Abstract
Objective
Dengue continues to cause significant global morbidity and mortality. Severe disease is characterized by cardiovascular compromise from capillary leakage. Cardiac involvement in dengue has also been reported, but has not been adequately studied.
Setting
Hospital for Tropical Diseases, Ho Chi Minh City, Viet Nam.
Design
Seventy-nine patients aged 8-46 years with different dengue severity grades were studied using echocardiography including tissue Doppler imaging. The patients were split into severity grades: dengue, dengue with warning signs and severe dengue. Changes in cardiac functional parameters and haemodynamic indices were monitored over the hospital stay.
Measurements and Main Results
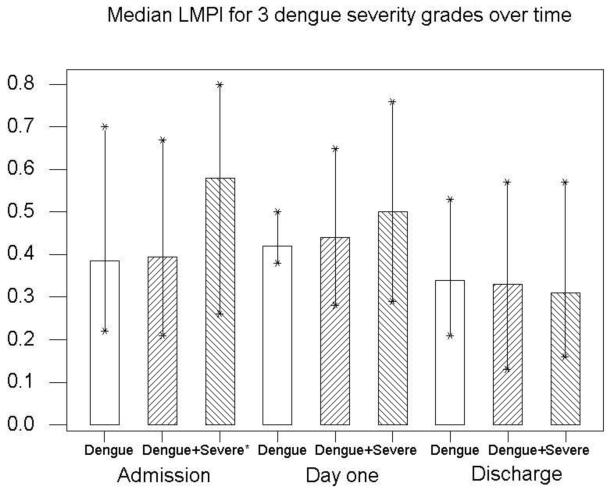
Patients with severe dengue had worse cardiac function compared to dengue, in the form of left ventricular (LV) systolic dysfunction with increased Left myocardial performance index (LMPI) (0.58 (0.26-0.80) vs. 0.38 (0.22-0.70), P=0.006). Septal myocardial systolic velocities (S′) were reduced, (6.4 (4.8-10) vs. 8.1 (6-13) cm/s, P=0.01) as well as right ventricular (RV) systolic (11.4 (7.5-17) vs. 13.5 (10-17) cm/s, P=0.016), and diastolic (E′) velocities (13 (8-23) vs. 17 (12-25) cm/s, P=0.0026). In the severe group, these parameters improved from hospital admission to discharge; septal S′ to 8.8 (7-11) cm/s (P=0.002), RV S′ to 15.0 (11.8-23) cm/s, (P=0.003) and diastolic velocity E′ to 21 (11-25) cm/s (P=0.002). Patients with cardiac impairment were more likely to have significant pleural effusions.
Conclusions
Patients with severe dengue have evidence of systolic and diastolic cardiac impairment, with septal and right ventricular wall being predominantly affected.
Keywords: Dengue, cardiac function, Haemodynamics, Vietnam, echocardiography, Tissue Doppler Imaging
Introduction
Dengue is the second most common vector born disease in the world with 50-100 million cases reported annually and 2.5 billion people at risk of the disease [1]. It is also the most rapidly spreading, with a fourfold increase in incidence in the last decade. Dengue causes a spectrum of disease processes ranging from dengue fever, a non-specific viral illness, to severe disease where patients can develop cardiovascular compromise secondary to increased capillary permeability and plasma leak. Cardiac complications have also been reported including; reduced myocardial function, acute myocarditis and conduction disturbances [2,3,4,5]. The nature and mechanism of the cardiac impairment and its contribution to severe disease has not been systematically defined.
Cardiac function as assessed by traditional echocardiographic parameters is dependent on preload. Severe dengue is characterized by reduced intravascular volume making accurate cardiac functional evaluation more difficult. Myocardial tissue Doppler imaging (TDI) is known for its sensitivity in detecting diastolic and early systolic dysfunction, and is considered to be relatively preload independent [6,7,8,9].
In this study we investigated in detail cardiac affection across a spectrum of dengue patients with different disease severities using echocardiographic techniques including TDI, coupled with intravascular volume measurements, and monitoring of any changes throughout the course of the hospital stay.
Patients, Materials and Methods
This observational study was conducted in the Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam, in September and October 2008. Children and adults admitted to the hospital with suspected dengue of any severity were eligible for inclusion in the study. Diagnosis of dengue was confirmed using standard IgM and IgG ELISA and NS1 assays [10]. Seventy nine patients were included in the study, 22 of whom had dengue, 42 dengue with warning signs (box 1) and 15 with severe dengue (box 2), according to the 2009 WHO Dengue Clinical Case definition [11]. Using the previous WHO classification, there was 1 dengue fever, 22 dengue hemorrhagic fever (DHF) grade 1, 43 DHF grade 2, 12 DHF grade 3 and 1 grade 4. Informed consent was obtained from all patients or their guardians. The study was approved by the Scientific and Ethical Committee of the Hospital for Tropical Diseases, Ho Chi Minh City. Clinical and Laboratory parameters were recorded, including, Blood pressure, respiratory rate, haematocrit, platelets, electrolytes and liver enzymes. Troponin I was measured on admission in a sub group of patients. Patients who met the criteria for severe dengue with shock received intravenous fluids, with an initial bolus of 15 ml/kg over 1 hour. Further boluses of 10ml/kg were given if signs of haemodynamic compromise persisted.
Box 1. Dengue warning signs.
Abdominal pain or tenderness
Persistent vomiting
Clinical fluid accumulation
Mucosal bleed
Lethargy/ restlessness
Liver enlargement >2 cm
Increase in HCT concurrent with rapid decrease in platelet count
Box 2. Severe Dengue.
- Evidence of plasma leakage
- -High or progressively rising HCT
- -Pleural effusions or ascites
- - Circulatory compromise or shock (tachycardia, cold extremities, pulse pressure <20 mmHg or unrecordable blood pressure)
Significant bleeding
Altered consciousness
Severe gastrointestinal involvement (persistent vomiting, increasing abdominal pain or jaundice)
Severe organ impairment
Taken from WHO 2009 dengue case classification
Echocardiography
Echocardiographic examinations were performed by two of the investigators (SY and AG), using a Phillips HD11 system with cardiac settings and linear array probe (4 - 2 MHz). In patients with shock the first echocardiogram was performed after initial resuscitation, or as soon as the patient was stable and able to be transferred to the ultrasound department. Echocardiograms were repeated at 24 hours, and before discharge, and included two-dimensional, M-mode, Doppler and tissue Doppler (TD) studies. Measurements were made from 3 consecutive cardiac cycles and the average was calculated. The 2D parasternal long axis view was used to measure the left ventricular end diastolic (LVEDD) and end systolic (LVESD) dimensions just below the tips of the mitral valve leaflets. The ejection fraction was calculated as; EF=(LVEDD3 – LVESD3)/LVEDD3%. Transmitral pulsed wave Doppler velocities were obtained from the apical four chamber view with the sample volume positioned at the tips of the mitral valve leaflets, and the aortic velocity from the apical five-chamber view with the sample volume positioned just below the aortic valve cusps, and the velocity time Integral (VTI) was measured. Left myocardial performance index (LMPI) was calculated from trans-aortic and trans-mitral pulsed Doppler velocities, and RMPI from tricuspid and pulmonary Doppler, using previously defined method [(isovolumic contraction time + isovolumic relaxation) time/ ejection time] [12]. Stroke volume (SV) was calculated as VTI × cross sectional area of the aortic valve (CSA), with (CSA= 0.785 × aortic diameter2) and cardiac output (CO) as SV × Heart Rate. Myocardial TD velocities were obtained in the standard manner, with the sector width and depth optimized to ensure maximum frame rate with optimum gain adjustment. Myocardial velocities were taken at the LV lateral (S′lat), septal (S′sept) and RV walls at the level of the mitral and tricuspid valve annulus, respectively. Peak systolic (S′) and early diastolic (E′) velocities were taken. Measurements of annular systole excursion at the mitral (MAPSE) and tricuspid (TAPSE) free wall were taken from nadir to peak. The inferior vena cava (IVC) was measured using the subcostal view, below the level of the hepatic veins, 5cm from the IVC-right atrial junction. The maximal diameter during expiration and minimal diameter in inspiration were measured from M-mode recordings and the collapsibility index (IVCCI) calculated using the formula IVCCI= (IVCmax-IVCmin/IVCmax)×100 [13]. Pericardial effusions were reported using conventional criteria. A left pleural effusion was reported if an echo-free space was seen around the heart and descending aorta, in the sub costal view. And a right pleural effusion was reported if an echo free space was seen posterior to the liver. The pleural effusions were quantified visually as; minimal, small, moderate or large. All images were stored digitally and reviewed by a consultant cardiologist in the UK (MH) independent to the clinical severity of disease. The inter-and intra-user variability was less than 10%.
Statistics
Medians, minimum and maximum values were calculated for continuous variates. Percentages for two groups were compared using a Fisher exact test and continuous variates were compared using a Mann Whitney test. The Wilcoxon signed ranks test was used to compare values at admission and discharge. Associations between continuous variates were measured using Spearman’s rank correlation coefficient. Significant evidence of a difference was indicated by p < 0.05.
Results
The median age of all the patients was 20 years (range 8 – 46 years) and the male: female ratio was 50:29. Length of hospital stay varied between 2- 7 days (median 3 days). Time from admission to first echo ranged between 2.5-72 hours (median 23 hours). Further admission laboratory parameters are shown in table 1. Fifty-one patients had a 12 lead ECG recorded; of which 18/51 (35%) were abnormal; 3/10 (30%) in dengue, 11/32 (34%) in dengue + and 4/9 (44%) in the severe group. The abnormalities included 1st degree AV block, sinus bradycardia, T wave changes and ST segment abnormalities. There were no deaths in the study group.
Table 1. Admission characteristics for patients with different dengue severity grades.
| Dengue 22 |
Dengue+ 42 |
p-value | Severe dengue 15 |
p-value | |
|---|---|---|---|---|---|
|
| |||||
| Age (yrs) | 18.5 (8 - 33) | 21.5 (14 – 46) | 0.007 | 18.0 (11 – 36) | 0.70 |
| Illness day | 6.0 (2 – 9) | 6.0 (4 – 8) | 0.13 | 5.0 (3 – 8) | 0.78 |
| Temp ( °C) | 37.5 (37 – 40) | 37.2 (37 – 40) | 0.22 | 37.2 (36 – 39) | 0.22 |
| Resp rate(b/min) | 20.0 (18 – 28) | 20.0 (18 – 24) | 0.98 | 22.0 (20 – 24) | 0.11 |
| Creat (μmol/l) | 89.5 (58 – 142) | 80.0 (42 – 112) | 0.32 | 79.0 (42 – 109) | 0.36 |
| ALT (mg/dl) | 62.0 (19 – 76) | 83.0 (14 – 331) | 0.056 | 73.0 (7 – 298) | 0.066 |
| K (mmol/l) | 3.5 (2.9 – 3.8) | 3.7 (3.1 – 5.1) | 0.053 | 3.7 (3.1 – 4.5) | 0.099 |
| HCT (%) | 43.8 (35 – 50) | 44.6 (36 – 56) | 0.13 | 44.0 (36 – 61) | 0.40 |
| WBC (g/dl) | 2.96 (1.6 – 5.4) | 3.18 (1.1 – 5.6) | 0.89 | 3.90 (1.7 – 7.1) | 0.24 |
| Platelets (/l) | 82.0 (5 – 233) | 36.0 (9 – 177) | 0.017 | 28.0 (3 – 119) | 0.006 |
| Pericardial effusion | 1 | 0 | 1 | ||
| Pleural effusion | 1/20 | 12/40 | 0.043 | 12/13 | <0.0001 |
Data are presented as median (minimum - maximum). The p-values refer to a test of dengue against dengue+ and dengue against severe dengue. They are obtained using Mann-Whitney (non-parametric) tests, except for pleural effusion where Fisher exact tests are used.
Clinical and biochemical findings
Admission clinical and biochemical data are shown in table 1. Platelets decreased parallel to disease severity (dengue: 82.0 (5-223) vs. severe: 28.0 (3-119) /l, P=0.006). Troponin I was tested in 17 patients at admission, one severe dengue patient had a borderline raised troponin of 0.31 ng/ml (normal<0.3 ng/ml), and the other 16 in different severity grades were normal. The amount of intravenous fluids received was slightly more in severe dengue compared to dengue +group (2350 (450-4800) vs. 1500 (500-3500)L, P=0.73). Dengue patients only received oral rehydration therapy, which was not possible to quantify.
Cardiac function between groups
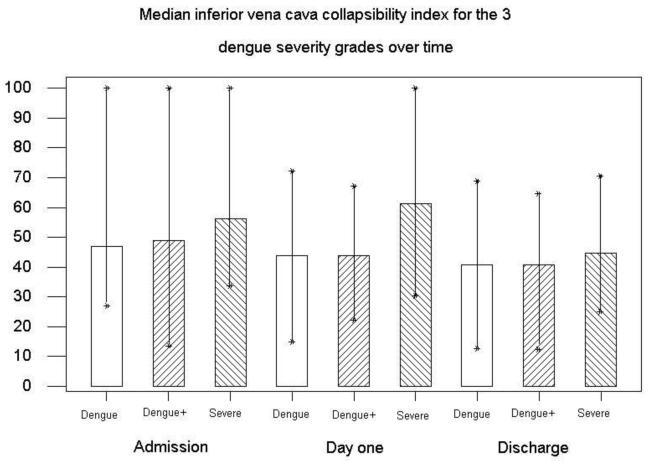
Heart rate was higher in dengue compared with the dengue + patients (88 (62-110) vs. 80(50-105) b/min, P=0.001) (table 2). This could be explained by the higher number of dengue+ patients with ECG abnormalities, particularly bradyarrythmias. Stroke volume (SV) was lowest in the severe group (table 2). The Inferior vena cava collapsibility index (IVCCI) increased with disease severity, but was not statistically significant (46.8 (27.1-100) vs. 56.3(33.7-100) P=0.19). (Fig 1, table 2). More patients in the severe group had pleural effusions compared to the other groups (92% vs. 30% and 5%, P<0.001) (table 1). The pleural effusions were larger in the severe group with 8/12 being quantified as moderate and 4/12 as small. In dengue+ 11/12 were small and 1/12 minimal, and dengue 1/1 had a minimal pleural effusion.
Table 2. Echocardiographic parameters by dengue grade at admission.
| n | Dengue 22 |
Dengue + 42 |
p-value | Severe dengue 15 |
p-value |
|---|---|---|---|---|---|
|
| |||||
| HR (b/min) | 88 (62 - 110) | 80 (50 – 105) | 0.001 | 88 (58 – 115) | 0.92 |
| PP (mmHg) | 40 (30 – 50) | 40 (20 – 50) | 0.99 | 30 (20 – 40) | 0.011 |
| SV (ml) | 42.6 (23.2 – 61.5) | 46.6 (19.0 – 75.1) | 0.12 | 35.7 (25.9 – 59.5) | 0.19 |
| CO (l/min) | 3.81 (2.15 – 5.39) | 3.47 (1.98 – 6.76) | 0.46 | 3.11 (1.87 – 5.21) | 0.16 |
| LVDd (cm) | 4.17 (3.39 – 5.03) | 4.29 (3.25 – 5.22) | 0.28 | 4.10 (2.97 – 4.87) | 1.00 |
| EF (%) | 68.8 (57.8 – 80.2) | 67.8 (54.6 – 79.1) | 0.60 | 66.0 (48.0 – 75.9) | 0.095 |
| S′ lat (cm/s) | 12.0 (9.0 – 16.5) | 11.2 (7.0 – 18.0) | 0.32 | 10.9 (7.0 – 16.0) | 0.11 |
| S′ sept (cm/s) | 8.1 (6.0 – 13.0) | 8.0 (6.0 – 12.2) | 0.43 | 6.4 (4.8 – 10.0) | 0.010 |
| S′ RV (cm/s) | 13.5 (10.0 – 17.0) | 13.0 (9.0 – 22.0) | 0.65 | 11.4 (7.5 – 17.0) | 0.016 |
| RMPI | 0.14 (0.06 – 0.38) | 0.23 (0.03 – 0.42) | 0.006 | 0.17 (0.04 – 0.43) | 0.18 |
| L MPI | 0.38 (0.22 – 0.70) | 0.39 (0.21 – 0.67) | 0.94 | 0.58 (0.26 – 0.80) | 0.006 |
| E′ (cm/s) | 17 (12 – 25) | 17 (8 – 27) | 0.33 | 13 (8 –23) | 0.0026 |
| MAPSE(cm/s) | 14.0 (11.0 – 18.9) | 16.0 (10.0 – 20.0) | 0.096 | 12.8 (9.0 – 17.0) | 0.026 |
| TAPSE (cm/s) | 20.0 (14.0 – 26.0) | 19.7 (13.0 – 29.0) | 0.62 | 16.3 (10.5 – 24.0) | 0.0089 |
| IVCCI | 46.79(27.10–100) | 49.03(13.60–100) | 0.86 | 56.25 (33.70 – 100) | 0.19 |
Data is presented as median (minimum - maximum). P values correspond to differences between dengue with dengue +, and dengue with severe dengue using Mann-Whitney (non-parametric) tests.
HR- heart rate, PP-pulse pressure, CO- Cardiac Output, SV- stroke volume, LVDd- Left ventricular diastolic dimension, EF- Ejection fraction, L MPI and RMPI- Left and right myocardial performance index, S′ lat, Sept, RV – myocardial systolic velocity of lateral wall, septum and right ventricle, MAPSE, TAPSE- Mitral, tricuspid annulus plane excursion, E′- Early diastolic mitral annular velocity, IVCCI- Inferior vena cava collapsibility Index.
Figure 1. The Inferior vena cava collapsibility index decreased significantly from admission to discharge in all groups, particularly severe dengue (P=0.03).
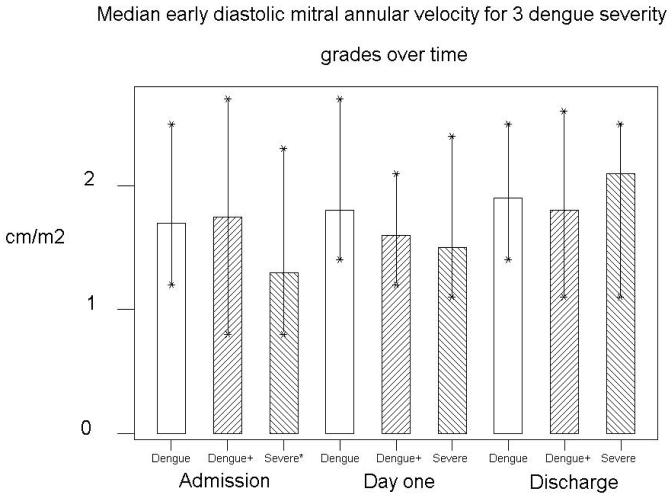
Left Ventricular (LV) systolic function in severe dengue was impaired compared to dengue, demonstrated by an increased LMPI (0.58 (0.26-0.8) vs. 0.38 (0.22-0.7), P=0.006) (Fig 2), and reduced septal myocardial systolic velocity (S′sept), (6.4 (4.8-10) vs. 8.1(6-13) cm/s, P=0.01). Right ventricular (RV) function was also impaired in the severe group with reduced S′RV (11.4 (7.5-17) vs. 13.5 (10-17) cm/s, P=0.016). Myocardial diastolic velocity (E′) was also reduced in severe dengue, (13 (8-23) vs. 17 (12-25) cm/s, P=0.003) (Fig 3) as was E/A ratio (1.33 (0.92-2.11 vs. 1.75 (1.09-2.6), P=0.002). LV filling pressures (defined as E-E′ <6 at the lateral wall) were normal [14,15] and did not alter with disease severity.
Figure 2. The left myocardial performance Index was increased in severe dengue compared with dengue (P=0.006), and improved significantly by hospital discharge in this group (P=0.002).
*indicates a significant difference from dengue patients.
Figure 3. The early mitral annular velocity (E′) was decreased in severe dengue compared to dengue (P=0.003), increasing significantly by hospital discharge (P=0.002).
*indicates a significant difference from dengue patients.
Cardiac function over time
SV and CO significantly increased by hospital discharge compared to admission, particularly in severe dengue, 35.7 (25.9-59.5) vs. 49.4 (30.1-69.2) ml, (P=0.004) and 3.1(1.9- 5.2) vs. 4.1 (2.7- 5.3) L/min (P=0.008) (table 3). In the same group, LV diastolic dimension (LVDd) increased by an average of 0.21cm (P=0.002). IVCCI decreased in all severity groups by discharge (Table 3).
Table 3. Haemodynamic and cardiac parameters in different dengue severity grades at hospital admission compared to discharge.
| Dengue and Dengue+ | Severe dengue | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Admission | Discharge | p-value | Admission | Discharge | p-value | |
| N | 64 | 11 | 15 | 14 | ||
|
| ||||||
| HR (b/min) | 82 (50 – 110) | 78 (52 – 94) | 0.46 | 88 (58 – 115) | 80 (58-110) | 0.005 |
| SV (ml) | 44.2 (19.0–75.1) | 51.3 (31.8-67.6) | 0.001 | 35.7 (25.9-59.5) | 49.4 (30.1-69.2) | 0.004 |
| CO (L/min) | 3.5 (2.0 – 6.8) | 4.0 (2.4-5.3) | 0.074 | 3.1 (1.9 – 5.2) | 4.1 (2.7-5.3) | 0.008 |
| LVDd (cm) | 4.24 (3.25-5.22) | 4.32 (3.74-5.44) | 0.60 | 4.10 (2.97-4.87) | 4.31 (3.64-5.05) | 0.002 |
| EF (%) | 68.4 (54.6-80.2) | 68.5 (57.3-79.2) | 0.27 | 66.0 (48.0-75.9) | 71.7 (56.9-78.8) | 0.033 |
| LMPI | 0.39 (0.21-0.70) | 0.33 (0.13-0.57) | 0.002 | 0.58 (0.26-0.80) | 0.31 0.16-0.57) | 0.002 |
| RMPI | 0.21 (0.03-0.42) | 0.19 (0.06-0.46) | 0.91 | 0.17 (0.04-0.43) | 0.17 (0.03-0.40) | 0.54 |
| S′ lat (cm/s) | 11.3 (7.0-18.0) | 11.2 (7.8-16.9) | 0.75 | 10.9 (7.0-16.0) | 12.2 (9.3-17.0) | 0.047 |
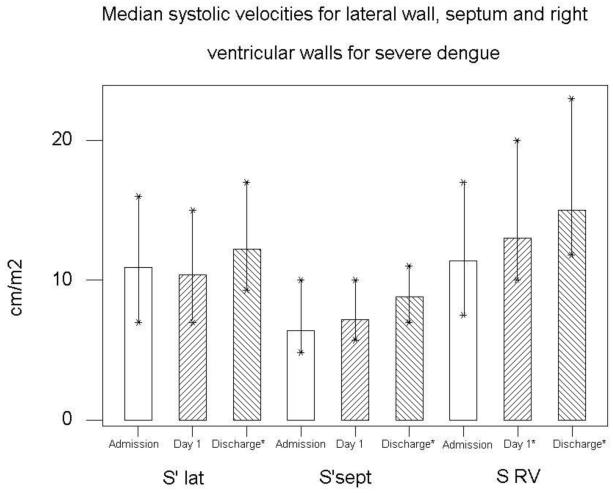
| S′ Sept (cm/s) | 8.0 (6.0-13.0) | 8.0 (6.3-15.0) | 0.89 | 6.4 (4.8-10.0) | 8.8 (7.0-11.0) | 0.002 |
| S′ RV (cm/s) | 13.2 (9.0-22.0) | 13.0 (8.2-25.0) | 0.62 | 11.4 (7.5-17.0) | 15.0 (11.8-23.0) | 0.003 |
| MAPSE (cm/s) | 15.0 (10.0-20.0) | 16.0 (12.3-20.0) | 0.008 | 12.8 (9.0-17.0) | 16.0 (13.0-18.1) | 0.003 |
| TAPSE (cm/s) | 20.0 (13.0-29.0) | 21.0 (17.0-27.0) | 0.003 | 16.3 (10.5-24.0) | 22.5 (17.7-25.0) | 0.002 |
| E′ (cm/s) | 17 (8-27) | 18 (11-26) | 0.074 | 13 (8-23) | 21 (11-25) | 0.002 |
| IVCCI | 47.9 (13.6-100) | 40.7 (12.5-68.7) | 0.001 | 56.2 (33.7-100) | 44.6 (25.0-70.6) | 0.058 |
The p-value relates to a (non-parametric) Wilcoxon test of zero median difference between admission and discharge.
Using TDI, 42% of patients at admission had evidence of LV systolic impairment (S′ lat <13 and S′ sept<7.5cm/s) and 45% impaired diastolic function (E′<16 cm/s).
In severe dengue, S′ velocities were all depressed on admission, particularly septal S′ which increased from 6.4 (4.8-8.8) to 8.8 (7-11) cm/s (P=0.002) by discharge (fig 4). In the same group RV S′ increased from 11.4 (7.5-17.0) to 15 (11.8-23.0) cm/s, (P=0.003) (Table 3). E′ also increased by an average of 8 cm/s (P=0.002) (Fig 3).
Figure 4. In severe dengue, systolic velocities were reduced at admission compared to discharge, most marked at the septal wall, (P=0.002).
* Indicates a significant difference between admission and the other time points.
At admission, patients with pleural effusions had worse cardiac function with higher LMPI (0.48 (0.26-0.8) vs.0.39 (0.21-0.74), P=0.01) and lower LV lateral S′ (10.8 (7-16) vs. 12.0 (7-18) cm/s, P=0.02) and RV S′ (12.2 (7.5-17) vs. 13.5 (9.3-22) cm/s, P=0.04) (table 4). Also, patients with pleural effusions were more likely to have abnormal septal S′ (<7.5cm/s) (P=0.04). Haematocrit and IVCCI at admission inversely correlated with ejection fraction (r=-0.39, P<0.001 and r=-0.24, P=0.04). None of the TDI parameters (S′ lat, sept and RV) correlated with IVCCI. There was no association between the amount of IV fluid given and cardiac function parameters, specifically S′lat, S′sept, S′RV, EF and LMPI. Nor was there any association between CO and systolic (r=0.082, P=0.49) or diastolic blood pressure (r=-0.086, P=0.47).
Table 4. Cardiac function in patients with and without pleural effusions.
| Without Pleural Effusion | With Pleural effusion | p-value | |
|---|---|---|---|
|
| |||
| n | 48 | 25 | |
|
| |||
| EF % | 68.32 (57.78 – 79.10) | 67.38 (49.80 – 80.23) | 0.63 |
|
| |||
| L MPI | 0.39 (0.21 – 0.74) | 0.48 (0.26 – 0.80) | 0.012 |
|
| |||
| S′lat cm/s | 12.0 (7.0 – 18.0) | 10.80 (7.0 – 16.0) | 0.023 |
|
| |||
| S′sept cm/s | 8.0 (6.0 – 13.0) | 7.0 (4.8 – 12.0) | 0.047 |
|
| |||
| S′RV cm/s | 13.5 (9.3 – 22.0) | 12.2 (7.5 – 17.0) | 0.036 |
The data are presented as median (minimum - maximum). The p-values refer to a test of without pleural effusion against with pleural effusion.
Discussion
We have shown that particularly in severe dengue, systolic and diastolic, left and right ventricular function was reduced. Patients with cardiac impairment were more likely to have pleural effusions. The cardiac dysfunction appeared to be transient, with significant improvements seen by the time of hospital discharge (2-7 days). These results could have therapeutic implications, as the current treatment of severe dengue is judicious replacement of intravenous fluids and careful fluid balance.[16] The severe dengue cases had been fluid resuscitated prior to the first echocardiogram, but still showed more signs of intravascular volume depletion than the other grades, likely representing ongoing vascular leakage. This made cardiac functional assessment in this group more challenging and hence our use of TDI parameters, some of which have been shown to be relatively load independent [15,17,18,19]. E′ was significantly reduced in the severe dengue group, suggesting impaired LV relaxation, and diastolic impairment [8,15]. The average E/E′ in the severe group was similar to the other grades, which may be due to the systolic dysfunction counteracting the reduced preload, thereby increasing LV pressures.
Systolic function was also impaired particularly in severe dengue, which confirms previous findings [2,3]. One study demonstrated 36% of dengue shock patients had reduced systolic function with an EF<50% [2]. We have demonstrated that myocardial velocities of the septal and right ventricular (RV) wall were predominantly affected. The exact cause for these regional wall differences requires further investigation. One possible mechanism could be differential regional myocardium vulnerability to coronary hypoperfusion [20].
Diastolic TDI parameters have been shown to correlate with outcome in a range of cardiovascular diseases, [6] and have prognostic value in previously healthy patients admitted to Intensive Care Units (ICU) [21,22]. Non-survivors of sepsis had significantly lower septal systolic velocities than survivors, with no difference on conventional echo parameters[23]. Although dengue and septic shock are different entities, there are several overlapping features, including increased capillary permeability and vascular leakage [24,25,26,27]. In sepsis a circulating myocardial depressant factor has been suggested as the cause [28], including pro-inflammatory cytokines [29,30,31,32,33]. The pathogenesis of severe dengue is poorly understood, but is thought to involve an overactive immune response[34], particularly in secondary infections, with a cytokine ‘storm’ being associated with the capillary permeability [35,36,37,38]. Our observed cardiac dysfunction may share the same underlying mechanism, with high circulating proinflammatory cytokines causing myocardial depression as well as the capillary permeability, particularly as they occur simultaneously. Other potential mechanisms may include altered intracellular calcium homeostasis [38] and coronary hypoperfusion. Direct viral invasion of the myocytes may be a possibility, but there was no evidence of myocardial injury, with no elevation of serum troponin I and little evidence from autopsy data [39]. There was electrical evidence of myocarditis with a third of patients having abnormal ECGs, which may have contributed to the functional impairment. This is consistent with previous findings where 70% of severe dengue patients had global hypokinesia using radionuclide ventriculography but no myocardial necrosis using 99m Tc-pyrophosphate imaging [40].
The clinical implications of these findings are potentially very important. Although the cardiac impairment is transient, it does occur at the same time as the vascular leakage, when the patients are most likely to be haemodynamically unstable. Patients with severe dengue are likely to have a degree of cardiac impairment in addition to intravascular volume loss, which combine to worsen cardiovascular instability and increase the risk of iatrogenic fluid overload [41]. The IVCCI used in our study has been shown to be a useful non-invasive method of volume assessment and determining preload [42]. It correlates well with CVP (a risky procedure in dengue patients with thrombocytopenia) and is useful in predicting fluid responsiveness [43,44]. Echocardiography, particularly portable machines are becoming more widely available worldwide, along with trained staff. We would therefore recommend severe dengue patients to have cardiac functional assessment particularly using the TDI parameters; S′ lat, sept and RV and IVCCI for intravascular volume evaluation and to estimate fluid responsiveness. This could guide fluid resuscitation and identify early, patients at risk of iatrogenic fluid overload and allow the use of alternative therapies including inotropes.
Limitations
The limitations include small patient numbers when separated into severity groups. The unstable cases were fluid resuscitated prior to first echo, so as not to interfere in the emergency management of the patients. This delayed time to first echo, which could have underestimated the cardiac involvement in this critical phase. Ideally the patients would have been recalled several months after discharge to check all parameters had normalized and investigate any long-term cardiac sequlae.
Conclusion
Dengue patients have evidence of systolic and diastolic myocardial impairment, with septal and right ventricular wall being predominantly affected. These changes are more common and pronounced in severe dengue and are associated with more pleural effusions. Echo screening should be considered for all severe dengue cases, to assess cardiac function and intravascular volume evaluation to tailor their management.
Acknowledgements
We thank Truong Thi Tho for help with patient recruitment, and the nursing and medical staff at the hospital for tropical diseases, Ho Chi Minh City for patient management. We also thank the staff in the ultrasound department for use of their facilities. Also, Derek Robinson and Clare Glover for the statistical analysis.
Funding: This work was supported by the Wellcome Trust, UK.
References
- 1.TDR research on dengue: recommendations of a scientific working group. TDR News. 2000;3:15. [PubMed] [Google Scholar]
- 2.Khongphatthanayothin A, Lertsapcharoen P, Supachokchaiwattana P, La-Orkhun V, Khumtonvong A, et al. Myocardial depression in dengue hemorrhagic fever: prevalence and clinical description. Pediatr Crit Care Med. 2007;8:524–529. doi: 10.1097/01.PCC.0000288672.77782.D4. [DOI] [PubMed] [Google Scholar]
- 3.Khongphatthanayothin A, Suesaowalak M, Muangmingsook S, Bhattarakosol P, Pancharoen C. Hemodynamic profiles of patients with dengue hemorrhagic fever during toxic stage: an echocardiographic study. Intensive Care Med. 2003;29:570–574. doi: 10.1007/s00134-003-1671-9. [DOI] [PubMed] [Google Scholar]
- 4.Kularatne SA, Pathirage MM, Kumarasiri PV, Gunasena S, Mahindawanse SI. Cardiac complications of a dengue fever outbreak in Sri Lanka, 2005. Trans R Soc Trop Med Hyg. 2007;101:804–808. doi: 10.1016/j.trstmh.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Khongphatthallayothin A, Chotivitayatarakorn P, Somchit S, Mitprasart A, Sakolsattayadorn S, et al. Morbitz type I second degree AV block during recovery from dengue hemorrhagic fever. Southeast Asian J Trop Med Public Health. 2000;31:642–645. [PubMed] [Google Scholar]
- 6.Meco M, Cirri S. The effects of load on systolic mitral annulus movements by tissue Doppler imaging in cardiac surgery patients. J Cardiovasc Surg. 51:277–281. [PubMed] [Google Scholar]
- 7.Mendes L, Ribeiras R, Adragao T, Lima S, Horta E, et al. Load-independent parameters of diastolic and systolic function by speckle tracking and tissue doppler in hemodialysis patients. Rev Port Cardiol. 2008;27:1011–1025. [PubMed] [Google Scholar]
- 8.Mogelvang R, Sogaard P, Pedersen SA, Olsen NT, Marott JL, et al. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation. 2009;119:2679–2685. doi: 10.1161/CIRCULATIONAHA.108.793471. [DOI] [PubMed] [Google Scholar]
- 9.Fijalkowski M, Koprowski A, Gruchala M, Galaska R, Debska-Slizien A, et al. Effect of preload reduction by hemodialysis on myocardial ultrasonic characterization, left atrial volume, and Doppler tissue imaging in patients with end-stage renal disease. J Am Soc Echocardiogr. 2006;19:1359–1364. doi: 10.1016/j.echo.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Hang VT, Nguyet NM, Trung DT, Tricou V, Yoksan S, et al. Diagnostic accuracy of NS1 ELISA and lateral flow rapid tests for dengue sensitivity, specificity and relationship to viraemia and antibody responses. PLoS Negl Trop Dis. 2009;3:e360. doi: 10.1371/journal.pntd.0000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. http://whqlibdoc.who.int/publications/2009/9789241547871_eng.pdf http://whqlibdoc.who.int/publications/2009.
- 12.Eidem BW, Tei C, O’Leary PW, Cetta F, Seward JB. Nongeometric quantitative assessment of right and left ventricular function: myocardial performance index in normal children and patients with Ebstein anomaly. J Am Soc Echocardiogr. 1998;11:849–856. doi: 10.1016/s0894-7317(98)70004-5. [DOI] [PubMed] [Google Scholar]
- 13.Haciomeroglu P, Ozkaya O, Gunal N, Baysal K. Venous collapsibility index changes in children on dialysis. Nephrology. 2007;12:135–139. doi: 10.1111/j.1440-1797.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 14.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 15.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 16.Wills BA, Nguyen MD, Ha TL, Dong TH, Tran TN, et al. Comparison of three fluid solutions for resuscitation in dengue shock syndrome. N Engl J Med. 2005;353:877–889. doi: 10.1056/NEJMoa044057. [DOI] [PubMed] [Google Scholar]
- 17.Vignon P, Allot V, Lesage J, Martaille JF, Aldigier JC, et al. Diagnosis of left ventricular diastolic dysfunction in the setting of acute changes in loading conditions. Crit Care. 2007;11:R43. doi: 10.1186/cc5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Boeck BW, Cramer MJ, Oh JK, van der Aa RP, Jaarsma W. Spectral pulsed tissue Doppler imaging in diastole: a tool to increase our insight in and assessment of diastolic relaxation of the left ventricle. Am Heart J. 2003;146:411–419. doi: 10.1016/S0002-8703(03)00322-3. [DOI] [PubMed] [Google Scholar]
- 19.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–480. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 20.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 21.Ikonomidis I, Nikolaou M, Dimopoulou I, Paraskevaidis I, Lekakis J, et al. Association of left ventricular diastolic dysfunction with elevated NT-pro-BNP in general intensive care unit patients with preserved ejection fraction: a complementary role of tissue Doppler imaging parameters and NT-pro-BNP levels for adverse outcome. Shock. 33:141–148. doi: 10.1097/SHK.0b013e3181ad31f8. [DOI] [PubMed] [Google Scholar]
- 22.Sturgess DJ, Marwick TH, Joyce CJ, Jones M, Venkatesh B. Tissue Doppler in critical illness: a retrospective cohort study. Crit Care. 2007;11:R97. doi: 10.1186/cc6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sturgess DJ, Marwick TH, Joyce C, Jenkins C, Jones M, et al. Prediction of hospital outcome in septic shock: a prospective comparison of tissue Doppler and cardiac biomarkers. Crit Care. 14:R44. doi: 10.1186/cc8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ranjit S, Kissoon N, Gandhi D, Dayal A, Rajeshwari N, et al. Early differentiation between dengue and septic shock by comparison of admission hemodynamic, clinical, and laboratory variables: a pilot study. Pediatr Emerg Care. 2007;23:368–375. doi: 10.1097/01.pec.0000278403.22450.a2. [DOI] [PubMed] [Google Scholar]
- 25.Subla MR, Khan SA, Behl D, Peters SG. Sepsis and myocardial depression in a young woman. Mayo Clin Proc. 2005;80:810–814. doi: 10.1016/S0025-6196(11)61537-1. [DOI] [PubMed] [Google Scholar]
- 26.Kumar A, Haery C, Parrillo JE. Myocardial dysfunction in septic shock. Crit Care Clin. 2000;16:251–287. doi: 10.1016/s0749-0704(05)70110-x. [DOI] [PubMed] [Google Scholar]
- 27.Merx MW, Weber C. Sepsis and the heart. Circulation. 2007;116:793–802. doi: 10.1161/CIRCULATIONAHA.106.678359. [DOI] [PubMed] [Google Scholar]
- 28.Reilly JM, Cunnion RE, Burch-Whitman C, Parker MM, Shelhamer JH, et al. A circulating myocardial depressant substance is associated with cardiac dysfunction and peripheral hypoperfusion (lactic acidemia) in patients with septic shock. Chest. 1989;95:1072–1080. doi: 10.1378/chest.95.5.1072. [DOI] [PubMed] [Google Scholar]
- 29.Pathan N, Hemingway CA, Alizadeh AA, Stephens AC, Boldrick JC, et al. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet. 2004;363:203–209. doi: 10.1016/S0140-6736(03)15326-3. [DOI] [PubMed] [Google Scholar]
- 30.Kumar A, Thota V, Dee L, Olson J, Uretz E, et al. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med. 1996;183:949–958. doi: 10.1084/jem.183.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chagnon F, Metz CN, Bucala R, Lesur O. Endotoxin-induced myocardial dysfunction: effects of macrophage migration inhibitory factor neutralization. Circ Res. 2005;96:1095–1102. doi: 10.1161/01.RES.0000168327.22888.4d. [DOI] [PubMed] [Google Scholar]
- 32.Krishnagopalan S, Kumar A, Parrillo JE, Kumar A. Myocardial dysfunction in the patient with sepsis. Curr Opin Crit Care. 2002;8:376–388. doi: 10.1097/00075198-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Chagnon F, Bentourkia M, Lecomte R, Lessard M, Lesur O. Endotoxin-induced heart dysfunction in rats: assessment of myocardial perfusion and permeability and the role of fluid resuscitation. Crit Care Med. 2006;34:127–133. doi: 10.1097/01.ccm.0000190622.02222.df. [DOI] [PubMed] [Google Scholar]
- 34.Simmons CP, Dong T, Chau NV, Dung NT, Chau TN, et al. Early T-cell responses to dengue virus epitopes in Vietnamese adults with secondary dengue virus infections. J Virol. 2005;79:5665–5675. doi: 10.1128/JVI.79.9.5665-5675.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pang T, Cardosa MJ, Guzman MG. Of cascades and perfect storms: the immunopathogenesis of dengue haemorrhagic fever-dengue shock syndrome (DHF/DSS) Immunol Cell Biol. 2007;85:43–45. doi: 10.1038/sj.icb.7100008. [DOI] [PubMed] [Google Scholar]
- 36.Dong T, Moran E, Vinh Chau N, Simmons C, Luhn K, et al. High pro-inflammatory cytokine secretion and loss of high avidity cross-reactive cytotoxic T-cells during the course of secondary dengue virus infection. PLoS One. 2007;2:e1192. doi: 10.1371/journal.pone.0001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chau TN, Quyen NT, Thuy TT, Tuan NM, Hoang DM, et al. Dengue in Vietnamese infants--results of infection-enhancement assays correlate with age-related disease epidemiology, and cellular immune responses correlate with disease severity. J Infect Dis. 2008;198:516–524. doi: 10.1086/590117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen TH, Lei HY, Nguyen TL, Lin YS, Huang KJ, et al. Dengue hemorrhagic fever in infants: a study of clinical and cytokine profiles. J Infect Dis. 2004;189:221–232. doi: 10.1086/380762. [DOI] [PubMed] [Google Scholar]
- 39.de Araujo JM, Schatzmayr HG, de Filippis AM, Dos Santos FB, Cardoso MA, et al. A retrospective survey of dengue virus infection in fatal cases from an epidemic in Brazil. J Virol Methods. 2009;155:34–38. doi: 10.1016/j.jviromet.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 40.Wali JP, Biswas A, Chandra S, Malhotra A, Aggarwal P, et al. Cardiac involvement in Dengue Haemorrhagic Fever. Int J Cardiol. 1998;64:31–36. doi: 10.1016/s0167-5273(98)00008-4. [DOI] [PubMed] [Google Scholar]
- 41.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, et al. Mortality after Fluid Bolus in African Children with Severe Infection. N Engl J Med. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 42.Ranjit S, Kissoon N, Jayakumar I. Aggressive management of dengue shock syndrome may decrease mortality rate: a suggested protocol. Pediatr Crit Care Med. 2005;6:412–419. doi: 10.1097/01.PCC.0000163676.75693.BF. [DOI] [PubMed] [Google Scholar]
- 43.Sefidbakht S, Assadsangabi R, Abbasi HR, Nabavizadeh A. Sonographic measurement of the inferior vena cava as a predictor of shock in trauma patients. Emerg Radiol. 2007;14:181–185. doi: 10.1007/s10140-007-0602-4. [DOI] [PubMed] [Google Scholar]
- 44.Charron C, Caille V, Jardin F, Vieillard-Baron A. Echocardiographic measurement of fluid responsiveness. Curr Opin Crit Care. 2006;12:249–254. doi: 10.1097/01.ccx.0000224870.24324.cc. [DOI] [PubMed] [Google Scholar]