Abstract
Rationale
Acute nicotine abstinence is associated with disruption of executive function and reward processes; however, the neurobiological basis of these effects has not been fully elucidated.
Methods
The effects of nicotine abstinence on brain function during rewardbased probabilistic decision making were preliminarily investigated by scanning adult smokers (n = 13) following 24 hours of smoking abstinence and in a smoking satiated condition. During fMRI scanning, participants completed the wheel of fortune task (Ernst et al. 2004), a decision making task with probabilistic monetary outcomes. Brain activation was modeled during selection of options, anticipation of outcomes, and outcome feedback.
Results
During choice selection, reaction times were slower, and there was greater neural activation in the postcentral gyrus, insula, frontal and parietal cortices in the abstinent compared to satiated condition. During reward anticipation, greater activation was observed in the frontal pole, insula, and paracingulate cortex in the abstinent compared to satiated condition. Greater activation was also shown in the precentral gyrus and putamen in the satiated condition compared to the abstinent condition. During the outcome phase, rewards (compared to no rewards) resulted in significant activation in the paracingulate cortex in the satiated compared to abstinent condition.
Conclusions
The results of this preliminary study suggest that smoking withdrawal results in greater recruitment of insular, frontal, and parietal cortical areas during probabilistic decision making.
Keywords: Smoking, abstinence, decision-making, risk, neuroimaging, fMRI
Introduction
Nicotine addiction and withdrawal have been linked to disruptions in decision making and reward-processing. Smokers have been reported to behave more impulsively than nonsmokers on tasks measuring a preference for smaller, sooner over larger, later monetary rewards (i.e., delayed discounting) (Baker et al. 2003; Bickel et al. 2008) and smokers have also been shown to discount cigarettes more rapidly than money (Bickel et al. 1999). In a probabilistic reversal learning task, smokers earned less money than nonsmokers, but had more activation in the right insula during monetary gain and less activation in the ventrolateral prefrontal cortex during monetary loss than controls (de Ruiter et al. 2009).
Smoking cessation results in the disruption of multiple forms of cognition including sustained attention (Gilbert et al. 2004; Hendricks et al. 2006; Myers et al. 2008) and working memory (Snyder et al. 1989; Mendrek et al. 2006; Myers et al. 2008). These abstinence-induced deficits begin soon after the last cigarette is smoked (Hendricks et al. 2006), can persist for weeks (Gilbert et al. 1999; Gilbert et al. 2004), and are predictive of cessation outcomes (Patterson et al. 2010). In rodents, nicotine withdrawal increased intracranial self-stimulation reward threshold, representing decreased function in brain reward systems (Epping-Jordan et al. 1998). Similarly, overnight abstinent smokers had reduced cue reactivity, pleasure expectancies, and responsiveness to financial incentives; whereas satiated smokers performed similarly to nonsmokers (Powell et al. 2002).
Numerous fMRI studies have shown that smoking and nicotine abstinence modulate brain activation during cognitive performance and results in modulation of regions known to specifically subserve these processes, such as the parietal cortex and caudate during a sustained attention task (Lawrence et al. 2002), the dorsolateral prefrontal cortex and anterior cingulate during n-back tasks (Xu et al. 2005; Loughead et al. 2010), and the ventrolateral prefrontal cortex, inferior parietal cortex, temporal poles, and medial frontal gyrus during verbal working memory tasks (Jacobsen et al. 2007; Sweet et al. 2010). In an fMRI study on the motivation to obtain cigarettes versus money, responding was not affected by nicotine-withdrawal, but a cluster of increased anticipatory brain activity was shown in the prefrontal cortex (Buhler et al. 2010). Additionally, in a recent study we observed that smoking abstinence decreased right frontal activation during periods of sustained attention; yet transiently increased activation in regions such as the precuneus and right superior frontal gyrus in responses to targets (Kozink et al. 2010).
Fewer studies have examined the effects of smoking abstinence on cognitive processes related to decision making. However, evidence is accumulating that within a group of smokers, smoking abstinence results in increased preference for smaller, sooner over larger, later rewards (Mitchell 2004; Field et al. 2006) and impaired ability to inhibit pre-potent responses (Powell et al. 2002; Pettiford et al. 2007; McClernon et al. 2008; Kozink et al. 2010). As such, the existing literature suggests that smoking abstinence biases the smoker toward more impulsive responding; however, the neurobiological basis of such a shift is unknown.
In an effort to preliminarily examine the effects of smoking abstinence on neural correlates of probabilistic decision making, we scanned smokers under satiated and abstinent conditions while they completed the Wheel of Fortune (WoF) task—a two-choice task with probabilistic monetary outcomes (Ernst et al. 2004). There are two types of probable outcome trials, the first type has a 30% chance of a large reward and a 70% chance of a small reward (30/70) and the second type has a 10% chance of a large reward and a 90% chance of a small reward (10/90). The ‘safe’ choice is the option with a large probability of a small reward and the ‘risky’ choice is the option with a small probability of a large reward. Following the selection phase, participants rate their confidence in their selection, and lastly, are informed whether they won and rate their hedonic response to the outcome (if the participant loses, no points are gained or lost). Previous studies have reported that the percentage of high-reward/risk options chosen for the 30/70 trials has been 21% and 63%, and for the 10/90 trials has been 27% and 53%, (Ernst et al. 2004; Dichter et al. 2009). Smith et al. (2009) found that participants chose the low probability of high-reward option 49% of the time. Previous neuroimaging studies observed activation in the prefrontal cortex, cingulate, and caudate nucleus during the selection phase, activation in the nucleus accumbens, parietal cortex, cingulate, insula, globus pallidus/putamen and temporal lobe during the anticipation phase (Ernst et al. 2004; Engelmann and Tamir 2009), and activation in the prefrontal cortex, striatum, and cingulate during the outcome phase (Engelmann and Tamir 2009). Given previous research, we hypothesized that 1) smokers would exhibit a greater preference for risky choices when abstinent, and 2) that abstinence would result in increased transient activation in the prefrontal cortex and striatum on probabilistic decision making trials. An understanding of the neural mechanisms that underlie the effects of smoking abstinence on probabilistic decision making could potentially inform the development of novel treatments that specifically address impulsive responding and could also provide insight into the neural mechanisms that govern decision making more broadly.
Methods
Participants
Thirteen adult nicotine-dependent smokers (6 women) completed the study. Potential participants were recruited via fliers, newspaper and internet advertisements, and by word-of-mouth. To be enrolled, participants had to report smoking ≥ 10 cigarettes per day for ≥ two years and have an afternoon expired-air carbon monoxide (CO) level > 10 ppm (in order to establish smoking status). Other inclusion criteria were age between 18 and 50, right-handedness, no serious health problems (e.g., hypertension), not currently undergoing treatment for a psychiatric illness, no current use of medications affecting the central nervous system, negative urinalysis for illicit drug use, no MRI contraindications, and a negative serum pregnancy test for women. Participants were compensated up to $250 for their time ($30 for the training session, $105 for each MRI scan, and $10 for the 20 minute quit check). Data from five additional subjects were excluded from the analyses due to incidental findings (n = 1), subjects reporting to have fallen asleep during the task (n = 3), or excessive head motion (>2.0 mm, n = 1) during the MRI scan. This study was approved by the Duke University Institutional Review Board.
Procedure
Participants first completed a 1.5-hr screening session in which they heard a description of the study and read and signed a consent form, completed questionnaires regarding smoking history and suitability for fMRI research and provided a breath sample for CO monitoring. A mock MRI scanner was used to habituate participants to the scanning environment. Participants who passed screening then completed a 1-hr training session in which they were familiarized with the behavioral task.
Following training, participants completed two fMRI sessions: once following smoking as usual (satiated condition) and once following a 24-hr period of smoking abstinence (abstinent condition). Session order was counterbalanced. Sessions were held no less than 2 days and no more than 19 days apart. Smoking abstinence compliance was verified using breath CO levels and confirmed with saliva nicotine levels collected at the beginning of the session. Participants were required to maintain smoking abstinence for an additional 24-hrs following the abstinent condition scanning session to prevent cigarette anticipation from influencing the data. Continued abstinence was also verified 24 hrs following the abstinent condition scanning session with breath and saliva samples. The Shiffman-Jarvik Smoking Withdrawal Questionnaire (Shiffman and Jarvik 1976) was administered upon arrival to the fMRI sessions.
fMRI Task
Participants completed the Wheel of Fortune (WoF) task during each scanning session. The WoF task is a two-choice decision making task with probabilistic outcomes (see Figure 1) (Ernst et al. 2004). Participants were instructed that they would earn a fixed amount for each scanning session, or whatever they made during the WoF task, whichever was more. In each trial participants chose between two options, each with an assigned probability of winning a certain amount of money. If the computer randomly selected the same option as the participant, the participant won the designated amount of money; if the computer randomly selected the other option, the participant won nothing.
Figure 1.
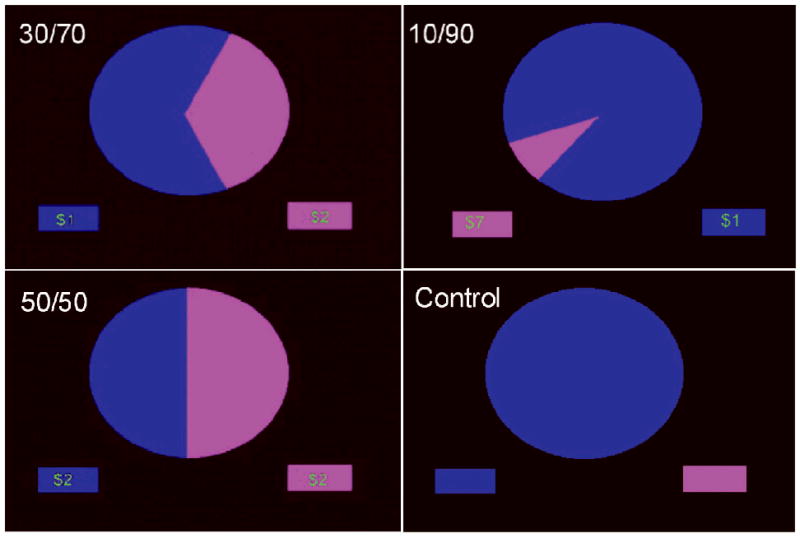
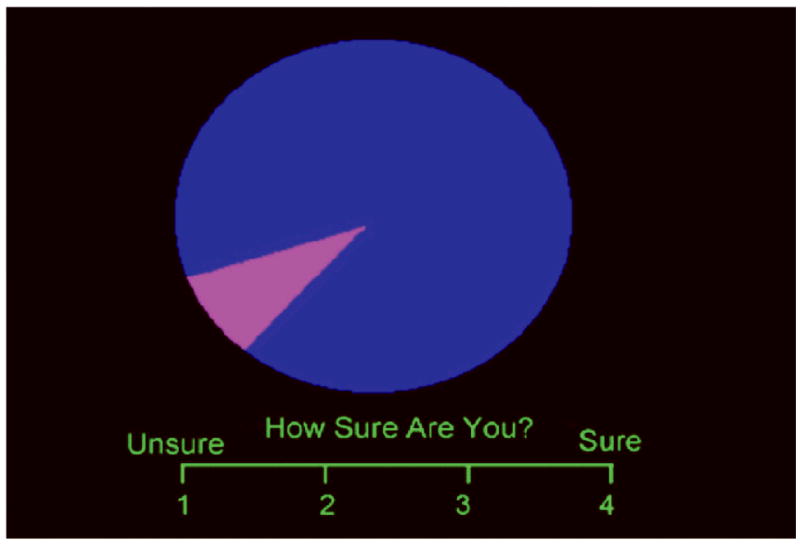

Screen displays for the three phases of the wheel of fortune (WoF) task. (a) The selection phase consisted of three money trials, in which participants chose to bet on either the blue or magenta slice, or else a control trial. (b) In the anticipation phase, participants rated their confidence in winning the placed bet. (c) In the outcome phase, participants were informed whether or not they won and rated their emotional valence.
Participants completed two runs of 53 trials, which lasted approximately 12.5 minutes each. Each trial lasted 12 – 16 s and consisted of three phases: a selection phase (3 s), an anticipation phase (5-9 s), and a feedback phase (4 s). Inter-trial intervals were 1-4 s. All stimuli were presented using E-Prime presentation software (PST Inc., Pittsburgh, PA) and displayed to participants in the scanner through magnetcompatible goggles (Resonance Technology, Inc., Northridge CA).
Three trial conditions were used (see Figure 1): selecting between (1) a 10% chance of winning $7 and a 90% chance of winning $1, (2) a 30% chance of winning $2 and a 70% chance of winning $1, and (3) two 50% chances of winning $2. Each of the three monetary outcomes was displayed as a two-slice WoF, with each slice representing a distinct option. The area of a slice matched the likelihood of winning an explicit amount of money (e.g., 10% chance of winning $7).
During the selection phase, participants viewed one of the 3 trial types and made a selection with a button press. During the anticipation phase, the wheel remained on the screen while a four-point rating scale appeared. Participants had to rate their level of confidence of winning (1 for “unsure” to 4 for “sure”). During the feedback phase, the amount won for that trial was shown (or $0 if a no-win trial) along with the cumulative earnings. Participants were also shown a four-point pictorial rating scale along which they rated how they felt (win trials: 1 - neutral, 4 - very happy; no-win trials: 1- very sad, 4 - neutral). The control condition consisted of a wheel of a single color (i.e., no slices), and participants were told to press the button corresponding to the color of the wheel. All responses were recorded on a four-key button-box placed under the right hand (Current Designs, Inc., Philadelphia, PA).
Data Analysis
Behavioral data was analyzed with repeated-measures analysis of variance (ANOVA) to investigate the effects of condition (satiated or abstinent), trial type (50/50, 30/70, and 10/90) on reaction time (RT) and responses. In the selection phase, RT and risky choice selection were analyzed with a 2 (condition) × 3 (trial type) ANOVA. In the anticipation phase, confidence ratings were analyzed with a 2 (condition) × 3 (trial type) × 2 (risk preference) ANOVA. In the outcome phase, valence ratings were analyzed with a 2 (condition) × 2 (outcome: win, no-win) ANOVA.
Imaging Methods
Scanning was preformed on a 4.0-T GE LX NVi scanner with 41 mT/m gradients (General Electric, Waukesha, Wisconsin). Blood-oxygen-level dependent (BOLD) functional images were collected in 34 contiguous slices (4 mm thick) parallel to the horizontal plane connecting the anterior and posterior commissures. An inverse spiral pulse sequence sensitive to BOLD contrast was used, with TR = 1.5 s, TE = 6 ms, FOV = 24 cm, matrix = 64 × 64, flip angle = 60°, and in-plane resolution = 3.75 mm2. After completion of the functional data collection, a T1-weighted 3D fast spoiled gradient recalled (FSPGR) structural image was collected for 68 slices (1.9 mm thick) with TR = 12.3 s, TE = 5.4 ms, FOV = 24 cm, matrix = 256 × 256, flip angle = 20°, and in-plane resolution = 0.9375 mm2
Functional data were preprocessed using FSL version 4.1.4 (Oxford FMRIB, Oxford, U.K.). Onset times of events were used to model a signal response containing a regressor for each response type, which was convolved with a double-γ function. Groupwise activation images were carried out using FEAT (FMRI Expert Analysis Tool) Version 5.98. Significant voxels passed a statistical threshold of p < 0.01 uncorrected and were required to be part of a 424 μl (53 voxel) cluster of contiguous significant voxels resulting in a cluster-corrected p < 0.05. Cluster-size for the comparisons was determined through Monte Carlo simulations (Ward 2000). Clusters were localized using the Montreal Neurological Institute (MNI) coordinates. Brain areas were identified using the Harvard-Oxford atlas. The imaging contrasts were modeled after Dichter et al., 2009 and consisted of the comparison between the smoking conditions (satiated versus abstinent) during each phase of the WoF task. During the selection and anticipation phases, decision making trials (50/50, 30/70, and 10/90) were contrasted against the control condition (monochrome wheel, no decision). During the outcome phase, win trials were contrasted against no-win trials.
A post hoc exploratory analysis was conducted to reduce potential variability in the BOLD response due to differences in RT between the abstained and satiated conditions. In the selection phase analysis, individual’s mean-centered RTs (calculated separately for abstained and satiated conditions) were entered as two nuisance regressors (abstinent RT, satiated RT) in the group-level paired t-test
Results
Participant Characteristics
The final sample (n = 13) was 46.2% female (n = 6). Reported racial/ethnic group membership was 92.3% Caucasian (n = 12) and 7.7% African-American (n = 1). On average, participants were 30.7 years of age (SD = 9.7) and smoked 16.8 cigarettes/day (SD = 4.0). They smoked for 13.5 years (SD = 8.1) and had Fagerström Test for Nicotine Dependence (FTND) scores of 4.7 (SD = 1.7). Their baseline afternoon CO was 25.8 (SD = 14.2) and salivary cotinine level was 269.8 ng/ml (SD = 149.3). During the abstinent condition, paired-samples t-tests revealed greater Schiffman-Jarvik subscale scores for craving (t = 9.7, p < 0.001) negative affect (t = 3.9, p < 0.005), arousal (t = 2.7, p < 0.05), and habit withdrawal (t = 6.1, p < 0.001) than during the satiated condition.
Abstinence Verification
Expired breath CO concentrations and salivary nicotine levels indicated compliance with study requirements. In the satiated condition, mean CO level was 30.5 ppm (SD = 21.6) whereas in the 24-hr abstinent condition mean CO was 2.4 ppm (SD = 0.8). Mean CO 24 hrs after the abstinent fMRI session was 2.3 ppm (SD = 2.1).
Mean salivary nicotine was 411.1 ng/ml (SD = 308.7) in the satiated condition and 14.7 ng/ml (SD = 9.0) in the abstinent condition. Salivary nicotine 24 hrs after the abstinent fMRI session was 23.4 ng/ml (SD = 23.6).
Behavioral Results
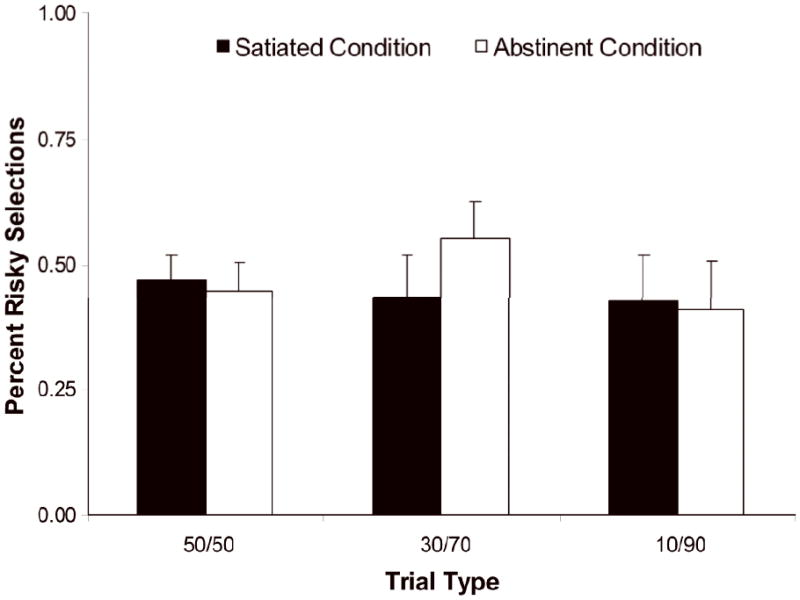
During the selection phase, RTs were slower under the abstinent compared to satiated condition (main effect of condition: F(1,12) = 14.3, p < 0.005); see Figure 2a. Abstinence did not affect the number of risky choices selected overall, and the condition × trial type interaction did not reach significance; see Figure 2b and Table 1 for ratios of risky choices by trial type. There was no difference in the amount of money earned between the abstinent and satiated conditions. Post-hoc correlations were performed between percentage of risky selections and FTND, cigs/day, and Shiffman-Jarvik scores in each condition. No significant associations were found.
Figure 2.
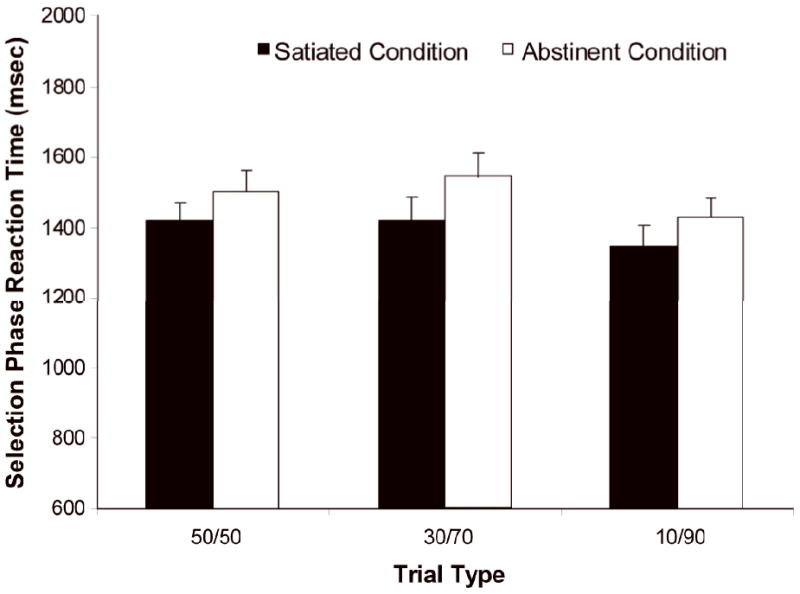
a Average reaction time for both safe and risky choices. Reaction time during the selection phase of the wheel of fortune (WoF) task was slower during the abstinent condition than during the satiated condition across the three money trials (p < 0.005).
b Percentage of risky (magenta) selections across the three money trials. Risky selections were not affected by smoking condition (satiated versus abstinent).
Table 1.
Ratio of red (risky) selections per subject in the satiated and abstinent conditions.
| Satiated | Abstinent | |||
|---|---|---|---|---|
| Subject | 30/70 | 10/90 | 30/70 | 10/90 |
| 1 | 0.33 | 0.1 | 0.25 | 0.09 |
| 2 | 0.39 | 0.09 | 0.82 | 0.03 |
| 3 | 0.96 | 0.97 | 0.96 | 1 |
| 4 | 0.21 | 0.28 | 0.57 | 0.55 |
| 5 | 0.07 | 0.39 | 0.5 | 0.38 |
| 6 | 0.78 | 0.69 | 0.85 | 0.13 |
| 7 | 0.46 | 0.53 | 0.56 | 0.52 |
| 8 | 0.54 | 0.78 | 0.11 | 0.1 |
| 9 | 0.36 | 0.06 | 0.54 | 0.28 |
| 10 | 0.93 | 0.97 | 0.85 | 0.94 |
| 11 | 0.54 | 0.31 | 0.41 | 0.28 |
| 12 | 0 | 0.36 | 0.61 | 0.94 |
| 13 | 0.07 | 0.03 | 0.15 | 0.1 |
Confidence ratings during the anticipation phase were greater for “safe” choices than for “risky” choices (main effect of risk preference: F(1,12)= 27.7, p < 0.001). Moreover, across the three trial types (50/50, 30/70, 10/90) confidence scores for safe choices increased (mean = 2.4, 2.8, 3.0, respectively) while scores for risky choices decreased (mean = 2.4, 2.2, 1.7, respectively), (interaction effect of trial type × risk preference: F(2,11) = 11.8, p < 0.005). No abstinence effects or interactions were observed.
Outcome phase valence ratings were analyzed as a function of whether the participant won money or not, irrespective of the trial type. Scores were higher after a win than after a loss (main effect of outcome: F(1,12) = 12.8, p < 0.005); average score after a win: 3.48 (SD = 0.38) and average after a no-win: 2.55 (SD = 0.85). There was no effect of abstinent condition on valence rating.
fMRI Results
Selection Phase
When comparing conditions, greater activation was observed during the abstinent condition (money > control) in the bilateral postcentral gyrus, right insula, left lingual gyrus, bilateral rolandic operculum, left middle frontal gyrus, bilateral paracingulate cortex, right precentral gyrus, right supplementary motor area (SMA), left precuneus, the right inferior and orbitofrontal gyri, as well as in the left cerebellum and middle occipital gyrus, and the right supracalcarine cortex. There were no regions in which activation was greater in the satiated as compared to the abstinent condition (see Table 2, Figure 3).
Table 2.
Areas showing significant activation during the Wheel of Fortune task.
| Brain Area | Side | # of voxels | Z max | MNI Coordinates | ||
|---|---|---|---|---|---|---|
| x | y | Z | ||||
| Selection Phase (Abstinent > Satiated, Money > Control) | ||||||
| Postcentral Gyrus | L | 1072 | 3.28 | -26 | -32 | 60 |
| Insula | R | 393 | 3.43 | 44 | -8 | -12 |
| Lingual Gyrus * | L | 385 | 2.80 | -16 | -56 | -4 |
| Rolandic Operculum * | R | 362 | 3.61 | 52 | -26 | 22 |
| Postcentral Gyrus | R | 333 | 2.97 | 34 | -30 | 54 |
| Cerebellum | L | 256 | 3.05 | -2 | -54 | -16 |
| Rolandic Operculum * | L | 241 | 3.07 | -60 | 4 | 4 |
| Insula | R | 227 | 2.88 | 36 | -8 | -8 |
| Middle Frontal Gyrus * | L | 183 | 3.10 | -34 | 52 | 8 |
| Precentral Gyrus | R | 164 | 2.67 | 34 | -8 | 46 |
| Middle Occipital Gyrus * | L | 163 | 3.04 | -40 | -70 | 6 |
| Supplementary Motor Area | R | 159 | 3.05 | 14 | -2 | 68 |
| Supracalcarine Cortex * | R | 155 | 3.08 | 22 | -66 | 16 |
| Middle Frontal Gyrus | L | 144 | 3.07 | -30 | 22 | 58 |
| Inferior Frontal Gyrus * | R | 131 | 3.19 | 52 | 36 | -14 |
| Precuneus * | L | 124 | 2.95 | -2 | -60 | 12 |
| Paracingulate Cortex | L | 121 | 2.92 | -12 | 16 | 36 |
| Paracingulate Cortex | R | 65 | 2.56 | 12 | -14 | 48 |
| Orbital Frontal Gyrus * | R | 55 | 2.89 | 4 | 54 | -24 |
| Anticipation Phase (Abstinent > Satiated, Money > Control) | ||||||
| Frontal Pole | R | 336 | 3.46 | 26 | 46 | 20 |
| Insula | L | 255 | 3.36 | -38 | 6 | -2 |
| Paracingulate Cortex | R | 139 | 2.77 | 14 | 40 | -6 |
| Anticipation Phase (Satiated > Abstinent, Money > Control) | ||||||
| Precentral Gyrus | L | 394 | 2.8 | -2 | -28 | 56 |
| Putamen | R | 53 | 3.05 | 28 | -14 | -2 |
| Outcome Phase (Satiated > Abstinent, Win > No Win) | ||||||
| Paracingulate Cortex | L | 122 | 3.04 | -2 | 48 | -6 |
Minimum cluster size = 53 voxels.
denotes area of overlapping activation in post hoc analysis with RT covariate.
Figure 3.
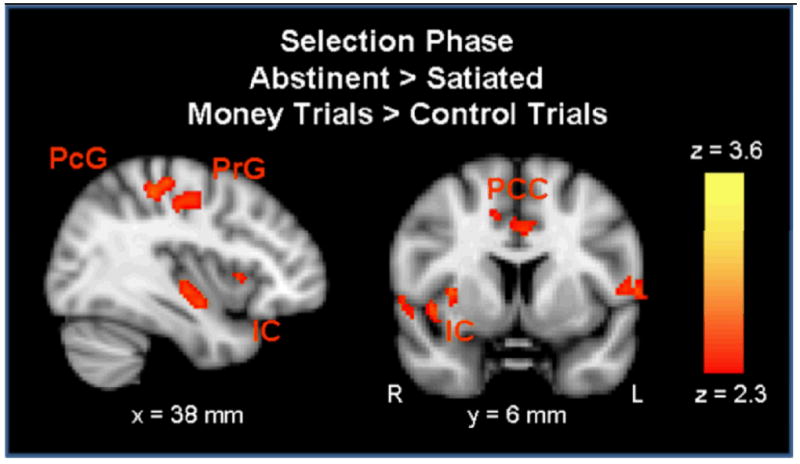
During the selection phase, activation was greater in the abstinent condition than the satiated condition in the right insular cortex (IC), the right precentral gyrus (PrG), the right postcentral gyrus (PcG), and the bilateral paracingulate cortex (PCC) (p < 0.01 uncorrected, extent threshold = 53 voxels; MNI brain template).
Anticipation Phase
During outcome anticipation, greater activation was observed on the abstinent day (relative to the satiated day) for money > control trials in the right frontal pole, left insula, right paracingulate gyrus. Activation was greater in the satiated condition than in the abstinent condition in the left precentral gyrus and the right putamen (see Figures 4a,b).
Figure 4.
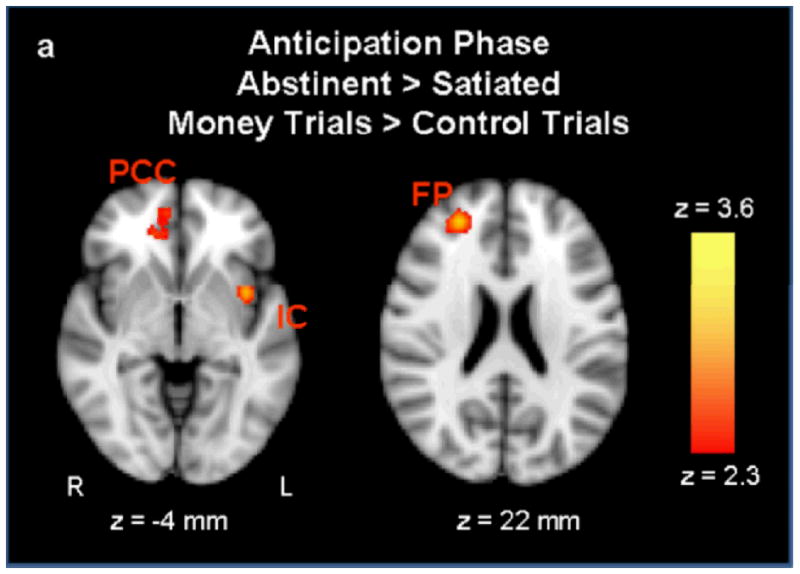
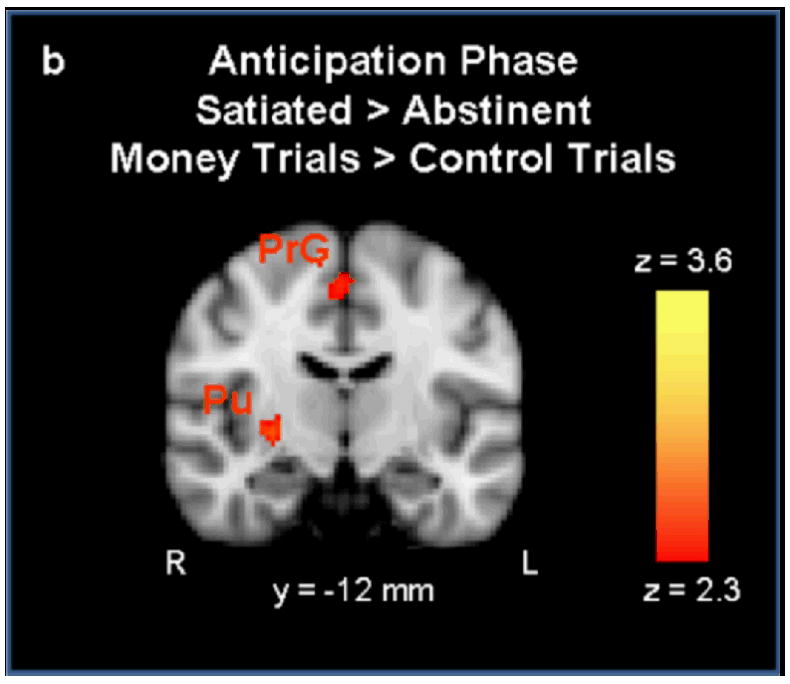
a During the anticipation phase, activation was greater in the abstinent condition than the satiated condition in the left insular cortex (IC), right paracingulate cortex (PCC), and the right frontal pole (FP). Figure 4b Activation was greater in the satiated condition than the abstinent condition in the right putamen (Pu) and the right precentral gyrus (PrG) (p < 0.01 uncorrected, extent threshold = 53 voxels; MNI brain template).
Outcome Phase
During the outcome phase, brain activation was compared between trials in which the participant won (win), versus trials in which they did not win (no-win). When participants won money, greater activation was observed in left paracingulate cortex in the satiated condition compared to the abstinent condition. No activation was greater during the abstinent condition relative to the satiated condition. In addition, no-win>win activation was not different between the two conditions (see Figure 5).
Figure 5.
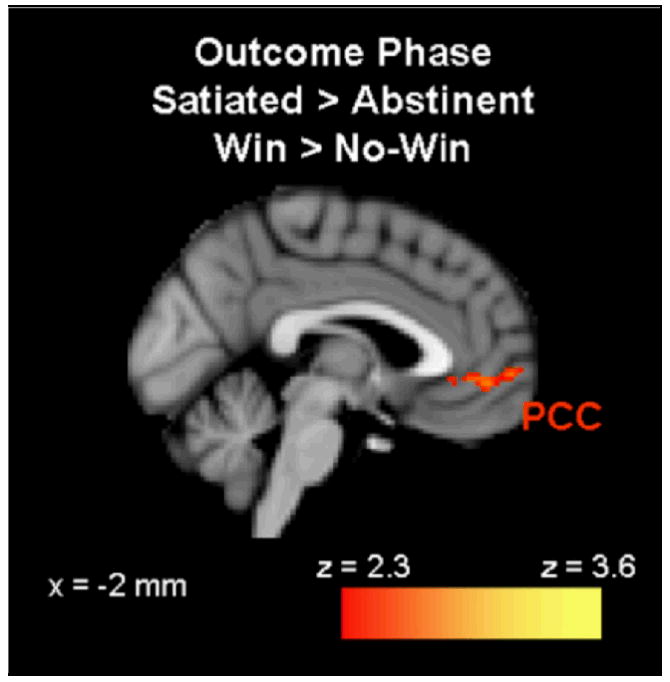
During the outcome phase, activation was greater during the satiated condition than during the abstinent condition in the left paracingulate cortex (PCC) (p < 0.01 uncorrected, extent threshold = 53 voxels; MNI brain template).
In the post hoc covariate analysis, the BOLD activation contrast remained robust; however, regressing out RT resulted in a reduced number of regions activated during the selection phase (Abstinent > Satiated, Money > Control). Areas of activation overlap between the analyses with and without the RT covariate are marked with an asterisk, see Table 2.
Discussion
The purpose of this study was to investigate the effects of smoking abstinence on probabilistic decision making behavior and brain activity among nicotine-dependent smokers. As we had hypothesized, the results showed that during the selection phase of the task there was greater activation in several brain regions including the insula, frontal, and parietal cortices during the abstinent condition. However, abstinence did not produce a greater activation in the striatum nor did it significantly affect risk preference. During the anticipation phase, confidence ratings changed according to the probability of the outcome and anticipation was associated with greater activity in the left insular cortex, right frontal pole and right paracingulate cortex during the abstinent condition, and with greater activity in the left precentral gyrus and right putamen during the satiated condition. Lastly, outcome valence was more positive on win trials than no-win trials and this phase of the task was associated with greater activity in the left paracingulate cortex during the satiated condition. Together these preliminary findings suggest that smoking abstinence modulates brain function associated with probabilistic decision making and the anticipation and receipt of outcomes outside of any behavioral effects on risk preference, confidence or hedonic ratings of outcomes.
Individuals with substance use disorders have been shown to have impaired decision making behavior (Barry and Petry 2008) similar to individuals with frontal lobe lesions (Bechara 2003), and neuroimaging studies support the idea of frontal lobe dysfunction in subjects with substance dependence (Bolla et al. 2003; Beck et al. 2009). While there is some evidence that nicotine-dependent smokers make riskier decisions (Lejuez et al. 2003; Businelle et al. 2009), many studies have not reported differences between smokers and non smokers on gambling task performance (Lejuez et al. 2003; Rotheram-Fuller et al. 2004; Harmsen et al. 2006), on pattern-recognition with reward outcomes (Martin-Soelch et al. 2003), or on delay-discounting tasks (Ohmura et al. 2005). It is possible that smokers in a satiated condition perform similar to non smokers, although differences may be detected in neural activation patterns (Martin-Soelch et al. 2003). To date, no study has investigated neural or behavioral differences between smokers and non smokers on the WoF task, and the absence of a control group limits the interpretation of our results. Differences in reward processing and probabilistic decision making strategies may be key mechanisms in the development of drug dependence and the inability to quit permanently.
During the selection phase, a previous neuroimaging study with healthy subjects revealed greater activation for money trials than control trials in the dorsal and middle frontal gyrus, cingulate gyrus, inferior parietal lobe, fusiform gyrus, and middle and inferior occipital lobe (Ernst et al. 2004). Similarly, we found increased activation in the abstinent condition in frontal, parietal, and occipital regions, as well as in the insula and paracingulate gyrus. In contrast, we did not find activation in the anterior cingulate, an area thought to be involved in conflict monitoring and shown to be activated during the WoF selection phase (Ernst et al. 2004; Engelmann and Tamir 2009). However, we did not report the activation caused by task performance in general, only the difference in activation between the abstinent and satiated conditions. Previous studies have also shown greater activation during risky selections than safe selections in the superior frontal gyrus, middle occipital gyrus, nucleus accumbens, caudate nucleus, and cingulate gyrus (Ernst et al. 2004; Engelmann and Tamir 2009; Smith et al. 2009). We were unable to compare risky versus safe selections because of an unequal number of trial types per subject, but in the future this may be possible with a larger sample size. This contrast analysis may be necessary to detect potential differences in striatal activation between the satiated and abstinent conditions.
In support of our hypothesis, there was greater activation in frontal regions during abstinence in the selection phase. This was accompanied by slower reaction times (100 ms slower, on average). This may be indicative of an increased cognitive effort needed for probabilistic decision making during nicotine withdrawal, which can impair concentration and working memory (Xu et al. 2005; Mendrek et al. 2006; Myers et al. 2008; Kozink et al. 2010). The RT regressor was included to explore whether the differences in neural activity between the abstained and satiated conditions were dependent upon corresponding differences in RT. Regressing out RT reduced activation in frontal, motor, and somatosensory regions. These regions may play a role in general psychomotor performance, not specific to risky decision-making. Several fMRI studies have used RT as a correlate of cognitive performance. Yarkoni et al. (2009) correlated demeaned RT with the hemodynamic response (HDR) from 5 data sets with different cognitive RT tasks. The authors reported BOLD activations in areas such as the insula, cerebellum, pre- and post-central gyrus (Yarkoni et al. 2009), which overlaps with areas reported in the present study. In another study, a RT regressor was used to identify brain areas modulated by task difficulty. During a word-naming task, areas that correlated with RT included the inferior and middle frontal gyrus, anterior insula, anterior cingulate gyrus, and SMA (Binder et al. 2005). In addition to representing sensorimotor function (pre-, postcentral gyrus, SMA, cerebellum), RT-related activation may also represent a “time-on-task” effect of general processes in frontal brain regions responsible for working memory, attention, decision, and response selection, instead of being task-specific (Binder et al. 2005; Yarkoni et al. 2009). However, in our study, the difference in RT is between a nicotine-satiated and abstinent state. Abstinence has been shown to produce slower RT during cognitive tasks (Shiffman et al. 1995; Parrott et al. 1996). Furthermore, there is evidence that abstinence decreases cognitive efficiency and other neural networks may be recruited to compensate for performance deficits (Kozink et al. 2010; Sweet et al. 2010). The brain regions that correlate with RT could be different between conditions, which makes the reduced activation following the RT covariate more difficult to interpret.
Some regions of increased activation reported here (e.g., insula, paracingulate, orbitofrontal cortex) have been identified as being involved in reward processing. Therefore, it is likely that the increased activation in fronto-parietal regions is related to nicotine-withdrawal modulation of probabilistic decision making. For instance, insula activity has been associated with cue-induced urges to take a drug (for review, see Naqvi and Bechara 2009) and specifically with cigarette cue-induced craving among smokers (Brody et al. 2002). Insula lesions have also been shown to interrupt smoking addiction; therefore, this area has been proposed to represent the interoceptive effects of drug use (Naqvi et al. 2007). Alternatively, in a gambling task, the insula may represent the excitement or internal arousal associated with anticipating a monetary win. Insula activity has also been associated with making and winning a gamble, and with a measure of trait impulsivity (Xue et al. 2010); in addition, hyperactivation of the insula was shown during monetary gain in a probabilistic reversal learning task in smokers compared to controls (de Ruiter et al. 2009). The internal representation of a reward outcome is an important motivating influence during probabilistic decision making. The increased insular activity during the abstained condition of this study could indicate greater emotive reward valuation, independent of the cognitive (behavioral) expectation of winning the reward.
The paracingulate cortex was activated in all three phases of the WoF task (selection, anticipation, and outcome). In contrast to the selection and anticipation phases, in the outcome phase there was greater activity in the satiated condition in the left paracingulate cortex. An adjacent region, the cingulate gyrus, has previously been reported to be activated during the selection (Ernst et al. 2004; Engelmann and Tamir 2009; Smith et al. 2009), anticipation, and outcome phases (Engelmann and Tamir 2009). The paracingulate was also a region that showed increased activation in subjects with major depression following behavioral therapy during the selection phase and decreased activation following therapy in the outcome phase of the WoF task (Dichter et al. 2009). Similarly, the paracingulate has been reported to be activated during the decision phase of a different task of probabilistic decision making (Rogers et al. 2004) and it appeared to be part of a larger network of brain regions (including the pregenual ACC and dorsal mPFC) that represented the reward magnitude while subjects decided between two gambles. In the outcome phase, a similar study found greater activation in the frontal cortex, caudate, putamen, and insula during a win compared to a loss (Liu et al. 2007). Our results suggest that the difference in outcome monitoring between the satiated and abstained conditions was limited to the paracingulate cortex.
In the selection phase, the right orbitofrontal cortex (OFC) had greater activation during the abstinent condition. The OFC is involved in reward-based learning and is thought to maintain and update stimulus-reward associations (Schoenbaum et al. 2003; Ghahremani et al. 2010), it has also been implicated in altering behavior when in response to a change in environmental reinforcement contingencies (for review, see Rolls 2000). For Instance, in rodents, lesions to the OFC impaired reversal learning (Schoenbaum et al. 2003). Evidence suggests that the OFC is functionally altered in drug addicted individuals (Schoenbaum et al. 2006). Therefore, the increased activation shown here in the abstinent condition may be indicative of an impairment in the ability to modify future decisions based on previous outcomes.
The relationship between the abstinent condition and increased activation in fronto-parietal regions during a task of probabilistic monetary rewards is complicated because the motivation to obtain a drug reward is not equivalent to the motivation to obtain a non-drug reward. Hypothetically, in a substance dependent individual, affective hedonic state is depressed during withdrawal (Koob and Le Moal 2008) and withdrawal may spur compulsive drug use, but primarily because it removes the negative affect (Koob 2009). In rodent studies, motivation to obtain non-drug rewards is typically diminished during withdrawal. In studies using delay discounting tasks in humans, withdrawn smokers respond more impulsively for cigarettes than when satiated, but delay discounting of monetary rewards is not consistently affected by nicotine state (Mitchell 2004; Field et al. 2006). However, in real-world situations, money is needed to purchase the desired drug, and the strength of this conditioned association needs to be tested in future studies.
To our knowledge, this WoF task has not been administered in conjunction with other probabilistic decision making tasks or other measures of risky/impulsive behavior for comparison. However, this study and another (Dichter et al. 2009) suggest that performance is stable within-groups across repeated measures; therefore, risk preference may be a relatively stable trait. A study by Engelmann and Tamir (2009) found that the proportion of risky choices ranged between 0.14 and 0.59 across 10 subjects. Similarly, we found evidence of individual differences in risk preference. Some individuals in this sample almost exclusively selected risky or safe options across all trials (Table 1). Identifying personality traits or characteristics that predict performance and neural activation would strengthen the utility of the WoF task in future studies.
Though providing a preliminary assessment of the effects of smoking withdrawal on neural circuits underlying probabilistic decision making, this study is limited by several factors, many of which will be addressed in future research. First, the sample size is small and our statistical threshold, though providing some protection against Type 1 error via cluster correction, is relatively liberal. As such, the findings should be considered preliminary and in need of replication with large samples. Second, only enough trials were presented to allow for an examination of brain responses during decision making (versus trials in which no decision was required). Since many subjects had an unequal number of risky/safe selections in each trial type (see Table 1), we were unable to assess neural circuitry specifically associated with the risk/reward balance of a trial or with the selection of high versus low risk options. Subjects were not screened regarding a history of Axis-I psychiatric disorders, and a history of depression or anxiety could have affected reward/decision-making processing. Finally, the lack of a non smoking control group makes it unclear whether brain function during the abstinent versus satiated condition more closely approximates normal functioning.
In conclusion, the WoF task elicited differences in neural activation between a satiated and abstinent condition among cigarette smokers. The increased activation in fronto-parietal brain areas during abstinence suggest a modulation of probabilistic decision-making cognition during nicotine withdrawal. From a clinical perspective, these findings suggest that smoking abstinence changes probabilistic decision making cognition, which might potentially have implications for understanding smoking lapse/relapse. Future studies could provide a more in-depth analysis of magnitude, probability, and risk in order to identify withdrawal-induced changes in behavioral decision making and individual differences that account for risk preference.
Supplementary Material
Acknowledgments
We thank Avery Lutz for her assistance with data acquisition. This research was supported by NIDA grants K23DA017261 and R01 DA023516 to FJM, R03 MH078145 and K23 MH081285 to GSD, and by K23 MH087754 to MJS.
Footnotes
Disclosure/Conflict of Interest
Dr. Addicott, Mr. Baranger, Ms. Kozink, Dr. Smoski and Dr. Dichter report no conflicts of interest. Dr. McClernon has received research funding from the National Institute on Drug Abuse, the Atkins Foundation, and from an unrestricted grant from Philip Morris USA to Duke University (Dr. Jed E. Rose, PI).
References
- Baker F, Johnson MW, Bickel WK. Delay discounting in current and neverbefore cigarette smokers: similarities and differences across commodity, sign, and magnitude. J Abnorm Psychol. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- Barry D, Petry NM. Predictors of decision-making on the Iowa Gambling Task: independent effects of lifetime history of substance use disorders and performance on the Trail Making Test. Brain Cogn. 2008;66:243–252. doi: 10.1016/j.bandc.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Risky business: emotion, decision-making, and addiction. Journal Gambl Stud. 2003;19:23–51. doi: 10.1023/a:1021223113233. [DOI] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hagele C, Knutson B, Heinz A, et al. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Kowal BR, Gatchalian KM. Cigarette smokers discount past and future rewards symmetrically and more than controls: Is discounting a measure of impulsivity? Drug Alcohol Depend. 2008;96:256–262. doi: 10.1016/j.drugalcdep.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E. Some neurophysiological constraints on models of word naming. Neuroimage. 2005;27:677–693. doi: 10.1016/j.neuroimage.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, et al. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. NeuroImage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Madsen D, et al. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Buhler M, Vollstadt-Klein S, Kobiella A, Budde H, Reed LJ, Braus DF, Buchel C, Smolka MN. Nicotine dependence is characterized by disordered reward processing in a network driving motivation. Biol Psychiatry. 2010;67:745–752. doi: 10.1016/j.biopsych.2009.10.029. [DOI] [PubMed] [Google Scholar]
- Businelle MS, Kendzor DE, Rash CJ, Patterson SM, Coffey SF, Copeland AL. Heavy smokers perform more poorly than nonsmokers on a simulated task of gambling. Subst Use Misuse. 2009;44:905–914. doi: 10.1080/10826080802484173. [DOI] [PubMed] [Google Scholar]
- de Ruiter MB, Veltman DJ, Goudriaan AE, Oosterlaan J, Sjoerds Z, van den Brink W. Response perseveration and ventral prefrontal sensitivity to reward and punishment in male problem gamblers and smokers. Neuropsychopharmacology. 2009;34:1027–1038. doi: 10.1038/npp.2008.175. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ. The effects of psychotherapy on neural responses to rewards in major depression. Biol Psychiatry. 2009;66:886–897. doi: 10.1016/j.biopsych.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JB, Tamir D. Individual differences in risk preference predict neural responses during financial decision-making. Brain Res. 2009;1290:28–51. doi: 10.1016/j.brainres.2009.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, Zarahn E, Leibenluft E, Zametkin A, Towbin K, et al. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42:1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, Zarahn E, Leibenluft E, Zametkin A, Towbin K, et al. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42:1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Field M, Santarcangelo M, Sumnall H, Goudie A, Cole J. Delay discounting and the behavioural economics of cigarette purchases in smokers: the effects of nicotine deprivation. Psychopharmacology. 2006;186:255–263. doi: 10.1007/s00213-006-0385-4. [DOI] [PubMed] [Google Scholar]
- Ghahremani DG, Monterosso J, Jentsch JD, Bilder RM, Poldrack RA. Neural components underlying behavioral flexibility in human reversal learning. Cereb Cortex. 2010;20:1843–1852. doi: 10.1093/cercor/bhp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D, McClernon J, Rabinovich N, Sugai C, Plath L, Asgaard G, Zuo Y, Huggenvik J, Botros N. Effects of quitting smoking on EEG activation and attention last for more than 31 days and are more severe with stress, dependence, DRD2 A1 allele, and depressive traits. Nicotine Tob Res. 2004;6:249–267. doi: 10.1080/14622200410001676305. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, Dibb WD, Plath LC, Hiyane S, Jensen RA, Meliska CJ, Estes SL, Gehlbach BA. EEG, physiology, and task-related mood fail to resolve across 31 days of smoking abstinence: relations to depressive traits, nicotine exposure, and dependence. Exp Clin Psychopharmacol. 1999;7:427–443. doi: 10.1037//1064-1297.7.4.427. [DOI] [PubMed] [Google Scholar]
- Harmsen H, Bischof G, Brooks A, Hohagen F, Rumpf HJ. The relationship between impaired decision-making, sensation seeking and readiness to change in cigarette smokers. Addict Behav. 2006;31:581–592. doi: 10.1016/j.addbeh.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology. 2006;187:385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Constable RT, Westerveld M, Pugh KR. Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology. 2007;193:557–566. doi: 10.1007/s00213-007-0797-9. [DOI] [PubMed] [Google Scholar]
- Koob GE, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56:18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozink RV, Lutz AM, Rose JE, Froeliger B, McClernon FJ. Smoking withdrawal shifts the spatiotemporal dynamics of neurocognition. Addict Biol. 2010;15:480–490. doi: 10.1111/j.1369-1600.2010.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36:539–548. doi: 10.1016/s0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Jones HA, Richards JB, Strong DR, Kahler CW, Read JP. The Balloon Analogue Risk Task (BART) differentiates smokers and nonsmokers. Exp Clin Psychopharmacol. 2003;11:26–33. doi: 10.1037//1064-1297.11.1.26. [DOI] [PubMed] [Google Scholar]
- Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. J Neurosci. 2007;27:4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, Gur RC, Lerman C. Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol Psychiatry. 2010;67:715–721. doi: 10.1016/j.biopsych.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Missimer J, Leenders KL, Schultz W. Neural activity related to the processing of increasing monetary reward in smokers and nonsmokers. Eur J Neurosci. 2003;18:680–688. doi: 10.1046/j.1460-9568.2003.02791.x. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kollins SH, Lutz AM, Fitzgerald DP, Murray DW, Redman C, Rose JE. Effects of smoking abstinence on adult smokers with and without attention deficit hyperactivity disorder: results of a preliminary study. Psychopharmacology. 2008;197:95–105. doi: 10.1007/s00213-007-1009-3. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: comparison to non-smokers and effects of abstinence. Addict Behav. 2006;31:833–844. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH. Effects of short-term nicotine deprivation on decision-making: delay, uncertainty and effort discounting. Nicotine Tob Res. 2004;6:819–828. doi: 10.1080/14622200412331296002. [DOI] [PubMed] [Google Scholar]
- Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology. 2008;33:588–598. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmura Y, Takahashi T, Kitamura N. Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacology. 2005;182:508–515. doi: 10.1007/s00213-005-0110-8. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Garnham NJ, Wesnes K, Pincock C. Cigarette smoking and abstinence: Comparative effects upon cognitive task performance and mood state over 24 hours. Hum Psychopharmacol. 1996;11:391–400. [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depend. 2010;106:61–64. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettiford J, Kozink RV, Lutz AM, Kollins SH, Rose JE, McClernon FJ. Increases in impulsivity following smoking abstinence are related to baseline nicotine intake and boredom susceptibility. Addict Behav. 2007;32:2351–2357. doi: 10.1016/j.addbeh.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J, Dawkins L, Davis RE. Smoking, reward responsiveness, and response inhibition: tests of an incentive motivational model. Biol Psychiatry. 2002;51:151–163. doi: 10.1016/s0006-3223(01)01208-2. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, Smith SM. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry. 2004;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rotheram-Fuller E, Shoptaw S, Berman SM, London ED. Impaired performance in a test of decision-making by opiate-dependent tobacco smokers. Drug Alcohol Depend. 2004;73:79–86. doi: 10.1016/j.drugalcdep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decisionmaking and drug addiction. Trends Neurosci. 2006;29:116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem. 2003;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Ramus SJ. A systems approach to orbitofrontal cortex function: recordings in rat orbitofrontal cortex reveal interactions with different learning systems. Behav Brain Res. 2003;146:19–29. doi: 10.1016/j.bbr.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Elash C, Kassel JD. Nicotine withdrawal in chippers and regular smokers - Subjective and cognitive effects. Health Psychol. 1995;14:301–309. doi: 10.1037//0278-6133.14.4.301. [DOI] [PubMed] [Google Scholar]
- Shiffman SM, Jarvik ME. Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology. 1976;50:35–39. doi: 10.1007/BF00634151. [DOI] [PubMed] [Google Scholar]
- Smith BW, Mitchell DGV, Hardin MG, Jazbec S, Fridberg D, Blair RJR, Ernst M. Neural substrates of reward magnitude, probability, and risk during a wheel of fortune decision-making task. NeuroImage. 2009;44:600–609. doi: 10.1016/j.neuroimage.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder FR, Davis FC, Henningfield JE. The tobacco withdrawal syndrome: performance decrements assessed on a computerized test battery. Drug Alcohol Depend. 1989;23:259–266. doi: 10.1016/0376-8716(89)90090-2. [DOI] [PubMed] [Google Scholar]
- Sweet LH, Mulligan RC, Finnerty CE, Jerskey BA, David SP, Cohen RA, Niaura RS. Effects of nicotine withdrawal on verbal working memory and associated brain response. Psychiatry Res. 2010;183:69–74. doi: 10.1016/j.pscychresns.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. 2000 Online, http://homepage.usask.ca/~ges125/fMRI/AFNIdoc/AlphaSim.pdf.
- Xu J, Mendrek A, Cohen MS, Monterosso J, Rodriguez P, Simon SL, Brody A, Jarvik M, Domier CP, Olmstead R, et al. Brain activity in cigarette smokers performing a working memory task: effect of smoking abstinence. Biol Psychiatry. 2005;58:143–150. doi: 10.1016/j.biopsych.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Bechara A. The impact of prior risk experiences on subsequent risky decision-making: the role of the insula. NeuroImage. 2010;50:709–716. doi: 10.1016/j.neuroimage.2009.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Barch DM, Gray JR, Conturo TE, Braver TS. BOLD correlates of trial-by-trial reaction time variability in gray and white matter: A multi-study fMRI analysis. Plos One. 2009;4 doi: 10.1371/journal.pone.0004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


