Abstract
Neuroimaging research has demonstrated that ventromedial prefrontal cortex (vmPFC) encodes value signals that can be modulated by top-down cognitive input such as semantic knowledge, price incentives, and monetary favors suggesting that such biases may have an identified biological basis. It has been hypothesized that mindfulness training (MT) provides one path for gaining control over such top-down influences; yet, there have been no direct tests of this hypothesis. Here, we probe the behavioral and neural effects of MT on value signals in vmPFC in a randomized longitudinal design of 8 weeks of MT on an initially naïve subject cohort. The impact of this within-subject training was assessed using two paradigms: one that employed primary rewards (fruit juice) in a simple conditioning task and another that used a well-validated art-viewing paradigm to test bias of monetary favors on preference. We show that MT behaviorally censors the top-down bias of monetary favors through a measurable influence on value signals in vmPFC. MT also modulates value signals in vmPFC to primary reward delivery. Using a separate cohort of subjects we show that 8 weeks of active control training (ACT) generates the same behavioral impact also through an effect on signals in the vmPFC. Importantly, functional connectivity analyses show that value signals in vmPFC are coupled with bilateral posterior insula in the MT groups in both paradigms, but not in the ACT groups. These results suggest that MT integrates interoceptive input from insular cortex in the context of value computations of both primary and secondary rewards.
Keywords: fMRI, Valuation, vmPFC, Insular cortex, Mindfulness training, Longitudinal design
Introduction
One hypothesis is central to the emerging field of decision neuroscience: that the ventromedial prefrontal cortex (vmPFC) encodes value signals at the time of choice in a range of decision-making tasks involving both primary and secondary rewards (Hampton et al., 2006; Lebreton et al., 2009; Padoa-Schioppa et al., 2006; Philiastides et al., 2010). In support of this hypothesis, recent neuroimaging work shows that value signals in vmPFC can be modulated by top-down cognitive input such as knowledge of price, monetary favors, brand and semantic knowledge (De Araujo et al., 2005; Harvey et al., 2010; Plassmann et al., 2008; McClure et al., 2004; Kirk et al., 2009, 2011a). Collectively, these studies have expanded the role of the vmPFC in value-based decision-making suggesting that biases may have a biological basis that subverts cognitive control. Building on this work, this study examines whether mindfulness training (MT) enables subjects to protect against top-down bias and whether simpler components of such training may be responsible for beneficial effects. Despite extensive work on the neural underpinnings and behavioral dynamics of value-based decision-making, it remains unknown whether value signals can be modulated by MT, and what neural networks provide input for these computations.
The current study addresses both questions using functional magnetic resonance imaging (fMRI) in the context of 1) a primary reward paradigm by probing value-related regions at the time of reward (fruit juice) delivery, and 2) a secondary reward paradigm, namely a previously deployed version of the art-viewing paradigm, which uses monetary favors to examine the effect of bias on subjective decision-making (Harvey et al., 2010).
Mindfulness research has demonstrated that MT seems to act through interoceptive mechanisms (Allen et al., 2012; Farb et al., 2007, 2010, 2013; Kirk et al., 2011b; Lutz et al., 2008; Zeidan et al., 2011). As the insular cortex mediates subjective awareness of internal bodily processes, it has been argued that a sense of ‘self-as-witness’ is grounded in homeostatic bodily responses (Craig 2002, 2003, 2009; Damasio, 2010; Gu et al., 2013). Indeed, MT enables practitioners to experience “space between one’s perception and response” (Shapiro et al., 2006), and recent work has suggested the neural implications for the psychological construct of such decentering abilities. For example, even a short training course of MT is effective in decoupling the activity of the insula from the activity of other regions involved in valuation and decision-making, such as the vmPFC (Falk 2014; Farb et al., 2007; Tang et al., 2009). Other studies have observed increases in insular cortex coupled with decreases in posterior parietal cortex and vmPFC (Creswell et al., 2007; Farb et al., 2010; Holzel et al., 2007). Given the link between interoceptive processes in the insular cortex and MT, the current study tested the hypothesis that in the context of MT, value computation acquires input from areas involved in interoception such as the insular cortex, by modulating value signals in the vmPFC. As several types of top-down inputs are integrated in value signals computed in the vmPFC at the time of choice (or reward delivery), we speculated that MT leads to an altered weighing of different value signals. Specifically, we predicted that value signals computed in vmPFC in the group assigned to MT relative to the group assigned to active control training (ACT) in both the primary and the secondary reward paradigms, would integrate inputs from the insular cortex based on this region’s role in interoceptive processing (Critchley et al., 2004; Craig 2002, 2003, 2009). To investigate these aims we employed an experimental setup consisting of a fully randomized longitudinal design including 8 weeks of either MT or ACT.
Materials and Methods
Subjects
Fifty-two subjects participated in the art-viewing paradigm. They were divided in two groups; both the ACT and the MT group consisted of 26 subjects. The ACT group included 15 women and 11 men (mean age 31.3; standard deviation (SD) 10.1), while the MT group included 14 women and 12 men (mean age 32.2; SD 10.4). The two groups did not differ in terms of mean age or gender distribution. A separate cohort of 33 subjects participated in the primary reward paradigm; 17 of these subjects were assigned to the MT group and 16 to the ACT group. The ACT group included 9 women and 8 men (mean age 32.4; SD 11.4), while the MT group included 10 women and 7 men (mean age 32.7; SD 11.1).
Recruitment procedures consisted of advertising for participants “who want to learn to deal with stress issues in everyday life”; the study was framed as a stress-management program lasting 8 weeks. This recruitment strategy was employed in order to reduce self-selection bias in order to gain volunteers from a broad demographic range. Subjects were recruited with the understanding that the study consisted of comparing two equally valid stress reduction interventions, which minimized motivation and placebo effects. In addition, subjects were notified that they would be assigned to a stress reduction intervention in a random manner, which eliminated any self-selection effects between the two intervention. The study was advertised for staff and students around Virginia Tech. This recruitment strategy resulted in 238 volunteers who signed up for the study. Of this initial number, 45 subjects were found to be ineligible (33 subjects were using psychiatric medication or had a medical history of psychiatric medication; 12 subjects were MRI ineligible due to either metal implants, claustrophobia or subjects who had previously suffered from concussions that included a loss of consciousness for more than 10 min). In addition an exclusion criteria for the study was prior experience (i.e. regular practice) with mindfulness meditation. The subjects included in the study were randomly selected from the eligible group, and the non-selected volunteers were put on a waitlist to participate in future studies involving stress-management training. The subjects who were included in the current study were subsequently randomly assigned to receive either MT or ACT. Subjects in the study received compensation for their participation according to the following payment scheme: Subjects were paid $20 for attendance in each of the 8 weekly group sessions independent of group modality (MT/ACT). In addition subjects were paid $20 for participation in the primary reward task, and $300 on each visit (pre and post) for participation in the art-viewing paradigm. The subjects received compensation associated with the fMRI-tasks immediately after each scanning session. However, attendance compensation for the 8 weekly group sessions was paid in total upon study completion. All subjects across the two experiments had normal or corrected-to-normal vision, and none had a history of neurological or psychiatric disorders. All procedures were conducted in accordance with the Institutional Review Board of Virginia Tech.
Procedure for MT
The MT consisted of 8 weeks of practice of mindfulness that mimic the canonical mindfulness program entitled Mindfulness Based Stress Reduction (MBSR) (Kabat-Zinn 1990). The MT program was taught by a certified MBSR instructor. The program includes introducing participants to moment-to-moment awareness and non-judgmental awareness. A structured group format was applied whereby participants attended weekly group sessions that introduced them to formal meditation practices. Each group session lasted 2.5 hours. The MT program also included a full day of meditation between the sixth and seventh meeting sessions. Participants were required to attend at least seven of the eight group sessions and the full-day session to be considered compliant with the training protocol. In addition to group meetings, participants were asked to practice meditation on non-class days for 20 minutes a day with the assistance of guided meditation CDs. The formal meditation practices included breath monitoring, body scans, and attention to sounds, thoughts, feelings and bodily sensations. Participants were instructed to maintain a daily log of practice completion, which was collected by the course instructors at every weekly session. In addition to class attendance, participants were required to complete at least 50% of the recommended daily homework.
Procedure for ACT
For the ACT, a structured group format was applied whereby participants attended weekly group sessions introducing them to progressive muscle relaxation. The ACT program was taught by a certified and experienced instructor in progressive muscle relaxation. The weekly sessions were 2.5 hours in duration and included 30 minutes of stretching and exercise. These moves could be easily completed in comfortable clothing and some positions performed whilst seated. Then there would be group discussion for 30 minutes. Participants would share their experience on a particular topic and give updates from previous weeks. Sometimes a question was asked to the group to facilitate conversation and each person in the group would take a turn to answer the question. This time was then followed by the introduction of a new topic by the facilitator. Topics included: time management, physical activity, sleep, healthy eating, organization, communication, and future goal setting. The facilitator provided information gathered from online sources about each topic. During the week in between classes, participants were expected to complete their stretching/exercise moves daily and to reflect on the topic for the week. The ACT program also included a full day of physical relaxation exercises between the sixth and seventh meeting sessions. Participants were required to attend at least seven of the eight group sessions and the full-day session to be considered compliant with the training protocol. In addition to group meetings, on non-class days participants were asked to practice stretching and relaxation exercises for 20 min a day with the assistance of guided CDs. Participants were instructed to maintain a daily log of practice completion, which was collected by the course instructors at every weekly session. In addition to class attendance, participants were required to complete at least 50% of the recommended daily homework.
fMRI task
Art-viewing paradigm
Prior to scanning, subjects were told they would be sponsored by one of two companies. In the scanner subjects were initially presented with two company logos, followed by a screen indicating which of the two companies would be sponsoring them, as well as their amount of compensation ($300). Subjects from both the ACT and the MT group participated in the task both pre- and post-training and were paid $300 on each visit. On each trial an image of a painting was presented centrally and the logos were positioned in the upper left and right corners of the screen. Each of the 60 paintings was paired with either the sponsor logo or another, nonsponsor logo. The procedure was presented in a pseudorandom fashion and counterbalanced across subjects. Likewise, the pairing of logo and sponsorship was counterbalanced across subjects. During the scanning session, subjects were instructed to passively view each painting. Post-scanning, subjects were asked to complete a behavioral run of the paintings, while making a subjective preference rating of each image using a Likert-scale (+3 to −3). Visual chromatic reproductions of original paintings served as stimuli. In total 120 paintings (60 abstract and 60 representational) were shown. Different paintings were shown to subjects on each of the two scanning visits to the lab. 50% of the paintings, i.e. 60 paintings (30 abstract and 30 representational) were shown on visit 1 and the remaining 50% were shown on visit 2. The logos were unfamiliar to the subjects in that logos were pre-fabricated by the experimenters, and different logos served as sponsor and nonsponsor within subjects across the two time points (pre- and post-training). The experimental protocol consisted of an event-related design. On each trial, a stimulus appeared for 5 sec followed by an inter-trial interval of 4–14 sec (Figure 1, top left). The stimuli were presented at a screen resolution of 1024 × 768 pixels, and centered in a 500 × 500 pixel resolution surrounded by a black background. Stimuli were presented and responses collected using NEMO (Human Neuroimaging Lab, Virginia Tech Carilion Research Institute). The stimuli were back-projected via an LCD projector on to a transparent screen positioned over the subjects’ head and viewed through a tilted mirror fixed to the head coil. Subjects were scanned both before the 8-week training intervention and immediately after the intervention was completed.
Figure 1. Art-viewing paradigm: pre-training behavioral and neural results.
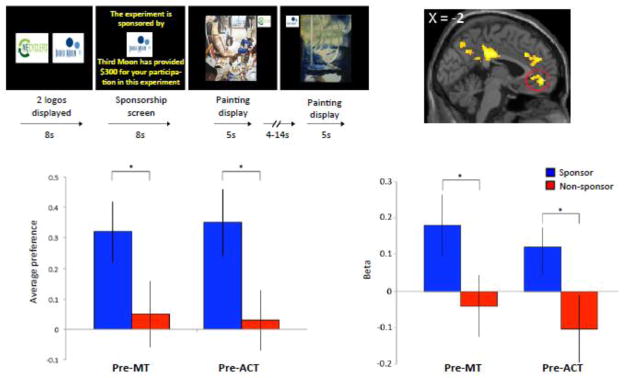
Top left: Art viewing paradigm. During fMRI scanning one of two company logos was associated with funds ($300) that participants received for study compensation. Subsequently, 60 paintings were presented that displayed either the sponsor or nonsponsor logos during a passive scanning run. In a subsequent behavioral run, participants provided preference responses for each painting.
Bottom left: Average preference responses across groups collected at the pre-training condition. Average preference responses grouped into sponsor (blue bars) and nonsponsor (red bars) conditions. The rating scale was a Likert-type scale (+3 to −3). Statistical analysis showed a significant difference between sponsor and nonsponsor conditions in the pre-training condition in both groups. Asterisks denote significance. Error bars represent SE.
Top right: Neural activity in the vmPFC encoding value signals emerged in a conjunction analysis in the pre-training condition between the contrast [sponsor > nonsponsor] for both the ACT and MT group.
Bottom right: ROI in vmPFC based on vmPFC MNI coordinates from our previous study (Harvey et al., 2010). β-values extracted for each group in the pre-training condition display significantly higher β-values for sponsor (blue bars) than nonsponsor (red bars) conditions in both ACT and MT group.
Primary reward paradigm
The task consisted of 4 scanning runs. The sequence in runs 1 and 2 consisted of presentation of a yellow light cue (1 sec) centrally positioned on an otherwise black screen. This cue was followed by juice delivery 6 sec later. The time between individual pairings was randomly selected from between 4 and 14 sec (at 2 s increments). In run 1 there were 23 such training events and in run 2 there were 22 events. In the subsequent runs 3 and 4 there were 18 events in each run of which 6 events were catch/testing events. For these catch events, the time from light cue to juice delivery was increased to 10 sec (Figure 4, top). The cue cue duration during catch events was identical to training events (1 sec). Subjects were instructed to focus on the light cue and swallow juice as it was delivered. No reference was made to the cue/juice pairings. The light cues were presented and responses collected using NEMO (Human Neuroimaging Lab, Virginia Tech Carilion Research Institute). Juice was delivered using a computer-controlled syringe pump (Harvard Apparatus, Holliston, MA). Juice delivery consisted of 0.8 ml juice per event. Subjects were asked to come into the lab in a thirsty state. This procedure was installed to ensure that there were no systematic differences between groups in satiety levels that might influence the results. Prior to scanning subjects were asked to select their preferred juice amongst three different flavors. Post-scanning, all subjects reported that they had enjoyed the juice. Subjects in the juice task completed the identical 8-week training programs as subjects who participated in the art-viewing paradigm, although subjects in the juice task were scanned only after the training intervention due to logistic issues at the scanning facility.
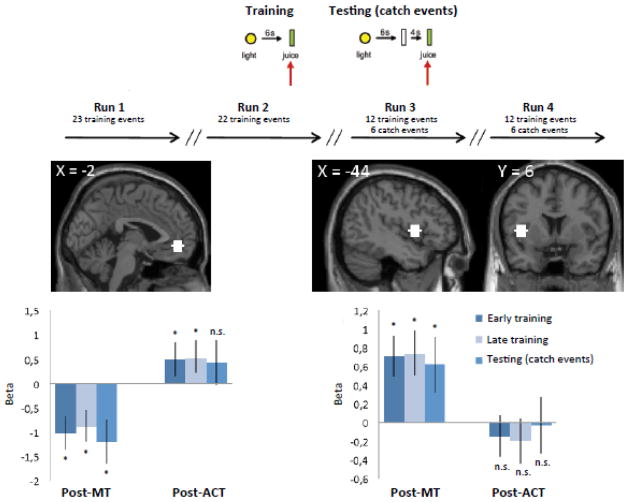
Figure 4. Primary reward paradigm: post-training neural results.
Top: Outline of the primary reward paradigm. A training event consisted of a yellow light (1 sec) predicting the oral delivery of fruit juice (0.8 ml) 6 s later. A catch event consisted of presentation of the light cue (1 sec) and juice delivery 10 sec later. During the MRI scanning session, catch events were interspersed among the standard (training) events in run 3 and run 4. Run 1 and run 2 consisted on training runs only.
Mid left: ROI analysis (6 mm spheres) based on MNI coordinates in the left vmPFC (x,y,z = 2 50 −6) taken form the art-viewing paradigm at the time of juice delivery.
Bottom left: β-values extracted from the vmPFC ROI in both groups show that the group difference is driven by elevated activity in the ACT group and significant deactivation in the ROI in the MT group across training and catch events.
Mid right: ROI analysis (6 mm spheres) based on MNI coordinates in the left mid/anterior insula (x,y,z = −44 6 4) taken form the art-viewing paradigm at the time of juice delivery.
Bottom right: β-values extracted from the left mid/anterior insula ROI in both groups show that the group difference in driven by elevated activity in the MT group only whereas the ACT group does not display changes average beta estimates from baseline.
fMRI data acquisition
The anatomical and functional imaging was performed using 3 Tesla Siemens Trio scanners. High-resolution T1-weighted scans were acquired using an MPRAGE sequence (Siemens). Functional imaging used an EPI sequence with a repetition time (TR) of 2000 ms, echo time (TE) = 30 ms, flip angle = 90°, 220 mm field of view (FOV), 64 × 64 matrix. Functional slices were oriented 30° superior-caudal to the plane through the anterior and posterior commissures in order to reduce signal drop-out due to magnetic field inhomogeneities (Deichmann et al., 2003). Each functional image was acquired in an interleaved way, comprising 34 4-mm axial slices for measurement of the blood oxygenation level-dependent (BOLD) effect (Ogawa et al., 1990), yielding 3.4 mm × 3.4 mm × 4.0 mm voxels.
fMRI data analysis
Image pre-processing and data analysis were performed using SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK). Motion correction to the first functional scan was performed using a six-parameter rigid-body transformation (Friston et al., 1996). The average of the motion-corrected images was co-registered to each individuals structural MRI using a 12-parameter affine transformation. Slice timing artifact was corrected, after which images were spatially normalized to the Montreal Neurological Institute (MNI) template provided in SPM8. Images were then spatially filtered with an 8 mm isotropic Gaussian kernel and for the analysis a high pass filter with a cut-off frequency at 1/128 Hz was applied. Following pre-processing a GLM was applied to the fMRI time-series where stimulus onset was modeled as single impulse response functions including stimulus duration and then convolved with the canonical hemodynamic response function (HRF) (Friston et al., 1998).
Art-viewing paradigm
A parametric regression analysis was used (Buchel et al., 1998) that allowed us to model linear first-order and nonlinear second-order hemodynamic responses using orthogonalized polynomial expansions. This was performed for each of the two conditions (sponsor and nonsponsor) using subject-specific preference ratings for each stimulus. Residual effects of head motion were corrected for by including the six estimated motion parameters for each subject as regressors of no interest. First-level analysis was performed on each subject to generate a single mean image corresponding to each term of the polynomial expansions. The mean images from the first-level analysis were entered into a second-level, random effects (RFX) analysis accounting for the between subject variance. An ANOVA model using the β-estimates of the two conditions for the first- and second-order expansions was applied. Equal variance was not assumed, thus SPM8’s option for non-sphericity correction was applied (Glaser & Friston 2004). Using t-contrasts allowed us to test for correlations of the fMRI BOLD signal and the parameters of interest performed respectively as first- and second-order parametric modulations. The resulting t maps were subsequently transformed to z-distributions to create a statistical parametric map for each contrast. Unless otherwise stated, statistical threshold was set at voxel level P < 0.001, uncorrected and a cluster size of 10 voxels. Bilateral insula cortex anatomical ROI: a gray matter ROI was selected according to the anatomical division of the insular cortex. The bilateral insula ROI was identified using the xjView toolbox software package and were masked exclusively to ensure that no overlapping voxels were selected (Figure S2). The coordinates of all activations are reported in MNI space. Data was displayed using the xjView toolbox.
Primary reward paradigm
Following pre-processing, a GLM was applied to the fMRI time-series where each event was modeled as single impulse response functions at light cue onset and juice delivery onset (for runs 1 and 2). For runs 3 and 4 the model included the light cue, juice delivery during normal events, juice delivery during catch events, the absence of juice delivery at 6 s during catch events, and the absence of juice delivery during normal events (10 s after light cue). The model was convolved with the HRF including its temporal derivative to account for slight discrepancies in juice delivery time and duration. Residual effects of head motion were corrected for by including the six estimated motion parameters for each subject as regressors of no interest. We constructed two ROI analyses in 1) the vmPFC (x,y,z = 2 50 −6) and 2) the left mid/anterior insula (x,y,z = −44 6 4). The MNI coordinates were identified using peak activations from the art-viewing paradigm. A spherical mask with a 6 mm radius centered at (x,y,z = 2 50 −6) and (x,y,z = −44 6 4) was used to extract the time-series from these two ROIs. Post hoc analyses were performed using subject-specific β-estimates of the regressors of interest. Significant results from a whole brain analysis will be summarized in a separate paper. In the current paper we report ROI analyses to supplement results from the art-viewing paradigm
Psychophysiological interaction analysis
For the functional connectivity analysis, we implemented psychophysiological interaction analyses (PPI) (Friston et al., 1997) by including data from both the primary and secondary reward paradigms. The PPI employed assess changes in functional connectivity between the seed region of vmPFC and other brain regions whose activities anti-correlated with the vmPFC. In the first PPI we included data from the secondary reward paradigm. The PPI model included a regressor representing the deconvolved time-series of neural activity within a 4-mm sphere centered on vmPFC (x,y,z = 2 50 −6), which constituted the physiological variable, a second regressor representing the psychological variable, which we collapsed across the sponsor and nonsponsor trials, and a third regressor representing the cross-product of the previous two (the PPI term). The model also included motion parameters as regressors of no interest. The PPI was carried out in each subject and entered into random-effects analysis separately for each of the two groups. In a second PPI we included data from the primary reward paradigm. The PPI analysis was performed using identical parameters and MNI coordinates as applied in the first PPI described above, except that all trials at the time of juice delivery were collapsed and constituted the psychological variable.
Results
The experimental setup in the art-viewing paradigm is such that there is no association between the logo and the displayed paintings. Therefore, increased preference for a painting presented next to the sponsoring logo indexes a behavioral sponsorship effect. The behavioral results pre-training conformed to our expectations (Figure 1, bottom left). In accordance with our previous research (Harvey et al., 2010; Kirk et al., 2011a) we observed a significant sponsorship effect—i.e. subjects rated those paintings that were presented next to a sponsor logo more preferable relative to those paintings that were presented next to a nonsponsor logo—in both the MT (paired t = 3.12; p < 0.004) and the ACT group (paired t = 3.41; p < 0.002). In the post-training condition the effect of sponsorship was not significant in either the MT group or the ACT group (Figure S1). The average daily amount of time spent on home exercises as measured by daily practice logs was 13.2 minutes for the MT group. The CT group spent an average of 15.3 minutes on home exercises. Note that daily practice logs from two subjects in the MT group were not collected due to technical issues and hence could not be included in this behavioral analysis (they were however both included in the neural analysis). In addition, weekly attendance to the group sessions was 5.5 (STD=1.2) out of a total of 8 sessions for the MT group, and 5.8 (STD=1.3) for the ACT group. There was no significant difference between groups for daily practice (p<0.8) or weekly group attendance (p<0.08).
Modulation of vmPFC by sponsorship in the pre-training condition
We expected that value signals in the vmPFC would exhibit a modulation reflecting the behavioral sponsorship effect (Harvey et al., 2010; Kirk et al., 2011a). We did observe such a relationship in both groups in the pre-training condition. Specifically, using a parametric regression model that computes correlations between regions in the brain that scale linearly with subjective painting preference, we found increased activity in the vmPFC in the contrast [sponsor > nonsponsor] when applying a conjunction analysis to identify common regions between the two groups (x,y,z = −2 50 −6; p < 0.0012, uncorrected) (Figure 1, top right). For completeness, all regions showing significant activity in the conjunction analysis are listed in Table S1. We subsequently extracted the average β-estimates in a 10mm sphere centered on the peak voxels of vmPFC reported in another study that used the art-viewing paradigm (Harvey et al., 2010). We found that the sponsor condition displayed increased activity compared with the nonsponsor condition in both the MT group (paired t = 3.04; p < 0.004) and in the ACT group (paired t = 3.28; p < 0.003) (Figure 1, bottom right). These results support our previous findings that the vmPFC is susceptible to modulation by sponsorship (Harvey et al., 2010; Kirk et al., 2011a).
Modulation of vmPFC by MT
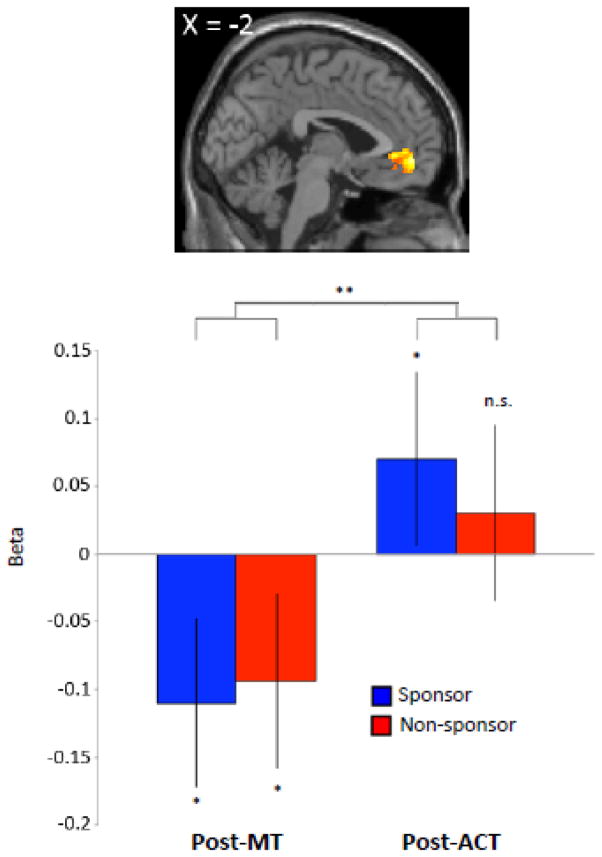
In the absence of behavioral differences between the sponsor and nonsponsor conditions in either of the two groups in the post-training condition, we performed the subsequent fMRI analysis independent of sponsor modality. That is, we conducted a direct comparison between [ACT > MT] by collapsing the sponsor and nonsponsor conditions. This contrast showed that the vmPFC activation was significantly decreased in the post-MT group compared to the post-ACT group (x,y,z = 2 50 −6; p < 0.001, uncorrected) (Figure 2, top). Furthermore, post hoc analyses using average β-estimates extracted in a 4 mm sphere centered on the peak voxels from the vmPFC showed that there were no significant differences between the sponsor and nonsponsor conditions in any of the two groups (Figure 2, bottom). Note that there was a substantial overlap between voxels in the vmPFC in the post-training condition (x,y,z = 2 50 −6) and the vmPFC region in the pre-training condition (x,y,z = −2 50 −6) when applying the 4mm sphere. This result convey two important points about the vmPFC: 1) that the modulation of the vmPFC observed in both groups in the pre-training condition dissipate in the absence of a behavioral sponsorship effect in both groups in the post-training condition, and 2) that value signals in the vmPFC in the MT group are suppressed in the post-training condition, leaving open the possibility that the vmPFC in the MT group might integrate input from other brain regions during value computation.
Figure 2. Art-viewing paradigm: post-training results in vmPFC.
Top: Main-effect of group post-training. The sponsor modality (sponsor/nonsponsor) was collapsed together resulting in the following contrast in the post-training condition: [ACT > MT]. The two conditions (sponsor and nonsponsor) were collapsed in that there was no significant behavioral effect of condition in the post-training condition in both groups. The SPM display increased activity in the vmPFC at p < 0.001, uncorrected (albeit displayed at p < 0.005 to show the extend of the activation). Note the peak MNI coordinates in the vmPFC (x,y,z = 2 50 −6), albeit show here at in a sagittal plane (X=−2) to allow comparison with Figure 1.
Bottom: β-values extracted for each group in the post-training condition in the vmPFC display the group difference (significance at p < 0.01 denoted by *; p < 0.001, uncorrected by **; non significance denoted by n.s.). Error bars indicate SE. Note that only the sponsor β-estimate in the ACT group is significantly different from zero, whereas the nonsponsor β-estimate is not significant from zero. In the post MT-group, both the sponsor and nonsponsor β-estimates are significantly different from zero, albeit in the opposite direction compared to the ACT group.
MT integrates interoceptive signals during value computation
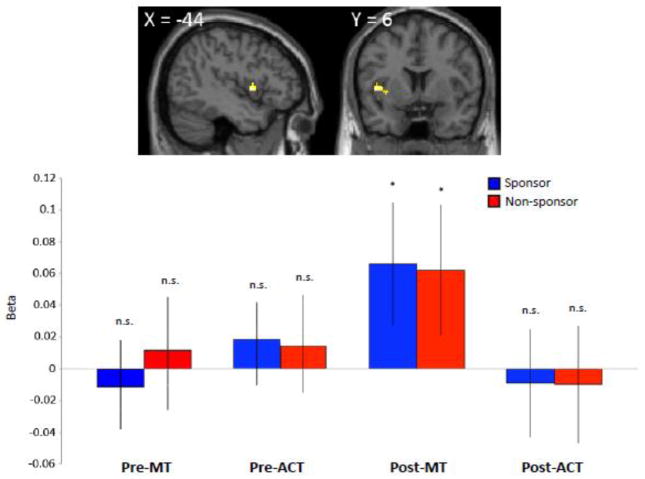
To explore the possibility that value signals in the vmPFC integrate input from other brain regions in the MT group in the post-training condition, we computed the contrast [MT > ACT] by collapsing across the two sponsorship conditions. A whole-brain analysis identified significant group differences solely in the left insular cortex, encompassing the mid/anterior insula (x,y,z = −44 6 4; p < 0.001, uncorrected) (Figure 3, top). Subsequent analyses using the average β-estimates from the left mid/anterior insula showed that the contrast [MT > ACT] was neither driven by differences within the sponsor modalities nor differences in the pre-training condition between groups, but that only the MT group in the post-training condition recruited the anterior insula region during value computation (Figure 3, bottom). In accordance with our a priori hypothesis we subsequently constructed an anatomical region of interest (ROI) analysis in bilateral insular cortex. Small-volume correction (SVC) (Worsley et al., 1996) was used to correct for multiple comparisons in reporting these results in the insular cortex. Applying an exclusive mask in the insular cortex (Figure S2) yielded significant voxels in the left mid/anterior insula (x,y,z = −44 6 4; p < 0.05, FDR-corrected, SVC). No other brain regions were observed in the insular cortex search volume.
Figure 3. Art-viewing paradigm: post-training results in mid/anterior insula.
Top: Binary group-specific comparison [MT > ACT] collapsing across sponsor modality (sponsor/nonsponsor) exhibit activity in the left mid/anterior insula.
Bottom: β-values extracted from the left mid/anterior insula in the pre and post-training condition do not exhibit differences across the sponsor modality in either group (denoted by n.s.). Only the β-values in the post-MT exhibit a significant effect. Error bars indicate SE.
Overlapping neural value signals in primary reward paradigm
We next searched for overlap between the secondary reward paradigm and an independent primary reward paradigm. Overlapping neural value signals between these two paradigms would argue for a general mechanism, whereby MT modulates value signals in vmPFC through input from the insular cortex. We used a primary reward paradigm in which fruit juice was delivered to subjects (Figure 4, top). From the conditioning paradigm we extracted ROIs based on activation clusters in the vmPFC and mid/anterior insula from the art-viewing paradigm (Figure 4, mid left/mid right). The vmPFC ROI showed significantly greater activity in the ACT group than in the MT group at the time of juice delivery (Figure 4, bottom left). By contrast, a reverse activation pattern emerged in the left mid/anterior insula ROI, where we found elevated activity in the MT group, but not in the ACT group (Figure 4, bottom right). Thus, across two independent experiments the results showed a consistent decrease in the vmPFC and a corresponding increase in the mid/anterior insula following MT.
Interestingly, the conditioning paradigm did not have any impact on trained expectation in the ROIs; that is, we did not find significant differences between early, late and catch events at the time of juice delivery.
Input from insular cortex interact with vmPFC
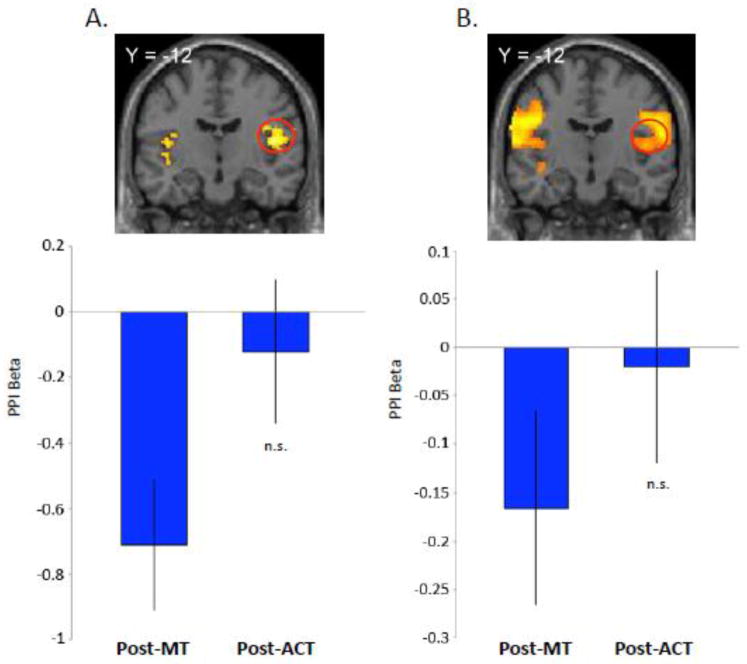
If the modulation of vmPFC in the post-MT condition were mediated by interoceptive signals, we might expect that vmPFC activity would show a negative coupling with interoceptive signals in regions such as the insular cortex. We implemented psychophysiological interaction (PPI) analyses to test the hypothesis that regions recruited during MT interact with networks that provide input to vmPFC and thereby influence value signals indirectly. We carried out the PPI analyses separately for the two paradigms using voxels in the vmPFC as the seed region. A whole-brain analysis from the secondary reward paradigm showed that activity in bilateral posterior insula, among other regions (Table S2), interacted negatively with vmPFC (x,y,z = 44 −14 12 and −40 −10 10; p < 0.005, uncorrected) (Figure 5A). We subsequently estimated a second PPI to identify regions exhibiting negative connectivity with the vmPFC at the time of juice delivery in the primary reward paradigm. We found that bilateral posterior insula (Table S3) showed negative functional connectivity with vmPFC (x,y,z = 48 −10 10 and −44 −14 10; p < 0.05, FDR-corrected) (Figure 5B). Taken together, these results support vmPFC-insula functional coupling under the modulation of both secondary and primary rewards.
Figure 5. Connectivity analyses or primary and secondary reward paradigms.
A) Secondary reward paradigm. PPI displaying decreased coupling between the vmPFC seed region and the bilateral posterior insula in the post-training condition for the MT group. The ACT group did not exhibit significant changes in connectivity with the posterior insula. β-values extracted from the right posterior insula measuring the degree of correlation between BOLD activity in the vmPFC and the right posterior insula in both groups. Error bars are SE.
B) Primary reward paradigm. PPI displaying decreased coupling between the vmPFC seed region and the bilateral posterior insula in the post-training condition for the MT group. The ACT group did not exhibit significant changes in connectivity with the posterior insula. β-values extracted from the right posterior insula measuring the degree of correlation between BOLD activity in the vmPFC and the right posterior insula in both groups. Error bars are SE.
Discussion
This study builds on previous work on the neurobiological basis of valuation (Harvey et al., 2010) and the impact of expertise training on the vmPFC valuation systems (Kirk et al., 2011a). In this randomized controlled design using MT and ACT, we wanted to study the potential impact that MT exert on valuation processes. Importantly, we were able to replicate previous findings (Harvey et al., 2010; Kirk et al., 2011a) by showing that both the ACT and the MT groups in the pre-training condition were susceptible to bias in that a monetary favor increased the valuation of paintings placed next to the sponsoring corporate logo relative to the paintings presented adjacent to the nonsponsoring logo. We demonstrate that the behavioral sponsorship effect in the pre-training condition correlates with vmPFC activity in both the ACT and MT group. This finding has two important implications. First, our finding supports the hypothesis that vmPFC encodes value signals related to a wide range of both primary and secondary reinforcers. Indeed, the region of vmPFC that we observed in the current study overlaps with regions of the vmPFC that have been shown in previous studies to encode the value of primary and secondary rewards at the time of decision making (Hampton et al., 2006; Lebreton et al., 2009; Padoa-Schioppa et al., 2006; Philiastides et al., 2010). Second, our finding supports the hypothesis that valuation in the vmPFC is susceptible to top-down cognitive input. In the context of the current results it is evident that a monetary favor can change perceived subjective value through modulation of value signals computed in the vmPFC. This finding has behavioral support from the psychological literature showing that value judgments can be affected by external manipulations such as familiarity (Monin 2003) or context-dependent framing effects (Ariely et al., 2006).
The results from the post-training condition show an interesting pattern both neurally and behaviorally. Both groups mitigate the behavioral influence of a monetary favor on behavioral preference during the second exposure (i.e. in the post training condition) in the art-viewing paradigm. One likely explanation for the mitigation of the bias effect in the post-training condition might be a priming effect. As such, it is likely that subjects during the second exposure of the art-viewing paradigm may have adopted value-neutral behavior, which is supported by the preference responses in the post-training condition showing that the average painting preference dropped compared to the pre-training condition and remained non-significantly different from zero in the two sponsor modalities in both groups. However, the behavioral results in the post-training condition might also be explained by other possible mechanisms. For example, differences in working memory retrieval might account for the differences between the pre and post exposure to the art-viewing paradigm, albeit the fact that we did not observe differences in the neural regions involved in working memory such as dlPFC from pre-training to post-training might preclude this possibility (Cohen et al., 1997; Fuster & Alexander 1971).
Based on our neural hypothesis for the post-training condition - that the MT group in addition to value signals computed in vmPFC integrates signals on the basis of input from the insula—we found three novel patterns in the results reported, which will be discussed next. First, we found that while neither group showed a sponsorship-bias effect in the post-training condition, the ACT group, relative to the MT group, maintained activity in the vmPFC that correlated with painting preference in the second exposure (albeit only in the sponsor condition), suggesting that value signals in the ACT group are computed primarily in the vmPFC. Second, the results show that in contrast to the ACT group, the MT group decoupled activity in the vmPFC during value computation, reflected by a suppression of vmPFC responding in this group. Third, the MT group recruited value signals that scaled linearly with painting preference in the left mid/anterior insula. This brain region has been proposed to play a role in attending to momentary self-reference (Farb et al., 2007, 2010, 2013) and attending to internal bodily states (Critchley et al., 2004; Craig, 2009) as well as the homeostatic state of the body (Craig 2003; Seth et al., 2011). These findings argue for the possibility that the MT group was better than the ACT group at maintaining interoceptive awareness, e.g., attending to internal bodily states, and integrated such signals during value computation. The PPI results further suggest the possibility that value signals in the MT group seem to be computed by incorporating input from the insular cortex, specifically the posterior insula. The decoupling between the posterior insula and the vmPFC valuation systems in the MT group in the context of both the primary and secondary reward tasks suggests a specific interaction mediated by MT between interoceptive networks and value computation systems. A recent study that found that 8 weeks of mindfulness training decoupled activity in the vmPFC from activity in the posterior insula in a task in which subjects were required to maintain momentary self-reference. This decoupling supposedly enabled mindfulness participants to shift focus to a more self-detached and objective analysis of interoceptive sensory events represented in elevated posterior insula activity as opposed to the subjective self-referential value of sensory events represented in decreased vmPFC activity (Farb et al., 2007).
Previous studies pertaining to the neural effects of mindfulness have been complicated by possible alternative explanations such as pre-existing group differences in the case of cross-sectional designs (e.g. Kirk et al., 2011b; Farb et al., 2007; Creswell et al., 2007, Farb et al, 2010; Holzel et al., 2007) and self-selection bias (e.g. Kirk et al., 2011b; Farb et al, 2007; Holzel et al., 2007). The use of a fully randomized longitudinal design minimizes these possible confounds (see Material and Methods). In the present study we did not explicitly ask subjects to enter into a meditative state during the task. Hence, we suspect that the neural differences observed are likely to be attributed as trait-like training effects. Future studies will be required to delineate potential impact of state vs. trait effects in these particular neural regions identified in the current study.
Our findings have implications for an ongoing debate on the extent to which individual decision-makers are able to employ self-regulatory mechanisms across sensory modalities. While the findings in the current study show that both ACT and MT curb behavioral bias effects, the neural data specifically in the MT group may have implications for self-regulation mechanisms. For example, it remains a possibility that MT may decouple the influence of the vmPFC valuation systems and integrate input from insula regions, which may indirectly guide decision-making on the basis of interoceptive signals. Thus, future studies should explore the extent to which MT may have clinical applications to areas such as both obesity and addiction whereby MT may provide choice formation based on allostatic input.
Supplementary Material
Highlights.
Within-subject design including active control group (ACT) show effects mindfulness training (MT) on value signals in the brain
MT effectively changes value signals in vmPFC in both primary and secondary reward tasks.
MT integrates interoceptive input from insular cortex during value computation
Acknowledgments
This work was supported by National Institute on Drug Abuse Grant R01DA011723-11 (PRM) and National Institute of Neurological Disorders and Stroke Grant R01 NS045790 (PRM).
Footnotes
Conflict of interest: The authors declare no competing financial interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen M, Dietz M, Blair KS, van Beek M, Rees G, Vestergaard-Poulsen P, Lutz A, Roepstorff A. Cognitive-affective neural plasticity following active-controlled mindfulness intervention. J Neurosci. 2012;31;32(44):15601–10. doi: 10.1523/JNEUROSCI.2957-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariely D, Loewenstein G, Prelec D. Tom Sawyer and the construction of value. J Econ Behavior & Organization Vol. 2006;60:1–10. [Google Scholar]
- Buchel C, Holmes AP, Rees G, Friston KJ. Characterizing stimulus response functions using nonlinear regressors in parametric fMRI experiments. Neuroimage. 1998;8:140–148. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;10;386:604–8. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Op Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel - now? The anterior insula and human awareness. Nat Nev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosom Med. 2007;69:560–565. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rothstein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio A. Self Comes to Mind: Constructing the Conscious Brain. New York, NY: Pantheon Books; 2010. [Google Scholar]
- De Araujo IE, Rolls ET, Velazco MI, Margot C, Cayeux I. Cognitive modulation of olfactory processing. Neuron. 2005;46:671–679. doi: 10.1016/j.neuron.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Falk EB. Mindfulness and the neuroscience of influence. In: Christelle CT, Ngnoumen, Langer EJ, editors. The Wiley handbook of mindfulness. John Wiley & Sons Ltd; 2014. [Google Scholar]
- Farb NA, Anderson AK, Mayberg H, Bean J, McKeon D, Segal ZV. Minding one’s emotions: mindfulness training alters the neural expression of sadness. Emotion. 2010;10:25–33. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Anderson AK. Mindfulness meditation training alters cortical representations of interoceptive attention. Soc Cogn Affect Neurosci. 2013 Jan;8(1):15–26. doi: 10.1093/scan/nss066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, Anderson AK. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci. 2007;2(4):313–322. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;13;173:652–4. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buchel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams R, Howard RS, Frackowiak RSJ, Turner R. Movement-related effects in fMRI time-series. Mag Res Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Joseph O, Holmes A, Rugg MD, Turner R. Event-related fMRI: Characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Glaser D, Friston KJ. Variance components. In: Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Price CJ, Zeki S, editors. Human brain function. Elsevier Academic Press; 2004. [Google Scholar]
- Gu X, Hof PR, Friston KJ, Fan J. Anterior insular cortex and emotional awareness. J Comp Neurol. 2013 Jun 8; doi: 10.1002/cne.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton AN, Bossaerts P, O’Doherty JP. The role of the ventromedial prefrontal cortex in abstract state-based inference during decision making in humans. J Neurosci. 2006;32:8360–7. doi: 10.1523/JNEUROSCI.1010-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A, Kirk U, Denfield G, Montague PR. Monetary favors and their influence on neural responses and revealed preference. J Neurosci. 2010;30:9597–9602. doi: 10.1523/JNEUROSCI.1086-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Ott U, Hempel H, Hackl A, Wolf K, Stark R. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neurosci Lett. 2007;421:16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Using the wisdom of your body and mind to face stress, pain and illness. Random House; 1990. Full catastrophe living. [Google Scholar]
- Kirk U, Skov M, Hulme O, Christensen MS, Zeki S. Modulation of aesthetic value by semantic context: An fMRI study. Neuroimage. 2009;44:1125–1132. doi: 10.1016/j.neuroimage.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Kirk U, Harvey A, Montague PR. Domain expertise insulates against judgment bias by monetary favors through a modulation of ventromedial prefrontal cortex. Proc Natl Acad Sci U S A. 2011a;21;108(25):10332–6. doi: 10.1073/pnas.1019332108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk U, Downar J, Montague PR. Interoception drives increased rational decision-making in meditators playing the ultimatum game. Front Neurosci. 2011b Apr 18;5:49. doi: 10.3389/fnins.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton M, Jorge S, Michel V, Thirion B, Pessiglione M. An automatic valuation system in the human brain: evidence from functional neuroimaging. Neuron. 2009;63:431–439. doi: 10.1016/j.neuron.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cogn Sci. 2008;12(4):163–9. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Li J, Tomlin D, Cypert KS, Montague LM, Montague PR. Neural correlates of behavioral preference for culturally familiar drinks. Neuron. 2004;44:379–387. doi: 10.1016/j.neuron.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Monin B. The warm glow heuristic: when liking leads to familiarity. J Pers Soc Psychol. 2003 Dec;85(6):1035–48. doi: 10.1037/0022-3514.85.6.1035. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci US A. 1990;87:9868–72. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philiastides MG, Biele G, Heekeren HR. A mechanistic account of value computation in the human brain. Proc Natl Acad Sci U S A. 2010;107:9430–9435. doi: 10.1073/pnas.1001732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O’Doherty J, Shiv B, Rangel A. Marketing actions can modulate neural representations of experienced pleasantness. Proc Natl Acad Sci USA. 2008;105:1050–1054. doi: 10.1073/pnas.0706929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth AK, Suzuki K, Critchley HD. An interoceptive predictive coding model of conscious presence. Front Psychol. 2011;2:395. doi: 10.3389/fpsyg.2011.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SL, Carlson LE, Astin JA, Freedman B. Mechanisms of mindfulness. J Clin Psychol. 2006;62:373–386. doi: 10.1002/jclp.20237. [DOI] [PubMed] [Google Scholar]
- Tang YY, Ma Y, Fan Y, Feng H, Wang J, Feng S, Lu Q, Hu B, Lin Y, Li J, Zhang Y, Wang Y, Zhou L, Fan M. Central and autonomic nervous system interaction is altered by short-term meditation. Proc Natl Acad Sci U S A. 2009;2;106(22):8865–70. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston K. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci. 2011 Apr 6;31(14):5540–8. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.