Abstract
The study objective was to evaluate the composition of a neutral and weakly acidic water-soluble extract from Echinacea purpurea (L.) Moench (EchNWA) previously shown to modify murine influenza infection, and to assess immunomodulatory effects on human T-cells. EchNWA extract from fresh aerial parts was extracted with water, ethanolic precipitation, and size-exclusion chromatography. The chemical profile of EchNWA was characterized by chromatography (size-exclusion, HPLC, GC–MS), and small molecule finger-print analysis performed by HPLC–PDA. Jurkat T-cells at high and low cell density were pretreated or not with doses of EchNWA, followed by activation with phorbol 12-myristate 13-acetate plus ionomycin (PMA+I). Interleukin-2 (IL-2) and interferon gamma (IFNg) cytokine secretions were measured by multi-cytokine luminex technology. Results showed that EchNWA contains 80% polysaccharides, predominantly a 10 kDa entity; phenolic compounds, cynarin, cichoric and caftaric acids, but no detectable alkylamides. Cytokine production required stimulation and was lower after PMA+I activation in high-density compared to low-density conditions. EchNWA mediated a strong dose-dependent enhancement of high-density T-cell production of IL-2 and IFNg response to PMA+I. EchNWA alone did not stimulate T-cells. EchNWA enhanced mean fluorescence intensity of IL-2 in Jurkat T-cells activated by PMA+1 or ionomycin alone. Conversely EchNWA mediated modest but significant suppression of IFNg response and reduced the percentage of CD25+ T-cells under low-density conditions. Conclusions are that EchNWA polysaccharides, but not phenolic compounds have dose-related adjuvant effects on human T-cell cytokine responses characterized by enhancing and suppressive effects that are regulated by T-cell density.
Keywords: Echinacea, Human T-cell density, Neutral and weakly acidic water-soluble extract, Polysaccharides and phenolic compounds, Small molecule fingerprint-analysis, Immune modulation, Cytokine response
1. Introduction
Echinacea purpurea (L.) Moench has a long history of medicinal use and along with Echinacea pallida and Echinacea angustifolia, is often consumed for the prevention and treatment of the common cold [1,2]. As a collection of nine related plant species indigenous to North America, Echinacea comprises the most frequently chosen neutraceutical in the US [3,4]. Comparison of the numerous clinical trials designed to test the efficacy of Echinacea species against upper respiratory tract infections has been hindered by differences in the plant species selected, the type of solvent used for extraction, and by variation in extraction procedures all of which affect both the chemical profile of the herbal preparation and the biological activity. Investigators seeking to ascertain the overall effects of Echinacea have excluded numerous trials from comparative analysis due to differences in trial design, sample size, analytical methodology, and choice of biomarker and placebo. However several authoritative meta-analyses have concluded, although not proven, that Echinacea has therapeutic potential against the severity or duration of rhinovirus symptoms and may also have preventive effects if taken early after exposure [3-6]. Meta-analyses of pooled data from three trials involving experimental exposure of volunteers to rhinovirus infection support the benefit of Echinacea [6,7], despite the inclusion of one trial in the analysis that reported no benefit from any of three different Echinacea extracts against either infection or illness compared to placebo [8]. Echinacea preparations have traditionally been made by extraction of roots, but more recently fresh pressed juice from flowering tops, often stabilized with alcohol, has been used as well [2]. A current study using a standardized extract of Echinacea has reported direct anti-viral effects against H5N1, H7N7 and H1N1 [9].
The development of chemical profiling and standardized extraction procedures for botanicals including bioactivity-guided fractionation has enabled greater precision in delineating active components and advanced our understanding of how different constituents and derivatives in Echinacea species may function [10-15]. While discovery of the bioactive constituents is far from complete, polysaccharides, glycoproteins, alkylamides, and caffeic acid derivatives (CAD) including caftaric acid, cichoric acid, and echinacoside, have emerged as potential modifiers of immune response to infection [16-19]. Generally ethanol extracts are expected to contain higher levels of alkylamides and phenolic compounds, while aqueous extracts contain more hydrophilic compounds such as polysaccharides and glycoproteins. Polysaccharides, glycoproteins, and cichoric acid have been identified as having immune-stimulating activity [11,20,21] in contrast to alkylamides and ketones that appear to be anti-inflammatory in vitro and in vivo [22-24]. Purified polysaccharide extracts from Echinacea increased macrophage chemotaxis, production of reactive oxygen intermediates (ROS), and inhibited growth of fungi in vivo [25]. Echinacea polysaccharides enhanced production and secretion of Tumor Necrosis Factor-α (TNF-α), Interleukin-10 (IL-10), IL-6, and IL-1-β [25,26]. In contrast, ethanol extracts of Echinacea have led to reduced monocyte and macrophage response to the main antigenic component of endotoxin, lipopolysaccharide (LPS), by suppressing TNF-α and prostaglandin E2 (PGE2) production [27,28].
Recent studies have shown that a neutral and weakly acidic water soluble extract of aerial parts from E. purpurea, a widely used and commercially available product, hereafter referred as EchNWA, modified the course of influenza infection in a mouse model of live H1N1 influenza without evident antiviral activity [17]. The objective of the present study was to evaluate the chemical composition of this same E. purpurea preparation (EchNWA) and to assess EchNWA’s immunomodulatory effects on human Jurkat T-cells. Determination of the small molecule chemical constitution and polysaccharide profile was carried out to identify and quantify compounds likely to exert immunomodulatory activity.
2. Methods
2.1. Plant material
The E. purpurea (L.) Moench extract was provided by Gaia Herbs Inc. (Brevard, NC, Certificate of Analysis WA10-12-1) via the botanical core facility at the Memorial Sloan Kettering Botanical Center. Voucher specimen (HK40433) was deposited at the Hong Kong Herbarium of the Agricultural & Fishery Conservation.
2.2. Extraction process
Fresh E. purpurea aerial parts were harvested, ground with spring water into a slurry and further passed through a hydraulic press. The liquid was filtered with a 100-micron screen and 20% ethanol was added. The mixture was allowed to precipitate overnight and filtered through a 100-micron screen. The filtrate was mixed with 70% ethanol. After overnight precipitation, the solid sediments were collected and freeze-dried to remove all solvent and water. The dried powder was further dissolved in deionized water, centrifuged to remove solid particles and the supernatant was passed through a G-50 column; fractions were collected and combined before freeze-drying to powder form subsequently referred to as Echinacea Neutral and Weak Acidic (EchNWA) extract. The preparation contained <0.5 endotoxin units (EU) of lipopolysaccharide (LPS) as per certificate of analysis.
2.3. Reagents and chemicals
Cichoric and caftaric acids, cynarin and the alkylamide (2E)-N-isobutylundeca-2-ene-8,10-diynamidewere purchased from Chromadex (Irvine, CA). d-Galactose, phorbol 12-myristate 13-acetate (PMA), ionomycin, and FK506 were from Sigma (St. Louis, MO). Methanol and acetonitrile (HPLC grade) were obtained from Merck (Darmstadt, Germany) and sodium dihydrogen phosphate-2-hydrate and disodium hydrogen orthophosphate (AR grade) from Sigma (MO, USA). All other solvents used for sample preparation were also from Fisher Scientific.
2.4. Monosaccharide composition
The monosaccharide composition of polysaccharides in the EchNWA extract was determined by GC-MS as follows: 200 mg of EchNWA extract was hydrolyzed under reflux with 38 mL of 2 M H2SO4 (100 °C, 6 h). The hydrolyzed samples were then acetylated as described [29] and analyzed by GC–MS on DB-1 capillary column (15 m–0.2 mm) with a flame ionization detector (Agilent 6890GC/5973MS instrument) in a temperature gradient of 100–280°C/min. Injector temperature was set at 250 °C. Injection volume was 1.0 μL with a 10:1 split ratio. Interface temperature was set at 280 °C, ion source temperature at 230 °C, and electron impact at 70 eV. Identification and quantification of monosaccharides were made in comparison with standards. Calibration standard solutions done by derivatizing rhamnose, arabinose, xylose, fucose, mannose, glucose and galactose as described above.
2.5. Total polysaccharides
The EchNWA obtained from Gaia in a powder form, was solubilized as a 5 mg/mL solution in distilled water and placed in a vortex for about 2 h. The dark solution was then passed through a 0.45 μm membrane filter (Millipore, Bedford, MA). The total carbohydrate content in this extract was assessed as described [30]. D-Galactose was used as standard. Briefly, 25 μL of the plant extract solution (1 mg/mL) in water was aliquoted into wells of a flat bottom 96 well plate non-tissue culture treated (BD-Falcon) and treated with 25 μL of an aqueous solution of phenol 5% w/w. After 30 second agitation, the 96 well plate was placed on a bed of ice and 125 μL of concentrated sulfuric acid was added to the wells. The plate was agitated for 30 s and incubated on ice for 30 min. After incubation, absorbance was read in a plate reader (MRX Dynex) at 490 nm.
2.6. Relative molecular weight
The relative molecular weights analysis of the EchNWA extract was done by gel filtration as described [31]. 100 mg of extract was solubilized in 100 mL of 0.05 M NaH2PO4–Na2HPO4 buffer (at pH 6.7 with 0.05% NaN3) and filtered (0.45 μm). The sample was run on a TSK-Gel G3000SWXL column (5 μm, 7.8 mm–300 mm (Tosoh Bioscience LLC, PA)) eluted with 0.05 M NaH2PO4–Na2HPO4 buffer. The flow rate was 0.5 mL/min. A calibration curve was constructed using standard dextran with molecular weights of 0.738-103, 5.8-103, 1.22-104, 2.37-104, 4.8-104, 1.0-105, 1.86-105, 3.8-105, 8.35-105 Daltons (Da).
2.7. Amino acid content
Amino acid content was determined as described previously [31]. Chromatographic analyses were done in an Agilent 1100 HPLC coupled to a HP 1046A fluorescence detector, with fluorescence excitation at 340 nm or 260 nm and emission at 450 nm or 305 nm, and a C18 column (Hypersil ODS, 5 μm, 125 mm–4.0 mm, Cheshire, UK). Mobile phase A consisted of 10 mM Na2HPO4–NaH2PO4, pH 7.2 (PB); and mobile phase B was a mixture of PB, methanol and acetonitrile in a ratio of 50:35:15 (v/v). The gradient was A:B (100:0 to 0:100) for 0–25 min. The flow rate was 1 mL/min. Each amino acid was quantified by the calibration curve of the authentic amino acid (Sigma, MO, USA).
2.8. Small molecule analysis
Chromatographic analyses were carried by gradient- mode elution with water plus 0.1% trifluoroacetic acid (HPLC grade) and acetonitrile plus 0.1% trifluoroacetic acid. Reference standards of cynarin, cichoric acid, caftaric acid and (2E)-N-isobutylundeca-2-ene-8, 10-diynamide were prepared as a 5 mg/mL solution in dimethylsulfoxide (DMSO) and then diluted to 1 mg/mL in methanol. For qualitative analyzes, EchNWA extract (5 mg/mL) was added to HPLC grade water and placed in a shaker for 2 h, and then passed through a 0.45 μm membrane filter (Millipore, Bedford, MA). Quantification was carried using the external standard calibration method. For the construction of calibration curves, appropriate aliquots of individual standard stock solutions of caftaric acid, cynarin and cichoric acid (1000 μg/mL) were prepared in DMSO and kept at −20 °C. Working solutions of 10.0, 50.0, 100.0 and 500.0 μg/mL were prepared daily in methanol. Each solution was analyzed in triplicate, and the resulting peak areas were plotted against the respective compound concentrations. For quantitative analyzes, 5.0 mg of EchNWA was diluted in 1.0 mL of distilled water as described above. All samples were analyzed in triplicate. All analyses were performed using a Waters 2695 HPLC coupled to a Waters 2996 PDA detector. The HPLC was equipped with a column oven set at 25 °C. Chromatographic separations were performed on a SunFire C18 column (Waters) with 4.6 × 100 mm and 5 μm particles. The mobile phase, a mixture of acetonitrile and 0.1% TFA (Solvent B) in water with 0.1% TFA (Solvent A) was applied as follows: 5% of Solvent B for 2 min; 5% of B to 95% of B for 58 min, followed by 95% of B for 5 min. Flow rate was set at 1 mL/min.
2.9. T-cell stimulation assays
For all biological analyses, EchNWA extract was prepared as a 5 mg/mL solution in distilled water and placed in a vortex for about 2 h. The dark solution was then filter-sterilized through a membrane filter of 0.45 μm and then 0.22 μm (Millipore, Bedford, MA) under aseptic conditions. The human T-cell line Jurkat E6-1 (American Type Culture Collection, Manassas, VA) was grown under standard conditions in RPMI 1640 medium (Gibco-Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated bovine calf serum (Hyclone, ThermoFisher Scientific, Pittsburgh, PA), penicillin/streptomycin (100 U/mL and 100 μg/mL, respectively, Gibco-Invitrogen) and Glutamax (2 mM, Gibco-Invitrogen) in a 37 °C 5% CO2 humidified cell culture incubator. Jurkat T-cells (0.5 × 106 or 5 × 106 cells/mL) were added per well of a 96 well plate and were incubated with EchNWA extract (195 μL was the total final volume per well) for 40 min at 37 °C in a 5% CO2, 37″ humidified incubator. After this pre-treatment step, 5 μL of PMA (200 ng/mL stock solution) plus 5 μL ionomycin at (40 μg/mL stock solution) in DMSO or DMSO alone were added to each well adding to a final volume of 200 μL per well. Cells were once more incubated at 37 °C (5% CO2, 95% air) for 24 h. FK506 (1 μg/mL in DMSO) was used as negative control. When indicated, cichoric and caftaric acids were used instead of the EchNWA extract.
2.10. Interleukin (IL)-2 and interferon gamma (IFN-γ) cytokine secretion
Jurkat T-cells at high (5 × 106 cells/mL) and low (0.5 × 106 cells/mL) cell density were pre-treated with different concentrations of EchNWA extract (0, 10, 25, 100 and 250 μg/mL) for 40 min in a humidified incubator with 5% CO2 at 37°C, followed by another incubation for 24 h with/without PMA (10, 50 ng/mL) and/or Ionomycin (1 μg/mL) as described above. After 24 h of incubation, 50 μL of the cell culture supernatants were removed and assayed for secretion of IL-2 and IFN-γ using Beadlyte Human Multi-Cytokine Beadmaster Kit (Millipore, Bedford, MA). Calibration curves from recombinant cytokine standards were prepared using threefold dilution steps in RPMI 1640 medium containing 10% heat-inactivated bovine calf serum. Samples were analyzed in triplicate, and blank values were subtracted from all readings. The assay was carried out directly in a 96-well multiscreen filter plate (Millipore, City, ST) at room temperature. During the incubation periods, the plates were kept in the dark at room temperature, under strong agitation. Briefly, a mixture containing 5000 microspheres of Human IL-2 Beadmates and Human IFN-γ Beadmates (both from Millipore) were incubated with sample and standards at room temperature in the dark for 2 h. After filtering, the conjugated cytokine-beads were retained on the 96 well plate, resuspended in a cocktail of biotinylated antibodies and incubated for 1.5 h, after which 100 ng of Streptavidin–Phycoerythrin was used to treat each well. Measurements and analyses were performed on a Luminex 200 Total System (Luminex Corporation, Austin, TX).
2.11. Flow cytometric determination of CD25 expression
In order to evaluate CD25 expression on the Jurkat T-cell surface, flow cytometric analysis was performed after staining of aliquots from both high (5 × 106 cells/mL) and low (0.5 × 106 cells/mL) concentrations of cells that had been pre-incubated or not with EchNWA extract (0, 10, 100 μg/mL) for 40 min, followed by another 24 hour incubation period with PMA (10, 50 ng/mL) plus Ionomycin (1 μg/mL) as described above for T-cell stimulation studies. For staining, 0.5 × 106 cells were washed twice with 0.01 mol/L phosphate-buffered saline (PBS) pH 7.4 buffer + 1% fetal bovine serum, (Gibco and Hyclone, respectively) and the cells were suspended with 50 μL PBS buffer and incubated in the dark at room temperature for 30 min withPE-Cy7-labeled mouse anti-human anti-CD3, PE-Cy5-labeled anti-human anti-CD8 and APC-Cy7-labeled mouse anti-human anti-CD25 (IL-2Rα) mABs (BD Biosciences, San Jose, CA). Cells were washed twice in PBS and flow cytometric acquisition and analysis were performed in triplicate on a Cyano ADP 9 Colors (Beckman Coulter, Fullerton, CA) flow cytometer. At least 2500 gated events were collected. Analysis was performed using Flowjo software (Tree Star, Inc., Ashland, CA).
2.12. Statistical analysis
The analyses were carried out using GraphPad Prism software version 5.01. Data are expressed as mean ± SD for n = 3 in all experiments. Statistics were calculated using either 1-way or 2-way ANOVA, as designated to determine significance of variance and followed by Tukey’s posttest for 1-way ANOVA or Bonferroni’s posttest for 2-way ANOVA as appropriate if ANOVA variance was significant.
3. Results
3.1. Chemical analyses of Echinacea NWA
3.1.1. Carbohydrate analysis
Monosaccharide analysis showed that the main carbohydrate units of polysaccharides present in EchNWA extract are galactose (43.5%) and glucose (27.9%), followed by smaller amounts of arabinose (14.0%), mannose (8.2%), rhamnose (3.3%) and xylose (3%), and the total polysaccharide content was 80% w/w. The total amino acid content in the extract was 3% (w/w) with the dominating units glutamic and aspartic acids, glycine and alanine (Table S-1 Supplemental Material). Characterization of the molecular weight range was determined by gel filtration chromatography (Table 1). Preliminary analysis showed that compounds with large molecular weight (56% of total extract) were spread between 2000 Da to 218 kDa, most of them, with an average of 10 kDa. The remainder consisted of molecules smaller than 2000 Da.
Table 1.
Relative molecular weight distribution of Echinacea NWA extract solubilized in water.
| Retention time (min) | Peak area (nRIU) | Mn/Mwa |
|---|---|---|
| Peaks 1 + 2 (9.7–14.7) | 354,102 | 91,892/218,890 |
| Peak 3 (15.7–20.6) | 1,198,370 | 9,177/9,914 |
| Peak 4 (20.6–22.0) | 585,707 | 1,933/2,038 |
| Peak 5 (after 22.0) | 1,675,586 | na |
Mw: weight average molecular weight; Mn: number average molecular weight.
3.1.2. Characterization of small molecules
HPLC analyses were performed in order to determine whether small molecules with immunomodulatory potential, known to be present in E. purpurea preparations, were also present in this extract. Fig. 1A shows a chromatogram and the UV spectrum of a commercial sample of the alkylamide (2E)-N-isobutylundeca-2-ene-8,10-diynamide. Fig. 1B shows a fingerprint chromatogram of EchNWA extract with representative UV spectra of all peaks absorbed at the UV–visible range. These chromatographic analyses, which were obtained with an optimized exploratory gradient, show the presence of several peaks eluting from 0% to 35% of acetonitrile in acidified water. The chromatographic analysis revealed several phenolic compounds, including cichoric and caftaric acids, as well as cynarin (Fig. 1B), but not alkylamides. Quantification analyzes were done in order to verify the concentration of cichoric and caftaric acids as well as cynarin present in EchNWA. These phenolic compounds were present at 1.2 ± 0.003%, 0.4 ± 0.004%, and 0.03 ± 0.001% w/w respectively.
Fig. 1.
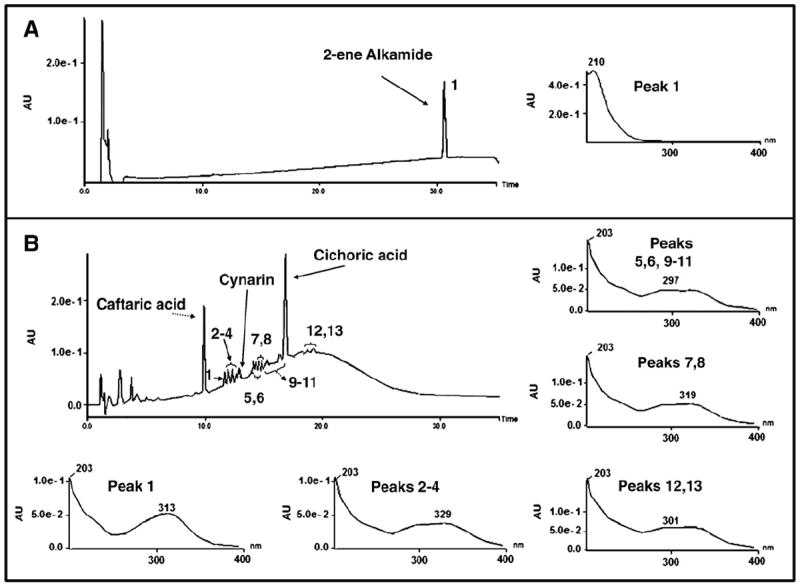
Panel A: 2-ene alkamide reference substance and the UV–vis peak spectrum. Panel B: Echinacea NWA extract and UV–vis spectra. The chromatogram for all resolved peaks.
3.2. Effect of EchNWA on cytokine response
To optimize experimental conditions, two different concentrations of PMA (10 and 50 ng/mL) plus ionomycin (1 μg/mL) were first tested on Jurkat T-cells plated at low (0.5 × 106 cells/mL) or high (5 × 106 cells/mL) cell densities in the absence of EchNWA. Viability of T-cells cultured at low and high density was the same (Guava Viacount, data not shown). Since cells treated with 10 ng/mL PMA showed a higher degree of IL-2 and IFN-γ production and the higher dose of PMA tended to cause loss of T-cell viability (data not shown), PMA 10 ng/mL was selected. The effect of pretreatment with varying doses of EchNWA extract on subsequent response to PMA + I was assessed. Controls included using the same concentrations of EchNWA extract but without subsequent addition of PMA + I, addition of IL-2 or PBS alone, and FK506 as a negative control. Pre-treatment with EchNWA extract alone did not lead to either IL-2 or IFN-γ secretion at any concentration (data not shown).
3.2.1. IL-2 response to EchNWA at high and low T-cell density
The relative effects of EchNWA on IL-2 cytokine response to PMA + I at high and low T-cell densities were markedly different. As shown in Fig. 2, overall response to PMA + I with and without EchNWA pretreatment varied significantly within each condition by 1-way ANOVA (p < 0.0001 for high density and p < 0.03 for low density) and across both the high and low cell density conditions (p < 0.0001 by 2-way ANOVA). At high density, IL-2 secretion in response to PMA + I was lower (1404 pg/mL ± 104.4) compared to response in low-density conditions (3427 pg/mL ± 256.5, p < 0.0001 by Bonferroni post-test) in the absence of EchNWA pretreatment. EchNWA pretreatment had a greater impact on subsequent response to PMA + I in the high-density setting compared to the low-density condition. The increase in T-cell secretion of IL-2 in the high-density cultures after pretreatment with EchNWA showed a dose–response relationship. Pair-wise differences showed that EchNWA pretreatment enhanced response to PMA + I at both 100 μg/mL and 250 μg/mL doses compared to PMA + I alone (p < 0.001, Tukey’s posttest) and compared to EchNWA pretreatment at 10 μg/mL (p < 0.001, Tukey’s posttest). The effect of the 250 μg/mL dose was greater than the 100 μg/mL dose (p < 0.01, Tukey’s posttest) after culture at high-density. IL-2 response in the high-density condition was lower compared to the low-density condition after EchNWA pretreatment at 10 and 100μg/mL (p < 0.001, p < 0.01, Bonferroni posttest), and achieved the same level of response only after EchNWA pretreatment at 250 μg/mL.
Fig. 2.
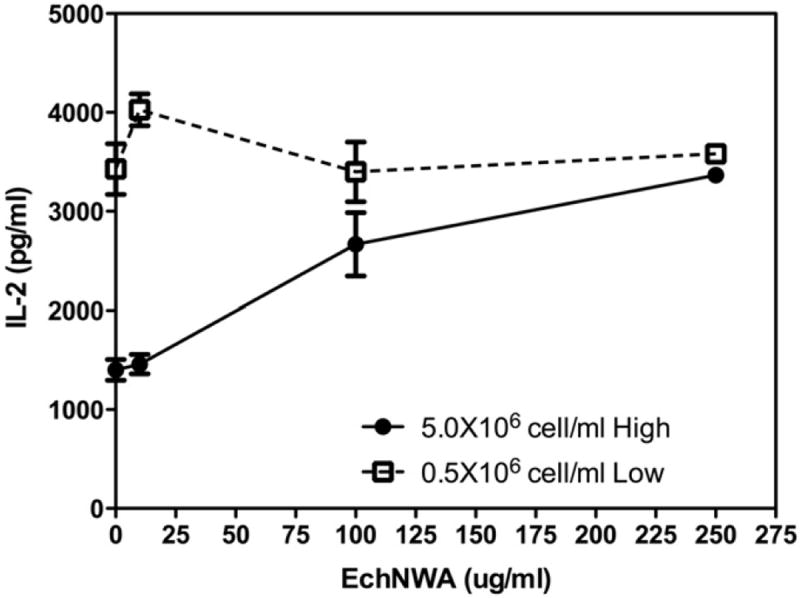
Effect of EchNWA pretreatment on IL-2 response: Jurkat T-cells were cultured at low (0.5 × 106 cells/mL) or high (5 × 106 cells/mL) cell density, pretreated with or without EchNWA, and then stimulated with PMA + I. At high density, IL-2 response to PMA + I was less without EchNWA compared to low-density conditions (2-way ANOVA, p < 0.0001 Bonferroni post-test). IL-2 response in high-density cultures showed optimal improvement after EchNWA pretreatment at 250 μg/mL. IL-2 response to EchNWA in the high-density condition showed a dose–response relationship. Differences were significant (1-way ANOVA, Tukey’s posttest) at 100 μg/mL and 250 μg/mL doses compared to PMA + I alone (p < 0.001) and also for EchNWA pretreatment at 10 μg/mL (p < 0.001).
As shown in Fig. 3, panel A, evaluation of the mean fluorescence intensity (MFI) of IL-2 secretion showed that EchNWA exerted a dose-related effect on IL-2 production in high density conditions when the calcium ionophore A23817 ionomycin alone was used as the stimulant suggesting an effect on calcium mobilization sufficient to increase the intensity of cellular IL-2 production. Overall variance was significant (p < 0.01 by 1-way ANOVA). The dose related increase in MFI at 100 μg/mL and 200 μg/mL of EchNWA was significant compared to ionomycin alone (p < 0.01, Tukey’s post test). In addition, as shown in panel B the addition of EchNWA also strongly affected the MFI of cytokine response to the combination of PMA + I (overall variance p < 0.0001 by 1-way ANOVA) in high-density conditions. Doses of 100 μg/mL and 200 μg/mL of EchNWA led to increased levels of MFI compared to PMA alone or to 10 μg/mL EchNWA (p < 0.0001, Tukey’s post test).
Fig. 3.
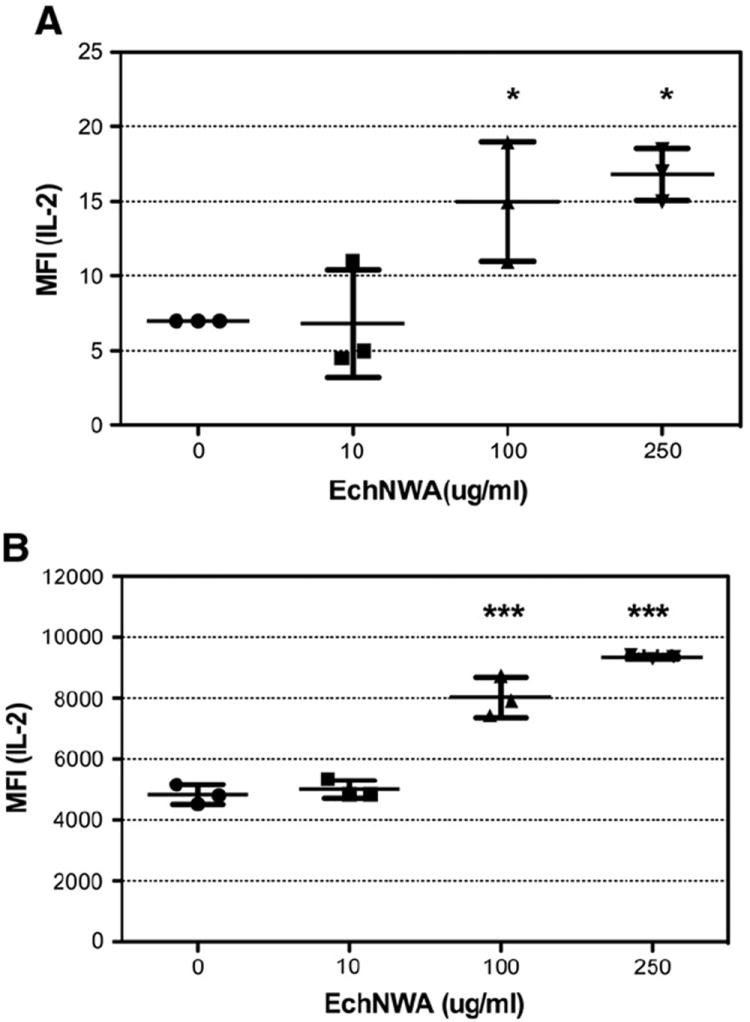
Effect of EchNWA pretreatment on T-cell response to ionomycin alone: Jurkat T-cells cultured at high density (5 × 106 cells/mL) were pretreated or not with EchNWA and stimulated with ionomycin (I) or phorbol ester + I (PMA + I) and evaluated for IL-2 production by multi-cytokine assay. The increase inmean fluorescence intensity (MFI) of the IL-2 response (MFI) at 100 μg/mL and 250 μg/mL of EchNWA was significant compared to ionomycin alone (p < 0.01, Tukey’s posttest) after 1-way ANOVA as shown in panel A, and after treatment with EchNWA at 100 μg/mL and 250 μg/mL compared to PMA + I alone (p < 0.0001, Tukey’s posttest) after 1-way ANOVA as shown in panel B.
3.2.2. IFN-γ response to EchNWA at high and low T-cell density
IFN-γ secretion in response to PMA + I was also influenced by T-cell density as shown in Fig. 4. Response to PMA + 1 varied significantly in Jurkat T-cells cultured at low-density and stimulated with PMA + I with or without EchNWA pretreatment compared to T-cells cultured at high-density (p < 0.0001, 2-way ANOVA). Low-density T-cells produced 45.5 ± 1.8 pg/mL IFN-γ and high-density T-cells produced substantially less (14.4 ± 1.4 pg/mL, p < 0.001, Bonferroni posttest). Both 100 μg/mL and 250 μg/mL doses of EchNWA enhanced response to PMA + I in high-density T-cells (p < 0.0001, Tukey’s post test after 1-way ANOVA,) compared to PMA + I alone, causing a fivefold increase in secretion of IFN-γ. In contrast the effect of EchNWA on T-cells cultured at low-density was a small dose-dependent suppression that was significant comparing the effects of the 100 μg/mL or 250 μg/mL EchNWA to PMA + I alone (p < 0.02, p < 0.05, Tukey’s posttest).
Fig. 4.
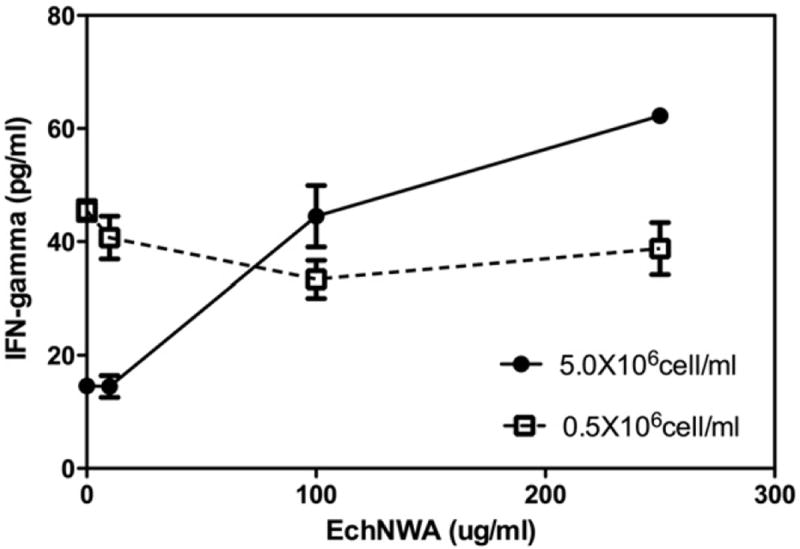
Effect of EchNWA pretreatment on IFN-γ response: Jurkat T-cells were cultured at low (0.5 × 106 cells/mL) or high (5 × 106 cells/mL) cell densities and pretreated with PMA + I. At high density, IFN-γ response to PMA + I was less without EchNWA pretreatment compared to low-density condition (2-way ANOVA, p < 0.0001 Bonferroni post-test). At 100 μg/mL and 250 μg/mL doses of EchNWA enhanced response in high-density T-cells (p < 0.0001, 1-way ANOVA, Tukey’s post test) compared to no pretreatment. Low-density T-cells showed mild dose dependent suppression that was significant at 100 μg/mL dose of EchNWA compared to PMI + I alone (p < 0.02, 1-way ANOVA, p < 0.05, Tukey’s posttest).
3.3. Effect of EchNWA on CD25 expression
Expression of the IL-2 receptor, (IL-2Rα, Tac), CD25 after PMA + I activation in the presence and absence of EchNWA was assessed (Fig. 5). As expected, very few unstimulated Jurkat T-cells expressed CD25 in either high or low-density conditions (less than 1%) while after PMA + I stimulation many more T-cells expressed CD25. PMA + I stimulation led to a significant increase in expression in both low-density (14.9 ± 0.9%) as well as in high-density (23.4 ± 0.8%) conditions compared to PBS or EchNWA alone. Differences between high-density and low-density T-cell response to PMA were significant (overall variance p < 0.001 by 1-way ANOVA, p < 0.001 by Tukey’s post test). EchNWA did not impact the percentage of T-cells expressing CD25 in high-density conditions. EchNWA reduced the percentage of T cells expressing CD25 in response to PMA + Ionomycin in low-density conditions (1-way ANOVA, p < 0.001 Tukey’s posttest). Supplementary Figs. S-1 and S-2 show representative individual flow cytometry plots for controls (S-1) and PMI + I activated cultures (S-2).
Fig. 5.
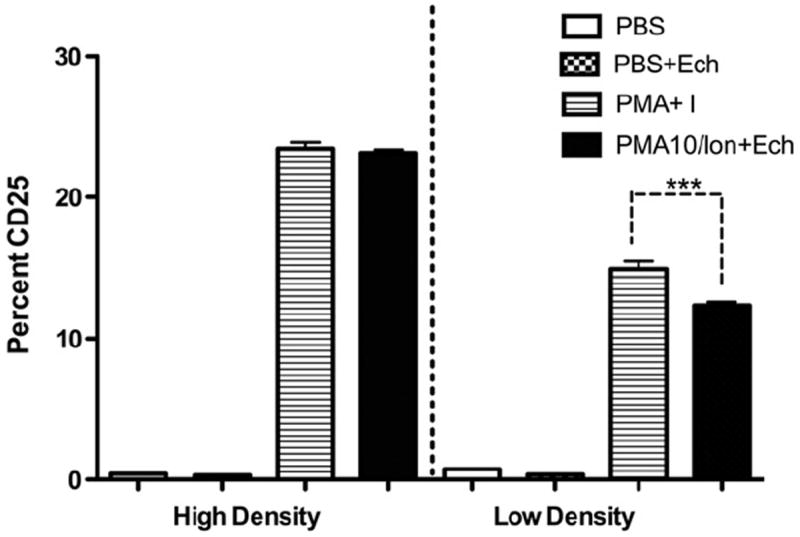
Effect of EchNWA on CD25 Expression in High and Low Cell Density Jurkat T-cells: EchNWA reduced the percentage of T-cells expressing CD25 in response to PMA + Ionomycin in low-density conditions (p < 0.0001 by 1-way ANOVA, Tukey’s posttest). This appears to correlate with the slight suppression of IL-2 and IFN-γ cytokine response when this dose of EchNWA was used to pretreat cell in Low-density cultures before PMA + Ionomycin stimulation. CD25 up regulation in response to PMA without EchNWA at 100 μg/mL was greater in the high density compared to the low-density conditions (p < 0.0001 by 1-way ANOVA, Tukey’s posttest) and did increase in response to EchNWA.
3.4. Cytokine response after pretreatment with phenylpropanoids
In order to establish whether the presence of cichoric and caftaric acids in the EchNWA extract (1.2% w/w and 0.4% w/w respectively) could be responsible for the modulation of IL-2 and IFN-γ in Jurkat T-cells, commercially available reference compounds were tested at different concentrations, including at doses matching those in our extract (0.12 g/mL; 1.2 g/mL and 12 μg/mL for cichoric acid and 0.4 μg/mL and 4 μg/mL for caftaric acid). Neither cichoric nor caftaric acids showed any modulatory effect on IL-2 or IFN-γ response when used to pretreat T-cells that had been cultured at high or low concentration. Data are not shown.
4. Discussion
Chemical analysis of Echinacea plant extracts has identified major components that may have medical potential including polysaccharides, flavonoids, caffeic acid derivatives, essential oils, polyacetylenes, and alkylamides. The objective of this study was to analyze the chemical constituents of a widely available neutral and weak acidic extract obtained from E. purpurea (EchNWA) and then to evaluate immune modulatory effects on human T-cells. Aerial parts of E. purpurea are known to contain alkylamides, such as caffeic acid derivatives, polysaccharides, glycoproteins and polyacetylines, among others [2]. As shown here, EchNWA extract was found to be rich in polysaccharides, predominantly a 10 kDa entity, but also contained 44% of molecules smaller than 2000 Da. E. purpurea extracts are known to have immunoactive small molecules such as alkylamides and cinnamic acid derivatives [32-34]. HPLC-PDA fingerprint analysis was performed to determine if these entities were present. Chromatographic analyses showed several peaks identifying relatively polar small molecules. The phenylpropanoidic moiety of cinnamoyl conjugates contributes significantly to UV absorption, and thus their spectra usually correspond to the free cinnamic acid, where the long wave-band of caffeoyl conjugates are generally recorded around 325–335 nm and may have characteristic absorption bands at 243 and 326 nm, with a distinctive shoulder at 300 nm to the long wave-band [35]. UV spectra of all the resolved peaks showed absorption characteristic of phenylpropanoids, with a long wave band around 325–335 nm and distinctive shoulder at 300 nm demonstrating that these compounds are phenolic derivatives. Some of the peaks coeluted with purified standards of cichoric and caftaric acids and cynarin. This study clearly shows that none of the spectra for the EchNWA extract revealed absorption bands characteristic of alkylamides. The absence of alkylamides was further indicated by comparing chromatographic analysis of the extract with a purified sample of (2E)-N-isobutylundeca-2-ene-8,10-diynamide, a typical alkylamide present in E. purpurea extracts. Analysis showed that this particular alkylamide did not co-elute with any chromatographic peak, and had other alkylamides been present in the extract preparation, they would have eluted in a similar chromatographic region to this reference standard. Since a small percentage of amino acids (3%), was detected, it is also likely that EchNWA contains oligoproteins. Therefore the EchNWA extract used in the biological experiments described here is composed mainly of large polysaccharides, the major one with 10 kDa and small phenolic compounds, including cynarin (0.03% w/w) and cichoric (1.2%w/w) and caftaric (0.4%w/w) acids, but no detectable alkylamides.
Polysaccharides have been considered as the primary component of Echinacea that are likely to have immune enhancing effects by experts [36,37], but delineation of specific constituents and corresponding immune activity has been limited to the work of a few groups [37]. Polysaccharides (heteroglycans) isolated from E. purpurea, such as arabinogalactan have shown activating effects on macrophages leading to cytokine production and cytotoxic effects against microorganisms and tumor cells in vitro [37,38]. Proksch and Wagner identified a 4-O-methyl-glucuronoarabinoxylan from E. purpurea that enhanced granulocyte phagocytosis in vitro [39]. A polysaccharide fraction from E. angustifolia that reduced edema of inflammation when applied topically was subsequently further characterized by Cozzolino et al. but immune studies have not been done [40]. Recently Morrazzoni et al. have reported on the immune-stimulating effects of a standardized E. angustifolia root extract (Polinacea) containing echinoside, high molecular weight polysaccharide, weight that enhanced T-cell IFN-γ production in murine T-lymphocyte cell cultures stimulated by anti-CD3 and reduced mortality in both normal and cyclosporine A-treated mice infected with Candida albicans [41].
A major goal of present studies was to examine the immunomodulatory effects of EchNWA on T-cell cytokine responses relevant to host defense against infection. Although current studies support the concept that Echinacea species have activity against infections associated with the common cold, little is known about the mechanisms of action including the basis of anti-inflammatory effects, potential for direct immune stimulating activity activator, or adjuvant activity. Members of our group have recently shown that treatment of mice with the same E. purpurea aerial polysaccharide extract used here was protective against morbidity in an established mouse model of live H1N1 influenza A [17]. Mice demonstrated less weight loss, lower systemic and pulmonary levels of the mouse analog to human IL-8 (KC), lower IL-10 levels and lower systemic IFN-gamma levels following influenza exposure after receiving EchNWA compared to controls. No direct anti-viral activity was observed. The studies described here provide the first evidence that EchNWA polysaccharides have adjuvant effects on human T cell cytokine response but do not directly activate T cells. In vivo confirmation of these results would be the next step to support future studies of EchNWA as a vaccine adjuvant in humans. Others have shown that alkylamide extracts given in tablet form do reach the blood stream, supporting in vivo relevance [42]. Another recent study has shown that Echinacea polysaccharide was a strong adjuvant for the stimulation of accelerated and enhanced effects on rabies-neutralizing antibody responses in mice and dogs [43].
We reported here that the immunomodulatory effects of EchNWA are strongly influenced by T-cell density, which may explain some of the variability in previous studies of Echinacea. These effects were not related to viability since cell viability was not affected by culture density in the range used here. EchNWA showed highly significant enhancing effects when T cells were sub-optimally responsive to stimulation due to high-density conditions that could be analogous to physiological inflammation and leukocytosis during infection. A limitation of the studies is that we did not assess the time course of optimal response in both culture conditions, which might have been shifted such that higher response would have occurred later under high-density conditions.
The human Jurkat leukemic T-cell line is one of the most widely used cellular systems for the study of cellular immune responses that require T-cell receptor signaling. Many key advances in T-cell biology were made using these cells [44] including the effects of Echinacea alkylamides on PMA stimulation of the NFκB pathway bioactivity and on IL-2 production [32-34]. Matthias et al. showed that the addition of Echinacea alkylamides and cichoric acid had concentration-dependent effects on NFκB expression after PMA stimulation of Jurkat T-cells. Cichoric acid and a 2,4-diene alkylamide induced higher NFκB levels, whereas a 2-ene alkylamide caused a significant inhibition [33]. Sasagawa et al. demonstrated that alkylamides in ethanolic extracts prepared from dried aerial parts of E. purpurea reduced IL-2 production of Jurkat T-cells that were sub maximally stimulated to produce half the amount of IL-2 that could be elicited with a higher dose of PHA in the presence of a fixed amount of PMA. Concentrations of their extract at 50 or 100 μg/mL were cytotoxic to Jurkat cells in contrast to EchNWA used here which had no effects on T cell viability at these doses [34]. Dong et al. reported that cynarin down-regulated CD28-dependent IL-2 production in T-cells and later showed that this was likely to occur in the “G-pocket” of CD28 and therefore to act by interrupting the site of interaction between CD28 and CD80 [32,45].
The mechanism whereby Echinacea alkylamides inhibit production of inflammatory mediators produced by activated phagocytic cells can involve binding to the endocannabinoid receptor CB2 on many immune cells including primary human monocytes/macrophages, and dendritic cells [46]. Alkylamides from E. purpurea were shown to have weak inhibitory effects on cytokine production in PMA and T cell receptor-stimulated T-cells and on human PBMC response to LPS response. The mechanism of action involved modulatory effects on calcium mobilization triggered by PMA rather than T cell receptor activation and was partly independent of CB2 [47]. Alkylamides from Echinacea species which lack affinity for the CB2 receptor, use the peroxisome proliferator activated receptor gamma (PPARγ) [48]. Recent studies have shown that PPARgamma is involved in alkylamide inhibition of T cell IL-2 response [48]. Few investigators have examined the mechanism of Echinacea polysaccharide action on the immune system and very few have undertaken comparative studies. Benson et al. have shown that the polysaccharide-rich root extract increased the expression of MHC class II, CD86, and CD54 while the alkylamide-rich leaf extract inhibited expression of both [21]. Thude et al. using flow cytometry showed that a polyclonal antibody to a glycoprotein, arabinogalactan-protein, from E. purpurea not only bound leukocytes extensively and non- specifically, but also did not interact with either CD4 or CD8 receptors [49].
Echinacea extracts also modify the bioactivity of other immune cells such as dendritic cells (DC) and B cells according to which plant tissue is extracted. Wang et al. reported that alkylamide-rich root extracts promote maturation of human DCs as shown by enhanced expression of CD83 [50]. Yin et al. have recently reported that the butanol fraction of E. purpurea stem and leaf extracts did not promote maturation of mouse bone marrow-derived DC’s but increased expression of cell adhesion or motility genes in association with increased migration in vivo [51] indicating the potential importance of species differences. Both glycerin and alcohol extracts of Echinacea enhanced the T cell dependent antibody response to immunization with sheep red blood cells in mice [52,53] and increased splenic T production of IL-4 and IL-10 suggesting that Echinacea stimulation of both Th-2 and Th-1 cytokine production improved B cell function increasing antibody formation [53].
Both immune stimulation and immune suppression are relevant to the putative benefits of Echinacea in infection [18,20,54]. Different components of Echinacea are likely responsible for suppressive or enhancing effects. Lalone et al. found that combining an alkylamide with a ketene from E. angustifolia led to a greater anti-inflammatory effect on LPS induced PGE2 production suggesting that interaction of different constituents influences bioactivity [24]. Recent studies reported by Cech et al. show that purified alkylamides from E. purpurea suppressed production of TNF-α and/or other cytokines and chemokines and PGE2 in RAW 264.7 macrophage cells infected with influenza A according to the concentration and structural type of the alkamides. In contrast the activity of extracts from the roots of indigenously growing Echinacea plants did not correlate with concentration of the same alkylamides, suggesting that the presence of diverse alkylamides with differing bioactivity or admixture other masking components in the crude extract [55]. The investigators noted that the cytokine enhancing activity of some crude extracts was lost from supernatants after ethanol precipitation. This observation generally supports our findings that enhancing activity was associated with the water-soluble polysaccharides. The immune-enhancing activity of alkylamide-rich extracts from some preparations of dried Echinacea roots for monocyte/macrophages has been shown to be substantially reduced by destruction of bacterial lipoproteins and lipopolysaccharides, but this treatment did not eliminate the enhancing activity from aerial parts [56]. EchNWA, as described here, is made from fresh aerial parts and does not contain any significant amount of LPS. The activity of the polysaccharide component in EchNWA appears to be the principal source of T cell enhancement in our studies.
As shown here, production of IL-2 and IFN-γ in Jurkat T-cells stimulated with PMA + I was strongly influenced by cell density. A similar effect was described for Jurkat T-cells stimulated with PHA [57]. PMA activates Jurkat T cells and stimulates both IL-2 and IFN-γ through a common pathway that requires up regulation of the high-affinity IL-2R corresponding to a complex composed of three distinct IL-2 binding subunits (α,β,γ) including the 55-kDa IL-2Rα subunit (IL-2Rα), also called CD25 or Tac antigen (5); all are required for activation and proliferation. PMA upregulates the activator protein AP-1 in vitro and this effect is somewhat enhanced in the presence of the calcium ionophore, ionomycin. PMA initially triggers and then down regulates NFκB in Jurkat T cells while ionomycin blocks this inhibition thus attenuating PMA suppression [58]. PMA has recently been shown to act through effects on protein kinase C, PKC, that lead to stabilization of IL-2mRNA [59]. In our studies EchNWA showed adjuvant activity on T-cell activation mediated by a soluble activator PMA and enhanced the effect of the calcium ionophore (ionomycin), while pre-treatment with EchNWA extract alone did not stimulate production of IL-2 and IFN-γ. EchNWA strongly enhanced both IL-2 and IFN-γ production in high-density conditions where cytokine production was substantially lower compared to lower density conditions. Conversely the mild but significant reducing effect of EchNWA pretreatment on IFN-γ production and on the relative number of cells expressing CD25 after exposure to PMA + I in low-density T-cell cultures may reflect time dependent effects on T cell activation and cytokine production. Others have shown that in vivo administration of E. purpurea lead to an increased percentage of circulating T cells expressing CD25 [60].
Cichoric acid and other phenylpropanoids have been reported to enhance IL-2 and NFκB production in Jurkat T-cells [32-34]. However in this study, neither cichoric nor caftaric acid showed effects on IL-2 or IFN-γ response when used to pre-treat Jurkat T-cells at any concentration. Since the EchNWA extract solubilized in water contained no detectable alkylamides, it is likely that only the polysaccharide fraction was responsible for the immune modulatory effects described here. The observation that EchNWA enhanced T-cell response to ionomycin in the high-density condition may be relevant to the mechanism of enhancement, indicating a possible role for EchNWA in enhanced Ca2+ mobilization and T cell activation.
In summary, our studies showed that an E. purpurea preparation composed mainly of not only polysaccharides, but also phenylpropanoids in a relatively significant amount, and exerted immunomodulatory effects on human T lymphocytes as an adjuvant. Immunomodulation is dependent on the dose of EchNWA as well as the cell density of the target cell. Due to the extensive use of Echinacea preparations and its complex mechanism of action, further studies are warranted to determine the immunological and pharmacologic potential of Echinacea’s polysaccharides.
Supplementary Material
Acknowledgments
The authors acknowledge with gratitude the expert guidance and support of our esteemed colleague, the late Dr. David Y. Gin (Sloan Kettering Institute, Molecular Pharmacology & Chemistry Laboratory).
Footnotes
Support: The studies were supported in part by NIH NCCAM and ODS: 1-P50-AT02779 Botanical Research Center for Botanical Immunomodulators, NIH NCI Cancer Education and Career Development R25 CA105012: Nutrition and Cancer Prevention and the Children’s Cancer and Blood Foundation.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.intimp.2013.12.019.
References
- 1.Bardia A, Nisly NL, Zimmerman MB, Gryzlak BM, Wallace RB. Use of herbs among adults based on evidence-based indications: findings from the National Health Interview Survey. Mayo Clin Proc. 2007;82:561–6. doi: 10.4065/82.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes J, Anderson LA, Gibbons S, Phillipson JD. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): a review of their chemistry, pharmacology and clinical properties. J Pharm Pharmacol. 2005;57:929–54. doi: 10.1211/0022357056127. [DOI] [PubMed] [Google Scholar]
- 3.Caruso TJ, Gwaltney JM., Jr Treatment of the common cold with Echinacea: a structured review. Clin Infect Dis. 2005;40:807–10. doi: 10.1086/428061. [DOI] [PubMed] [Google Scholar]
- 4.Shah SA, Sander S, White CM, Rinaldi M, Coleman CI. Evaluation of Echinacea for the prevention and treatment of the common cold: a meta-analysis. Lancet Infect Dis. 2007;7:473–80. doi: 10.1016/S1473-3099(07)70160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linde K, Barrett B, Wolkart K, Bauer R, Melchart D. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD000530.pub2. CD000530. [DOI] [PubMed] [Google Scholar]
- 6.Woelkart K, Linde K, Bauer R. Echinacea for preventing and treating the common cold. Planta Med. 2008;74:633–7. doi: 10.1055/s-2007-993766. [DOI] [PubMed] [Google Scholar]
- 7.Schoop R, Klein P, Suter A, Johnston SL. Echinacea in the prevention of induced rhinovirus colds: a meta-analysis. Clin Ther. 2006;28:174–83. doi: 10.1016/j.clinthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Turner RB, Bauer R, Woelkart K, Hulsey TC, Gangemi JD. An evaluation of Echinacea angustifolia in experimental rhinovirus infections. N Engl J Med. 2005;353:341–8. doi: 10.1056/NEJMoa044441. [DOI] [PubMed] [Google Scholar]
- 9.Pleschka S, Stein M, Schoop R, Hudson JB. Anti-viral properties andmode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV) Virol J. 2009;6:197. doi: 10.1186/1743-422X-6-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binns SE, Hudson J, Merali S, Arnason JT. Antiviral activity of characterized extracts from Echinacea spp. (Heliantheae: Asteraceae) against herpes simplex virus (HSV-I) Planta Med. 2002;68:780–3. doi: 10.1055/s-2002-34397. [DOI] [PubMed] [Google Scholar]
- 11.Birt DF, Widrlechner MP, Lalone CA, Wu L, Bae J, Solco AK, et al. Echinacea in infection. Am J Clin Nutr. 2008;87:488S–92S. doi: 10.1093/ajcn/87.2.488S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binns SE, Livesey JF, Arnason JT, Baum BR. Phytochemical variation in Echinacea from roots and flowerheads of wild and cultivated populations. J Agric Food Chem. 2002;50:3673–87. doi: 10.1021/jf011439t. [DOI] [PubMed] [Google Scholar]
- 13.Woelkart K, Bauer R. The role of alkamides as an active principle of Echinacea. Planta Med. 2007;73:615–23. doi: 10.1055/s-2007-981531. [DOI] [PubMed] [Google Scholar]
- 14.Cech NB, Tutor K, Doty BA, Spelman K, Sasagawa M, Raner GM, et al. Liver enzymemediated oxidation of Echinacea purpurea alkylamides: production of novel metabolites and changes in immunomodulatory activity. Planta Med. 2006;72:1372–7. doi: 10.1055/s-2006-951718. [DOI] [PubMed] [Google Scholar]
- 15.Ramasahayam S, Baraka HN, Abdel Bar FM, Abuasal BS, Widrlechner MP, El Sayed KA, et al. Effects of Chemically Characterized Fractions fromAerial Parts of Echinacea purpurea and E. angustifolia on Myelopoiesis in Rats. Planta Med. 2011;77:1883–9. doi: 10.1055/s-0031-1279990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woelkart K, Koidl C, Grisold A, Gangemi JD, Turner RB, Marth E, et al. Bioavailability and pharmacokinetics of alkamides from the roots of Echinacea angustifolia in humans. J Clin Pharmacol. 2005;45:683–9. doi: 10.1177/0091270004273493. [DOI] [PubMed] [Google Scholar]
- 17.Fusco D, Liu X, Savage C, Taur Y, Xiao W, Kennelly E, et al. Echinacea purpurea aerial extract alters course of influenza infection in mice. Vaccine. 2010;28:3956–62. doi: 10.1016/j.vaccine.2010.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma M, Arnason JT, Burt A, Hudson JB. Echinacea extracts modulate the pattern of chemokine and cytokine secretion in rhinovirus-infected and uninfected epithelial cells. Phytother Res. 2006;20:147–52. doi: 10.1002/ptr.1824. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan AM, Laba JG, Moore JA, Lee TD. Echinacea-induced macrophage activation. Immunopharmacol Immunotoxicol. 2008;30:553–74. doi: 10.1080/08923970802135534. [DOI] [PubMed] [Google Scholar]
- 20.Altamirano-Dimas M, Hudson JB, Cochrane D, Nelson C, Arnason JT. Modulation of immune response gene expression by Echinacea extracts: results of a gene array analysis. Can J Physiol Pharmacol. 2007;85:1091–8. doi: 10.1139/Y07-110. [DOI] [PubMed] [Google Scholar]
- 21.Benson JM, Pokorny AJ, Rhule A, Wenner CA, Kandhi V, Cech NB, et al. Echinacea purpurea extracts modulate murine dendritic cell fate and function. Food Chem Toxicol. 2010;48:1170–7. doi: 10.1016/j.fct.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaLone CA, Rizshsky L, Hammer KD, Wu L, Solco AK, Yum M, et al. Endogenous levels of Echinacea alkylamides and ketones are important contributors to the inhibition of prostaglandin E2 and nitric oxide production in cultured macrophages. J Agric Food Chem. 2009;57:8820–30. doi: 10.1021/jf901202y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou CC, Huang CC, Shyur LF. Echinacea alkamides prevent lipopolysaccharide/D-galactosamine-induced acute hepatic injury through JNK pathway-mediated HO-1 expression. J Agric Food Chem. 2011;59:11966–74. doi: 10.1021/jf202958r. [DOI] [PubMed] [Google Scholar]
- 24.LaLone CA, Huang N, Rizshsky L, Yum L, Singh N, Hauck C, et al. Enrichment of Echinacea angustifolia with Bauer alkylamide 11 and Bauer ketone 23 increased anti-inflammatory potential through interference with cox-2 enzyme activity. J Agric Food Chem. 2009;58:8573–84. doi: 10.1021/jf1014268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roesler J, Steinmuller C, Kiderlen A, Emmendorffer A, Wagner H, Lohmann-Matthes ML. Application of purified polysaccharides from cell cultures of the plant Echinacea purpurea to mice mediates protection against systemic infections with Listeria monocytogenes and Candida albicans. Int J Immunopharmacol. 1991;13:27–37. doi: 10.1016/0192-0561(91)90022-y. [DOI] [PubMed] [Google Scholar]
- 26.Steinmuller C, Roesler J, Grottrup E, Franke G, Wagner H, Lohmann-Matthes ML. Polysaccharides isolated from plant cell cultures of Echinacea purpurea enhance the resistance of immunosuppressed mice against systemic infections with Candida albicans and Listeria monocytogenes. Int J Immunopharmacol. 1993;15:605–14. doi: 10.1016/0192-0561(93)90078-d. [DOI] [PubMed] [Google Scholar]
- 27.Stevenson LM, Matthias A, Banbury L, Penman KG, Bone KM, Leach DL, et al. Modulation of macrophage immune responses by Echinacea. Molecules. 2005;10:1279–85. doi: 10.3390/10101279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaLone CA, Hammer KD, Wu L, Bae J, Leyva N, Liu Y, et al. Echinacea species and alkamides inhibit prostaglandin E(2) production in RAW264.7 mouse macrophage cells. J Agric Food Chem. 2007;55:7314–22. doi: 10.1021/jf063711a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yue GG, Chan BC, Hon PM, Kennelly EJ, Yeung SK, Cassileth BR, et al. Immunostimulatory activities of polysaccharide extract isolated from Curcuma longa. Int J Biol Macromol. 2010;47:342–7. doi: 10.1016/j.ijbiomac.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox J, DaR JF. Miniaturization of three carbohydrate analyses using a microsample plate reader miniaturization of three carbohydrate analyses using a microsample plate reader. Anal Chem. 1991;195:09–96. doi: 10.1016/0003-2697(91)90300-i. [DOI] [PubMed] [Google Scholar]
- 31.Zheng J, Gu YJ, Yan SP, Ma LG, Mu DH, Li GX. Pre-column derivatization and RP-HPLC determination of free amino acids in plasma and its application in inborn aminoacidopathies screening. J Instrum Anal. 2005;24:22–5. [Google Scholar]
- 32.Dong GC, Chuang PH, Forrest MD, Lin YC, Chen HM. Immuno-suppressive effect of blocking the CD28 signaling pathway in T-cells by an active component of Echinacea found by a novel pharmaceutical screening method. J Med Chem. 2006;49:1845–54. doi: 10.1021/jm0509039. [DOI] [PubMed] [Google Scholar]
- 33.Matthias A, Banbury L, Bone KM, Leach DN, Lehmann RP. Echinacea alkylamides modulate induced immune responses in T-cells. Fitoterapia. 2008;79:53–8. doi: 10.1016/j.fitote.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Sasagawa M, Cech NB, Gray DE, Elmer GW, Wenner CA. Echinacea alkylamides inhibit interleukin-2 production by Jurkat T cells. Int Immunopharmacol. 2006;6:1214–21. doi: 10.1016/j.intimp.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Ibrahim R, Barron D. Plant phenolics. In: Harborne JB, editor. Methods in Plant Biochemistry. London, IK: Academic Press; 1989. p. 75. [Google Scholar]
- 36.Wagner H, Jurcic K. Immunological studies of Revitonil, a phytopharmaceutical containing Echinacea purpurea and Glycyrrhiza glabra root extract. Phytomedicine. 2002;9:390–7. doi: 10.1078/09447110260571616. [DOI] [PubMed] [Google Scholar]
- 37.Melchart D, Clemm C, Weber B, Draczynski T, Worku F, Linde K, et al. Polysaccharides isolated from Echinacea purpurea herba cell cultures to counteract undesired effects of chemotherapy–a pilot study. Phytother Res. 2002;16:138–42. doi: 10.1002/ptr.888. [DOI] [PubMed] [Google Scholar]
- 38.Luettig B, Steinmuller C, Gifford GE, Wagner H, Lohmann-Matthes ML. Macrophage activation by the polysaccharide arabinogalactan isolated from plant cell cultures of Echinacea purpurea. J Natl Cancer Inst. 1989;81:669–75. doi: 10.1093/jnci/81.9.669. [DOI] [PubMed] [Google Scholar]
- 39.Proksch A, Wagner H. Structural analysis of a 4-0-methyl-glucuronoarabinoxylan with immune-stimulating activity from Echinacea purpurea. Phytochemistry. 1987;26:1989–93. [Google Scholar]
- 40.Cozzolino R, Malvagna P, Spina E, Giori A, Fuzzata N, Anelli A, et al. Structural analysis of the polysaccharides from Echinacea angustifolia radix. Carbohydr Polym. 2006;65:263–72. [Google Scholar]
- 41.Morazzoni P, Cristoni A, Di Pierro F, Avanzini C, Ravarino D, Stornello S, et al. In vitro and in vivo immune stimulating effects of a new standardized Echinacea angustifolia root extract (Polinacea) Fitoterapia. 2005;76:401–11. doi: 10.1016/j.fitote.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Matthias A, Penman KG, Matovic NJ, Bone KM, De Voss JJ, Lehmann RP. Bioavailability of Echinacea constituents: Caco-2 monolayers and pharmacokinetics of the alkylamides and caffeic acid conjugates. Molecules. 2005;10:1242–51. doi: 10.3390/10101242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Zhang S, Zhang F, Hu R. Adjuvant activity of Chinese herbal polysaccharides in inactivated veterinary rabies vaccines. Int J Biol Macromol. 2012;50:598–602. doi: 10.1016/j.ijbiomac.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 44.Harborne JB. In: Advances in Flavonoid Research since 1986. Harborne JB, editor. London, UK: Chapman and Hall; 1994. p. 589. [Google Scholar]
- 45.Dong GC, Chuang PH, Chang KC, Jan PS, Hwang PI, Wu HB, et al. Blocking effect of an immuno-suppressive agent, cynarin, on CD28 of T-cell receptor. Pharm Res. 2009;26:375–81. doi: 10.1007/s11095-008-9754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gertsch J. Immunomodulatory lipids in plants: plant fatty acid amides and the human endocannabinoid system. Planta Med. 2008;74:638–50. doi: 10.1055/s-2008-1034302. [DOI] [PubMed] [Google Scholar]
- 47.Raduner S, Majewska A, Chen JZ, Xie XQ, Hamon J, Faller B, et al. Alkylamides from Echinacea are a new class of cannabinomimetics. Cannabinoid type 2 receptor-dependent and -independent immunomodulatory effects. J Biol Chem. 2006;281:14192–206. doi: 10.1074/jbc.M601074200. [DOI] [PubMed] [Google Scholar]
- 48.Spelman K, Iiams-Hauser K, Cech NB, Taylor EW, Smirnoff N, Wenner CA. Role for PPARgamma in IL-2 inhibition in T cells by Echinacea-derived undeca-2E-ene-8,10-diynoic acid isobutylamide. Int Immunopharmacol. 2009;9:1260–4. doi: 10.1016/j.intimp.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Thude S, Classen B, Blaschek W, Barz D, Thude H. Binding studies of an arabinogalactan-protein from Echinacea purpurea to leucocytes. Phytomedicine. 2006;13:425–7. doi: 10.1016/j.phymed.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 50.Wang CY, Chiao MT, Yen PJ, Huang WC, Hou CC, Chien SC, et al. Modulatory effects of Echinacea purpurea extracts on human dendritic cells: a cell- and gene-based study. Genomics. 2006;88:801–8. doi: 10.1016/j.ygeno.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 51.Yin SY, Wang WH, Wang BX, Aravindaram K, Hwang PI, Wu HM, et al. Stimulatory effect of Echinacea purpurea extract on the trafficking activity of mouse dendritic cells: revealed by genomic and proteomic analyses. BMC Genomics. 2010;11:612. doi: 10.1186/1471-2164-11-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freier DO, Wright K, Klein K, Voll D, Dabiri K, Cosulich K, et al. Enhancement of the humoral immune response by Echinacea purpurea in female Swiss mice. Immunopharmacol Immunotoxicol. 2003;25:551–60. doi: 10.1081/iph-120026440. [DOI] [PubMed] [Google Scholar]
- 53.Zhai Z, Liu Y, Wu L, Senchina DS, Wurtele ES, Murphy PA, et al. Enhancement of innate and adaptive immune functions by multiple Echinacea species. J Med Food. 2007;10:423–34. doi: 10.1089/jmf.2006.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chicca A, Raduner S, Pellati F, Strompen T, Altmann KH, Schoop R, et al. Synergistic immunomopharmacological effects of N-alkylamides in Echinacea purpurea herbal extracts. Int Immunopharmacol. 2009;9:850–8. doi: 10.1016/j.intimp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Cech NB, Kandhi V, Davis JM, Hamilton A, Eads D, Laster SM. Echinacea and its alkylamides: effects on the influenza A-induced secretion of cytokines, chemokines, and PGE from RAW 264.7 macrophage-like cells. Int Immunopharmacol. 2010;10:1268–78. doi: 10.1016/j.intimp.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 56.Tamta H, Pugh ND, Balachandran P, Moraes R, Sumiyanto J, Pasco DS. Variability in in vitro macrophage activation by commercially diverse bulk Echinacea plant material is predominantly due to bacterial lipoproteins and lipopolysaccharides. J Agric Food Chem. 2008;56:10552–6. doi: 10.1021/jf8023722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gillis S, Watson J. Biochemical and biological characterization of lymphocyte regulatory molecules. V. Identification of an interleukin 2-producing human leukemia T cell line. J Exp Med. 1980;152:1709–19. doi: 10.1084/jem.152.6.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khalaf H, Jass J, Olsson PE. Differential cytokine regulation by NF-kappaB and AP-1 in Jurkat T-cells. BMC Immunol. 2010;11:26. doi: 10.1186/1471-2172-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu P, Jiang W, Cao L, Yu W, Pei Y, Yang X, et al. IL-2 mRNA stabilization upon PMA stimulation is dependent on NF90-Ser647 phosphorylation by protein kinase CbetaI. J Immunol. 2010;185:5140–9. doi: 10.4049/jimmunol.1000849. [DOI] [PubMed] [Google Scholar]
- 60.Zwickey H, Brush J, Iacullo CM, Connelly E, Gregory WL, Soumyanath A, et al. The effect of Echinacea purpurea, Astragalus membranaceus and Glycyrrhiza glabra on CD25 expression in humans: a pilot study. Phytother Res. 2007;21:1109–12. doi: 10.1002/ptr.2207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


