Abstract
Activation of Th2 CD4+ T cells is necessary and sufficient to elicit allergic airway disease, a mouse model with many features of human allergic asthma. Effectively controlling the activities of these cells could be a panacea for asthma therapy. Blood-feeding parasites have devised remarkable strategies to effectively evade the immune response. For example, ticks such as Ixodes scapularis, which must remain on the host for up to 7 days to feed to repletion, secrete immunosuppressive proteins. Included among these proteins is the 15-kDa salivary protein Salp15, which inhibits T cell activation and IL-2 production. Our objective for these studies was to evaluate the T cell inhibitory properties of Salp15 in a mouse model of allergic asthma. BALB/cJ mice were Ag sensitized by i.p. injection of OVA in aluminum hydroxide, with or without 50 µg of Salp15, on days 0 and 7. All mice were challenged with aerosolized OVA on days 14 –16 and were studied on day 18. Compared with control mice sensitized with Ag, mice sensitized with Ag and Salp15 displayed significantly reduced airway hyperresponsiveness, eosinophilia, Ag-specific IgG1 and IgE, mucus cell metaplasia, and Th2 cytokine secretion in vivo and by CD4+ T cells restimulated with Ag in vitro. Our results demonstrate that Salp15 can effectively prevent the generation of a Th2 immune response and the development of experimental asthma. These studies, and those of others, support the notion that a lack of ectoparasitism may contribute to the increasing prevalence of allergic asthma.
The prevalence and incidence of asthma and other atopic diseases continue to increase in the Western world (1). The hygiene hypothesis proposes a mechanism to explain the elevation in asthma prevalence, which states that decreased exposure to parasites and pathogens, particularly during early life, promotes the development of atopic immune responses against foreign Ags (2). The pathologic features of asthma are driven by an imbalance of CD4+ Th cells (T cells) favoring atopic Th2 responses (3). Th2 cells produce cytokines such as IL-4, IL-5, and IL-13, all of which have been implicated in the disease state. The overproduction of these cytokines in asthma results in a number of outcomes, including isotype class switching in B cells to produce IgE, eosinophilpoiesis, an increase in eosinophils within the airway, mucus production and secretion, as well as airway hyper-reactivity (4). Therefore, modulating the activities of Th2 cells represents a mechanism by which prominent features of the disease may be ameliorated.
Microorganisms and their products, including live or killed bacteria (5, 6), endotoxin (7), CpG DNA (8), as well as helminths (9), have all proven to be effective in the prevention, and even in some instances the treatment, of experimental models of allergic asthma. Currently, CpG DNA is in clinical trials for the treatment of allergic rhinitis and asthma (10). Although the mechanisms by which these potential therapeutics may work for the treatment of atopic disease remain incompletely understood, they center upon the modulation of the pathogenic Th2 immune response. Potential mechanisms of their action include the induction of a Th1 response, the IDO-dependent killing of pathogenic T cells, induction of T regulatory cells, or the promotion of the development of a Th2 response in the appropriate microenvironment.
Ectoparasites that feed on blood secrete a number of molecules into the host to prevent coagulation, promote the transmission of vector-borne pathogens, and modulate the host immune response (11, 12). The saliva of Ixodes scapularis ticks contains a mixture of proteins that have immunomodulatory activity. One such protein, Salp15, has been shown to inhibit early events in the activation of CD4+ T cells (13, 14). Anti-CD3 plus anti-CD28-activated CD4+ T cells pretreated with Salp15 demonstrate a reduction in Lck and Zap70 phosphorylation, which culminate in repression of calcium signaling within the cell (13, 15). Salp15 inhibits IL-2 production as well as CD25 (IL-2Rα) expression by CD4+ T cells, but has no effect on CD8+ T cells. It has recently been described that the specificity of Salp15 for CD4+ T cells is through its capacity to bind CD4 (13, 14), thereby presumably inhibiting the subsequent downstream signaling cascades leading to T cell activation.
Because Salp15 has been proven to inhibit early T cell signaling events, we hypothesized that Salp15 could represent a novel immunomodulatory molecule capable of preventing the pathologic activities of CD4+ T cells in a mouse model of allergic airways disease. We found that Salp15 reduced all of the features of allergic asthma that we examined, including airway hyperresponsiveness, eosinophilia, and IL-4, IL-5, and IL-13 production from effector CD4+ T cells restimulated with Ag in vitro. We also found that OVA-specific IgE and IgG1, mucus metaplasia, and bronchoalveolar lavage (BAL)3 levels of IL-4, IL-5, and inflammatory cytokines in the BAL were all reduced in mice that were treated with Salp15. These findings indicate that Salp15 may have potential utility in the prevention or treatment of allergic asthma and that ectoparasites, such as ticks, may be yet another of the factors diminished in the modern hygienic environment that normally prevents the development of atopic immune responses.
Materials and Methods
Mice
Six- to 8-wk-old female BALB/cJ mice were purchased from The Jackson Laboratory and housed in the American Association of Laboratory Animal Care accredited animal facility at the University of Vermont. The Institutional Animal Care and Use Committee granted approval for all studies.
Purification of recombinant His-tagged Salp15
His-tagged Salp15 was purified from Drosophila S2 cells as previously described (13, 15).
Experimental model of Ag sensitization and challenge
For the induction of allergic airway disease, mice were sensitized by administering OVA (20 µg, grade V; Sigma-Aldrich) with aluminum hydroxide (alum, 2.25 mg, ImjectAlum; Pierce) in a 100-µl total volume via i.p. injection on days 0 and 7. Mice were treated on days 0 and 7 via i.p. injection with a PBS vehicle or 50 µg Salp15 in a total volume of 100 µl ~2 min before alum plus OVA. All mice were challenged using three doses of aerosolized 1% OVA for 30 min on days 14–16 and were studied on day 18. In some studies, mice were unsensitized and unchallenged to establish baseline levels of several variables. Mice were euthanized by a lethal dose of pentobarbital via i.p. injection.
Pulmonary function assessment to measure airway hyperresponsiveness
Mice anesthetized with 90 mg/kg pentobarbital and tracheotomized were mechanically ventilated for the assessment of pulmonary function using the forced oscillation technique as previously described (16, 17). In brief, a tracheotomy tube was inserted and then connected to the inspiratory and expiratory ports of a volume-cycled ventilator (flexiVent; SCIREQ Scientific Respiratory Equipment). Mice were ventilated at a rate of 160–200 breaths/min, with a tidal volume of 0.2 ml, using a computer-controlled volume ventilator with 3 cm H2O positive end-expiratory pressure. Data from regular ventilation was collected to establish the baseline values for each animal. Pressure, flow, and volume were used to calculate the peak responses for airway resistance (RN), tissue damping (G), and tissue stiffness (H) (18) after challenge with inhaled doses of saline or aerosolized methacholine (Sigma-Aldrich) in saline, ranging from 3.125 to 50 mg/ml in half-log increments, as previously described (19). The percentage change from baseline after each methacholine dose (ΔRN, ΔG, and ΔH) is reported (16, 18, 20).
BAL, cell enumeration, and bio-plex analysis
BAL fluid was immediately collected from euthanized mice by instillation and recovery of 800 µl of 0.9% NaCl plus protease inhibitor mixture (Sigma-Aldrich) into the lungs through the tracheal cannula using a tuberculin syringe. The BAL fluid was centrifuged and the total cells in the pellet were resuspended in PBS and enumerated by counting with an Advia 120 Hematology System (Bayer). For cytospins, 2 × 104 cells were centrifuged onto glass slides at 800 rpm. Cytospins were stained using the Hema3 kit (Biochemical Sciences) and differential cell counts were performed on at least 500 cells. For the simultaneous quantitation of multiple analytes, undiluted BAL fluid was analyzed in duplicate using a mouse cytokine 23-plex kit on the Bio-Plex suspension array system (Bio-Rad) according to the manufacturer’s instructions. Standards were diluted in 0.9% NaCl plus protease inhibitor mixture. Standard curves were calculated and samples were analyzed using the Bio-Plex Manager software (Bio-Rad).
Serum collection and Ig analysis
Following euthanasia, ~300 µl of blood was collected via cardiac puncture of the right ventricle using a 26-gauge needle attached to a 1-ml syringe into serum separator tubes (BD Biosciences) and centrifuged, and serum was kept frozen at −80°C. For Ig ELISAs, 96-well plates were coated overnight at 4°C with 2 µg/ml OVA in PBS (pH 7.2–7.4), washed with 0.05% Tween 20 in PBS, and blocked for 2 h at 4°C with 1% BSA in PBS. Plates were washed and serum diluted in blocking solution was applied to the wells in triplicate at dilutions of 1/8 to 1/4096 and incubated overnight at 4°C. Plates were washed, and 2 µg/ml biotinylated secondary Abs (BD Pharmingen) in 1% BSA/PBS were incubated in the plates at room temperature for 1 h. Plates were washed, and 0.05 U/ml streptavidin/peroxidase (Roche) was incubated in the plates at room temperature for 1 h. Plates were washed, developed using reagents from R&D Systems, and ODs were read using a PowerWaveX (Bio-Tek Instruments) at 450 nm with background subtraction at 570 nm. Data are reported as OD values from identical dilutions in the linear range of the readings (1/1024).
Histopathology and morphometry
Following euthanasia and BAL, the left lobe of the lungs was instilled with 4% paraformaldehyde in PBS (4% paraformaledehyde) for 10 min at a pressure of 25 cm H2O and placed into 4% paraformaldehyde at 4°C overnight for fixation of the tissue. Fixed lungs were then mounted in paraffin, 7-µm sections were cut, affixed to glass microscope slides, deparaffinized, and stained with H&E and periodic acid-Schiff (PAS), coverslipped, and examined by light microscopy. Sections were morphometrically assessed for inflammatory cell infiltration and PAS (mucin) positivity of the air-ways. Multiple airways of similar size with a length-to-diameter ratio of <2:1 were assessed per section, and the type, number, and location of inflammatory cells, as well as the percentage of PAS-positive airway epithelial cells were recorded.
Semiquantitative RT-PCR
Total RNA isolated from lungs by RNeasy columns (Qiagen) was DNase-treated and reverse transcribed into cDNA using SuperscriptII (Invitrogen Life Technologies). Real-time quantitative RT-PCR was performed using TaqMan Universal PCR Master Mix (Applied Biosystems) and intron-spanning primers and probe (Applied Biosystems) designed and validated for mouse Muc5AC, an indicator of mucus cell metaplasia and mucus production, and the housekeeping gene HPRT. Forty cycles of PCR were performed on an Applied Biosystems Prism 7900HT Sequence Detection System using universal cycling conditions: denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 min. The level of Muc5AC expression was normalized to HPRT levels and relative Muc5AC mRNA levels were determined according to the comparative cycle threshold method (Applied Biosystems Prism 7700 Sequence Detection System, User Bulletin No. 2). In brief, the threshold cycle (CT) was determined for Muc5AC and HPRT in each sample. The ΔCT was calculated for each sample by subtracting the CT of HPRT from the CT of Muc5AC. The ΔΔCT values were calculated by subtracting the ΔCT of the OVA/OVA mice from the ΔCT of the OVA/OVA plus Salp15 samples. The ΔΔCT values were transformed into absolute values using the equation: 2−ΔΔCT.
Preparation, stimulation, and ELISA analysis of single-cell CD4+ lymphocyte suspensions
Single-cell suspensions were generated from spleens by passing the tissues through a 70-µm mesh, and lymphocytes were enriched on LSM Lymphocyte Separation Medium (MP Biomedicals). CD4+ T cells were isolated by positive selection using CD4 magnetic beads (Miltenyi Biotec) according to the manufacturer’s protocol. Isolated CD4+ T cells were >95% pure, as assessed by CD4 surface staining and FACS analysis. CD4+ T cells (4 × 106 cells/ml) were activated either by plate-bound anti-CD3ε (5 µg/ml; BD Pharmingen) and soluble anti-CD28 (1 µg/ml; BD Pharmingen) as a positive control for T cell stimulation or with 100 µg/ml OVA in the presence of syngeneic APCs (4 × 106 cells/ml) obtained by splenic T cell depletion by negative selection using Abs to CD4 (GK1.5) (21), CD8, and Thy-1 (22) and treatment with rabbit complement and mitomycin C, as previously described (23). Following 96 h of stimulation, supernatants were collected and analyzed for IL-2, IL-4, IL-5, IL-13, and IFN-γ using reagents and instructions from R&D Systems. ODs from triplicate samples and duplicate standards were read using a Bio-Tek Instruments PowerWaveX at 450 nm, with background subtraction at 570 nm.
Statistical analysis
For comparisons between the OVA/OVA and OVA/OVA plus Salp15 groups, data were analyzed by a two-tailed unpaired t test. For comparison of the control, OVA/OVA, and OVA/OVA plus Salp15 groups, data were analyzed by two-way ANOVA followed by comparison between groups by a two-tailed unpaired t test with Welch’s correction. Statistical calculations were performed using GraphPad Prism 4 for Windows (GraphPad). A p < 0.05 was considered to be statistically significant.
Results
Sensitized and challenged Salp15-treated mice have reduced numbers of inflammatory eosinophils and lymphocytes in their lungs
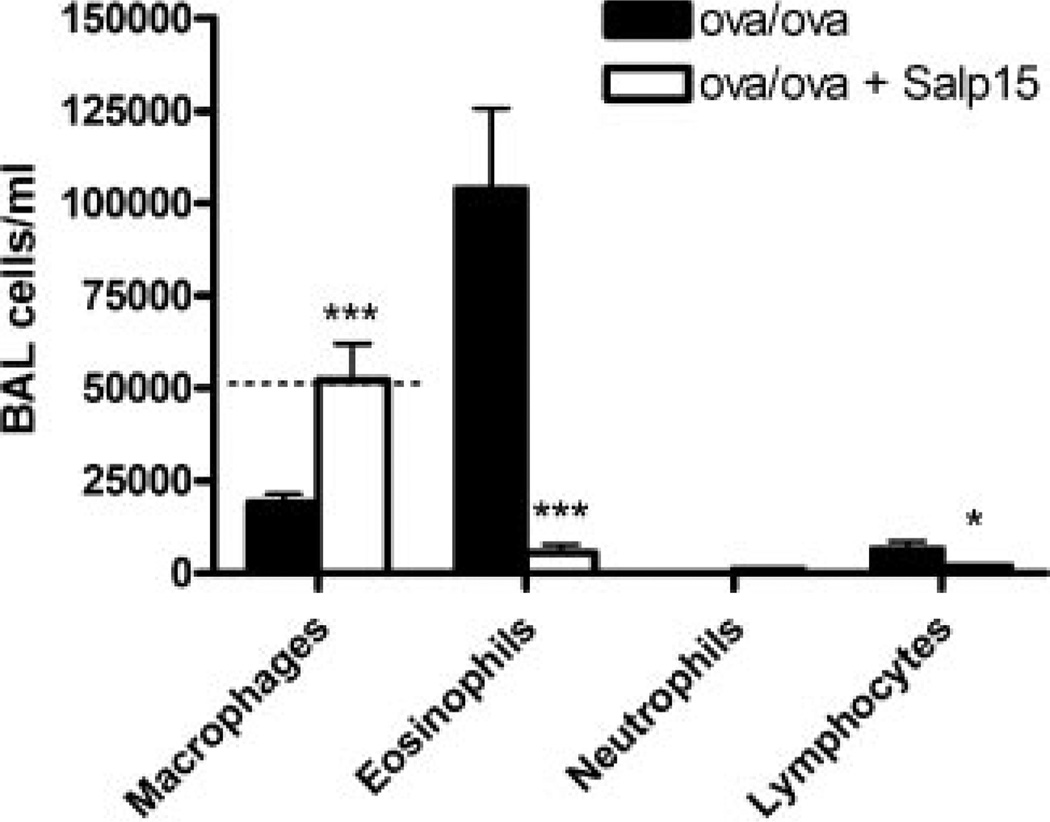
Salp15 inhibits CD4+ T cell activation in vitro and in vivo (15). Therefore, we sought to determine whether Salp15 could inhibit the activation of CD4+ T cells in a model in which they are both necessary and sufficient for the pathophysiology of disease, a mouse model of allergic asthma. Using an OVA model of allergic airway disease, mice were treated with 50 µg of Salp15 by i.p. injection immediately before i.p. immunization with OVA plus alum on days 0 and 7. Fifty micrograms of Salp15 was used because previous work has demonstrated that this dose and route of administration inhibits the activity of CD4+ T cells and the generation of Ag-specific IgG responses in vivo (15). As shown in Fig. 1, compared with control mice that received PBS vehicle instead of Salp15 and were immunized with OVA plus alum, the mice that received Salp15 had a statistically significant reduction in the numbers of eosinophils (p < 0.01) and lymphocytes (p < 0.05) present within the BAL fluid (BALF). The reduction in these cells contributed to the significant reduction in the total number of cells present within the BALF (Salp15-treated mice: 72,220 ± 7,395 vs controls: 190,600 ± 41,140; p < 0.05). Higher numbers of macrophages were present in the BALF of the mice that received Salp15 (p < 0.001) and these levels were similar to those in BALF from control mice. Furthermore, the macrophages from the Salp15-treated mice had the morphologic appearance of unactivated resident alveolar macrophages, with a dense cytoplasm that lacked extensions and vacuoles, when examined from cytospin preparations (data not shown).
FIGURE 1.
Reduced eosinophils and lymphocytes in the BALF following Ag sensitization and challenge in Salp15-treated mice compared with control mice. Mice were administered vehicle (OVA/OVA) or 50 µg of Salp15 (OVA/OVA + Salp15) immediately before OVA sensitization on days 0 and 7 and were challenged with OVA on days 14–16. BALF was collected 48 h after the third aerosolized OVA challenge, and cell counts were performed. Dotted line (where visible) indicate levels of cells in the BALF from unsensitized mice. Values are means (±SEM) of seven mice from the OVA/OVA group and six mice from the OVA/OVA + Salp15 group. Data were analyzed by a two-tailed unpaired t test. *, p ≤ 0.05; ***, p ≤ 0.001.
Reduced airway inflammation and mucus production in sensitized and challenged Salp15-treated mice
We assessed airway inflammation after OVA sensitization and challenge in Salp15-treated and untreated mice by examining the number and type of cells that were present in the lung tissue, as revealed by H&E staining (Fig. 2A). In the lungs of the Salp15-treated mice, we observed a reduction in the number of inflammatory cells present within the peribronchiolar and perivascular regions, compared with vehicle-treated control mice that had been Ag sensitized and challenged. In addition to the reductions in inflammatory cells, there were also reductions in the number of mucin-producing (PAS+) cells in the airways of the Salp15-treated mice (Fig. 2A). Real-time quantitative PCR of cDNA from the lungs also demonstrated a reduction in the expression of Muc5AC (p < 0.01; Fig. 2B), further indicating that Salp15-treated mice have a reduction in mucus production compared with controls.
FIGURE 2.
Salp15-treated mice have lower numbers of inflammatory cells and less mucus production within the airways. Sections from paraffin-embedded lung were stained using H&E and PAS reagents (A) to visualize inflammatory and mucus-producing airway cells. Original magnification, ×100. Samples are representative of six mice per group. B, RNA was collected from the whole lung, reverse transcribed, and analyzed using quantitative real-time PCR for Muc5AC relative to HPRT. Values are means (±SEM) of 10 mice from the OVA/OVA group and nine mice from the OVA/OVA + Salp15 group. Data were analyzed by a two-tailed un-paired t test. **, p ≤ 0.01.
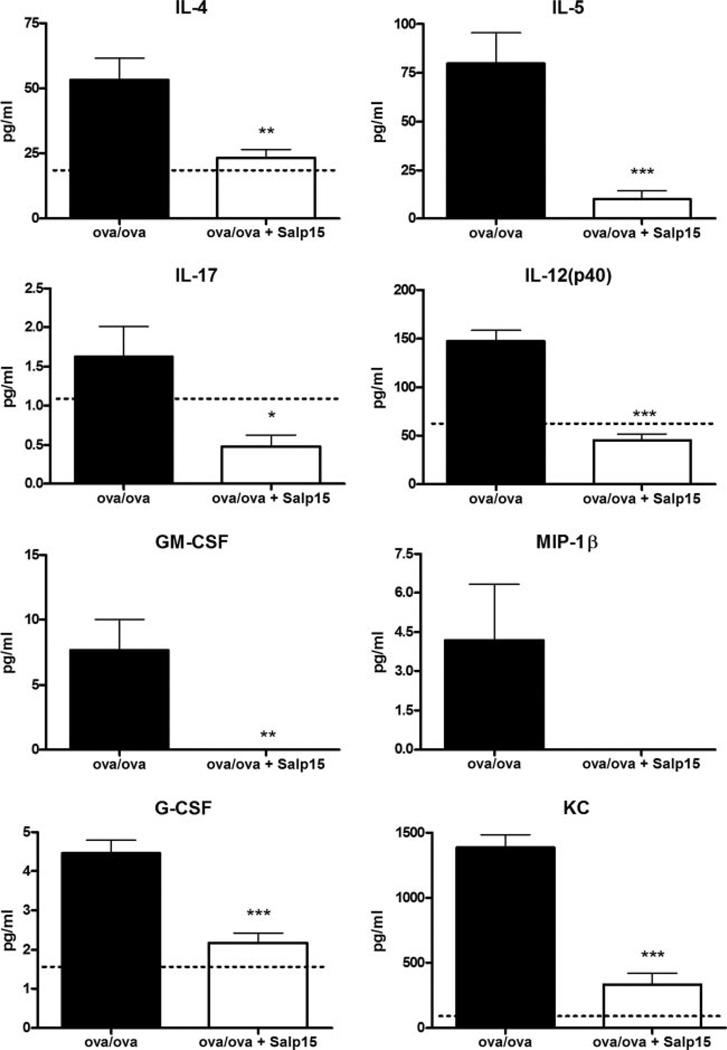
Salp15-treated mice secrete lower levels of Th2 and inflammatory cytokines into the airspaces compared with sensitized and challenged control mice
Following the allergen challenge of Ag-sensitized mice, a number of inflammatory cytokines are elaborated into the airspaces, which can be quantitated by a multianalyte approach, such as the Bio-Plex. We collected BALF from control or Salp15-treated Ag-sensitized and challenged mice 48 h after the final aerosolized challenge and measured cytokines using the mouse 23-plex kit. Although a number of cytokines were undetectable in the BALF from sensitized and challenged mice, including eotaxin, IFN-γ, IL-10, and IL-13, detectable levels of IL-4, IL-5, IL-17, IL-12p40, GM-CSF, keratinocyte chemoattractant, G-CSF, and MIP-1β were present (Fig. 3). However, in the BALF of the mice treated with Salp15, reduced levels of IL-4, IL-5, IL-17, IL-12p40, KC, and G-CSF were present. In addition, MIP-1β and GM-CSF were undetectable in the BALF of the Salp15-treated mice. These results support the hypothesis that the activities of Ag-specific CD4+ cells are diminished by treatment with Salp15, resulting in diminished Th2 cytokine production and Ag-induced inflammatory cytokine production.
FIGURE 3.
Salp15-treated mice produce lower levels of Th2 and inflammatory cytokines after Ag challenge compared with control mice. BALF was collected from Ag-sensitized and challenged control and Salp15-treated mice 48 h following the third aerosolized Ag challenge and was analyzed by Bio-Plex using the mouse cytokine 23-plex kit. Dotted lines indicate levels of cytokines in the BALF from unsensitized mice. The absence of a dotted line indicates that the cytokine was undetectable in the BALF from unsensitized mice. Values are means (±SEM) of 10 OVA/OVA mice and 9 OVA/OVA + Salp15 mice. Data were analyzed by two-tailed un-paired t test. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001.
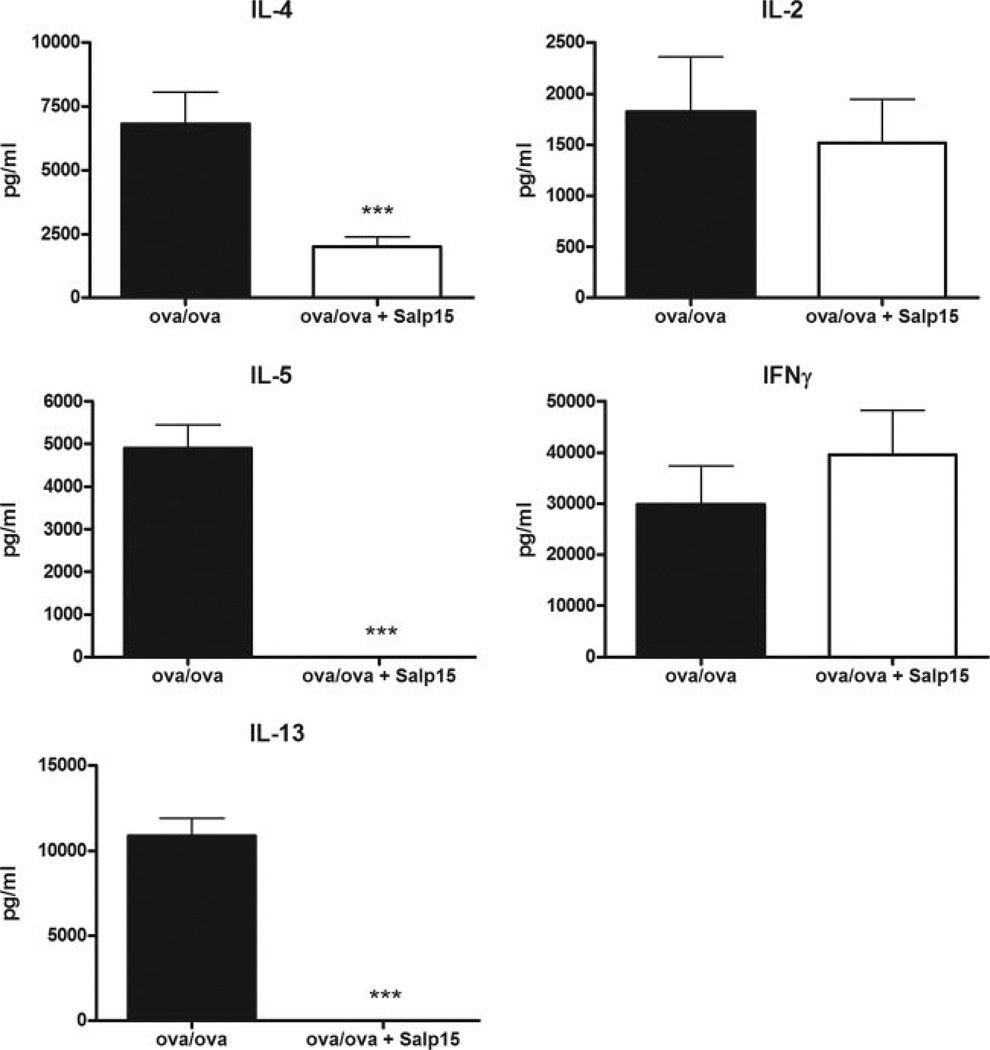
Ag-specific CD4+ T cells from sensitized and challenged Salp15-treated mice produce less IL-4, IL-5, and IL-13
Because prototypical Th2 cytokines may also be generated in the lung by cells besides CD4+ T cells, including eosinophils (24), mast cells, and basophils (25), we sought to determine whether treatment of mice with Salp15 results in a reduced capacity of their OVA-specific CD4+ T cells to generate the prototypic Th2 cytokines, IL-4, IL-5, and IL-13, compared with control mice. Therefore, purified CD4+ T cells from spleens were isolated from allergen-sensitized (with or without Salp15) mice 48 h after the final OVA challenge, and stimulated in vitro for 72 h with OVA in the presence of syngeneic APCs from naive mice. As measured by ELISA, CD4+ T cells from the Salp15-treated mice produced significantly lower levels of the Th2 cytokines IL-4, IL-5, and IL-13 than cells from the vehicle-treated mice (Fig. 4). Despite reductions in Th2 cytokines, however, there was no reduction in IFN-γ. Because Ag sensitization with alum and OVA promotes the generation of Th2 immunity in BALB/c mice, we were not surprised that the production of IFN-γ was not altered in the Salp15-treated mice. Furthermore, because treatment of the effector cells with Salp15 does not modulate the production of IL-2 (15), the fact that the levels of IL-2 were not reduced in the Ag-restimulated CD4+ T cells from Salp15-treated mice was also not surprising.
FIGURE 4.
CD4+ T cells from Salp15-treated mice produce lower levels of Th2 cytokines after Ag stimulation in vitro. Positive selection was performed from splenocytes to isolate CD4+ T cells from Ag-sensitized (with or without Salp15) mice ~48 h after the final OVA challenge. The cells were cultured for 72 h in the presence of OVA, and naive syngeneic APCs and ELISAs were performed from the cleared cellular supernatant. Values are means (±SEM) of 3–10 mice. Data were analyzed by a two-tailed unpaired t test. ***, p ≤ 0.001.
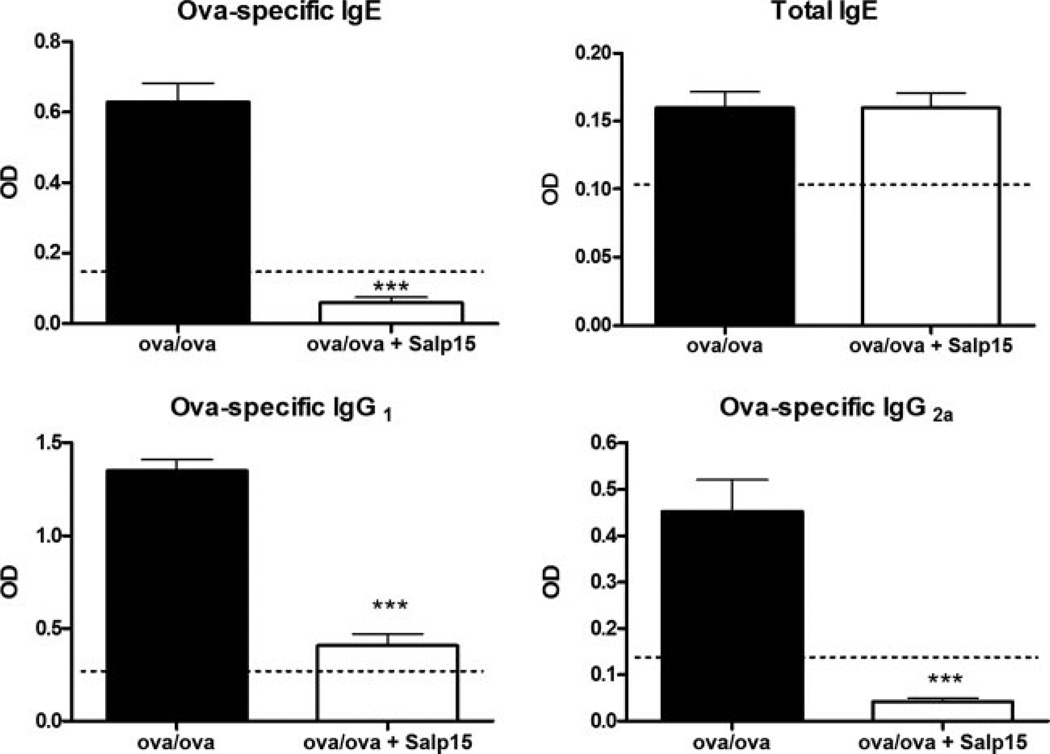
Sensitized and challenged Salp15-treated mice have reduced levels of OVA-specific IgG1 and IgE
Increased circulating levels of Ag-specific IgE and IgG1 are additional hallmarks of allergic disorders, including allergic asthma, and of mouse models of the disease. The isotype class switch of B lymphocytes to generate IgE and IgG1 is controlled by the Th2 cytokine IL-4 (26, 27). Therefore, having demonstrated reduced levels of IL-4 production in vivo and in vitro by Salp15-treated mice, we determined whether Salp15-treated mice also had a reduction in the circulating levels of these Igs. ELISA analysis of serum revealed that both OVA-specific IgG1 and IgE levels were reduced in the Salp15-treated mice (Fig. 5). We also measured serum OVA-specific IgG2a, class switch to which is controlled by IFN-γ (28), and found that its level was reduced in the mice that received Salp15. However, Salp15 treatment did not change the relatively low levels of total serum IgE measured at this time point.
FIGURE 5.
Serum Ig levels from vehicle control-treated Ag-sensitized and challenged mice (OVA/OVA) or Salp15-treated Ag-sensitized and challenged mice (OVA/OVA + Salp15). Diluted serum was analyzed 48 h after the third aerosolized OVA challenge for OVA-specific IgE (1/10), IgG1 (1/1000), IgG2a (1/50), as well as for total IgE (1/5000) by ELISA. Dotted lines indicate Ig levels in the serum from control (unsensitized) mice. Values are mean ODs (±SEM) from nine OVA/OVA mice and eight OVA/OVA + Salp15 mice. The serum dilutions were the same for the OVA/OVA and OVA/OVA + Salp15 groups. Data were analyzed by a two-tailed unpaired t test. ***, p ≤ 0.001.
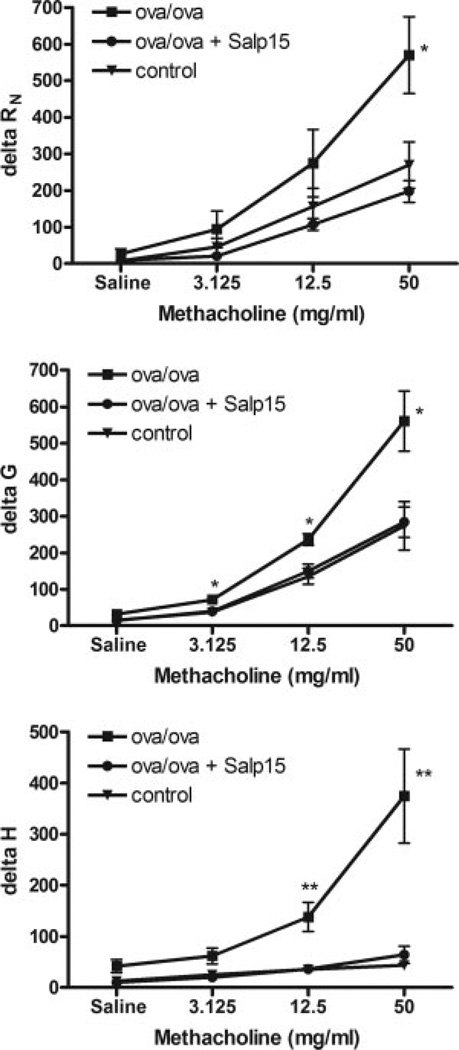
Salp15 protects mice from developing airway hyperresponsiveness
Arguably, the most important diagnostic criteria of allergic asthma is airway hyperresponsiveness, the enhanced reaction of the airway smooth muscle to agonists, such as methacholine or histamine, which promote bronchoconstriction. Therefore, we sought to determine whether Salp15 treatment would attenuate airway hyperresponsiveness. Anesthetized and tracheally cannulated mice were mechanically ventilated and, using a forced-oscillation technique, airway resistance (RN), tissue damping (G), and tissue stiffness (H) were calculated, as changes in these variables can implicate the subanatomical alterations in pulmonary function (17, 29). Ag-challenged Salp15-treated mice had significantly less methacholine-induced airway resistance, tissue elastance, and parenchymal hyperresponsiveness compared with Ag-sensitized and challenged mice. In fact, all of the measured parameters of airway hyperresponsiveness of the Salp15-treated mice were no different from naive control mice, which in this case had not been immunized or challenged with Ag. These results demonstrate that the effect of Salp15 treatment is not limited to either the conducting or distal airways and that the responsiveness to methacholine is that of control animals in each of the anatomical compartments tested, which is consistent with the overall diminishment in the inflammatory response in the lungs.
Discussion
In order for ectoparasites, such as wood ticks, to feed to repletion, they must remain on their host for as long as 7 days (12). Therefore, they possess inherent mechanisms, including the secretion of immunomodulatory agents, to combat the host immune response during the extended periods of blood feeding. Ixodes scapularis, the tick that transmits Borrelia burgdorferi, the causative agent of Lyme disease, secretes a number of proteins in its saliva, one of which is Salp15. Salp15 is a potent immunomodulatory protein thought to contribute to the ability of the tick to prevent the generation of immune responses to saliva Ags (15). Interestingly, Salp15 is also used by B. burg-dorferi to facilitate the transfer of the spirochete from the tick to the blood of the host (30).
Salp15 elicits its immunomodulatory properties through the inhibition of CD4+ T cell activation, proliferation, and IL-2 production (15). We have recently demonstrated that Salp15 binds selectively to the D1–D2 domains of the CD4 molecule (13), thereby preventing the activation of Lck and downstream substrates essential to the initiation of naive CD4+ T cell differentiation into effector T cells. Because CD4+ T cells play critical roles in allergic asthma (3), we hypothesized that Salp15 may prevent the development of the pathologic features in a mouse model of asthma by inhibiting the generation of CD4+ effector T cells. Our results demonstrate that Salp15 prevents the development of the atopic immune response modulated by CD4+ Th2 cells in the setting of allergic sensitization with OVA plus alum. Consequently, the fundamental features of allergic asthma, including airway eosinophilia, mucus cell metaplasia, Ag-specific Igs, Th2 and inflammatory cytokines, and airway hyperresponsiveness, were not generated in Ag-challenged mice that were Ag sensitized in the presence of Salp15. Although the reduction of IgE and IgG1 correlate with the suppression of IL-4 production by Salp15-treated animals, it remains incompletely understood why the in vivo generation of IgG2a was diminished in Salp15-treated mice, whereas the in vitro production of IFN-0γ and IL-2 were not diminished by Ag-restimulated CD4+ T cells from these same animals. As we observed, inhaled Ag challenge elicits a complex inflammatory response, including the generation of IL-12, a potent modulator of IgG2a production (31). Because the levels of IL-12 were reduced in the BALF of Salp15-treated mice, perhaps these reductions account for the decreased IgG2a measured in these mice.
Other molecules secreted into the host by ectoparasites, in addition to I. scapularis Salp15, may be potentially beneficial in combating inflammatory conditions, such as asthma. Recent studies have implicated the coagulation cascade, especially the formation of fibrin in the lung (32), as an important contributor to the pathophysiologic airway hyperreactivity response in an experimental model of allergic asthma. The medicinal leech (Hirudo medicinalis) secretes an extraordinarily potent anticoagulant, hirudin, which inhibits the activity of thrombin (33). Thrombin is elevated in the sputum from asthmatic individuals (34), where it may play a role in airway remodeling (35). Interestingly, hirudin has been used to alleviate pathophysiologies associated with remodeling in experimental models of allergic asthma, including diminishment of fibroblast proliferation (36). I. scapularis saliva also contains a potent anticoagulation protein, Salp14 (37), which may be capable of modulating airway hyperresponsiveness or remodeling. The tick, Rhipicephalus appendiculatus, secretes high-affinity histamine-binding proteins that inhibit histamine-induced effects (38). Although the neutralization of histamine is thought to reduce the removal of ticks by the host, the potentially beneficial effects of tick-derived histamine-neutralizing proteins have also been demonstrated in experimental models of allergic asthma (39). In fact, many of the effects observed were similar to those we observed with Salp15, including reduced Th2 cytokines, inflammation, and airway hyperreactivity (39). Finally, Salp25D from I. scapularis is homologous to various eukaryotic glutathione peroxidases (40), which could dampen the production of reactive oxygen species, thought to be important modulators of the inflammatory response in asthma (41). Recent evidence suggests that reactive oxygen species production may also be important for the generation of adaptive immune responses by providing a third signal for the activation of naive T cells (42). These data suggest that the salivary proteins from a number of hematophagous parasites have been demonstrated to or may be beneficial in the prevention or treatment of asthma. Of these proteins, Salp15 may be unique, through its mechanism of CD4 binding and inhibition, in its capacity to prevent the allergic sensitization necessary for the development of allergic asthma. However, the capacity of Salp15 to inhibit CD4+ T cells is likely not limited to Th2 responses, as the Ig-inducing effects of Ag plus CFA immunization are also diminished by Salp15 (15).
The reasons for the relatively recent rises in the prevalence of asthma (1) remain unclear, but the hygiene hypothesis states that decreased exposure to infectious microorganisms may play an important role in the development of atopic immune responses (2). In fact, bacteria and bacterial-derived molecules themselves can prevent the development of or even treat established asthma-like disease in an experimental setting (5–7), while infection with helmin-thic parasites is also able to suppress the deleterious atopic immune response that promotes allergic airway pathologies (9). Like helminthic enteroparasites, ectoparasites may also be capable of effectively modulating atopic disease. Our finding that the tick-derived salivary protein Salp15 prevents the development of experimental allergic asthma suggests that ticks or their secreted products may also be important and underappreciated contributors to the hygiene hypothesis.
FIGURE 6.
Reduced airway hyperresponsiveness in Salp15-treated mice. Mice were treated with vehicle or Salp15, then sensitized and challenged with Ag, as described above. Airway hyperresponsivness was assessed in mice that were treated with Salp15 (●) compared with mice that were treated with vehicle (■). These two groups of mice were also compared with mice that had not been sensitized or challenged, control (▼). Hyperresponsiveness to increasing amounts of methacholine was measured from forced oscillations and determined as airway resistance (Rn), tissue damping (G), and tissue stiffness (H). As derived from the constant phase model, data are expressed as the percent change from baseline measurements (±SEM) for each of the parameters measured. Data are inclusive of three separate experiments containing a total of eight OVA/OVA mice, nine Salp15-treated mice, and three controls. Differences in the curves for the three groups of mice were analyzed by two-way ANOVA (RN, p ≤ 0.01; G, p ≤ 0.01; H, p ≤ 0.0001) and differences at each dose of methacholine between the control and OVA/OVA or OVA/OVA + Salp15 groups were compared using a two-tailed unpaired t test with Welch’s correction. *, p ≤ 0.05; **, p ≤ 0.01.
Acknowledgment
We thank Dr. Mercedes Rincon (University of Vermont, Burlington, VT) for innumerable helpful discussions.
Footnotes
This work was supported by National Institutes of Health/National Heart, Lung, and Blood Institute Predoctoral Pulmonary Training Grant T32 HL076122 (to S.A.P.), by Institutional Research Support from the University of Vermont, College of Medicine (to M.E.P.), by National Institutes of Health National Center for Research Resources Center of Biomedical Research Excellence Grant RR15557 (to M.E.P. and L.A.W.), and by National Institutes of Health Grant AI053064 (to J.A.).
Abbreviations used in this paper: BAL, bronchoalveolar lavage; alum, aluminum hydroxide; BALF, BAL fluid; PAS, periodic acid-Schiff; CT, threshold cycle.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N. Engl. J. Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 2.Schaub B, Lauener R, von Mutius E. The many faces of the hygiene hypothesis. J. Allergy Clin. Immunol. 2006;117:969–977. doi: 10.1016/j.jaci.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Mazzarella G, Bianco A, Catena E, De Palma R, Abbate GF. Th1/Th2 lymphocyte polarization in asthma. Allergy. 2000;55(Suppl. 61):6–9. doi: 10.1034/j.1398-9995.2000.00511.x. [DOI] [PubMed] [Google Scholar]
- 4.Taube C, Dakhama A, Gelfand EW. Insights into the pathogenesis of asthma utilizing murine models. Int. Arch. Allergy Immunol. 2004;135:173–186. doi: 10.1159/000080899. [DOI] [PubMed] [Google Scholar]
- 5.Yeung VP, Gieni RS, Umetsu DT, DeKruyff RH. Heat-killed Listeria monocytogenes as an adjuvant converts established murine Th2-dominated immune responses into Th1-dominated responses. J. Immunol. 1998;161:4146–4152. [PubMed] [Google Scholar]
- 6.Hopfenspirger MT, Agrawal DK. Airway hyperresponsiveness, late allergic response, and eosinophilia are reversed with mycobacterial antigens in ovalbumin-presensitized mice. J. Immunol. 2002;168:2516–2522. doi: 10.4049/jimmunol.168.5.2516. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez D, Keller AC, Faquim-Mauro EL, de Macedo MS, Cunha FQ, Lefort J, Vargaftig BB, Russo M. Bacterial lipopolysaccharide signaling through Toll-like receptor 4 suppresses asthma-like responses via nitric oxide synthase 2 activity. J. Immunol. 2003;171:1001–1008. doi: 10.4049/jimmunol.171.2.1001. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi T, Beck L, Rossetto C, Gong X, Takikawa O, Takabayashi K, Broide DH, Carson DA, Raz E. Inhibition of experimental asthma by indoleamine 2,3-dioxygenase. J. Clin. Invest. 2004;114:270–279. doi: 10.1172/JCI21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitagaki K, Businga TR, Racila D, Elliott DE, Weinstock JV, Kline JN. Intestinal helminths protect in a murine model of asthma. J. Immunol. 2006;177:1628–1635. doi: 10.4049/jimmunol.177.3.1628. [DOI] [PubMed] [Google Scholar]
- 10.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 11.Wikel SK. Host immunity to ticks. Annu. Rev. Entomol. 1996;41:1–22. doi: 10.1146/annurev.en.41.010196.000245. [DOI] [PubMed] [Google Scholar]
- 12.Binnington KC, Kemp DH. Role of tick salivary glands in feeding and disease transmission. Adv. Parasitol. 1980;18:315–339. doi: 10.1016/s0065-308x(08)60403-0. [DOI] [PubMed] [Google Scholar]
- 13.Garg R, Juncadella IJ, Ramamoorthi N, Ashish, Ananthanarayanan SK, Thomas V, Rincon M, Krueger JK, Fikrig E, Yengo CM, Anguita J. Cutting edge: CD4 is the receptor for the tick saliva immunosuppressor, Salp15. J. Immunol. 2006;177:6579–6583. doi: 10.4049/jimmunol.177.10.6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juncadella IJ, Garg R, Ananthnarayanan SK, Yengo CM, Anguita J. T-cell signaling pathways inhibited by the tick saliva immunosuppressor, Salp15. FEMS Immunol. Med. Microbiol. 2007;49:433–438. doi: 10.1111/j.1574-695X.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 15.Anguita J, Ramamoorthi N, Hovius JW, Das S, Thomas V, Persinski R, Conze D, Askenase PW, Rincon M, Kantor FS, Fikrig E. Salp15, an ixodes scapularis salivary protein, inhibits CD4+ T cell activation. Immunity. 2002;16:849–859. doi: 10.1016/s1074-7613(02)00325-4. [DOI] [PubMed] [Google Scholar]
- 16.Poynter ME, Cloots R, van Woerkom T, Butnor KJ, Vacek P, Taatjes DJ, Irvin CG, Janssen-Heininger YM. NF-κB activation in airways modulates allergic inflammation but not hyperresponsiveness. J. Immunol. 2004;173:7003–7009. doi: 10.4049/jimmunol.173.11.7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomioka S, Bates JH, Irvin CG. Airway and tissue mechanics in a murine model of asthma: alveolar capsule vs. forced oscillations. J. Appl. Physiol. 2002;93:263–270. doi: 10.1152/japplphysiol.01129.2001. [DOI] [PubMed] [Google Scholar]
- 18.Lundblad LK, Thompson-Figueroa J, Leclair T, Sullivan MJ, Poynter ME, Irvin CG, Bates JH. Tumor necrosis factor-α overexpression in lung disease: a single cause behind a complex phenotype. Am. J. Respir. Crit. Care Med. 2005;171:1363–1370. doi: 10.1164/rccm.200410-1349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J. Exp. Med. 1997;186:449–454. doi: 10.1084/jem.186.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poynter M, Irvin C, Janssen-Heniniger Y. Rapid activation of nuclear factor-κB in airway epithelium in a murine model of allergic airway inflammation. Am. J. Pathol. 2002;160:1325–1334. doi: 10.1016/s0002-9440(10)62559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dialynas DP, Wilde DB, Marrack P, Pierres A, Wall KA, Havran W, Otten G, Loken MR, Pierres M, Kappler J, et al. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol. Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 22.Jones B. Evidence that the Thy-1 molecule is the target for T cell mitogenic antibody against brain-associated antigens. Eur. J. Immunol. 1983;13:678–684. doi: 10.1002/eji.1830130813. [DOI] [PubMed] [Google Scholar]
- 23.Cohn L, Homer RJ, MacLeod H, Mohrs M, Brombacher F, Bottomly K. Th2-induced airway mucus production is dependent on IL-4Rα, but not on eosinophils. J. Immunol. 1999;162:6178–6183. [PubMed] [Google Scholar]
- 24.Woerly G, Roger N, Loiseau S, Capron M. Expression of Th1 and Th2 immunoregulatory cytokines by human eosinophils. Int. Arch. Allergy Immunol. 1999;118:95–97. doi: 10.1159/000024038. [DOI] [PubMed] [Google Scholar]
- 25.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu. Rev. Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 26.Severinson E, Sideras P, Bergstedt-Lindqvist S. IgG1 induction factor. Int. Rev. Immunol. 1987;2:143–156. doi: 10.3109/08830188709044751. [DOI] [PubMed] [Google Scholar]
- 27.Coffman RL, Savelkoul HF, Lebman DA. Cytokine regulation of immunoglobulin isotype switching and expression. Semin. Immunol. 1989;1:55–63. [PubMed] [Google Scholar]
- 28.Jurado A, Carballido J, Griffel H, Hochkeppel HK, Wetzel GD. The immunomodulatory effects of interferon-γ on mature B-lymphocyte responses. Experientia. 1989;45:521–526. doi: 10.1007/BF01990501. [DOI] [PubMed] [Google Scholar]
- 29.Wagers SS, Haverkamp HC, Bates JH, Norton RJ, Thompson-Figueroa JA, Sullivan MJ, Irvin CG. Intrinsic and antigen-induced airway hyperresponsiveness are the result of diverse physiological mechanisms. J. Appl. Physiol. 2007;102:221–230. doi: 10.1152/japplphysiol.01385.2005. [DOI] [PubMed] [Google Scholar]
- 30.Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, Anguita J, Norgard MV, Kantor FS, Anderson JF, et al. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature. 2005;436:573–577. doi: 10.1038/nature03812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rempel JD, Wang M, HayGlass KT. In vivo IL-12 administration induces profound but transient commitment to T helper cell type 1-associated patterns of cytokine and antibody production. J. Immunol. 1997;159:1490–1496. [PubMed] [Google Scholar]
- 32.Wagers S, Norton R, Bates J, Irvin CG. Role of fibrin in determining airway closure. Chest. 2003;123:362S–363S. doi: 10.1378/chest.123.3_suppl.362s. [DOI] [PubMed] [Google Scholar]
- 33.Markwardt F, Sturzebecher J, Glusa E. Antithrombin effects of native and recombinant hirudins. Biomed. Biochim. Acta. 1990;49:399–404. [PubMed] [Google Scholar]
- 34.Gabazza EC, Taguchi O, Tamaki S, Takeya H, Kobayashi H, Yasui H, Kobayashi T, Hataji O, Urano H, Zhou H, et al. Thrombin in the airways of asthmatic patients. Lung. 1999;177:253–262. doi: 10.1007/pl00007645. [DOI] [PubMed] [Google Scholar]
- 35.Panettieri RA, Jr, Hall IP, Maki CS, Murray RK. α-thrombin increases cytosolic calcium and induces human airway smooth muscle cell proliferation. Am. J. Respir. Cell Mol. Biol. 1995;13:205–216. doi: 10.1165/ajrcmb.13.2.7626288. [DOI] [PubMed] [Google Scholar]
- 36.Terada M, Kelly EA, Jarjour NN. Increased thrombin activity after allergen challenge: a potential link to airway remodeling? Am. J. Respir. Crit. Care Med. 2004;169:373–377. doi: 10.1164/rccm.200308-1156OC. [DOI] [PubMed] [Google Scholar]
- 37.Pedra JH, Narasimhan S, Deponte K, Marcantonio N, Kantor FS, Fikrig E. Disruption of the salivary protein 14 in Ixodes scapularis nymphs and impact on pathogen acquisition. Am. J. Trop. Med. Hyg. 2006;75:677–682. [PubMed] [Google Scholar]
- 38.Paesen GC, Adams PL, Harlos K, Nuttall PA, Stuart DI. Tick histamine-binding proteins: isolation, cloning, and three-dimensional structure. Mol. Cell. 1999;3:661–671. doi: 10.1016/s1097-2765(00)80359-7. [DOI] [PubMed] [Google Scholar]
- 39.Couillin I, Maillet I, Vargaftig BB, Jacobs M, Paesen GC, Nuttall PA, Lefort J, Moser R, Weston-Davies W, Ryffel B. Arthropod-derived histamine-binding protein prevents murine allergic asthma. J. Immunol. 2004;173:3281–3286. doi: 10.4049/jimmunol.173.5.3281. [DOI] [PubMed] [Google Scholar]
- 40.Das S, Banerjee G, DePonte K, Marcantonio N, Kantor FS, Fikrig E. Salp25D, an Ixodes scapularis antioxidant, is 1 of 14 immunodominant antigens in engorged tick salivary glands. J. Infect. Dis. 2001;184:1056–1064. doi: 10.1086/323351. [DOI] [PubMed] [Google Scholar]
- 41.Andreadis AA, Hazen SL, Comhair SA, Erzurum SC. Oxidative and nitrosative events in asthma. Free Radical Biol. Med. 2003;35:213–225. doi: 10.1016/s0891-5849(03)00278-8. [DOI] [PubMed] [Google Scholar]
- 42.Tse HM, Milton MJ, Schreiner S, Profozich JL, Trucco M, Piganelli JD. Disruption of innate-mediated proinflammatory cytokine and reactive oxygen species third signal leads to antigen-specific hyporesponsiveness. J. Immunol. 2007;178:908–917. doi: 10.4049/jimmunol.178.2.908. [DOI] [PubMed] [Google Scholar]