Abstract
Most riboswitches are metabolite-binding RNA structures located in bacterial messenger RNAs where they control gene expression. We have discovered a riboswitch class in many bacterial and archaeal species whose members are selectively triggered by fluoride but reject other small anions, including chloride. These fluoride riboswitches activate expression of genes that encode putative fluoride transporters, enzymes that are known to be inhibited by fluoride, and additional proteins of unknown function. Our findings indicate that most organisms are naturally exposed to toxic levels of fluoride and that many species use fluoride-sensing RNAs to control the expression of proteins that alleviate the deleterious effects of this anion.
Fluoride has been widely used as an additive in oral hygiene products and water since the 1950s because of its usefulness in preventing tooth decay. Fluoride ions present at millimolar concentrations in bacterial culture media inhibit cell growth (1–3), and this has been proposed as one of the mechanisms for its efficacy (4–7). However, little has been reported on how organisms respond to toxic levels of fluoride.
We identified a fluoride-responsive riboswitch class by analyzing a group of noncoding RNA structures that carry a conserved domain called the crcB motif (Fig. 1A) (8, 9). crcB motif RNAs are located upstream of genes encoding proteins of diverse functions and presumably regulate these genes. Some of the gene products are annotated as ion transporters (for example, chloride, sodium, proton) and some others are involved in various physiological (e.g., universal stress adaptation, DNA repair) or metabolic (e.g., enolase, formate-hydrogen lyase) processes (fig. S1).
Fig. 1.
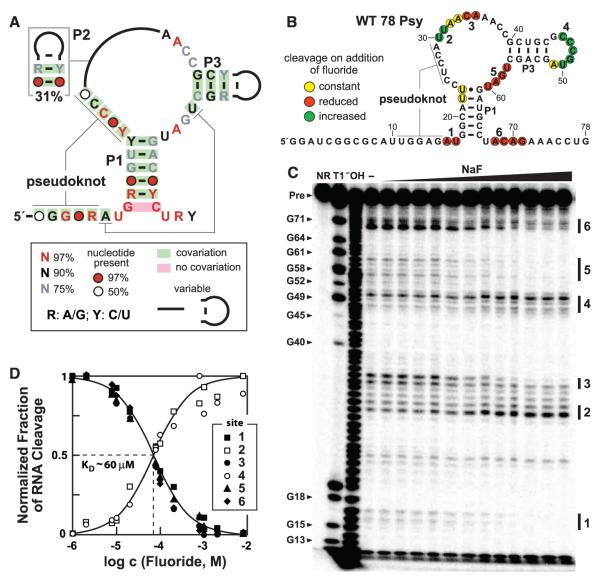
Fluoride binding by crcB motif RNAs. (A) Consensus sequence and structural model for crcB RNAs based on 2188 representatives from bacterial and archaeal species. P1, P2, P3 and pseudoknot labels identify extended base-paired substructures. (B) Sequence and secondary structure model for the WT 78 Psy RNA from P. syringae. Colored circles summarize the in-line probing results presented in (C). The two G residues preceding nucleotide 1 were added to facilitate RNA production. (C) Polyacrylamide gel electrophoresis analysis of an in-line probing assay with 78 Psy RNA and various amounts of fluoride. NR, T1, and −OH designate no reaction, partial digestion with RNase T1 (cleaves after guanosines), or partial digestion with hydroxide ions (cleaves after any nucleotide), respectively. Precursor RNA (Pre) band and some RNase T1 product bands are labeled (left). Locations of fluoride-mediated spontaneous RNA cleavage suppression (regions 1, 3, 5, 6) and enhancement (regions 2, 4) are identified by vertical bars. (D) Plot of the normalized fraction of RNA cleavage versus fluoride ion concentration from the data in (C). Curves represent those expected for one-to-one binding with a KD of 60 μM.
We used a method called in-line probing (10, 11) to assess the binding of various possible metabolites, which unexpectedly revealed that fluoride contaminating a commercial compound sample caused extensive conformational changes in crcB RNAs (fig. S2). Using a 78-nucleotide RNA called 78 Psy that encompasses the crcB motif from Pseudomonas syringae (Fig. 1B and table S1), we determined that the most highly conserved nucleotides of this RNA class undergo structural change on addition of NaF (Fig. 1C), which suggests that these nucleotides help form a ligand-binding aptamer for fluoride. The pattern of products resulting from in-line probing at various fluoride concentrations reveals an apparent dissociation constant (KD) of ~60 μM (Fig. 1D). We obtained similar KD values with crcB motif RNAs from other organisms (fig. S3).
Ca2+ forms a strong complex with fluoride ions (12), and the addition of Ca2+ in excess over fluoride precludes RNA structure changes (fig. S4). This demonstrates that free fluoride is important for binding by the RNA. The 78 Psy RNA rejects other halogen anions (chloride, bromide, iodide), even when they are added at high concentrations (fig. S5). Likewise, hydroxide ions (up to pH 9.7) and carbon monoxide and nitric oxide as dissolved gasses do not induce RNA folding changes.
Mutations that alter the aptamer’s conserved substructures (fig. S6) or conserved nucleotides (fig. S7) adversely affect fluoride binding, indicating that the features common to all crcB motif RNAs are necessary for the selective recognition of fluoride. Certain RNAs are known to directly bind chloride ions (13), and therefore it seems conceivable that RNA could form a fluoride-specific pocket without the use of a cofactor. Selectivity could be based on the ionic radius of fluoride (0.133 nm), which is smaller than those of other chemical species tested, including chloride (0.181 nm); also, fluoride has unique hydrogen-bonding abilities (9). Alternatively, the polyanionic crcB motif RNAs may exploit one or more Mg2+ ions to form bridging contacts between anionic fluoride and nucleotides.
A reporter construct was created by joining a representative crcB motif RNA from the Bacillus cereus crcB gene to a lacZ gene (transcriptional fusion). The observed activation in B. subtilis of the reporter gene by fluoride (figs. S8 and S9) and additional in-line probing experiments (fig. S10) indicate that this representative is a fluoride-responsive riboswitch that operates by controlling the formation of an intrinsic transcription terminator stem.
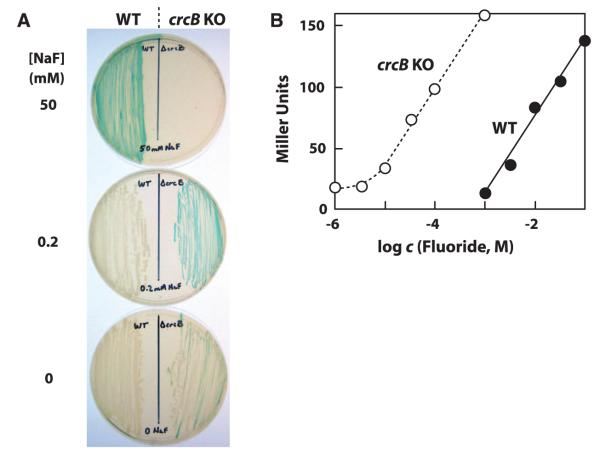
The homologous fluoride riboswitch from a P. syringae eriC gene was joined (translational fusion) to lacZ, and the plasmid-based construct was transformed into an Escherichia coli strain with its natural lacZ gene disabled. High expression occurred when cells were grown on a medium supplemented with 50 mM fluoride, whereas lower fluoride concentrations resulted in little or no expression (Fig. 2A, left side). Results of mutational analysis of this representative (fig. S11) also are consistent with our hypothesis that the crcB motif RNA from P. syringae is a fluoride-responsive riboswitch that controls gene expression by regulating translation initiation.
Fig. 2.
Fluoride riboswitch-mediated gene control. (A) Solid media cultures of WT E. coli cells or crcB KO E. coli cells transformed with a riboswitch reporter fusion construct carrying the P. syringae eriC fluoride riboswitch. (B) Plot of the β-galactosidase reporter activity versus fluoride concentration (c) in liquid media supporting growth of transformed E. coli cells [see (A)] as quantified using Miller assays. WT and crcB KO E. coli cells grown in media supplemented with 50 mM NaCl (no added fluoride) yielded 0.06 and 15.5 Miller units, respectively.
The crcB genes, which are most commonly associated with fluoride riboswitches, have previously been implicated in chromosome condensation and camphor resistance (14). However, crcB genes are predicted to code for membrane proteins (15) belonging to a superfamily that is predominantly composed of transporters (16). Therefore, we speculated that CrcB proteins might function as fluoride transporters to reduce cellular concentrations of this anion. An E. coli strain carrying a genetic knockout (KO) of its crcB gene could not grow at 50 mM fluoride and exhibited high reporter gene expression even at low (for example, 0.2 mM) fluoride concentrations (Fig. 2A, right side).
We recorded a series of growth curves for wild-type (WT) and KO cells at various fluoride concentrations in liquid media to assess whether there is a correlation between cell growth and reporter gene activity as fluoride concentrations in the medium are increased. For WT E. coli cells, growth was noticeably reduced at 30 mM NaF, and the minimum inhibitory concentration (MIC) for fluoride was ~200 mM (Fig. 3A and fig. S12A). In contrast, the growth of the E. coli crcB KO strain is inhibited by micromolar amounts of fluoride and exhibits an MIC of slightly higher than 1 mM (Fig. 3B and fig. S12A). Fluoride sensitivities of both strains are heightened under acidic conditions (fig. S12B) due to the increased membrane permeability of hydrogen fluoride.
Fig. 3.
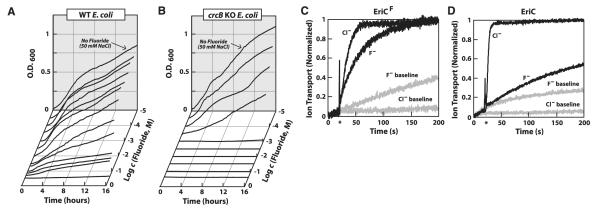
Evaluation of putative fluoride transport proteins. (A) Liquid cultures supplemented with specific amounts of NaF were inoculated with identical amounts of WT E. coli cells and the optical density (O.D.) at 600 nm was periodically recorded over a 16-hour period. (B) Growth curve plots for the E. coli crcB KO strain. (C) Anion efflux of an EriCF protein associated with the P. syringae fluoride riboswitch. Gray and black lines depict ion-transport measurements from liposomes in the absence or presence of protein, respectively. The high fluoride baseline is due to the relative membrane permeability of HF (pKa of 3.4) compared with chloride (pKa of −7). Asterisks in (C) and (D) identify the times at which protein-mediated anion transport is initiated. (D) Anion efflux by an EriC protein from E. coli that is known to serve as a chloride transporter.
A comparison of the growth curves (Fig. 3 and fig. S12) with the reporter gene expression driven by fluoride riboswitches (Fig. 2) indicates that reporter expression increases in proportion to the amount of fluoride in the culture media until the anion concentration becomes toxic to cells. Both growth inhibition (Fig. 3B) and reporter gene expression (Fig. 2B) phenotypes are similarly shifted to lower fluoride concentrations in crcB KO cells. These findings suggest that CrcB protein is important for reducing fluoride concentrations in cells, thus reducing its toxicity.
Another gene family commonly associated with fluoride riboswitches is a distinct subset of highly related eriC genes (hereafter called eriCF) coding for ClC-type ion channel proteins. The prototypic eriC representatives exhibit specificity for chloride (fig. S13) (17–19). However, EriCF protein homologs commonly associated with fluoride riboswitches carry a distinct set of amino acids in their putative channels compared with validated chloride-specific EriC proteins (fig. S14). This finding suggests that members of the EriCF subgroup could be channels for fluoride anions. Anion flux assays conducted with the EriCF protein from P. syringae reveal similar efficiency for chloride and fluoride transport (Fig. 3C), whereas a typical EriC protein from E. coli greatly favors chloride over fluoride (Fig. 3D).
We assessed the biological function of the EriCF variant from P. syringae by expressing the protein in the E. coli strain lacking the CrcB protein. Consistent with fluoride transport activity, the P. syringae eriCF gene rescues growth of the E. coli crcB KO strain to yield cells with restored resistance to high fluoride concentrations in both liquid and solid media (fig. S15). The functional equivalency of EriCF and CrcB proteins is likewise suggested by their distributions among bacterial species (fig. S16). The genes for these putative fluoride transport proteins are rarely observed in the same species under the control of fluoride riboswitches, suggesting that their biochemical roles may be identical.
crcB genes associated with fluoride riboswitches are distributed broadly among bacteria and archaea (fig. S16). Riboswitches are associated with genes for CrcB proteins that vary greatly in amino acid sequence (fig. S17), suggesting that all CrcB proteins might have the same function in mitigating fluoride toxicity. If true, a surprisingly large number of organisms are predicted to contend with fluoride toxicity, including eukaryotic lineages such as fungi and plants. Moreover, the bacterium Streptococcus mutans (a causative agent of dental caries) encodes EriCF proteins in the same genomic location where other Streptococcus species encode CrcB proteins (fig. S18). This arrangement again supports the hypothesis that the proteins are functionally equivalent and reveals the importance of fluoride toxicity resistance for S. mutans.
Though the vast majority of species encode at most 2 fluoride riboswitches, the bacterium Methylobacterium extorquens DM4 encodes at least 10 fluoride riboswitches in its genome. This organism, known for its ability to consume halogenated hydrocarbons as a food source (20), has been shown to survive on dichloromethane. The pertinent halogenase enzyme can catalyze the degradation of dibromomethane (21), suggesting that this organism also might degrade fluorinated hydrocarbons, which would generate fluoride anions and require a more robust fluoride sensor and toxicity mitigation response system for rapid growth on fluorinated food sources.
Our findings resolve a long-standing mystery regarding why some species carry sensor and mitigation systems for toxic metals such as arsenic, cadmium, lead, and silver, whereas an analogous fluoride-specific system had been notably absent (22). The pervasive occurrence of these fluoride toxicity mitigation systems is consistent with the fact that fluorine is the 13th most abundant element in Earth’s crust. Given their wide distributions, fluoride-specific riboswitches and commonly associated proteins such as CrcB may represent components of an ancient system by which cells have contended with toxic levels of this anion.
Supplementary Material
Acknowledgments
We thank members of the Breaker laboratory for helpful discussions and G. L. Ryan at Oligos Etc. for information on synthetic dinucleotide preparations. Ion channel flux experiments were conducted by R.B.S. in the laboratory of C. Miller, Brandeis Univ., and we thank him for his support of this project. We also thank N. Carriero and R. Bjornson for assisting our use of the Yale Life Sciences High Performance Computing Center (NIH grant RR19895-02). This work was supported by NIH (grant GM022778) and by the Howard Hughes Medical Institute. R.R.B. is a cofounder of and consults for BioRelix, a biotechnology company that has licensed intellectual property on riboswitches from Yale University Yale University has filed for patent protection on aspects of the crcB motif and fluoride riboswitches.
Footnotes
Supporting Online Material www.sciencemag.org/cgi/content/full/science.1215063/DC1
References and Notes
- 1.Lesher RJ, Bender GR, Marquis RE. Antimicrob. Agents Chemother. 1977;12:339. doi: 10.1128/aac.12.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maltz M, Emilson CG. J. Dent. Res. 1982;61:786. doi: 10.1177/00220345820610062701. [DOI] [PubMed] [Google Scholar]
- 3.Marquis RE, Clock SA, Mota-Meira M. FEMS Microbiol. Rev. 2003;26:493. doi: 10.1111/j.1574-6976.2003.tb00627.x. [DOI] [PubMed] [Google Scholar]
- 4.Levine RS. Br. Dent. J. 1976;140:9. doi: 10.1038/sj.bdj.4803690. [DOI] [PubMed] [Google Scholar]
- 5.Koo H. Adv. Dent. Res. 2008;20:17. doi: 10.1177/154407370802000105. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton IR. J. Dent. Res. 1990;69(spec. no. 660):682. doi: 10.1177/00220345900690S128. discussion. [DOI] [PubMed] [Google Scholar]
- 7.Van Loveren C. Caries Res. 2001;35:65. doi: 10.1159/000049114. [DOI] [PubMed] [Google Scholar]
- 8.Weinberg Z, et al. Genome Biol. 2010;11:R31. doi: 10.1186/gb-2010-11-3-r31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Supporting text and materials and methods are available as supporting material on Science Online.
- 10.Soukup GA, Breaker RR. RNA. 1999;5:1308. doi: 10.1017/s1355838299990891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regulski EE, Breaker RR. Methods Mol. Biol. 2008;419:53. doi: 10.1007/978-1-59745-033-1_4. [DOI] [PubMed] [Google Scholar]
- 12.Dobbins JT, Ljung HA. J. Chem. Educ. 1935;12:586. [Google Scholar]
- 13.Auffinger P, Bielecki L, Westhof E. Structure. 2004;12:379. doi: 10.1016/j.str.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Hu KH, et al. Genetics. 1996;143:1521. doi: 10.1093/genetics/143.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rapp M, Granseth E, Seppälä S, von Heijne G. Nat. Struct. Mol. Biol. 2006;13:112. doi: 10.1038/nsmb1057. [DOI] [PubMed] [Google Scholar]
- 16.Finn RD, et al. Nucleic Acids Res. 2010;38:D211. doi: 10.1093/nar/gkp985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matulef K, Maduke M. Mol. Membr. Biol. 2007;24:342. doi: 10.1080/09687680701413874. [DOI] [PubMed] [Google Scholar]
- 18.Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. Nature. 2002;415:287. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- 19.Accardi A, Miller C. Nature. 2004;427:803. doi: 10.1038/nature02314. [DOI] [PubMed] [Google Scholar]
- 20.Gälli R, Leisinger T. Conserv. Recy. 1985;8:91. [Google Scholar]
- 21.Vuilleumier S, Sorribas H, Leisinger T. Biochem. Biophys. Res. Commun. 1997;238:452. doi: 10.1006/bbrc.1997.7309. [DOI] [PubMed] [Google Scholar]
- 22.Silver S. Gene. 1996;179:9. doi: 10.1016/s0378-1119(96)00323-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.