isolated in cervical samples of women presenting cytological abnormalities and attending the cytological screening.
Design and Methods
Study population
Cervical scrape specimens were obtained from 650 women [mean age 37.22 years, standard deviation (SD) 9.3 years, range 15-76 years] of Cagliari, the main town of Sardinia, an island of Southern Italy, during the period 2008-2011. Age class distribution among the recruited women is reported in Table 1.
Table 1.
Distribution of age classes among the studied 650 women and positivity prevalence for any type of human papillomavirus (95% confidence intervals).
| Age classes (years) | No. | HPV-DNA positives | |
|---|---|---|---|
| No. (%) | 95% C.I. | ||
| ≤24 | 50 | 33 (66.0) | 52.9-79.1 |
| 25-35 | 244 | 148 (60.7) | 54.5-66.8 |
| 36-45 | 229 | 104 (45.4) | 38.9-51.9 |
| ≥46 | 127 | 57 (44.9) | 36.2-53.5 |
| Total | 650 | 342 (52.6) | 48.8-56.5 |
Samples were collected from 175 women attending the screening programme at the Prevention Department of the Local Health District, and from 475 women attending private gynaecologist clinics for the treatment of cervical lesions. All the specimens were tested for HPV at the laboratory of Molecular Biology of the University Hospital of Cagliari but only for a subgroup of 175 women [mean age 38.18, SD 9.04 years; range 20-64 years] a cytological diagnosis was available; the result of cytological test for the other women was unavailable. The diagnosis ware based on the Bethesda system classification: Atypical Squamous Cells of Undeterminated Significance (ASCUS), Low-grade Squamous Intraepithelial Lesion (L-SIL) and High-grade Squamous Intraepithelial Lesion (H-SIL).
Extraction of DNA from cervical swab specimens
The cervical swab specimen was collected from each patient using a cervix brush, suspended in a 20 mL PreservCyt vial (ThinPrep Pap Test Kit; Cytec Corporation, Malborough, MA, USA) and stored at 4°C until the DNA extraction. Each vial was vortexed for 15s before the use. Starting material (1 mL) was centrifugated at 14,000 rpm for 1 min, the supernatant was removed and the obtained pellet was processed by lysis and digestion with proteinase K, according to the manufacturer’s protocol (Blood and Body Fluid Spin Protocol, QIAamp DNA Mini Kit; Qiagen Inc. Hilden, Germany). The resultant DNA was stored at -20°C until use.
Human Papillomavirus-DNA detection and typing
Eluted DNA was used for the PCR amplification of HPV sequences from the L1 region, using INNO-LiPA HPV Genotyping Extra Amp (Innogenetics N.V., Gent, Belgium). Particularly the test uses 5’-biotynilated SPF10 primers to amplify a 65 bp fragment within the L1 open reading frame in HPV genome. An additional primer pair target human HLA-DBP1 gene to provide a control for sampling and cell adequacy, extraction and amplification. PCR was performed in a final reaction volume of 50 L, containing 10 L of the isolated DNA, 10 mM Tris/HCl, pH 9.0, 50 mM KCl, 2.0 mM MgCl2, 0.1% Triton X-100 0.01% gelatine, 200 M of biotinylated primers in buffer with dNT/dUTP, 15 pmol of forward and reverse primers each, and 1.5 U of Ampli Taq Gold DNA polymerase (Roche Molecular Systems Inc, USA). HPV type-specific sequences were detected by the INNO-LiPA Genotyping Extra (Innogenetics N.V., Gent, Belgium) assay according to the manufacturer’s instructions: the biotinylated PCR products were reverse hybridized to genotype-specific probes immobilized as parallel lines on a nitrocellulose strip. After stringent washing, streptavidin-conjugated alkaline phosphatase was added and a non-radioactive reaction performed using a chromogenic substrate. After drying the strips, the resulting purple precipitates were visually interpreted according to manufacturer’s protocol by using an interpretation grid supplied with the kit.
The current version of the assay allows the simultaneous and separate detection of the following HPV types: HPV-6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68, 69/71, 70, 73, 74 and 82. HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73 and 82 were defined high-risk (HR) HPV types; HPV-26, 53 and 66 were considered as probable high-risk (pHR), whereas the other genotypes were defined low-risk (LR) HPVs. HPV amplimers which did not hybridize to any probe were considered as uncharacterized.
Statistical analysis
Statistical analysis were performed using software SPSS version 18.0. SD was calculated for the mean values. Fisher’s exact test was used to compare proportions. Trends in proportion were tested using the Chi-square test for trend. Proportions were computed with the corresponding 95% confidence intervals (95% C.I.) according to the Poisson distribution.
A P value less than 0.05 was considered as statistically significant.
Results
Overall, 342 of the 650 women (52.6%) were positive for any type of HPV; prevalence according to age classes is reported in Table 1. The prevalence was significantly higher in women aged 25-35 years than in the 35-45 and >45 years age classes (P<0.01).
Of the 342 samples positive for HPV-DNA, 43 (12.6%) could not be genotyped because they did not match any of the specific probes of the strips (uncharacterized genotype); thus HPV genotyping was available for 299 women. Particularly, 206 of the 299 genotyped women (68.9%) were infected by HR-HPV, 60 (20.1%) by pHR-HPV and 33 (11.0%) by LR-HPV. The age point prevalence are reported in Table 2: HR-HPV recurrence was not significantly different among the age classes (P>0.05).
Table 2.
Positivity rates of HR-HPV, pHR-HPV and LR HPV (95% confidence intervals) stratified for age classes in 299 women with typeable human papillomavirus.
| Age classes (years) | Tot. positives | HR-HPV positives | pHR-HPV positives | LR-HPV positives | |||
|---|---|---|---|---|---|---|---|
| No. (%) | 95% C.I | No. (%) | 95% C.I | No. (%) | 95% C.I | ||
| <24 | 33 | 21 (63.6) | 39.4-96.9 | 5 (15.2) | 6.1-36.4 | 7 (21.2) | 9.1-42.4 |
| 25-35 | 133 | 95 (71.4) | 57.9-87.2 | 27 (20.3) | 13.5-29.3 | 11 (8.3) | 3.7-15.0 |
| 36-45 | 90 | 63 (70.0) | 53.3-90.0 | 16 (17.8) | 10.0-28.9 | 11 (12.2) | 5.5-22.2 |
| >46 | 43 | 27 (62.8) | 41.9-90.7 | 12 (27.9) | 13.9-48.8 | 4 (9.3) | 2.3-23.2 |
| Total | 299 | 206 (68.9) | 59.9-78.9 | 60 (20.1) | 15.4-25.8 | 33 (11.0) | 7.7-16.1 |
Overall, out of 299 infections, 191 (63.9%) were sustained by a single genotype and 108 (36.1%) by multiple HPV genotypes; genotypes distribution in single and multiple infections is shown in Table 3. The proportion of infections sustained by genotypes 16, 52 and 53 was significantly higher than that for all the other genotypes (P<0.05).
Table 3.
Human papillomavirus (HPV) genotyping of single or multiple infections in 299 women with detectable HPV DNA.
| Genotype | Single infection no. (%) | Multiple infection no. (%) | Total number of genotype recurrence | Proportion (%) |
|---|---|---|---|---|
| 6 | 25 (13.1) | 12 (11.1) | 37 | 12.4 |
| 11 | 0 | 2 (1.8) | 2 | 0.7 |
| 16 | 21 (10.9) | 25 (23.1) | 46 | 15.4 |
| 18 | 12 (6.3) | 6 (5.5) | 18 | 6.0 |
| 31 | 1 (0.5) | 22 (20.4) | 23 | 7.7 |
| 33 | 0 | 13 (12.0) | 13 | 4.3 |
| 35 | 1 (0.5) | 0 | 1 | 0.3 |
| 39 | 9 (4.7) | 10 (9.3) | 19 | 6.4 |
| 44 | 4 (2.1) | 3 (2.8) | 7 | 2.3 |
| 45 | 1 (0.5) | 12 (11.1) | 13 | 4.3 |
| 51 | 18 (9.4) | 18 (16.7) | 36 | 12.0 |
| 52 | 33 (17.3) | 37 (34.3) | 70 | 23.4 |
| 53 | 24 (12.6) | 23 (21.3) | 47 | 15.7 |
| 54 | 2 (1.0) | 31 (28.7) | 33 | 11.0 |
| 56 | 6 (3.1) | 9 (8.3) | 15 | 5.0 |
| 58 | 2 (1.0) | 15 (13.9) | 17 | 5.7 |
| 66 | 23 (12.0) | 14 (12.9) | 37 | 12.4 |
| 68 | 1 (0.5) | 11 (10.2) | 12 | 4.0 |
| 70 | 2 (1.0) | 13 (12.0) | 15 | 5.0 |
| 74 | 6 (3.1) | 11 (10.2) | 17 | 5.7 |
| 69/71 | 0 | 2 (1.8) | 2 | 0.7 |
| Total | 191 | 108 | - | - |
Among women with multiple genotypes there were 58 with dual infection (53.7%), 29 with triple infection (26.8%), 8 women with 4 genotypes each (7.4%) and 13 women with 5 or more genotypes each (12%).
HPV-52 was the most frequent genotype both in single and multiple infections; overall, it was detected in 70 cases (23.4%), HPV-53 was detected in 47 (15.7%), HPV-16 in 46 (15.4%), HPV 6 in 37 (12.4%), HPV-66 in 37 (12.4%) and HPV-51 in 36 (12.0%). HPV-18 was identified in 18 subjects (6.0% of infections) (Figure 1).
Figure 1.
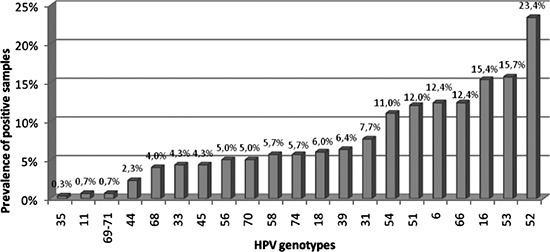
Prevalences of human papillomavirus (HPV) genotypes, in single or in multiple infections, among 299 HPV genotyped samples.
In the subgroup of 175 women for whom a cytological diagnosis according to the Bethesda system was available, 160 could be genotyped (15 were non-typable): 75 (46.9%) were affected by ASCUS (mean age 39.3 years), 66 (41.2%) by L-SIL (mean age 35.8) and 19 (11.9%) by H-SIL lesions (mean age 37.0). Overall, HPV DNA was found in 79/160 samples (49.4%, C.I. 95% 41.2-50.0): in 74 infections (93.7%) HR-HPV or pHR HPV genotypes were detected (Table 4).
Table 4.
Human papillomavirus types in 79 women whit known cytological diagnosis.
| Single infection | Multiple infections | High-risk types | pHigh-risk types | Low-risk types | |
|---|---|---|---|---|---|
| ASCUS | 17 | 1 | 11 | 0 | 7 |
| L-SIL | 29 | 11 | 28 | 10 | 2 |
| H-SIL | 5 | 6 | 10 | 0 | 1 |
| Total | 51 | 18 | 49 | 10 | 10 |
Among women with cytological diagnosis of ASCUS, L-SIL and H-SIL, 28 (37.3%), 40 (60.1%) and 11 (57.9%) respectively were HPV infected. The frequency of HPV infection was not significantly different in the 3 groups of cytological abnormalities.
Proportions of infections sustained by high risk and probable high risk HPV genotypes and by low risk genotypes is reported in Figure 2; the presence of HR HPV was significantly more frequent in women affected by L-SIL and H-SIL than in ASCUS cases (P<0.05).
Figure 2.
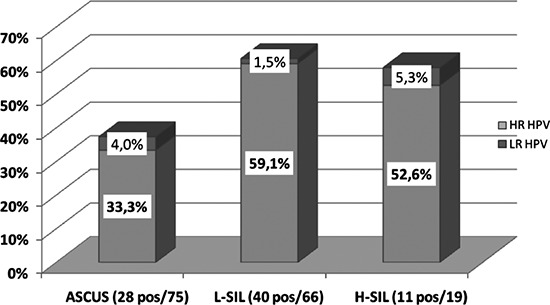
Proportion of positivity to HR/pHR HPV and LR HPV genotypes according to cytological abnormalities among 160 women for whom a cytological diagnosis according to Bethesda system classification was available.
ASCUS=Atypical Squamous Cells of Undeterminated Significance.
L-SIL=Low-grade Squamous Intraepithelial Lesion.
H-SIL=High-grade Squamous Intraepithelial Lesion.
The most frequent isolated HPV genotypes were: genotype 53 in 6 out of the 28 women affected by an ASCUS lesions (21.4%), genotype 52 in 9 out of the 40 HPV positive L-SIL cases (22.5%), genotype 16 in 3 out of 11 cases of H-SIL (27.3%). The distribution and proportion of the HPV genotypes in different classes of cytological abnormalities are reported in Figure 3.
Figure 3.
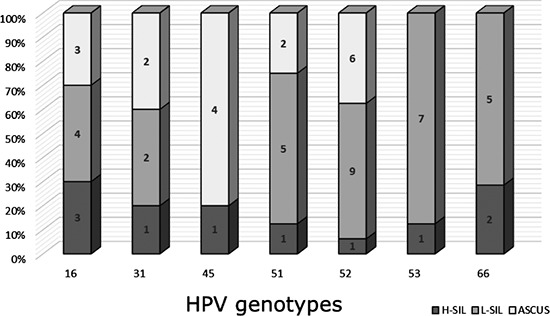
Distribution and proportion of the most frequent genotypes in ASCUS, L-SIL and H-SIL cytological lesions.
Discussion and Conclusions
The distribution of HPV genotypes varies greatly worldwide likely in relation to the complex geographical and biological interplay between different HPV types and host immunogenetic factors.24,25
The HPV genotype spreading in the pre-vaccination era was investigated in several European countries.26-28 However, comparison of the different results is problematic as it must consider the characteristics of the target population, particularly the age of the enrolled women and the evaluation of women with cervical abnormalities or with normal cervical cytology; the prevalence rates of HPV infection are expected to be higher in women with cervical abnormalities considering that HPV is unanimously recognized as the main risk factor for cervical lesions.29,30
Furthermore, HPV DNA testing methods are responsible for different interpretation of results reported in literature, as the different assays used in surveys have different type-specific sensitivities. In fact, although the Hybride Capture method (Digeneò HPV Test) is the only one approved by the Food and Drug Administration (FDA) to evaluate the presence of high/low-risk oncogenic types, several other technologies have been used, like Linear Array and standard PCR with different primers (SPF10, GP05+/GP06+, MY09/11) and real-time PCR. Although a good concordance has been observed in studies using different diagnostical tools, real-time PCR seems to be more sensitive than array methods to detect positive results.31-33
Our study involved only women with cytological abnormalities of the cervix who were HPV infected in 52.6% of cases, showing that HPV is highly endemic in Sardinia. This result is consistent with previous Italian studies, reporting HPV prevalence ranging from 35.3% to 88.8% among women with known cervical lesions.18,19,21,27,29,34
The peak of HPV infection was observed in 25-35 years old women where the prevalence rate was significantly higher than in older women (60.7% vs 45.4% and 44.9%). This finding is similar with data reported in other Italian areas, Germany and Denmark,21,26,27,34 reflecting a higher risk in young sexually active women who, however, mostly have a transient and asymptomatic infection, with a spontaneous HPV clearance in 70-90% of cases within 12-13 months.24
In our study a spectrum of 21 genotypes were identified, of which 12 were HR-HPV and 2 pHR-HPV accounting together for 89 % of infections, in line with rates reported in other Italian studies carried out in similar populations.18,21,27,29,34
Remarkably, HPV-52 was the most common genotype being present in 23.4% of all HPV DNA positive women; the high prevalence of this genotype was reported also in other Italian studies where it was detected as the second (together with HPV-18),21 the third and the fourth (together with HPV-51) most frequent genotype in females affected by H-SIL living in Southern Italy27 or in women with different risk of infection.35-37 Worldwide HPV-52, together with HPV-16, HPV-18, HPV-31, HPV-58 is considered one of the five most common types contributing to the 50% of all HPV infections.27 HPV-52 is considered as the most common type in Eastern Africa, Japan and Taiwan and the fourth common type in Eastern Asia27 among women with normal cytology.
The reason of the high prevalence of HPV-52 observed in our study might be also explained by the sexual promiscuity among immigrated women from high risk areas and the local community, likely proving the active role of men in spreading this infection.30,38
The second most frequent type was HPV-53 (15.7%) in accordance with other Italian34 and Spanish studies,28,39,40 both in general population and in females with abnormal smears. In fact, although it is not recurrent among the most prevalent types worldwide, it was found as the second and the fifth,21,30 most frequent genotype isolated in Italian studies which considered general female population and females with abnormal smears. The underestimation of the prevalence of HPV-53 infections in many studies may be related to the lack of sensitivity of the testing methods, particularly those in which amplification with GP05+\GP06+ primers has been used as screening for HPV positive samples, while the combination of amplification and hybridization used in our study for all samples seems to improve its detection. Another important reason is the absence of the specific probes for HPV-53 detection when using the Hybride Capture II current high-risk kit.41
Unexpectedly, HPV-16 was detected only in 15.4% of our series, at the third place in descendent order; however it was predominant among women affected by H-SIL, confirming that HPV-16 is the most frequent HPV type associated with high-grade lesions. The lower prevalence of HPV-16 infection in our cohort compared with the majority of literature reports could be ascribed to the small proportion of women with advanced precancerous lesions. Moreover we didn’t include any case of cervical cancer where HPV-16 is well-known to be predominant.12,13,24,25
Our survey showed that at least 36.1% of women were infected by two or more genotypes, in accordance with other studies reporting a prevalence of multiple infections in 30-53% of cases.18,21,42 The clinical significance of multiple infections remains uncertain; in our cases they were more frequently found in younger women suggesting a link with sexual promiscuity. It’s not clear whether multiple infections with several HPV types increases the progression risk: some reports show that in immunocompetent women the HPV clearance rate is independent of the number of genotypes involved in the infection and that multiple infections are not associated with a greater risk of cervical cancer compared with single infections.43
Concerning the association between HPV and cytological results, overall 46.9% of women were HPV infected and 93.7% of them harboured HR-HPV genotypes. According to current studies the presence of HR-HPV was significantly higher in women affected by L-SIL and HSIL than in those affected by ASCUS, strengthening the role of persistent infection sustained by HR genotypes in cervical lesions.
The predominance of HPV-53 in ASCUS cases of our observations, is consonant with the absence of this genotype in advanced cervical lesions reported in other studies,18,21 while the majority of HPV 52 infection in L-SIL cases is similar to the prevalence reported in another Italian study.18,29
In conclusion, the present study investigated about the prevalence of HPV infection and the HPV genotypes distribution in women with abnormal cytological test living in an area of Southern Sardinia; however, in our survey the proportion of women affected by high grade cervical lesions was lower than those of women with low grade or mild lesions. This situation could explain the relatively low prevalence of HPV-16 and the predominance of HPV-52. The target of current HPV vaccines, containing HPV-16 and 18 Viral Like Particles, is the prevention of severe pre-neoplastic cervical lesions and cervical cancer; both these conditions are strongly correlated with the presence of HPV-16 and also in our series this genotype was prevalent in high grade lesions. Nevertheless, surveillance programs are aimed to disclose the emergence of local differences in the prevalence of HPV genotypes not covered by current vaccines and present in persistent infections, such as HPV-52 in Southern Sardinia. Thus the assessment of cross-protective properties of HPV vaccines is an extremely important issue. So far, the cross-protection studies have shown that the efficacy in prevention of 6-months persistent infection in HPV naïve women vaccinated with current vaccines does not exceed 30% against non vaccine genotypes, namely HPV-31, 33, 45, 52, 58.22,23,44-46 It is clear the need to increase cross immunity studies in order to better assess the effectiveness of the current HPV vaccines; a better knowledge about cross protection molecular basis could be useful to formulate a second generation of HPV vaccines by including other HPV-types frequently detected in persistent infections.22,23
References
- 1.Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. Mar J Clin Virol 2005;32(Suppl 1):S16-24 [DOI] [PubMed] [Google Scholar]
- 2.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States 2007. JAMA 2007;297:813-9 [DOI] [PubMed] [Google Scholar]
- 3.Ebrahim SH, McKenna MT, Marks JS. Sexual behaviour: related adverse health burden in the United States feb sex. Transm Infect 2005;81:38-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho GY, Burk RD, Klein S, et al. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia J Natl Cancer Inst 1995;87:1365-71 [DOI] [PubMed] [Google Scholar]
- 5.Liaw KL, Hildesheim A, Burk RD, et al. A prospective study of humanpapillomavirus (HPV) type 16 DNA detection by polymerase chain reaction and its association with acquisition and persistence of other HPV types. J Infect Dis 2001;183:8-15 [DOI] [PubMed] [Google Scholar]
- 6.Remmink AJ, Walboomers JM, Helmerhorst TJ, et al. The presence ofpersistent high-risk HPV genotypes in dysplastic cervical lesions is associated with progressive disease: natural history up to 36 months. Int J Cancer 1995;61:306-11 [DOI] [PubMed] [Google Scholar]
- 7.Arbyn M, Castellsague X, de Sanjose S, et al. Worldwide burden of cervical cancer in 2008. Ann Oncol 2011;22:2675-86 [DOI] [PubMed] [Google Scholar]
- 8.Gillison ML. Human papillomavirus-related diseases: oropharynx cancers and potential implications for adolescent HPV vaccination J Adolesc Health 2008. :43(4Suppl):S52-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Souza G, Agrawal Y, Halpern J, et al. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis 2009;199:1263-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003;95:1772-83 [DOI] [PubMed] [Google Scholar]
- 11.IARC. Human papillomaviruses. IARC Monographs on the evaluation of carcinogenic risks to humans. Leon: IARC press; 2009 [Google Scholar]
- 12.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens - part B: biological agents. Lancet Oncol 2009;10:321-2 [DOI] [PubMed] [Google Scholar]
- 13.De Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010;11:1048-56 [DOI] [PubMed] [Google Scholar]
- 14.Lacey CJN, Lowndes CM, Shah KV. Burden and management of noncancerous HPV-related conditions: HPV-6/11 disease. Vaccine 2006;24(Suppl 3):S3/35-41 [DOI] [PubMed] [Google Scholar]
- 15.De Francesco MA, Gargiulo F, Schreber C, et al. Detection and genotyping of human papillomavirus in cervical samples from italian patients. J Med Virol 2007;75:588-92 [DOI] [PubMed] [Google Scholar]
- 16.Ronco G, Ghisetti V, Segnan N, et al. Prevalence of human papillomavirus infection in women in Turin, Italy. Eur J Cancer 2005;41:297-305 [DOI] [PubMed] [Google Scholar]
- 17.Masia G, Mazzoleni AP, Contu G, et al. Epidemiology and genotype distribution of human papillomavirus (HPV) in women of Sardinia (Italy). Vaccine 2009;27:11-6 [DOI] [PubMed] [Google Scholar]
- 18.Gargiulo F, De Francesco MA, Schreiber C, et al. Prevalence and distribution of single and multiple HPV infection in cytologically abnormal samples from Italian women. Vir Res 2007;125:176-82 [DOI] [PubMed] [Google Scholar]
- 19.Rassu M, Bertoloni G, Mengoli C, et al. HPv genotype prevalence in cervical specimens with abnormal cytology: a report from northeast Italy. Scand J Infect Dis 2005;37:476-81 [DOI] [PubMed] [Google Scholar]
- 20.Tornesello ML, Duraturo ML, Botti G, et al. Prevalence of alphapapillomavirus genotypes in cervical squamous intraepithelial lesions and invasive cervical carcinoma in the Italian population. J Med Virol 2006;78:1663-72 [DOI] [PubMed] [Google Scholar]
- 21.Capra G, Giovannelli L, Bellavia C, et al. HPV genotype prevalence in cytologically abnormal cervical samples from women living in South Italy. Vir Res 2008;133:195-200 [DOI] [PubMed] [Google Scholar]
- 22.De Vincenzo R, Ricci C, Conte C, Scambia G. HPV vaccine crossprotection: highlights on additional clinical benefit. Gynecolog Oncol 2013;130:642-51 [DOI] [PubMed] [Google Scholar]
- 23.Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16,and 18) L1 viruslike particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years. J Infect Dis 2009;199:926-35 [DOI] [PubMed] [Google Scholar]
- 24.Stanley MA. Immune responses to human papilloma viruses. Indian J Med Res 2009;130:266-76 [PubMed] [Google Scholar]
- 25.Stanley MA. Epithelial cell responses to infection with human papillomavirus. Clin Microbiol Rev 2012;25:215-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kjaer SK, Breugelmans G, Munk C, et al. Population-based prevalence, type and age-specific distribution of HPV in women before introduction of an HPV-vaccination program in Denmark. Int J Cancer 2008;123:1864-70 [DOI] [PubMed] [Google Scholar]
- 27.De Sanjosè S, Diaz M, Castellasaguè X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis 2007;7:453-9 [DOI] [PubMed] [Google Scholar]
- 28.Gomez-Roman JJ, Ecchevaria C, Salas S, et al. A type-specific study of human papilloma virus prevalence in cervicovaginal samples in three different Spanish regions. APMIS 2009;117:22-7 [DOI] [PubMed] [Google Scholar]
- 29.Sandri MT, Riggio D, Salvatici M, et al. Typing of human papilloma virus in women with cervical lesions: prevalence and distribution of different genotypes. Med Virol 2009;81:271-7 [DOI] [PubMed] [Google Scholar]
- 30.Giovannelli L, Vassallo R, Matranga D, et al. Prevalence of cervical human papilloma virus infection and types among women immigrated to Sicily, Italy. Acta Ostet Gynecol Scand 2009;88:737-42 [DOI] [PubMed] [Google Scholar]
- 31.Coutlee F, Rouleau D, Ghatas G, et al. Confirmatory real-time PCR assay for HPV-52 infection in anogenital specimens screened for HPV infection with the LINEAR ARRAY HPV genotyping test. J Clin Microbiol 2007;45:3821-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang CC, Qiu JT, Kashima ML, et al. Generation of type-specific probes for the detection of single-copy human papillomavirus by a novel in situ hybridization methods. Mod Pathol 1998;11:971-7 [PubMed] [Google Scholar]
- 33.Kleter B, Van Doorn LJ, Schrauwen L, et al. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol 1999;37:2508-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ammatuna P, Giovannelli L, Matranga D, et al. Prevalence of genital human papilloma virus infection and genotypes among young women in Sicily, South Italy. Cancer Epidemiol Biomarkers Prev 2008;17:2002-6 [DOI] [PubMed] [Google Scholar]
- 35.Clifford GM, Smith JS, Plummer M, et al. Human papillomavirus types in invasive cervical cancer worldwide: a metanalysis. Br J Cancer 2003;88:63-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clifford GM, Gallus S, Herrero R, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Reasearch on Cancer HPV prevalence surveys: a pooled analysis. Lancet 2005;366:991-8 [DOI] [PubMed] [Google Scholar]
- 37.Munoz N, Bosch FX, De Sanjose S, et al. Epidemiologic classification of human papillomavirus types associate with cervical cancer. N Engl J Med 2003;348:518-27 [DOI] [PubMed] [Google Scholar]
- 38.Tornesello ML, Duraturo ML, Buonaguro L, et al. Prevalence of human papillomavirus genotypes and their variants in high risk West Africa women immigrants in South Italy. Infect Agent Cancer 2007;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conesa-Zamora P, Ortiz-Reina S, Moya-Biosca J, et al. Genotype distribution of human papillomavirus (HPV) and co-infections in cervical cytologic specimens from two outpatient gynecological clinics in a region of southeast Spain. BMC Infect Dis 2009;9:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cobo F, Concha A, Ortiz M. Human papilloma virus (HPV) type distribution in females with abnormal cervical cytology. A correlation with histological study. Open Virol J 2009;3:60-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poljak M, Marin IJ, Seme K, Vince A. Hybrid capture IIHPV test detects at least 15 human papillomavirus: comparison between MY09/MY11 and GP5+/GP6+ primer systems. J Clin Virol 1997;35:1304-10 [Google Scholar]
- 42.Sammarco ML, Del Riccio I, Tamburro M, et al. Type-specific persistence and associated risk factors of human papillomavirus infections in women living in central Italy. Eur J Obstetr Gynecolo Reproduct Biol 2013;168:222-6 [DOI] [PubMed] [Google Scholar]
- 43.Molano M, Van den Brule A, Plummer M, et al. HPV study group. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am J Epidemiol 2003;158:486-94 [DOI] [PubMed] [Google Scholar]
- 44.Lu B, Kumar A, Castellsague X, Giuliano AR. Efficacy and safety of prophylactic vaccines against cervical HPV infection in disease among women: a systematic review and meta-analysis. BMC Infect Dis 2011;11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV) – 16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomized study in young women. Lancet 2009;374:301-14 [DOI] [PubMed] [Google Scholar]
- 46.Lehtinen M, Paavonen J, Wheeler CM, HPV PATRICIA Study Group. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year and-of-study analysis of the randomised double-blind PATRICIA trial. Lancet Oncol 2012;13:89-99 [DOI] [PubMed] [Google Scholar]


