Abstract
PURPOSE
To compare accuracy of intraocular lens (IOL) power calculation formulae in infantile eyes with primary IOL implantation.
DESIGN
Comparative case series.
METHODS
The Hoffer Q, Holladay 1, Holladay 2, Sanders-Retzlaff-Kraff (SRK) II, and Sanders-Retzlaff-Kraff theoretic (SRK/T) formulae were used to calculate predicted postoperative refraction for eyes that received primary IOL implantation in the Infant Aphakia Treatment Study. The protocol targeted postoperative hyperopia of +6.0 or +8.0 diopters (D). Eyes were excluded for invalid biometry, lack of refractive data at the specified postoperative visit, diagnosis of glaucoma or suspected glaucoma, or sulcus IOL placement. Actual refraction 1 month after surgery was converted to spherical equivalent and prediction error (predicted refraction – actual refraction) was calculated. Baseline characteristics were analyzed for effect on prediction error for each formula. The main outcome measure was absolute prediction error.
RESULTS
Forty-three eyes were studied; mean axial length was 18.1 ± 1.1 mm (in 23 eyes, it was <18.0 mm). Average age at surgery was 2.5 ± 1.5 months. Holladay 1 showed the lowest median absolute prediction error (1.2 D); a paired comparison of medians showed clinically similar results using the Holladay 1 and SRK/T formulae (median difference, 0.3 D). Comparison of the mean absolute prediction error showed the lowest values using the SRK/T formula (1.4 ± 1.1 D), followed by the Holladay 1 formula (1.7 ± 1.3 D). Calculations with an optimized constant showed the lowest values and no significant difference between the Holladay 1 and SRK/T formulae (median difference, 0.3 D). Eyes with globe AL of less than 18 mm had the largest mean and median prediction error and absolute prediction error, regardless of the formula used.
CONCLUSIONS
The Holladay 1 and SRK/T formulae gave equally good results and had the best predictive value for infant eyes.
Primary implantation of an intraocular lens (IOL) remains controversial for infants, and the selection of an appropriate IOL power is challenging. The Infant Aphakia Treatment Study was a multi-center, randomized, controlled clinical trial sponsored by the National Eye Institute to determine whether primary IOL implantation in infants younger than 7 months of age with a unilateral congenital cataract results in improved visual outcomes over contact lens correction of aphakia.1,2 Half of the 114 infants enrolled in this multicenter study were randomized to receive an IOL with a lens power determined using the Holladay 1 formula. The mean absolute prediction error was 1.8 ± 1.3 diopters (D), with greater error noted in eyes with shorter globe axial length (AL).3
There are known challenges in obtaining accurate biometry and refractive measures in children and infants because of the lack of patient cooperation, limitations in equipment, and errors induced or exaggerated by the small size of the infant eye.3–11 Additionally, by the time the postoperative refraction is obtained, rapid growth of an infant’s eye may result in a myopic shift from the predicted postoperative refraction calculated at the time of surgery.12,13 The Haigis, Hoffer Q, and Holladay formulae have been found to give the lowest prediction error in pediatric or short eyes with a globe AL of less than 22 mm,14–17 but most studies have very few extremely short eyes (AL <20 mm) for analysis.
In this study, we analyzed the prediction error of the commonly used IOL power calculation formulae Hoffer Q, Holladay 1, Holladay 2, Sanders-Retzlaff-Kraff (SRK) II, and Sanders-Retzlaff-Kraff theoretic (SRK/T) as applied to the eyes of infants who received primary IOL implantation in the Infant Aphakia Treatment Study and looked for associations between eye characteristics and formula predictability.
METHODS
THE STUDY FOLLOWED THE TENETS OF THE DECLARATION of Helsinki, was approved by the institutional review boards of all participating centers (Appendix 1), and was in compliance with the Health Insurance Portability and Accountability Act. The off-label research use of the AcrySof IOLs (Alcon Laboratories, Fort Worth, Texas, USA) was covered by United States Food and Drug Administration investigational device exemption G020021.
The design, surgical techniques, optical correction regimens, follow-up schedule, examination methods, and baseline characteristics of patients enrolled in the Infant Aphakia Treatment Study (ClinicalTrials.gov identifier, NCT00212134) have been reported previously, and therefore are only summarized briefly in this report.1 Infants with a unilateral visually significant cataract (≥3 mm central opacity) and an age of 28 to 209 days at the time of cataract surgery were eligible for enrollment in the study. The main exclusion criteria were persistent fetal vasculature associated with stretching of ciliary processes or involvement of the optic nerve or retina, corneal diameter less than 9 mm, premature birth (<36 weeks gestational age), presence of a medical condition that may interfere with later visual acuity testing, or acquired cataract. Patients were randomized either to have an IOL placed at the time of the initial surgery (with spectacle correction) or to be left aphakic (with contact lens correction).
Data were collected prospectively. All available A-scans were reviewed by a certified echographer to assess quality as described previously; one scan was invalid, and this eye was excluded from analysis.3 Additional eyes were excluded from analysis for lack of refractive data at the 1 month postoperative visit, diagnosis of glaucoma or suspected glaucoma by the 3-month postoperative visit (which could result in an unpredictable and early myopic shift), or sulcus IOL implantation (which could result in alterations in the effective lens position).
Glaucoma was defined as intraocular pressure (IOP) of more than 21 mm Hg with 1 or more of the following anatomical changes: (1) corneal enlargement; (2) asymmetrical progressive myopic shift coupled with enlargement of the corneal diameter, AL, or both; (3) increased optic nerve cupping defined as an increase of 0.2 or more in the cup-to-disc ratio; or (4) the use of a surgical procedure for IOP control. A patient was designated a glaucoma suspect if he or she either (1) had 2 consecutive IOP measurements of more than 21 mm Hg on different dates after topical corticosteroids had been discontinued without any of the anatomic changes listed previously or (2) took glaucoma medications to control IOP without experiencing any of the anatomic changes listed previously.
SCREENING EXAMINATION UNDER ANESTHESIA
Before randomization, each infant underwent an examination under anesthesia to confirm study eligibility and to perform biometry of both eyes. Keratometry was performed with a hand-held keratometer, with an average of at least 2 readings that varied by less than 1 D. A-scan ultrasonography was performed using immersion in most cases. Measurements were obtained from the scan with the best waveforms (i.e., highest peaks with a perpendicular retinal spike) using the phakic setting. If applanation A-scan ultra-sonography was used, the A-scan with the greatest anterior chamber depth was used.
SURGICAL TECHNIQUE AND INTRAOCULAR LENS POWER DETERMINATION
The IOL power was determined in the operating room based on A-scan ultrasonography and keratometry readings using the Holladay 1 formula. According to the Infant Aphakia Treatment Study protocol, an IOL power was chosen that was closest to the power predicted to produce a +8.0 postoperative refraction for infants 4 to 6 weeks of age and a +6.0 D postoperative refraction for infants older than 6 weeks. Because the normal fellow eye in an infant was expected to be hyperopic (typically about +2.0 D),18 some degree of anisometropia was expected. In patients in whom the calculated IOL power was more than 40.0 D, a 40.0-D IOL (the maximum power available) was selected. Infants randomized to the IOL group had the lens aspirated followed by the implantation of an AcryS of SN60AT IOL (Alcon Laboratories). After IOL placement, a posterior capsulectomy and an anterior vitrectomy were performed.
POSTOPERATIVE REFRACTION
Follow-up examinations were performed by an Infant Aphakia Treatment Study certified investigator as described in detail elsewhere.1 Retinoscopy was performed under cycloplegia to determine residual refractive error at the 1-month postoperative examination. This measure was converted to spherical equivalent (half of the cylinder added to the sphere) and was compared with the predicted refraction.
CALCULATION OF PREDICTION ERROR
The predicted refraction was calculated for each of the formulae studied (Hoffer Q, Holladay 1, Holladay 2, SRK/T, and SRK II)14,19–21 using the IOL power implanted and the patient’s AL, anterior chamber depth, lens thickness, corneal diameter, and average keratometry recorded at the time of surgery. The anterior chamber depth and lens thickness values were not available for all eyes, because this information was not collected on original study forms, but instead was gathered later from available A-scans that had been performed and collected at the time of surgery. Holladay IOL consultant software was used to calculate prediction error for Hoffer Q, Holladay 1, Holladay 2, and SRK/T formulae. Results of SRK II formula calculations were obtained using Microsoft Excel (Microsoft Corp, Redmond, Washington, USA). The prediction error and absolute prediction error were calculated as follows:
ADJUSTMENTS TO PREDICTION ERROR CALCULATION
Means of absolute prediction error and prediction error between groups were compared. Because absolute prediction errors often follow a non-Gaussian distribution, comparison of medians also was performed. To optimize analysis of formula performance, back calculations were performed until the mean error was 0 for each of the theoretic formulae (Holladay 1, Holladay 2, Hoffer Q, and SRK/T). This resulted in the ability to evaluate results using a customized or adjusted constant. Using this adjusted constant, the prediction error was recalculated for each subject and comparisons were made using group medians.
STATISTICAL METHODS
The distributions of age at surgery, baseline globe AL, baseline keratometry, IOL power, refractive error at 1 month after surgery, prediction error, and absolute prediction error were examined. For each prediction formula, a Wilcoxon signed-rank test was performed to determine whether the median absolute prediction error was more than 0 D, and a 1-sample t test was performed to determine whether the mean absolute prediction error was more than 0 D. Associations between prediction error or absolute prediction error and baseline characteristics that included globe AL (<18.0 vs ≥18.0 mm), keratometry (<46.5 vs ≥46.5 D), and IOL power implanted (<30.0 vs ≥30.0 D) were examined using 2-sample t tests to compare means and Wilcoxon rank-sum tests to compare medians. Absolute prediction errors were compared for the Hoffer Q, Holladay 1, Holladay 2, and SRK/T formulae by calculating within-patient differences in absolute prediction error for each pair of formulae and using a Wilcoxon signed-rank test to determine whether the median difference was 0 for each pair; Bonferroni adjustments were applied to P values to control type I error rates across the 6 paired tests performed. Analyses were performed using SAS software version 9.3 (SAS Institute, Cary, North Carolina USA) and JMP software version 10.0 (SAS Institute). A significance level of .05 was used in all hypothesis tests.
RESULTS
STUDY PATIENTS AND BASELINE AND SURGERY CHARACTERISTICS
Fifty-seven of the 114 patients in the Infant Aphakia Treatment Study were randomized to receive an IOL, and IOL implantation was completed in 56 patients. Of these 56 patients, 13 were excluded for 1 or more of the following reasons: lack of accurate refraction at the 1-month visit (n = 7; 1 of whom had invalid biometry), glaucoma or suspected glaucoma (n = 7; 3 of whom had lack of accurate refraction at the 1-month visit), or sulcus IOL placement (n = 4; 1 of whom had lack of accurate refraction and 1 of whom had glaucoma). The remaining 43 eyes were included in the analysis. Of the 13 excluded eyes, 7 were in the younger age group (all had AL <18 mm) and 6 were in the older age group (1 had AL <18 mm and 5 had AL ≥18 mm). There were no identifiable variations in baseline characteristics in the group of eyes that were excluded compared with those that were studied.
The baseline characteristics of these 43 pseudophakic eyes are reported in Table 1. Overall, the mean age at surgery was 2.5 ± 1.5 months. The mean globe AL was 18.1 ±1.1 mm; all but 2 of the eyes in this series had a globe AL measurement of 20.0 mm or less. Globe AL was measured using immersion ultrasound in 32 eyes and using contact ultrasound in 11 eyes. Anterior chamber depth and lens thickness measurements were available for 33 eyes.
TABLE 1.
Intraocular Lens Power Calculation Formulae in Infantile Eyes with Unilateral Congenital Cataract: Baseline globe axial length, keratometry, and intraocular lens power implanted for studied eyes, based on age group at surgery.
| Variable | Age Group | n | Mean (Standard Deviation) |
|---|---|---|---|
| Axial Length (mm)a | All ages | 43 | 18.1 (1.1) |
| 28–48 Days | 17 | 17.3 (0.7) | |
| >48 Days | 26 | 18.7 (1.4) | |
| Keratometry (diopters)b | All ages | 43 | 46.2 (2.5) |
| 28–48 Days | 17 | 47.4 (1.6) | |
| >48 Days | 26 | 45.5 (2.7) | |
| Intraocular Lens Power (diopters)c | All ages | 43 | 29.6 (5.4) |
| 28–48 Days | 17 | 30.8 (4.2) | |
| >48 Days | 26 | 28.8 (6.0) |
P-value <0.05 for a two-sample t-test comparing age-group means.
P-value <0.01 for a two-sample t-test comparing age-group means.
Difference not significant; since the post-operative target refraction was +8.0 diopters for the younger age group and +6.0 diopters for the older age group, the mean intraocular lens power implanted was similar between the age groups.
INTRAOCULAR LENS POWER AND PLACEMENT
The mean IOL power implanted was 29.6 ± 5.4 D (Table 1; range, 11.5 to 40 D). Twenty-two eyes were implanted with an IOL power of 30.0 D or more, and 7 of these were implanted with an IOL power of 35.0 D or more. All 43 patients had an SN60AT IOL placed within the capsular bag. There were no significant differences in mean IOL power implanted by age group at surgery.
POSTOPERATIVE REFRACTION
The overall mean refraction 1 month after surgery was +6.1 ± 1.9 D (median, +6.3 D; range, +2.75 to +10.0 D). In the younger age group, the mean refraction was +6.8 ± 2.0 D (median, +6.8 D; range, +3.5 to +10.0 D); in the older age group, the mean refraction was +5.6 ± 1.7 D (median, +5.8 D; range, +2.75 to +8.5 D).
ABSOLUTE PREDICTION ERROR AND BASELINE CHARACTERISTICS
The mean and median absolute prediction error using each formula studied is reported in Table 2. For all 5 formulae, the mean and median absolute prediction error were greater than 0 D (P < .0001 for each formula). Overall, the lowest mean absolute prediction error was obtained using the SRK/T formula (1.4 ± 1.1 D), and the lowest median absolute prediction error was found using the Holladay 1 formula (1.2 D; first and third quartiles, 0.7 D and 2.5 D, respectively).
TABLE 2.
Intraocular Lens Power Calculation Formulae in Infantile Eyes with Unilateral Congenital Cataract: Mean and Median Absolute Prediction Errora in Diopters, Calculated Using the Formulae Hoffer Q, Holladay 1, Holladay 2, Sanders-Retzlaff-Kraff Theoretic, and Sanders-Retzleff-Kraff II
| Group | Absolute Prediction Error (Diopters)
|
||||
|---|---|---|---|---|---|
| Hoffer Q | Holladay 1 | Holladay 2 | SRK/T | SRK II | |
| All eyes (n = 43) | |||||
| Mean ± SD | 2.6 ± 2.0 | 1.7 ± 1.3 | 1.9 ± 1.5 | 1.4 ± 1.1 | 2.4 ± 1.8 |
| Median | 2.1 (0.7, 4.0) | 1.2 (0.7, 2.5) | 1.4 (0.6, 2.9) | 1.3 (0.3, 2.1) | 2.2 (0.9, 3.6) |
| Baseline axial length (mm) | |||||
| <18.0 (n = 23) | |||||
| Mean ± SD | 3.7 ± 2.0 | 2.1 ± 1.4 | 2.5 ± 1.7 | 1.7 ± 1.3 | 3.3 ± 1.9 |
| ≥18.0 (n = 20) | |||||
| Mean ± SD | 1.3 ± 1.1 | 1.1 ± 0.8 | 1.2 ± 0.8 | 1.1 ± 0.8 | 1.5 ± 1.1 |
| P value | <.005b | <.01c | <.005b | <.005b | |
| <18.0 (n = 23) | |||||
| Median | 3.8 (2.1, 5.5) | 1.7 (0.8, 3.5) | 2.2 (1.2, 4.1) | 1.6 (0.3, 3.0) | 3.3 (2.2, 4.1) |
| ≥18.0 (n = 20) | |||||
| Median | 1.4 (0.4, 1.8) | 1.0 (0.6, 1.6) | 1.0 (0.5, 1.7) | 1.0 (0.4, 1.7) | 1.4 (0.4, 2.2) |
| P value | <.005b | <.05d | <.01c | <.005b | |
SD = standard deviation; SRK/T = Sanders-Retzlaff-Kraff theoretic; SRK II = Sanders-Retzleff-Kraff II.
Data are mean ± standard deviation and median (first quartile, third quartile) unless otherwise indicated.
Predicted refraction minus actual refraction at 1 month. The performance of each formula was compared based on category of baseline globe axial length (<18.0 mm vs ≥18.0 mm). Less error was found using all the formulae for the group of eyes with longer globe axial length, with a significant difference in performance found for each formula except Sanders-Retzlaff-Kraff theoretic formula.
P < .005 for a 2-sample t test comparing group means or a Wilcoxon signed-rank test comparing group medians.
P < .01 for a 2-sample t test comparing group means or a Wilcoxon signed-rank test comparing group medians.
P < .05 for a 2-sample t test comparing group means or a Wilcoxon signed-rank test comparing group medians.
Median and mean absolute prediction error were significantly different when comparing eyes with a globe AL of less than 18 mm versus 18.0 mm or more using any formula except the SRK/T (all P < .05), with smaller prediction error for eyes with longer globe ALs. No significant differences in median or mean prediction error were found by comparison of keratometry (<46.5 D vs ≥46.5 D). Differences were noted by comparison of implanted IOL with power of less than 30.0 D versus 30.0 D or more using any formula except the SRK/T (P < .05).
PREDICTION ERROR AND BASELINE CHARACTERISTICS
Overall, the SRK/T formula showed the lowest mean prediction error (0.3 ± 1.8 D) and median prediction error (0.0 D; 1st quartile, −1.1 D; 3rd quartile, 1.8 D). The highest mean prediction errors were found using the Hoffer Q formula, which tended to overcorrect eyes (2.3 ± 2.4 D; less residual hyperopia than expected) and the SRK II formula, which tended to undercorrect eyes (−2.3 ± 2.0 D; more residual hyperopia than expected). Comparison of median prediction errors showed similar results (Hoffer Q, 1.8 D; first quartile, 0.3 D; 3rd quartile, 4.0 D; and SRK II, −2.2 D, first quartile, −4.0 D; 3rd quartile, −0.6 D). In bivariate analyses, there were statistically significant differences in mean prediction error values between groups for the factors globe AL (all formulae, P < .01) and IOL power (all formulae, P < .01).
PREDICTION ERROR AND ABSOLUTE PREDICTION ERROR BY DIOPTER CATEGORIES
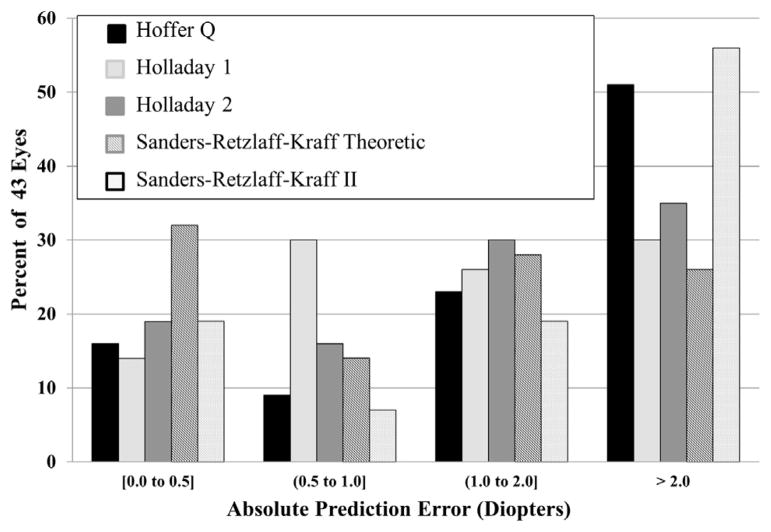
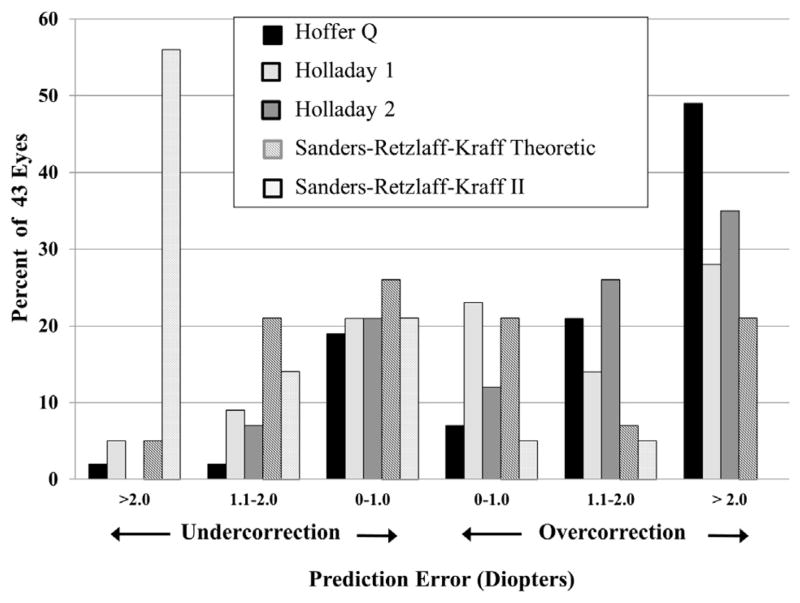
Figures 1 and 2 show the distribution of absolute prediction error and prediction error, respectively, by diopter increments. Almost half of the eyes would have been within 1.0 D of the predicted refraction by using either the Holladay 1 (44%) or SRK/T (46%) formulae, with 25% to 35% of eyes calculated to be within 1.0 D of the predicted refraction using the other formulae. The percent of eyes with prediction error of 2.0 D or less was 65% for the Holladay 2 formula, 70% for the Holladay 1 formula, and 74% for the SRK/T formula, compared with 45% and 48% for the SRK II and Hoffer Q formulae, respectively. A higher percent of eyes had prediction error of 2.0 D or more using the SRK II or Hoffer Q formulae, although as reflected in Figure 2, the direction of error was very different. Ninety percent of eyes had more residual hyperopia than expected using the SRK II formula, and 76% had less residual hyperopia than expected using the Hoffer Q formula. Very large absolute prediction error of more than 3.0 D would have occurred in only 4 eyes using either the Holladay 1 or SRK/T formulae, compared with 9 eyes using the Holladay 2 formula, 14 eyes using the Hoffer Q formula, and 15 eyes using the SRK II formula.
FIGURE 1.
Bar graph showing the distribution of absolute prediction error (diopters) for each formula studied (Hoffer Q, Holladay 1, Holladay 2, Sanders-Retzlaff-Kraff theoretic [SRK/T], and Sanders-Retzlaff-Kraff II [SRK II]) for 43 eyes from the Infant Aphakia Treatment Study.
FIGURE 2.
Bar graph showing the distribution of prediction error (diopters) for each formula studied (Hoffer Q, Holladay 1, Holladay 2, Sanders-Retzlaff-Kraff Theoretic [SRK/T], and Sanders-Retzlaff-Kraff II [SRK II]) for 43 eyes from the Infant Aphakia Treatment Study.
COMPARISON OF MEDIAN ABSOLUTE PREDICTION ERRORS, WITH ADJUSTED CONSTANTS
Table 3 shows the results of paired Wilcoxon signed-rank tests comparing the median absolute prediction error for the Hoffer Q, Holladay 1, Holladay 2, and SRK/T formulae. Results are shown for prediction error using both using the manufacturer’s constant (used clinically in the Infant Aphakia Treatment Study) and the adjusted A-constant. Although overall median absolute prediction error values appeared to be similar for the Holladay 1, Holladay 2, and SRK/T formulae (1.2 D, 1.4 D, and 1.3 D, respectively), in paired comparisons of SRK/T versus other formulae, the median paired differences in absolute prediction error (other formula minus SRK/T) was more than 0 (P < .05, Bonferroni-adjusted in each comparison), indicating greater accuracy for the SRK/T formula using the standard constant. For example, the median of the paired differences in absolute prediction error between the Holladay 1 and SRK/T formulae was 0.3 D (P < .05; first quartile, −0.3; third quartile, 0.8). When using the adjusted constant, the SRK/T and Holladay 1 formulae were not significantly different from each other, but were more accurate than each other methods (P < .05, Bonferroni adjusted in each pairwise comparison). Of the 4 methods compared, the Hoffer Q formula had the largest absolute prediction error in paired comparisons (P < .005 for each comparison). For example, the median of the paired differences, Hoffer Q absolute prediction error minus SRK/T absolute prediction error, was 1.4 D (P < .005; first quartile, −0.4; third quartile, 2.5) using the standard constant.
TABLE 3.
Paired Comparisons of Absolute Prediction Errorsa for the Theoretic Prediction Formulae Hoffer Q, Holladay 1, Holladay 2, and Sanders-Retzlaff-Kraff Theoretic for 43 Studied Infant Eyes from the Infant Aphakia Treatment Study
| Row Formula and A-Constant Type | Column Formula
|
||
|---|---|---|---|
| Holladay 1 | Holladay 2 | SRK/T | |
| Hoffer Q | |||
| Manufacturer’s A-constant | 1.1 (−0.1, 1.8)b | 0.7 (0.1, 1.3)b | 1.4 (−0.4, 2.5)b |
| Adjusted A-constant | 1.1 (−0.1, 1.8)b | 0.6 (0.1, 1.3)b | 1.2 (−0.4, 2.3)b |
| Holladay 1 | |||
| Manufacturer’s A-constant | −0.4 (−0.7, 0.3)c | 0.3 (−0.3, 0.8)c | |
| Adjusted A-constant | −0.4 (−0.7, 0.3) | 0.3 (−0.4, 0.8) | |
| Holladay 2 | |||
| Manufacturer’s A-constant | 1.0 (−0.6, 1.3)d | ||
| Adjusted A-constant | 0.9 (−0.7, 1.3)c | ||
SRK/T = Sanders-Retzlaff-Kraff theoretic formula.
Data are median (first, third quartiles) of paired differences (in diopters) from: (row formula minus column formula) in absolute prediction error.
Predicted refraction minus actual refraction at 1 month. The comparisons of formula absolute prediction errors were performed using paired Wilcoxon signed-rank tests with Bonferroni adjustment. Negative values in the table indicate smaller absolute prediction errors for the row formula; positive values indicate smaller absolute prediction errors for the column formula.
P < .005.
P < .05.
P < .01.
DISCUSSION
PAST REPORTS ON PREDICTABILITY OF IOL CALCULATION formulae have focused on comparisons of mean absolute prediction error and mean prediction error to determine accuracy and predictability. As such, in this cohort, we noted that a simple calculation of means suggests that the SRK/T formula would yield the best prediction results, followed closely by the Holladay 1 formula, which was used clinically in the Infant Aphakia Treatment Study. However, comparison of median absolute prediction errors showed clinically similar results using the Holladay 1 or SRK/T formulae, with and without use of a customized constant. For eyes of more than 18 mm AL, clinically similar results were obtained using the Holladay 1, Holladay 2, or SRK/T formulae.
In the initial report on early refractive status for Infant Aphakia Treatment Study eyes, we noted that many sources of measurement error may contribute to difficulties in IOL calculation accuracy in the infant population.3 However, in this study, we used uniform surgical technique, similar IOL type (SN60AT) and placement (within the capsular bag), and standard timing of postoperative refraction. We did not identify any relationship with a particular eye characteristic correlating with higher prediction error for individual formulae; rather, when higher prediction error occurred, it tended to occur across formulae. A typical rate of refractive growth for an infant eye based on age (in months)13,22 would result in a 0.4- to 0.7-D myopic shift by the 1-month postoperative refraction, with slower but continued shifts after the first year and anticipation of low to moderate myopia by the second decade. Thus, normal eye growth of an infant during the early postoperative period may contribute to prediction error across formulae. The refractive strategy of implanting an IOL to leave residual hyperopia was dealt with by the addition of spectacles correcting for near focus. For most children, with the expected myopic shift of the operated eye and hyperopia of the fellow eye, the induced anisometropia equilibrates quickly. Notably, we excluded eyes with a diagnosis of glaucoma or that were glaucoma suspects from this analysis (n = 7 eyes; 13% of IOL cohort), because early axial elongation resulting from elevated intraocular pressure results in an early and unpredictable amount of myopic shift. For infants in this study who underwent IOL implantation and who were not diagnosed with glaucoma or suspected glaucoma, the mean rate of change in AL by 1 year of age was 0.24 mm/month.23
There are few studies that include a significant number of eyes with globe AL of less than 20 mm.4,17,24,25 One small series of microphthalmic adult eyes demonstrated the superiority of the theoretic SRK/T formula over SRK regression formulae, though overall the Hoffer Q formula was most accurate.26 Hoffer’s original series14 included 36 eyes of less than 22 mm, but the mean AL of this group was 21.4 ± 0.7 D, which is significantly longer than that found in the infant population. In a separate adult study, Hoffer found that the Holladay 2 and Hoffer Q formulae perform better for eyes with AL of less than 22 mm.16 Gavin and Hammond found better absolute prediction error using the Hoffer Q formula compared with the SRK/T formula for 41 eyes with an AL of less than 22 mm, but similarly, the mean AL in this series was 21.51 mm (range, 20.29 to 21.96 mm).25 MacLaren and associates studied 76 eyes with a mean AL of 20.79 ± 0.07 mm (no eye had AL <19 mm) and found both the Haigis and Hoffer Q formulae to perform well.17 Jung and associates recently studied 17 nanophthalmic eyes with an AL of less than 20.5 mm and found the Holladay 1 formula to give the lowest mean predictive error.24 Day and associates found the Holladay 1 and Hoffer Q formulae to perform significantly better in adult eyes with an AL of less than 22 mm (group mean AL, 21.2 ± 0.6 mm) compared with the Haigis and SRK/T formulae, until IOL constant adjustments were made, and then there was no difference among formulae.27 Aristodemou and associates studied a large cohort of adult eyes, obtaining biometry by partial coherence interferometry and using optimized constants.28 The Hoffer Q formula gave the lowest mean absolute prediction error for eyes with an AL of 20.00 to 20.99 mm, whereas the Hoffer Q and Holladay 1 formulae both performed well for eyes with an AL of 21.00 to 21.49 mm, and a similar performance was found using the Hoffer Q, Holladay 1, or SRK/T formulae for eyes with an AL of 21.5 to 22.00 mm. These studies are not comparable because data were obtained by different biometry techniques, IOL types implanted, and variable use of customized surgeon factors.
Prior studies of pediatric eyes have similarly conflicting results regarding formula predictability, and most reports include few eyes with an AL of less than 20 mm, whereas the Infant Aphakia Treatment Study series includes only 2 eyes with an AL of more than 20 mm. Nihalani and VanderVeen found the Hoffer Q and Holladay 1 formulae to give similar absolute prediction error for eyes with an AL of less than 22 mm (n = 69), but only 12 eyes had an AL of less than 20 mm.5,29 Andreo and associates found little difference among the IOL calculation formulae studied, but only 17 eyes had an AL of less than 22 mm (range, 18.6 to 22.0 mm).11 Neely and associates showed that formulae were increasingly less accurate with shorter ALs, particularly those less than 19 mm, but found the least variability using the SRK II formula.6 Tromans and associates also found greater error with eyes with an AL of less than 20 mm, but used only the SRK/T or SRKII formulae.9 Kekunnaya and associates suggested that SRK II is superior for pediatric eyes, as calculated for their cohort of 128 eyes with a mean AL of 19.9 ± 1.7 mm, including 15 eyes with an AL of less than 18.00 mm (compared with 23 eyes with an AL of less than 18 mm in our cohort).4 However, the mean absolute prediction error for all formulae in the Kekunnaya and associates study was higher for all formulae (SRK II, 2.27 ±1.69 D; SRK/T, 3.23 ±2.24 D; Holladay 1, 3.62 ± 2.42 D; Hoffer Q, 4.61 ± 3.12 D), suggesting other possible sources of error. Eibschitz-Tsimhoni and associates demonstrated discrepancies between formulae for a variety of keratometry and AL measures and concluded that it was unclear under which circumstances each formula may be preferred in the pediatric population.30,31
There are several limitations to this study. The accuracy of each formula depends on optimized values and measures of the formula components, including factors such as actual anterior chamber depth, lens thickness, vertex distance, and use of a personalized surgeon factor or A-constant.17,32 Although we performed customized calculations for this cohort, there were multiple surgeons, and the sample size is too low to assume reliability for standardized use of our modified constant. Additionally, formulae are based on the anatomic features of adult eyes, but the anterior segment of an infant is significantly smaller, eyes with congenital cataract may have greater anatomic variation in anterior segment structures (with variable anterior chamber depth and lens thickness), and the anterior segment of an infant is proportionally larger to the posterior segment compared with an adult eye. These structural variations may alter the expected effective lens position, and therefore the power. The capsular bag of an infant eye is smaller and will contract earlier, which may result in greater posterior IOL displacement. Implantation of high-power IOLs (particularly ≥30 D), often required in infantile eyes, can magnify measurement and calculation errors as well as the errors induced by changes in IOL position. Another limitation of this study is that we did not have all biometry data for all eyes; however, the results did not change when we analyzed data by comparing only eyes with complete data sets that included anterior chamber depth and lens thickness. Future research may allow development of a formula specifically designed for infantile eyes.
IOL implantation for eyes of infants younger than 7 months of age presents many challenges, including selection of an appropriate IOL calculation formula. We note that SRK/T calculations yielded good results in this cohort, and the Holladay 1 formula, used clinically in the Infant Aphakia Treatment Study, gave equally good results, particularly when medians and a customized constant were used, with no clinically significant difference between the 2 formulae even for eyes with the shortest ALs. When choosing a formula for IOL selection, clinical judgment should prevail, depending on the specific characteristics of the operative eye, surgeon experience, ability to customize calculations, and limitations of all formulae, which should be recognized within the range of very short ALs found in these children. We continue to endorse the use of the Holladay 1 formula for infant IOL calculations.
Biographies
 Deborah K. VanderVeen, MD, is an Associate in Ophthalmology at Boston Children’s Hospital and Associate Professor of Ophthalmology at Harvard Medical School, where she serves as Fellowship Director for the Harvard Pediatric Ophthalmology and Strabismus Program. Clinical research interests include topics in pediatric cataract and retinopathy of prematurity.
Deborah K. VanderVeen, MD, is an Associate in Ophthalmology at Boston Children’s Hospital and Associate Professor of Ophthalmology at Harvard Medical School, where she serves as Fellowship Director for the Harvard Pediatric Ophthalmology and Strabismus Program. Clinical research interests include topics in pediatric cataract and retinopathy of prematurity.
 Scott R. Lambert, MD is the Chief of Pediatric Ophthalmology at the Emory Eye Center and the R. Howard Dobbs Professor of Ophthalmology and Pediatrics at Emory University. He is the chairman of the Infant Aphakia Treatment Study and the Pediatric Ophthalmic Technology Assessment Committee for the American Academy of Ophthalmology. He is on the editorial board of the Journal of AAPOS. His clinical interests include pediatric cataract surgery, pediatric neuroophthalmology and adult strabismus surgery.
Scott R. Lambert, MD is the Chief of Pediatric Ophthalmology at the Emory Eye Center and the R. Howard Dobbs Professor of Ophthalmology and Pediatrics at Emory University. He is the chairman of the Infant Aphakia Treatment Study and the Pediatric Ophthalmic Technology Assessment Committee for the American Academy of Ophthalmology. He is on the editorial board of the Journal of AAPOS. His clinical interests include pediatric cataract surgery, pediatric neuroophthalmology and adult strabismus surgery.
APPENDIX 1: THE INFANT APHAKIA TREATMENT STUDY GROUP
ADMINISTRATIVE UNITS AND PARTICIPATING CLINICAL CENTERS
Clinical Coordinating Center (Emory University). Scott R. Lambert (Study Chair) and Lindreth DuBois (National Coordinator).
Data Coordinating Center (Emory University). Michael Lynn (Director), Betsy Bridgman, Marianne Celano, Julia Cleveland, George Cotsonis, Carey Drews-Botsch, Nana Freret, Lu Lu, Azhar Nizam, Seegar Swanson, and Thandeka Tutu-Gxashe.
Visual Acuity Testing Center (University of Alabama, Birmingham). E. Eugenie Hartmann (Director), Clara Edwards, Claudio Busettini, Samuel Hayley, and Anna Carrigan.
Steering Committee. Scott R Lambert, Edward G. Buckley, David A. Plager, M. Edward Wilson, Michael Lynn, Lindreth DuBois, Carolyn Drews-Botsch, E. Eugenie Hartmann, and Donald F. Everett.
Contact Lens Committee. Buddy Russell and Michael Ward.
PARTICIPATING CLINICAL CENTERS (IN ORDER BY THE NUMBER OF PATIENTS ENROLLED)
Medical University of South Carolina, Charleston, South Carolina (14). M. Edward Wilson, Margaret Bozic, and Carol Bradham.
Harvard University, Boston, Massachusetts (14). Deborah K. VanderVeen, Theresa A. Mansfield, and Kathryn Bisceglia Miller.
University of Minnesota, Minneapolis, Minnesota (13). Stephen P. Christiansen, Erick D. Bothun, Ann Holleschau, Jason Jedlicka, and Patricia Winters.
Cleveland Clinic, Cleveland, Ohio (10). Elias I. Traboulsi, Susan Crowe, Heather Hasley Cimino.
Baylor College of Medicine, Houston, Texas (10). Kimberly G. Yen, Maria Castanes, Alma Sanchez, Shirley York, Margaret Olfson, and Stacy Malone.
Oregon Health and Science University, Portland, Oregon (9). David T. Wheeler, Ann U. Stout, Paula Rauch, Kimberly Beaudet, and Pam Berg.
Emory University, Atlanta, Georgia (9). Scott R. Lambert, Amy K. Hutchinson, Lindreth DuBois, Rachel Robb, and Marla J. Shainberg.
Duke University, Durham, North Carolina (8). Edward G. Buckley, Sharon F. Freedman, Lois Duncan, B. W. Phillips, and John T. Petrowski.
Vanderbilt University, Nashville, Tennessee (8). David Morrison, Sandy Owings, Ron Biernacki, and Christine Franklin.
Indiana University (7). David A. Plager, Daniel E. Neely, Michele Whitaker, Donna Bates, and Dana Donaldson.
Miami Children’s Hospital (6). Stacey Kruger, Charlotte Tibi, and Susan Vega. University of Texas Southwestern, Dallas, Texas (6). David R. Weakley, David R. Stager, Jr., Joost Felius, Clare Dias, Debra L. Sager, and Todd Brantley.
Data and Safety Monitoring Committee. Robert Hardy (Chair), Eileen Birch, Ken Cheng, Richard Hertle, Craig Kollman, Marshalyn Yeargin-Allsopp (resigned), Cindy Bachman, and Donald F. Everett.
Medical Safety Monitor. Allen Beck.
Footnotes
ALL AUTHORS HAVE COMPLETED AND SUBMITTED THE ICMJE FORM FOR DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST and the following were reported. Drs Lambert and VanderVeen receive research grant support from Alcon, and Dr Trivedi’s institution receives royalties paid by Springer. Supported by Grants U10 EY13272 and U10 EY013287 and Departmental Core Grant EY06360 from the National Institutes of Health, Bethesda, Maryland; and by Research to Prevent Blindness, Inc, New York, New York. Involved in Design and conduct of study (D.K.V., R.H.T., A.N., M.J.L., S.R.L.); Collection, management, analysis, and interpretation of data (D.K.V., R.H.T., A.N., M.J.L., S.R.L.); and Preparation, review, or approval of manuscript (D.K.V., R.H.T., A.N., M.J.L., S.R.L.). Investigators in the Infant Aphakia Treatment Study, listed in Appendix A, contributed to the design and conduct of the study and collection and management of the data for this report. Michael Lynn, Department of Biostatistics and Bioinformatics, School of Public Health, Emory University, Atlanta, Georgia, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Lambert SR, Buckley EG, et al. Infant Aphakia Treatment Study Group. The infant aphakia treatment study: design and clinical measures at enrollment. Arch Ophthalmol. 2010;128(1):21–27. doi: 10.1001/archophthalmol.2009.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambert SR, Buckley EG, et al. Infant Aphakia Treatment Study Group. A randomized clinical trial comparing contact lens with intraocular lens correction of monocular aphakia during infancy: grating acuity and adverse events at age 1 year. Arch Ophthalmol. 2010;128(7):810–818. doi: 10.1001/archophthalmol.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.VanderVeen DK, Nizam A, Lynn MJ, et al. Predictability of intraocular lens calculation and early refractive status: the Infant Aphakia Treatment Study. Arch Ophthalmol. 2012;130(3):293–299. doi: 10.1001/archophthalmol.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kekunnaya R, Gupta A, Sachdeva V, Rao HL, Vaddavalli PK, Om Prakash V. Accuracy of intraocular lens power calculation formulae in children less than two years. Am J Ophthalmol. 2012;154(1):13–19. e12. doi: 10.1016/j.ajo.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 5.Nihalani BR, VanderVeen DK. Accuracy of intraocular lens power calculation formulae in children less than two years. Am J Ophthalmol. 2012;154(4):759–760. doi: 10.1016/j.ajo.2012.06.017. author reply 760. [DOI] [PubMed] [Google Scholar]
- 6.Neely DE, Plager DA, Borger SM, Golub RL. Accuracy of intraocular lens calculations in infants and children undergoing cataract surgery. J AAPOS. 2005;9(2):160–165. doi: 10.1016/j.jaapos.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Moore DB, Ben Zion I, Neely DE, et al. Accuracy of biometry in pediatric cataract extraction with primary intraocular lens implantation. J Cataract Refract Surg. 2008;34(11):1940–1947. doi: 10.1016/j.jcrs.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Mezer E, Rootman DS, Abdolell M, Levin AV. Early postoperative refractive outcomes of pediatric intraocular lens implantation. J Cataract Refract Surg. 2004;30(3):603–610. doi: 10.1016/j.jcrs.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Tromans C, Haigh PM, Biswas S, Lloyd IC. Accuracy of intra-ocular lens power calculation in paediatric cataract surgery. Br J Ophthalmol. 2001;85(8):939–941. doi: 10.1136/bjo.85.8.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutchinson AK, Wilson ME, Saunders RA. Outcomes and ocular growth rates after intraocular lens implantation in the first 2 years of life. J Cataract Refract Surg. 1998;24(6):846–852. doi: 10.1016/s0886-3350(98)80142-9. [DOI] [PubMed] [Google Scholar]
- 11.Andreo LK, Wilson ME, Saunders RA. Predictive value of regression and theoretical IOL formulas in pediatric intraocular lens implantation. J Pediatr Ophthalmol Strabismus. 1997;34(4):240–243. doi: 10.3928/0191-3913-19970701-12. [DOI] [PubMed] [Google Scholar]
- 12.McClatchey SK, Dahan E, Maselli E, et al. A comparison of the rate of refractive growth in pediatric aphakic and pseudophakic eyes. Ophthalmology. 2000;107(1):118–122. doi: 10.1016/s0161-6420(99)00033-0. [DOI] [PubMed] [Google Scholar]
- 13.McClatchey SK, Parks MM. Theoretic refractive changes after lens implantation in childhood. Ophthalmology. 1997;104(11):1744–1751. doi: 10.1016/s0161-6420(97)30032-3. [DOI] [PubMed] [Google Scholar]
- 14.Hoffer KJ. The Hoffer Q formula: a comparison of theoretic and regression formulas. J Cataract Refract Surg. 1993;19(6):700–712. doi: 10.1016/s0886-3350(13)80338-0. Errata: J Cataract Refract Surg 1994;20(6):677 and 2007;33(1):2–3. [DOI] [PubMed] [Google Scholar]
- 15.Holladay JT. Standardizing constants for ultrasonic biometry, keratometry, and intraocular lens power calculations. J Cataract Refract Surg. 1997;23(9):1356–1370. doi: 10.1016/s0886-3350(97)80115-0. [DOI] [PubMed] [Google Scholar]
- 16.Hoffer KJ. Clinical results using the Holladay 2 intraocular lens power formula. J Cataract Refract Surg. 2000;26(8):1233–1237. doi: 10.1016/s0886-3350(00)00376-x. [DOI] [PubMed] [Google Scholar]
- 17.MacLaren RE, Natkunarajah M, Riaz Y, Bourne RR, Restori M, Allan BD. Biometry and formula accuracy with intraocular lenses used for cataract surgery in extreme hyperopia. Am J Ophthalmol. 2007;143(6):920–931. doi: 10.1016/j.ajo.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 18.Mayer DL, Hansen RM, Moore BD, Kim S, Fulton AB. Cycloplegic refractions in healthy children aged 1 through 48 months. Arch Ophthalmol. 2001;119(11):1625–1628. doi: 10.1001/archopht.119.11.1625. [DOI] [PubMed] [Google Scholar]
- 19.Holladay JT, Prager TC, Chandler TY, Musgrove KH, Lewis JW, Ruiz RS. A three-part system for refining intraocular lens power calculations. J Cataract Refract Surg. 1988;14(1):17–24. doi: 10.1016/s0886-3350(88)80059-2. [DOI] [PubMed] [Google Scholar]
- 20.Retzlaff J. Posterior chamber implant power calculation: regression formulas. J Am Intraocul Implant Soc. 1980;6(3):268–270. doi: 10.1016/s0146-2776(80)80076-0. [DOI] [PubMed] [Google Scholar]
- 21.Retzlaff JA, Sanders DR, Kraff MC. Development of the SRK/T intraocular lens implant power calculation formula. J Cataract Refract Surg. 1990;16(3):333–340. doi: 10.1016/s0886-3350(13)80705-5. [DOI] [PubMed] [Google Scholar]
- 22.McClatchey SK, Hofmeister EM. The optics of aphakic and pseudophakic eyes in childhood. Surv Ophthalmol. 2010;55(2):174–182. doi: 10.1016/j.survophthal.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Lambert SR, Lynn MJ, DuBois LG, et al. Axial elongation following cataract surgery during the first year of life in the Infant Aphakia Treatment Study. Invest Ophthalmol Vis Sci. 2012;53(12):7539–7545. doi: 10.1167/iovs.12-10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung KI, Yang JW, Lee YC, Kim SY. Cataract surgery in eyes with nanophthalmos and relative anterior microphthalmos. Am J Ophthalmol. 2012;153(6):1161–1168. e1161. doi: 10.1016/j.ajo.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Gavin EA, Hammond CJ. Intraocular lens power calculation in short eyes. Eye (Lond) 2008;22(7):935–938. doi: 10.1038/sj.eye.6702774. [DOI] [PubMed] [Google Scholar]
- 26.Inatomi M, Ishii K, Koide R, Kora Y, Ozawa T. Intraocular lens power calculation for microphthalmos. J Cataract Refract Surg. 1997;23(8):1208–1212. doi: 10.1016/s0886-3350(97)80317-3. [DOI] [PubMed] [Google Scholar]
- 27.Day AC, Foster PJ, Stevens JD. Accuracy of intraocular lens power calculations in eyes with axial length <22. 00 mm. Clin Experiment Ophthalmol. 2012;40(9):855–862. doi: 10.1111/j.1442-9071.2012.02810.x. [DOI] [PubMed] [Google Scholar]
- 28.Aristodemou P, Knox Cartwright NE, Sparrow JM, Johnston RL. Formula choice: Hoffer Q, Holladay 1, or SRK/T and refractive outcomes in 8108 eyes after cataract surgery with biometry by partial coherence interferometry. J Cataract Refract Surg. 2011;37(1):63–71. doi: 10.1016/j.jcrs.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 29.Nihalani BR, VanderVeen DK. Comparison of intraocular lens power calculation formulae in pediatric eyes. Ophthalmology. 2010;117(8):1493–1499. doi: 10.1016/j.ophtha.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 30.Eibschitz-Tsimhoni M, Tsimhoni O, Archer SM, Del Monte MA. Discrepancies between intraocular lens implant power prediction formulas in pediatric patients. Ophthalmology. 2007;114(2):383–386. doi: 10.1016/j.ophtha.2006.06.063. [DOI] [PubMed] [Google Scholar]
- 31.Eibschitz-Tsimhoni M, Tsimhoni O, Archer SM, Del Monte MA. Effect of axial length and keratometry measurement error on intraocular lens implant power prediction formulas in pediatric patients. J AAPOS. 2008;12(2):173–176. doi: 10.1016/j.jaapos.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Norrby S. Sources of error in intraocular lens power calculation. J Cataract Refract Surg. 2008;34(3):368–376. doi: 10.1016/j.jcrs.2007.10.031. [DOI] [PubMed] [Google Scholar]