Abstract
Polycyclic aromatic hydrocarbons (PAHs) on virgin polystyrene (PS) and PS marine debris led us to examine PS as a source and sink for PAHs in the marine environment. At two locations in San Diego Bay, we measured sorption of PAHs to PS pellets, sampling at 0, 1, 3, 6, 9 and 12 months. We detected 25 PAHs using a new analytical method with comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry. Several congeners were detected on samples before deployment. After deployment, some concentrations decreased (1,3-dimethylnaphthalene and 2,6-methylnaphthalene) while most increased (2-methylanthracene and all parent PAHs (PPAHs) except fluorene and fluoranthene), suggesting PS debris is a source and sink for PAHs. When comparing sorbed concentrations of PPAHs on PS to the five most common polymers (polyethylene terephthalate (PET), high-density polyethylene (HDPE), polyvinyl chloride (PVC), low-density polyethylene (LDPE), and polypropylene (PP)), PS sorbed greater concentrations than PP, PET and PVC, similar to HDPE and LDPE. Most strikingly, at 0 months, PPAHs on PS ranged from 8-200 times greater than on PET, HDPE, PVC, LDPE, and PP. The combination of greater PAHs in virgin pellets and large sorption suggests that PS may pose a greater risk of exposure to PAHs upon ingestion.
Introduction
Plastics debris is ubiquitous across several habitats in the marine environment from beaches1 to the open ocean2 extending to the depths of the sea3. Priority pollutants (e.g. persistent organic pollutants) are consistently found sorbed to this debris from seawater4 and are associated with plastics as ingredients and/or byproducts of manufacturing5. Thus, when determining the risk of plastic marine debris to an organism, it is important to consider the chemical ingredients and the sorbed priority pollutants6,7. For example, we found similarly large levels of polycyclic aromatic hydrocarbons (PAHs) on polystyrene (PS) foam packaging materials as we did on PS foam debris recovered from beaches8, suggesting that PAHs are associated with plastic debris via absorption and manufacturing. Here, we examine this further and use PS pellets to measure PS debris as both a source and sink for PAHs in the marine environment.
PAHs are ubiquitous contaminants generated during the incomplete combustion of organic material9,10 and are considered a priority due to their persistence, bioaccumulation and toxicity11,12. In water, PAHs tend to associate with particles rather than dissolve due to their hydrophobic nature13, and thus plastics are used as passive samplers to measure PAHs in seawater14. The large sorption of PAHs to polyethylene14 and polyurethane foam15 is well known. Thus, it is expected that plastic debris will act as a sink for PAHs in aquatic habitats and it is no surprise that plastic debris recovered globally contains measurable PAHs4.
To understand sorption of PAHs to different types of plastic debris, we conducted the first long-term controlled field experiment designed to measure sorption of several priority pollutants, including PAHs, in the marine environment to the six most commonly mass-produced polymers (polyethylene terephthalate (PET), high-density polyethylene (HDPE), polyvinyl chloride (PVC), low-density polyethylene (LDPE), polypropylene (PP) and PS).6,16 Virgin pre-production pellets of each polymer were deployed at five locations throughout San Diego Bay, CA for five time periods up to one year and showed that HDPE, LDPE, PP and PVC sorbed significantly different concentrations of PAHs.6 We were unable to determine sorption to PS because chemical analyses were unsuccessful using a conventional one-dimensional GC/MS method due to the complexity of the sample matrix. Here, we applied a recently developed method based on comprehensive two-dimensional gas chromatography (GC×GC/ToF-MS)17 to successfully analyze parent-PAHs (PPAHs), alkyl-PAHs (MPAHs), nitro-PAHs (NPAHs), oxy-PAHs (OPAHs) and thio-PAHs (SPAHs) within one single chromatographic run.
Here, we analyzed multiple classes of PAHs on PS pellets deployed in San Diego Bay for up to one year to test the hypotheses that 1) PAHs are associated with virgin PS, 2) PAHs will sorb to PS in the marine environment from several sources and 3) concentrations will differ from other plastic types. This work provides insight into potential hazards associated with PS marine debris. PS is a common marine debris item18 and has been found in the gut contents of fish.19 In the absence of PAHs, PS poses a hazard to marine organisms due to its hazardous styrene monomer, both carcinogenic and disruptive to the endocrine system.20 Here, we show that several PAHs are associated with PS before deployment and when littered in the marine environment sorb greater concentrations of these hazardous chemicals. Thus, individual hazards associated with both PS and PAHs make PS marine debris a potential multiple stressor in marine habitats when available for ingestion by marine life.
Experimental section
Experimental Design
PS virgin pre-production plastic pellets (3 mm long, 2 mm diameter) were deployed from floating docks in San Diego Bay.6 Here, we focus on two locations (Figure S1): San Diego Harbor Excursions in the central bay and Shelter Island near the mouth of the bay. Details regarding experimental design can be found in Rochman et al.6 Briefly, at each location, two replicate samples were deployed for collection at the end of five time periods: 1, 3, 6, 9, and 12 months (20 total samples). Each replicate consisted of 5 g of pellets in individual Nitex mesh (1.3 mm) bags within a nylon mesh (10 mm) bag. Replicate samples were deployed by hanging each nylon bag on one of two identical PVC frames suspended from each dock (approximately 2 meters from each other, and at a depth approximately 0.5 m below the surface). At the end of their randomly assigned deployment time, samples were collected and stored at -20°C until analysis. We also analyzed three replicate samples of virgin pellets never deployed in the bay (i.e., 0-month samples). Methods for preparing samples for chemical analyses were established previously in our laboratory8 and are described by Rochman et al.6. For more information regarding chemical standards and solvent materials, sample prep, chemical analyses and QA/QC refer to Supporting Information.
GC×GC/ToF-MS Analysis for PAHs
A GC×GC/ToF-MS Pegasus 4D (LECO, St. Joseph, MI, USA) equipped with an Agilent 6890 GC with a secondary oven, a split/splitless injector, and a non-moving quad-jet dual stage modulator was used. Two GC columns, LC-50 (10 m × 0.15 mm × 0.10 μm) in the first dimension and NSP-35 (1.2 m × 0.10 mm × 0.10 μm) in the second dimension (from J&K Scientific; Edwardsville, Nova Scotia, Canada), were connected using an Agilent CPM union (part no. 188-5361) for 0.1-0.25 mm I.D. columns. The data processing was performed using ChromaTOF version 4.33. The optimization parameters are described in previous studies17,24 and optimized conditions in Table S1. Five-point calibration curves with range 5-1000 pg/uL were used, using the same approach that has been described previously24.
Statistical Methods
We quantified temporal patterns by fitting a first-order approach to equilibrium model25 when concentrations appeared to be increasing and an exponential decay model when concentrations appeared to be decreasing26. SigmaPlot 10 (SYSTAT Software, Chicago, IL) was used to fit the exponential rise to maximum, Ct=Ceq(1-e-kt), and the exponential decay, Ct=(C0-Ceq)e-kt + Ceq, equations where Ct is the concentration at time t, Ceq is the predicted equilibrium concentration, C0 is the initial concentration and k is the rate constant. We examined spatial patterns using principle components analysis (PCA) run with IBM SPSS version 19 and potential sources using molecular ratios of several PAHs at each location. This method involves comparing concentration ratios of frequently found PAHs to identify possible sources27 and should be used with caution because molecular ratios can easily be altered by different factors such as reactivity of PAHs and degradation.27-29 The combination of five molecular ratios containing fluorene (FLO), pyrene (PYR), anthracene (ANT), phenanthrene (PHE), benzo[a]pyrene (BaP), chrysene (CHR), benz[a]anthracene (BaA), Triphenylene (TRI), benzo[b]fluoranthene (BbF) and benzo[k]fluoranthene (BkF) were used to generate bi-variate plots (Figure 5).30-33 Two-sample student t-tests run in SigmaPlot 10 (SYSTAT Software, Chicago, IL) determined if concentrations of PAHs and molecular ratios were statistically different between locations. Concentrations of total sorbed PPAHs were log-transformed to achieve normality. We tested for differences among plastic types and locations in San Diego Bay by performing a 2-factor ANOVA on data from each sampling period individually with SYSTAT 12 (SYSTAT Software, Chicago, IL). Data for PPAHs sorbed to HDPE, LDPE, PP, PET and PVC deployed simultaneously with PS was included in this analysis.6 Homogeneity of variance was verified by a Levene's test. Post-hoc Tukey's tests were used to distinguish significantly different treatment means.
Figure 5.
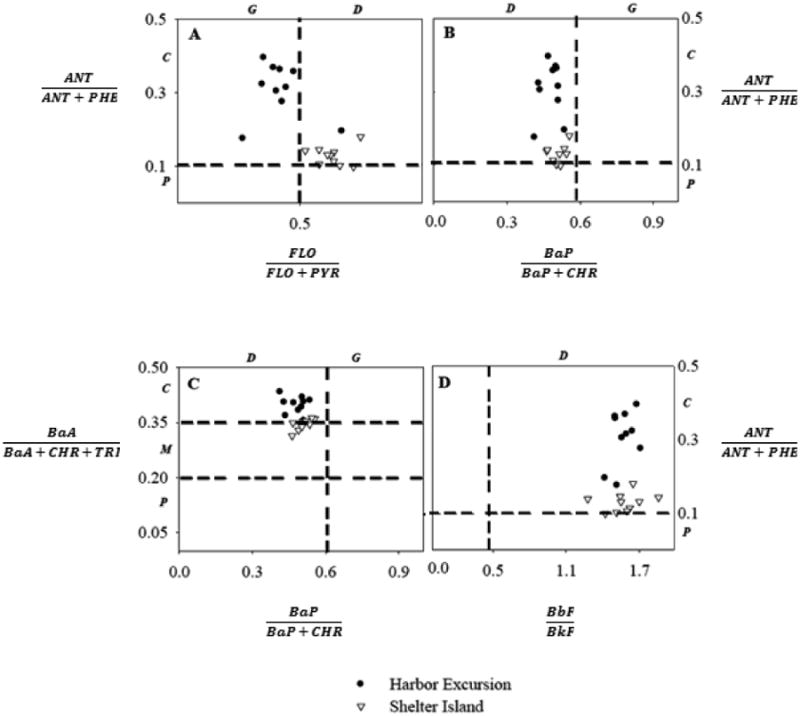
Bi-variate plots of PAH diagnostic ratios for PS pellets deployed in both sampling sites. (A) FLO/(FLO+PYR) vs. ANT/ (ANT+PHE), (B) BaP/(BaP+CHR) vs. ANT/(ANT+PHE), (C) BaP/ (BaP+CHR) vs. BaA/(BaA+CHR+TRI), (D) BbF/BkF vs. ANT/ (ANT+PHE). Dashed lines represent threshold values and letters in italics represent possible sources: G = gasoline, D = diesel, C = combustion of petroleum derivatives, P = PAHs from petroleum, M = mix sources.
Results and Discussion
PAH analysis achieved by the GC×GC/ToF-MS method
The one-dimensional GC-MS method6 previously used for analysis of PAHs in other plastic types was unsuccessful for the PS samples. The column was sacrificed due to column overload and strong matrix interferences that can be seen as very large peaks eluting at the beginning of the run in selected ion monitoring (SIM) chromatogram (Figure 1). The inner boxes in Figure 1 show PAHs co-eluting with interfering components represented by large peaks that do not correspond to any PAH or internal standard.
Figure 1.
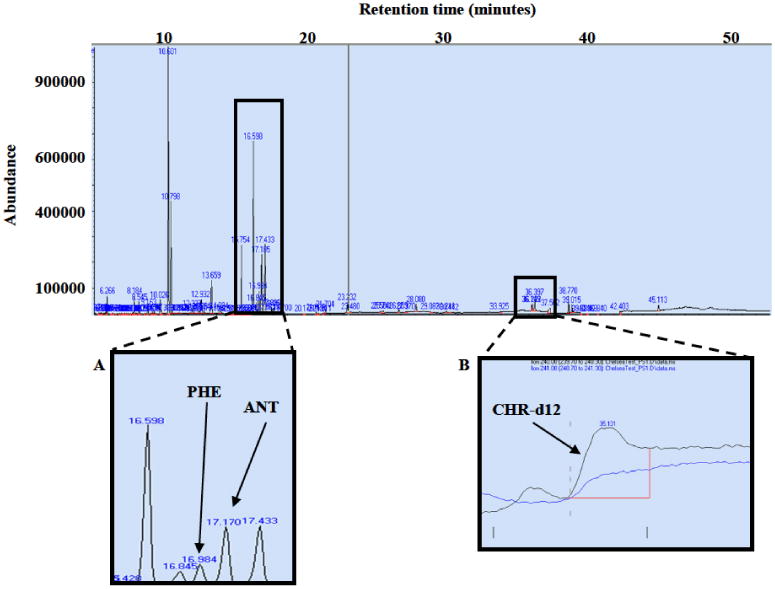
One-dimensional chromatogram of the sum of selected ions for a polystyrene extract analyzed using a 30 m DB-5 column after solid phase extraction (SPE). The inner boxes show some of the PAHs with co-elution problems. (A) Phenanthrene (PHE) and anthracene (ANT) co-eluting with interfering components represented by large peaks that do not correspond to PAHs or internal standards. (B) Chrys-ene-d12 (CHR-d12) showing peak broadening and baseline drifting possibly due to matrix co-eluting with target compound.
Figure 2 shows the total ion chromatogram (TIC) for the analysis of a PS sample using the GC×GC/ToF-MS method developed for simultaneous analysis of multiple groups of PAHs,17,24 where the sample matrix (represented by dashed lines) elutes in regions that do not interfere with most of the PAHs (represented by a dotted line). This two-dimensional method was successful to analyze a total of 85 PAHs for identification and quantitation in PS samples, including 18 PPAHs, 9 MPAHs, 15 ClPAHs, 6 BrPAHs, 17 OPAHs and 2 SPAHs (See Table S2 for a complete list of targeted PAHs).
Figure 2.
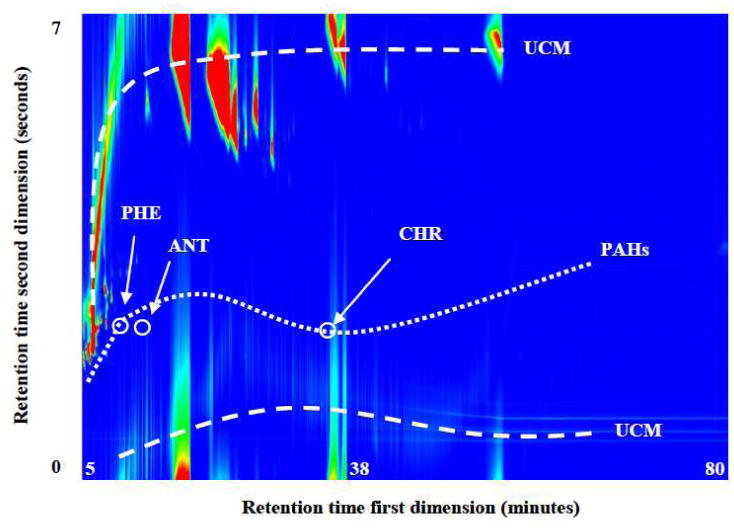
GC×GC/ToF-MS contour map of the total ion chromatogram (TIC) for a PS extract. The x-axis represents the retention time in the first dimension (min) and the y-axis the retention time in the second dimension (s). The dotted line represents the elution profile for the PAHs in the sample, which is isolated from most of sample matrix and UCM, represented by the dashed line below and above the PAHs line. PAHs that had co-elution problems when using a one-dimensional GC are labeled, phenanthrene (PHE), anthracene (ANT) and chrysene (CHR).
PAHs in PS
A total of 25 PAHs (15 PPAHs, 7 MPAHs, 2 OPAHs and 1 SPAH) were detected in the PS samples. Table 1 shows the concentrations of all PAHs detected (in ng/g) at each sampling period (0, 1, 3, 6, 9, and 12 months) from each location. Congeners from each group were found in virgin PS pellets before deployment. Of the PPAHs, acenaphthene, acenaphthylene, anthracene, fluoranthene, fluorene, phenanthrene and pyrene were found in virgin PS pellets. These seven PAHs have the lowest molecular weight (MW) of the PPAHs targeted and have a log Kow (octanol-water partitioning coefficient) less than or equal to five. Low molecular weight PAHs are characterized to come from direct petrogenic sources34, including the raw material petroleum. Of the MPAHs, 1,3-dimethylnaphthalene, 2,6-dimethylnaphthalene, 1-methylphenanthrene and 2-methylphenanthrene, were found in virgin PS pellets. MPAHs are an associated byproduct of petroleum emissions.35 Both OPAHs, 9-fluorenone and 1,4-naphthoquinone, were measured in virgin PS pellets and can be formed from the incomplete combustion of organic material36, including petroleum. Dibenzothiophene, also found in virgin PS pellets, occurs naturally in the production of oil.37 Because PAHs are associated with petroleum, the raw material of plastics, this is expected.
Table 1.
Concentration of PAHs found in PS pellets (in ng/g) deployed for 0, 1, 3, 6, 9 and 12 months in Harbor Excursion and Shelter Island sampling sites, determined using GC×GC/ToF-MS.
| Harbor Excursion | Shelter Island | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| months | 0 (blank) | 1 month | 3 months | 6 months | 9 months | 12 months | 1 month | 3 months | 6 months | 9 months | 12 months | |||||||||||
| n=3 | SD | n=2 | n=2 | n=2 | n=2 | n=2 | n=2 | n=2 | n=2 | n=2 | n=2 | |||||||||||
| 1,3-Dimethylnaphthalene | 24.3 | 4.24 | 29.8 | 17.9 | 22.9 | 23.1 | 15.4 | 22.3 | 18.2 | 17.4 | 17.8 | 14.8 | 18.5 | 21.7 | 17.0 | 17.8 | 14.9 | 14.8 | 9.64 | 12.3 | 13.4 | 8.93 |
| 1-Methylphenanthrene | 12.2 | 0.18 | 29.2 | 16.6 | 17.2 | 16.0 | 16.4 | 15.1 | 19.3 | 16.9 | 18.1 | 17.1 | 13.2 | 13.9 | 13.4 | 13.6 | 14.5 | 12.7 | 14.5 | 12.9 | 13.4 | 13.7 |
| 1-Methylpyrene | n.d. | 0 | 17.6 | 16.2 | 16.3 | 21.0 | 15.6 | 14.9 | 17.5 | 18.1 | 16.3 | 17.8 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2,6-Dimethylnaphthalene | 9.30 | 0.5 | 8.33 | 6.67 | 6.73 | 7.33 | 6.56 | 8.21 | 6.83 | 6.26 | 6.94 | 6.92 | 7.45 | 7.98 | 6.51 | 6.90 | 6.30 | 6.04 | 6.30 | 5.67 | 5.97 | 5.75 |
| 2-Methylanthracene | n.d. | 0 | 17.0 | 18.4 | 17.3 | 16.7 | 17.9 | 15.8 | 18.9 | 18.7 | 18.1 | 18.9 | 13.8 | 14.1 | 13.9 | 13.9 | 15.2 | 13.7 | 15.1 | 14.5 | 14.9 | 15.0 |
| 2-Methylphenanthrene | 13.5 | 0.15 | 19.7 | 15.5 | 15.6 | 15.5 | 15.1 | 14.5 | 14.9 | 15.9 | 15.8 | 15.3 | 13.9 | 14.0 | 14.1 | 13.9 | 14.2 | 13.4 | 14.4 | 13.4 | 13.9 | 13.8 |
| Triphenylene | n.d. | 0 | 10.9 | 8.60 | 8.63 | 7.56 | 8.82 | 6.32 | 9.23 | 9.68 | 8.09 | 10.2 | 5.84 | 5.41 | 5.38 | 5.59 | 6.33 | 5.65 | 6.32 | 5.65 | 6.53 | 6.71 |
| ΣMPAHs | 59.2 | 4.71 | 132 | 99.9 | 105 | 107 | 95.9 | 97.1 | 105 | 103 | 101 | 101 | 72.6 | 77.1 | 70.3 | 71.7 | 71.4 | 66.1 | 66.3 | 64.4 | 68.1 | 63.9 |
| Acenaphthene | 5.23 | 0.49 | 38.7 | 13.3 | 13.5 | 14.8 | 14.6 | 13.2 | 15.2 | 14.2 | 15.5 | 14.0 | 5.40 | 5.66 | 6.19 | 5.93 | 5.73 | 5.45 | 6.21 | 5.41 | 5.81 | 5.96 |
| Acenaphthylene | 6.76 | 0.83 | 18.7 | 19.3 | 15.8 | 17.8 | 22.8 | 13.9 | 18.8 | 22.8 | 18.7 | 22.1 | 9.33 | 9.74 | 9.80 | 10.4 | 11.2 | 10.3 | 15.2 | 10.6 | 11.1 | 13.3 |
| Anthracene | 5.41 | 0.14 | 44.1 | 42.2 | 40.1 | 39.2 | 43.8 | 25.4 | 45.9 | 53.5 | 44.3 | 49.4 | 10.1 | 10.6 | 11.0 | 13.7 | 15.6 | 14.3 | 18.1 | 13.5 | 16.7 | 18.1 |
| Benz[a]anthracene | n.d. | n.d. | 34.2 | 21.2 | 17.6 | 17.1 | 18.9 | 12.5 | 21.2 | 18.3 | 16.0 | 19.6 | 6.08 | 6.95 | 6.32 | 7.29 | 8.51 | 6.73 | 8.67 | 7.51 | 7.85 | 9.91 |
| Benzo[a]pyrene | n.d. | n.d. | 24.0 | 21.4 | 18.9 | 19.2 | 17.1 | 13.5 | 16.6 | 24.7 | 16.1 | 18.2 | 6.42 | 7.76 | 7.17 | 8.32 | 9.48 | 7.58 | 11.3 | 8.99 | 9.62 | 10.3 |
| Benzo[b]fluoranthene | n.d. | n.d. | 33.0 | 35.0 | 44.2 | 35.0 | 38.8 | 22.6 | 35.7 | 40.0 | 27.7 | 31.5 | 12.0 | 13.5 | 13.8 | 15.0 | 19.0 | 15.4 | 18.6 | 18.1 | 16.6 | 22.1 |
| Benzo[e]pyrene | n.d. | n.d. | 24.9 | 25.1 | 22.8 | 23.1 | 25.2 | 14.5 | 22.0 | 33.6 | 20.7 | 22.6 | 8.78 | 9.41 | 9.32 | 10.7 | 12.6 | 10.3 | 13.7 | 14.5 | 11.7 | 14.9 |
| Benzo[ghi]perylene | n.d. | n.d. | n.d. | 38.0 | 36.6 | 36.3 | 40.0 | 33.6 | 38.6 | 41.9 | 37.9 | 37.5 | 34.3 | 34.2 | 34.8 | 33.8 | 35.5 | 33.1 | 34.9 | 34.8 | 34.4 | 34.6 |
| Benzo[k]fluoranthene | n.d. | n.d. | 21.7 | 23.3 | 27.9 | 23.2 | 23.1 | 15.9 | 21.7 | 23.3 | 17.4 | 20.2 | 9.43 | 8.73 | 8.54 | 10.6 | 11.9 | 10.2 | 11.3 | 10.6 | 10.8 | 11.9 |
| Chrysene | n.d. | n.d. | 33.9 | 20.9 | 18.7 | 20.0 | 19.2 | 11.7 | 22.0 | 23.6 | 15.4 | 23.5 | 7.45 | 7.30 | 7.50 | 7.72 | 9.25 | 7.49 | 9.09 | 7.55 | 8.36 | 11.8 |
| Fluoranthene | 23.0 | 13.6 | 126 | 33.2 | 41.6 | 35.2 | 39.4 | 24.8 | 46.4 | 36.0 | 33.5 | 43.9 | 18.4 | 19.5 | 18.3 | 19.4 | 21.7 | 18.2 | 25.7 | 21.3 | 23.0 | 26.1 |
| Fluorene | 25.4 | 3.41 | 74.0 | 40.1 | 48.3 | 54.1 | 37.2 | 43.2 | 38.8 | 43.1 | 47.1 | 46.5 | 31.6 | 36.8 | 25.6 | 38.9 | 31.5 | 34.9 | 40.6 | 32.1 | 24.0 | 30.1 |
| Indeno[1,2,3-cd]pyrene | n.d. | n.d. | 30.4 | 33.7 | 33.8 | 32.6 | 34.3 | 27.7 | 33.2 | 39.6 | 32.9 | 32.4 | 27.6 | 28.4 | 27.9 | 29.8 | 30.6 | 27.9 | 28.0 | 29.2 | 29.8 | 30.7 |
| Phenanthrene | 12.6 | 0.64 | 208 | 74.2 | 68.9 | 70.8 | 67.1 | 105 | 96.4 | 141 | 96.9 | 113 | 63.0 | 71.1 | 85.8 | 126 | 133 | 127 | 82.3 | 89.6 | 98.4 | 109 |
| Pyrene | 15.7 | 6.42 | 203 | 55.5 | 74.6 | 59.5 | 68.3 | 21.0 | 73.0 | 57.5 | 58.9 | 68.4 | 17.8 | 22.0 | 14.5 | 15.1 | 22.9 | 17.7 | 13.6 | 20.2 | 17.4 | 27.6 |
| ΣPPAHs | 94.2 | 14.5 | 915 | 496 | 523 | 498 | 510 | 398 | 545 | 613 | 499 | 563 | 268 | 292 | 286 | 353 | 378 | 347 | 337 | 324 | 325 | 376 |
| 9-Fluorenone | 7.90 | 1.2 | 10.0 | 10.1 | 10.5 | 11.2 | 9.08 | 10.5 | 10.8 | 10.6 | 12.9 | 12.0 | 9.63 | 10.9 | 9.47 | 9.64 | 12.0 | 9.83 | 11.0 | 8.97 | 9.11 | 11.1 |
| 1,4-Naphthoquinone | 45.4 | 0.79 | 47.0 | 45.9 | 47.8 | 47.5 | 46.6 | 45.8 | 47.4 | 48.0 | 48.7 | 47.8 | 46.9 | 46.7 | 45.6 | 46.7 | 46.9 | 44.0 | 48.8 | 46.0 | 46.5 | 51.1 |
| ΣOPAHs | 53.3 | 1.93 | 57.0 | 56.0 | 58,3 | 58.7 | 55.7 | 56.3 | 58,2 | 58.6 | 61.6 | 59.8 | 56.5 | 57.6 | 55.1 | 56.3 | 58.9 | 53.9 | 59.8 | 54.9 | 55.6 | 62.2 |
| Dibenzothiophene | 4.35 | 0.06 | 19.3 | 6.90 | 7.41 | 7.30 | 6.19 | 5.99 | 7.40 | 6.92 | 7.13 | 7.02 | 3.98 | 3.99 | 3.87 | 4.01 | 4.08 | 3.92 | 4.15 | 3.93 | 3.97 | 4.03 |
| ΣSPAHs | 4.35 | 0.06 | 19.3 | 6.90 | 7.41 | 7.30 | 6.19 | 5.99 | 7.40 | 6.92 | 7.13 | 7.02 | 3.98 | 3.99 | 3.87 | 4.01 | 4.08 | 3.92 | 4.15 | 3.93 | 3.97 | 4.03 |
| Total PAHs | 211.0 | 21 | 1123 | 659 | 694 | 671 | 667 | 558 | 716 | 782 | 669 | 730 | 401 | 430 | 416 | 485 | 512 | 470 | 468 | 447 | 453 | 506 |
n.d.: compound not detected
The detection of PAHs in virgin PS pellets adds additional information to previous work showing that PS virgin pellets have up to two orders of magnitude greater concentrations of PPAHs than other polymers.8 When comparing PAHs in virgin PS pellets quantified here to those measured in other types of plastic by Rochman et al. (2013)6 we find that PPAHs in virgin pellets range from nd-2 ng/g on PVC, nd -1 ng/g in PET, 2-6 ng/g in PP, 3-6 ng/g on HDPE and nd-13 ng/g in LDPE; however, in virgin PS pellets PPAHs range from 79-97 ng/g (n=3), approximately 8 to 200 times greater than other polymers. This large difference in PPAHs in PS virgin pellets strengthens our hypothesis that PAHs are associated with the manufacturing process, likely related to the aromaticity of the styrene monomer.8,38
PAH formation may arise from multiple stages in the life cycle of PS. To manufacture polystyrene, ethylene and benzene are produced from crude oil under applied heat.39 The styrene monomer is contrived by reacting benzene with ethylene to make ethyl benzene which is dehydrogenated to styrene at 550-680°C.39 Both benzene and styrene are precursors of PAH formation.40 Once polymerized, polystyrene is more capable of depolymerizing into its individual monomers than other polymers20, and because polymerization reactions are rarely complete, the residual monomer styrene is likely to be found in the polymeric product.20 Thus, the monomer styrene is likely available for PAH formation during and post-manufacturing. Once PS pre-production pellets are produced, manufacturers apply heat again to process and shape end products from PS pellets39, likely resulting in emission of more PAHs. Lastly, combustion of PS waste products results in greater PAH emissions than other polymers.41
Temporal patterns among individual congeners of PAHs were examined to better understand PS as a source and sink for PAHs in the marine environment. We quantified temporal patterns by fitting a first-order approach to equilibrium model25 when concentrations of PAHs increased over time and an exponential decay model when concentrations decreased26. Fitting these equation assumes a relatively constant background concentration of PAHs. Although this assumption likely does not hold during a field deployment, the equations fit our data relatively well over the long time scales of our experiment despite temporal variability. Where concentrations of PAHs were relatively constant during deployment, neither model could be fit. Because sorption is related to the hydrophobicity of each specific congener, we quantified temporal patterns for individual PAHs sorbed to PS at each location (Figure 3, S2, S3). To compare sorption trends of PS analyzed here to the HDPE, LDPE, PP, PET and PVC analyzed previously6, we also quantified temporal patterns for sorption of total priority PPAHs at Harbor Excursion where concentrations are greatest (Figure S4).
Figure 3.
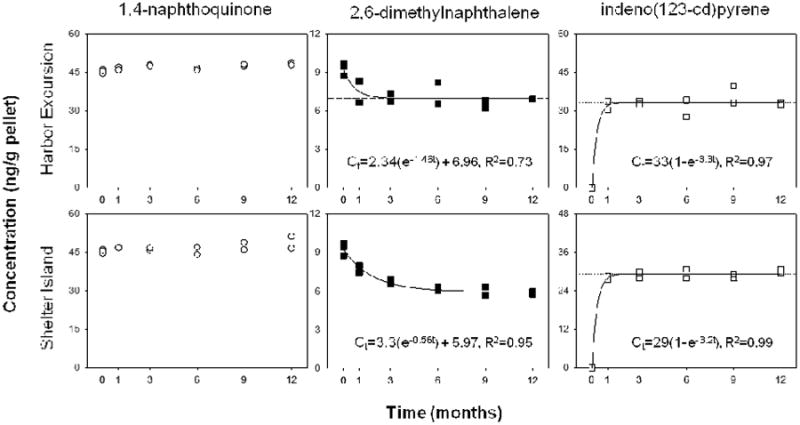
Concentrations of 1,4-naphthoquinone (no change over time; left), 2,6-dimethylnaphthalene (decreasing over time; middle), indeno(123-cd)pyrene (increasing over time; right) in ng/g of pellets vs. time for PS at Harbor Excursion (top rows) and Shelter Island (bottom rows). Please note that vertical axes differ among graphs. Data were fit to the first-order approach to equilibrium model25 using the exponential rise to maximum equation Ct=Ceq(1-e-kt) or the exponential decay model26 using the equation Ct=(C0 - Ceq)e-kt + Ceq, where Ct is the concentration at time t, Ceq is the predicted equilibrium concentration, C0 is the initial concentration and k is the rate constant. The horizontal dotted line denotes the predicted Ceq for each plastic type. Where the equation and lines are missing, the data could not be fit to the equation.
We observed PS behaving as a source or a sink for several PAH congeners in San Diego Bay. Yet, for some congeners, we did not observe PS behaving as either. Concentrations of 1-methylphenanthrene, 2-methylphenanthrene, 9-fluorenone, 1,4-naphthoquione, dibenzothiophene, fluorene and fluoranthene remained relatively constant over time (Figure 3, S2, S3). Concentrations of 1,3-dimethylnaphthalene and 2,6-methylnaphthalene decreased upon deployment (Figure 3, S2), suggesting that PS may be a source of these PAHs to the marine environment. While, these low molecular weight 2-ring PAHs do have a greater solubility in water than other PAH congeners, another explanation may be that these compounds underwent degradation due to exposure to sunlight and/or marine microorganisms. We observed PS behaving as a sink for several PAHs measured in this study, including 1-methylpyrene, 2-methylanthracene and all measured PPAHs except fluorene and fluoranthene (Figure 3, S2, S3). At Harbor Excursion, where concentrations of PAHs were greatest, these PAHs fit the first-order kinetics model well; whereas, at Shelter Island, sorption trends for 1-methylpyrene (non-detect), acenaphthylene, acenaphthene and pyrene could not be fit to the equation.
For PAHs sorbed to PS from ambient seawater, we expected individual congeners to behave differently, as chemicals with less hydrophobicity and a lighter MW are expected to reach saturation faster than those with greater hydrophobicity and a heavier MW.42 While we observed this for PPAHs sorbing to HDPE, LDPE and PP at Harbor Excursion6, similar to PET and PVC6 we did not see obvious differences in sorption patterns among individual congeners for PS at either location (Figure S3). Differences in sorption patterns among congeners may not be expected for the glassy polymers, PET, PVC and PS, where diffusion into the polymer is not expected.43 Thus, these polymers may exhibit a relatively rapid adsorption onto the surface that is not followed by a slower diffusion into the polymeric matrix as is expected for polyethylene.42
While sorbed concentrations of total PPAHs on PS are similar to HDPE and LDPE, the time to reach predicted equilibrium happens much faster (Figure S4). After the 1-month sampling period, concentrations of total PPAHs sorbed to PS changed little over time at both locations (Table 1). Temporal patterns for PS at Harbor Excursion are similar to what is observed for PET and PVC.6 In contrast, at this location HDPE, LDPE and PP reached their predicted equilibriums by 6 months.6 Thus, the relatively large concentrations of PPAHs sorbing to PS occurs relatively quickly after deployment into the marine environment (Figure S4). Management of PS may hold a greater priority than debris composed of HDPE, LDPE, PP, PET or PVC because our data suggests that PS acquires relatively large concentrations of hazardous chemicals after a short period of time at sea.
Site difference for PAHs
Total PAH concentrations were greater at Harbor Excursion compared to Shelter Island by a factor of approximately two (Table 1). Concentrations of 21 of the 25 individual PAHs quantified were significantly greater (p < 0.05) at Harbor Excursion than Shelter Island, whereas 2,6-dimethylnaphthalene, phenanthrene, 9-fluorenone, and 1,4-naphthaquinone were not statistically different (p > 0.05) between locations. Concentrations of these four PAHs, with the exception of phenanthrene, decreased or did not change over time. Significant differences among the remaining PAHs are probably related to different sources of contamination between locations.
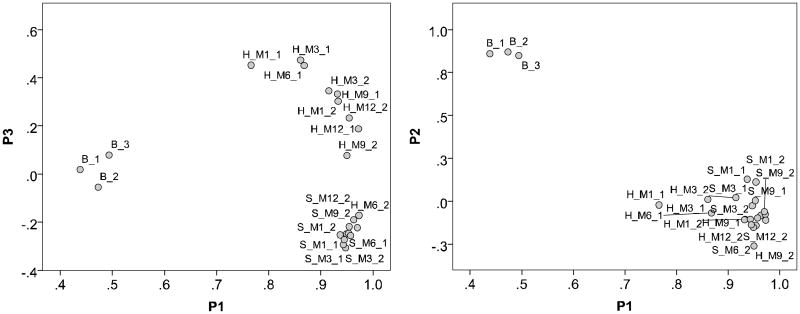
Table S3a and S3b show the PAH compositional difference (%) of the two locations compared to the virgin blanks (0-month exposure). The PAH composition of each sample was compared using principle component analysis (PCA) due to the many variables (25 PAHs). The analysis shows that the top three components (P1-P3) explain 97% of the variance, with P1 = 79%, P2 = 11%, and P3 = 7.5%. The PAH composition of the virgin PS pellets was clearly different from the deployed PS pellets (Figure 4). Among the deployed PS pellets, the PAH composition was slightly different between the two sites except one sample from Harbor Excursion (sampled at 6 months; Figure 4). Differences in PAH compositions suggest different sources of PAHs.
Figure 4.
Principal component analysis of the concentration of PAHs. Three principal components are shown. Generalized grouping: B – blank, virgin PS pellets not deployed, H_M – Harbor Excursion sampling site, with 1, 3, 6, 9 and 12 months of exposure, and S_M – Shelter Island sampling site, with 1, 3, 6, 9 and 12 months of exposure
To further examine sources of PAHs at each location, we used molecular ratios. The FLO/(FLO+PYR) ratio was significantly different between sites (p < 0.001), averaging 0.421 (±0.106) at Harbor Excursion and 0.633 (±0.067) at Shelter Island, suggesting a gasoline origin for PAHs at Harbor Excursion and a diesel origin for PAHs at Shelter Island (Figure 5A). The ANT/(ANT+PHE) ratio was also significantly different between sites (p < 0.001), averaging 0.307 (±0.073) for Harbor Excursion and 0.128 (±0.025) for Shelter Island, suggesting a pyrogenic origin for PAHs at Harbor Excursion and a more petrogenic origin at Shelter Island (Figure 5A, 5B and 5D) which is reinforced by the ratio BaA/(BaA+CHR+TRI) that was also significantly different between locations (p < 0.001), averaging 0.397 (±0.023) for Harbor Excursion and 0.346 (±0.015) for Shelter Island (Figure 5C). The two ratios BbF/BkF and BaP/(BaP+CHR) were not significantly different between locations. These results suggest that PAHs at Shelter Island and Harbor Excursion come from different sources, with Shelter Island showing ratios closer to those found in petroleum and Harbor Excursion closer to those found in the generation of pyrogenic PAHs. The suggestion that sources of PAHs to Harbor Excursion are more pyrogenic in origin is further confirmed by our data. For example, dibenzothiophene and 1-methylpyrene, with greater concentration at Harbor Excursion, are indicators of fossil fuels such as gasoline or diesel exhaust.44,45 In addition, MPAHs are indicative of direct petroleum emissions35, and we found greater concentrations of 1-methylpyrene and 2-mehtylanthracene at Harbor Excursion relative to Shelter Island likely due to the greater shipping activity at Harbor Excursion.
PPAH concentrations in PS compared to other mass-produced polymers
Upon comparing sorbed concentrations of PPAHs in PS to HDPE, LDPE, PP, PET and PVC previously reported, we found similar patterns confirming our past results showing that HDPE and LDPE sorb significantly greater PAHs than PP, PET and PVC and that PP sorbs an intermediate concentration.6 The inclusion of PS in a 2-factor ANOVA for each sampling period reveals a consistently significant interaction (p < 0.05) between location and plastic type. Still, over space and time, HDPE, LDPE and PS consistently sorb the greatest concentration of PPAHs. At Shelter Island PS sorbs the greatest concentration of PPAHs overall, but at Harbor Excursions differences among HDPE, LDPE and PS are less conspicuous (Figure 6).
Figure 6.
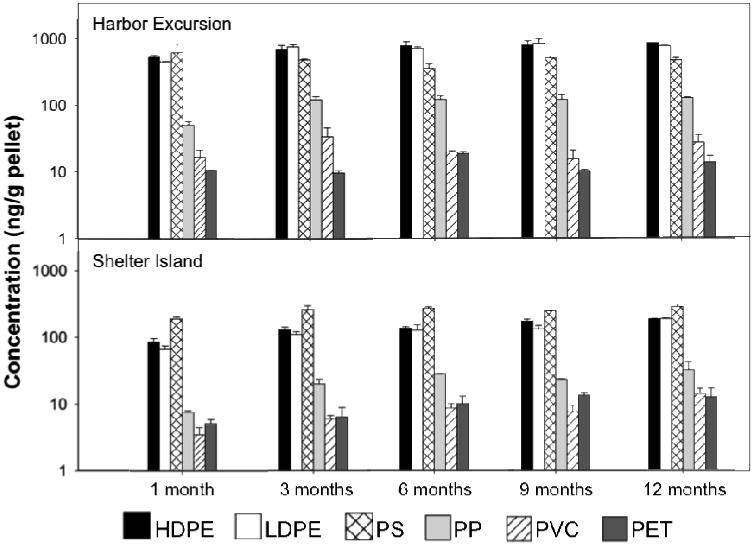
Mean concentrations (+/- S.E.) of ΣPPAHs (ng/g) sorbed to each plastic type at each location during each sampling period (1, 3, 6, 9 and 12 months; n=2). Harbor Excursion (HE-site) is shown on the top and Shelther Island (SI-site) on the bottom. At each sampling period, 2-factor ANOVA showed significant differences among plastic types (p<0.001) and locations (p<0.001), and post-hoc Tukey comparisons consistently distinguished HDPE, LDPE and PS as a group of plastics with the largest PPAH concentrations and PET and PVC with the smallest PPAH concentrations.
Sorption of PPAHs to PS is relatively large (up to 925.6 ng/g; Table 1) when comparing among the other five most commonly produced polymers (HDPE, LDPE, PP, PET and PVC).16 This result may be unexpected based upon the physical nature of PS. Non-expanded PS pellets are in a glassy state, similar to PET and PVC, suggesting a lower diffusivity than polyethylene, a rubbery polymer.43 Moreover, the polymeric backbone of polystyrene has a benzene molecule where polyethylene has a hydrogen, restricting segmental mobility within the polystyrene chains.43 In contrast, the presence of benzene increases the distance between adjacent polymeric chains, which can make it easier for a chemical to diffuse into the polymer.43 Therefore, although polyethylene has greater segmental mobility than PS, PS has a greater distance between polymeric chains, and may explain why we observed similar concentrations of PAHs in PS as we did polyethylene. Moreover, polystyrene foam is one of the most common materials used for solid phase extraction (SPE)46 due to the contribution of π-π and strong hydrophobic interactions to retention47, suggesting that environmental sorption to PS may be large, specifically for aromatic compounds such as PAHs. Because several factors influence the uptake of a compound to a polymer, including physical and chemical properties of the chemical sorbent, measuring sorption of other groups of chemicals to PS is recommended.
Hazards of PS littered in habitats
The mixture of several PAHs, including oxy-, methyl- and thio-PAHs, in virgin PS pellets may pose a risk to organisms immediately upon being discarded into marine habitats due to the mixture of PAHs in the absence of environmental sorption and its carcinogenic and potentially endocrine disrupting styrene monomer.20 Thus, it is important to consider risks to terrestrial and aquatic wildlife from PS litter. In addition, the combination of greater PAHs on virgin PS pellets and relatively large concentrations of sorbed PAHs from ambient seawater, suggests that PS may pose a greater risk of exposure to PAHs when it is ingested by marine animals than the other most commonly produced plastic-types (HDPE, LDPE, PP, PET and PVC). Future work should measure sorption of other priority pollutants (e.g. PCBs, metals) to PS. The mixture of the PS monomer itself, chemicals from the manufacturing process and those sorbed from the environment may act as a multiple stressor to several species19,48 that ingest PS debris. Testing this theory requires additional research that measures adverse health effects from dietary exposure of virgin PS and PS deployed in the marine environment to organisms.
Supplementary Material
Table S1 shows GC×GC/ToF-MS optimized parameters, Table S2 shows the list of PAHs targeted by GC×GC/ToF-MS, Table S3a and S3b shows the percent distribution of PAHs on PS at Harbor Excursion and Shelter Island, Figure S1 shows a map of our study sites in SD Bay, Figure S2 shows time trends for MPAHs, OPAHs and SPAHs over time, Figure S3 shows PPAHs over time and Figure S4 shows total PPAHs over time for each of the 6 major polymer types.
Acknowledgments
This material is based on work supported by SoCal SETAC, PADI Foundation, SDSU Division of Research Affairs, National Science Foundation Grant No. 0548190, and a National Science Foundation Graduate Research Fellowship (Grant No. 2010101195). This publication was made possible in part by grant number P30ES00210 from the National Institute of Environmental Health Sciences (NIEHS), NIH and NIEHS Grant P42 ES016465, and the National Science Foundation (ATM-0841165). The American Chemistry Council donated virgin plastic pellets. We thank Harbor Excursion and Michelson Yachts at Shelter Island for donating dock space. S. Kaye and M. Oei assisted with chemical analyses, and Z. Schakner, S. Wheeler, M. Colvin, C. Mazloff, J. Barr, M. Moore and S. Celustka assisted in the field. We thank K. Watanabe for assistance with Figure S1, G. Cherr for advising on experimental design and A. J. Underwood for advice regarding statistical analyses. The authors thank the analytical chemistry core of OSU's Superfund Research Program for supplying standards and Prof. Takeshi Ohura at the University of Shizouka in Shizouka, Japan for supplying ClPAH and BrPAH standards. Its contents are solely responsibility of the authors and do not necessarily represent the official view of NIEHS, NIH.
Footnotes
The authors declare no conflicts of interest.
Supplementary Materials This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Browne MA, Crump P, Niven SJ, Teuten EL, Tonkin A, Galloway T, Thompson RC. Accumulations of microplastic on shorelines worldwide: sources and sinks. Environ Sci Technol. 2011;45:9175–9179. doi: 10.1021/es201811s. [DOI] [PubMed] [Google Scholar]
- 2.Law KL, Moret-Ferguson S, Maximenko NA, Proskurowski G, Peacock EE, Hafner J, Reddy CM. Plastic Accumulation in the North Atlantic Subtropical Gyre. Science. 2010;329:1185–1188. doi: 10.1126/science.1192321. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg ED. Plasticizing the seafloor: An overview. Environ Technol. 1997;18:195–201. [Google Scholar]
- 4.Hirai H, Takada H, Ogata Y, Yamashita R, Mizukawa K, Saha M, Kwan C, Moore C, Gray H, Laursen D, Zettler E, Farrington J, Reddy C, Peacock E, Ward M. Organic micropollutants in marine plastic debris from the open ocean and remote and urban beaches. Mar Pollut Bull. 2011;62:1683–1692. doi: 10.1016/j.marpolbul.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Rochman CM, Browne MA, Halpern BS, Hentschel BT, Hoh E, Karapanagioti HK, Rios-Mendoza LM, Takada H, Teh S, Thompson RC. Classify plastic waste as hazardous. Nature. 2013;494:169–171. doi: 10.1038/494169a. [DOI] [PubMed] [Google Scholar]
- 6.Rochman CM, Hoh E, Hentschel BT, Kaye S. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: Implications for plastic marine debris. Environ Sci Technol. 2013;47:1646–1654. doi: 10.1021/es303700s. [DOI] [PubMed] [Google Scholar]
- 7.Rochman CM. Plastics and priority pollutants: a multiple stressor in aquatic habitats. Environ Sci Technol. 2013;47:2439–2440. doi: 10.1021/es400748b. [DOI] [PubMed] [Google Scholar]
- 8.Van A, Rochman CM, Flores EM, Hill KL, Vargas E, Vargas SA, Hoh E. Persistent organic pollutants in plastic marine debris found on beaches in San Diego, California. Chemosphere. 2012;86(3):258–263. doi: 10.1016/j.chemosphere.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 9.Lima ALC, Farrington JW, Reddy CM. Combustion-Derived Polycyclic Aromatic Hydrocarbons in the Environment—A Review. Environ Forensics. 2005;6(2):109–131. [Google Scholar]
- 10.Harvey RG. Environmental Chemistry of PAHs. In: Nielson AH, editor. PAHs and Related Compounds-Chemistry. Vol. 3.1 Springer; New York: 1998. [Google Scholar]
- 11.Horii Y, Ok G, Ohura T, Kannan K. Occurrence and Profiles of Chlorinated and Brominated Polycyclic Aromatic Hidrocarbons in Waste Incinerators. Environ Sci Technol. 2008;42:1904–1909. doi: 10.1021/es703001f. [DOI] [PubMed] [Google Scholar]
- 12.Ma J, Horii Y, Cheng J, Wang W, Wu Q, Ohura T, Kannan K. Chlorinated and Parent Polycyclic Aromatic Hydrocarbons in environmental Samples from an Electronic Waste Recycling Facility and a Chemical Industrial Complex in China. Environ Sci Technol. 2009;43:643–649. doi: 10.1021/es802878w. [DOI] [PubMed] [Google Scholar]
- 13.Lima ALC, Farrington JW, Reddy CM. Combustion-derived polycyclic aromatic hydrocarbons in the environment—a review. Environ Forensics. 2005;6(2):109–131. [Google Scholar]
- 14.Lohmann R. Critical review of low-density polyethylene's partitioning and diffusion coefficients for trace organic contaminants and implications for its use as a passive sampler. Environ Sci Technol. 2012;46:606–618. doi: 10.1021/es202702y. [DOI] [PubMed] [Google Scholar]
- 15.Dmitrienko SG, Gurariy EY, Nosov RE, Zolotoz YA. Solid-phase extraction of polycyclic aromatic hydrocarbons from aqueous samples using polyurethane foams in connection with solid-matrixs spectrofluorimetry. Anal Lett. 2001;34:425–438. [Google Scholar]
- 16.PlasticsEurope. Plastics—the Facts 2012: An analysis of European plastics production, demand and waste data for 2011. http://www.plasticseurope.org/Document/plastics-the-facts-2012.aspx?FolID=2.
- 17.Manzano C, Hoh E, Simonich SLM. Improved Separation of Complex Polycyclic Aromatic Hydrocarbon Mixtures Using Novel Column Combinations in GC × GC/ToF-MS. Environ Sci Technol. 2012;46(14):7677–7684. doi: 10.1021/es301790h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pruter AT. Sources, quantities and distribution of persistent plastics in the marine environment. Mar Pollut Bull. 1987;18:305–310. [Google Scholar]
- 19.Carpenter E, Anderson S, Harvey G, Miklas H, Peck B. Polystyrene spherules in coastal waters. Science. 1972;178:749–750. doi: 10.1126/science.178.4062.749. [DOI] [PubMed] [Google Scholar]
- 20.Lithner D, Larsson A, Dave G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci Total Environ. 2011;409:3309–3324. doi: 10.1016/j.scitotenv.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 21.Kitazawa A, Amagai T, Ohura T. Temproal Trends and Relationships of Particulate Chlorinated Polycyclic Aromatic Hydrocarbons and Their Parent Compounds in Urban Air. Environ Sci Technol. 2006;40:4592–4598. doi: 10.1021/es0602703. [DOI] [PubMed] [Google Scholar]
- 22.Ohura T, Kitazawa A, Amagai T, Makino M. Ocurrence, Profiles and Photoestabilities of Chlorinated Polycyclic Aromatic Hydrocarbons Associated with Particulates in Urban Air. Environ Sci Technol. 2005;39:85–91. [PubMed] [Google Scholar]
- 23.Wang W, Jariyasopit N, Schrlau J, Jia Y, Tao S, Yu TW, Dashwood RH, Zhang W, Wang X, Simonich SLM. Concentration and Photochemistry of PAHs, NPAHs, and OPAHs and Toxicity of PM2.5 during the Beijing Olympic Games. Environ Sci Technol. 2011;45(16):6887–6895. doi: 10.1021/es201443z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manzano C, Hoh E, Simonich SLM. Quantification of Complex Polycyclic Aromatic Hydrocarbon Mixtures in Standard Reference Materials Using Comprehensive Two-dimensional Gas Chromaography with Time-of-flight Mass Spectrometry. J Chrom A. 2013 doi: 10.1016/j.chroma.2013.07.093. Online publication complete: 8-AUG-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allan IJ, Booij K, Paschke A, Vrana B, Mills GA, Greenwood R. Field performance of seven passive sampling devices for monitoring hydrophobic substances. Environ Sci Technol. 2009;43:5383–5390. doi: 10.1021/es900608w. [DOI] [PubMed] [Google Scholar]
- 26.Schrap SM, Sleijpen GLG, Seinen W, Opperhuizen A. Sorption kinetics of chlorinated hydrophobic organic chemicals, Part II. Environ Sci & Pollut Res. 1994;1(2):81–92. doi: 10.1007/BF02986511. [DOI] [PubMed] [Google Scholar]
- 27.Ravindra K, Sokhi R, Van Grieken R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmospheric Environment. 2008;42(13):2895–2921. [Google Scholar]
- 28.Robinson AL, Donahue NM, Rogge WF. Photochemical oxidation and changes in molecular composition of organic aerosol in the regional context. J Geophys Res. 2006a;111(D3):D03302. [Google Scholar]
- 29.Robinson AL, Subramanian R, Donahue NM, Rogge WF. Source Apportionment of Molecular Markers and Organic Aerosol1. Polycyclic Aromatic Hydrocarbons and Methodology for Data Visualization. Environ Sci & Technol. 2006b;40(24):7803–7810. doi: 10.1021/es0510414. [DOI] [PubMed] [Google Scholar]
- 30.Rogge WF, Hildemann LM, Mazurek MA, Cass GR, Simoneit BRT. Sources of fine organic aerosol. 2. Noncatalyst and catalyst-equipped automobiles and heavy-duty diesel trucks. Environ Sci & Technol. 1993;27(4):636–651. [Google Scholar]
- 31.Guo H, Lee SC, Ho KF, Wang XM, Zou SC. Particle-associated polycyclic aromatic hydrocarbons in urban air of Hong Kong. Atmos Environ. 2003;37(38):5307–5317. [Google Scholar]
- 32.Park SS, Kim YJ, Kang CH. Atmospheric polycyclic aromatic hydrocarbons in Seoul, Korea. Atmos Environ. 2002;36(17):2917–2924. [Google Scholar]
- 33.Sofowote UM, Allan LM, McCarry BE. Evaluation of PAH diagnostic ratios as source apportionment tools for air particulates collected in an urban-industrial environment. J Environ Monitor. 2010;12(2):417–424. doi: 10.1039/b909660d. [DOI] [PubMed] [Google Scholar]
- 34.Soclo HH, Garrigues PH, Ewald M. Origin of polycyclic aromatic hydrocarbons (PAHs) in coastal marine sediments: case studies in Cotonou (Benin) and Aquitaine (France) areas. Mar Pollut Bull. 2000;40(5):387–396. [Google Scholar]
- 35.Dickhut RM, Canuel EA, Gustafson KE, Liu K, Arzayus KM, Walker SE, Edgecombe G, Gaylor MO, MacDonald EH. Automotive sources of carcinogenic polycyclic aromatic hydrocarbons associated with particulate matter in the Chesapeake Bay Region. Environ Sci Technol. 2000;34:4635–4640. [Google Scholar]
- 36.Sienra M. Oxygenated polycyclic aromatic hydrocarbons in urban air particulate matter. Atmos Environ. 2006;40(13):2374–2384. [Google Scholar]
- 37.Hughes WB, Holba AG, Dzou LI. The ratios of dibenzothiophene to phenanthrene and pristane to phytane as indicators of depositional environment and lithology of petroleum source rocks. Geochim Cosmochim Ac. 1995;59(17):3581–3598. [Google Scholar]
- 38.Durlak SK, Biswas P, Shi J, Bernhard MJ. Characterization of Polycyclic Aromatic Hydrocarbon Particulate and Gaseous Emissions from Polystyrene Combustion. Environmental Science & Technology. 1998;32(15):2301–2307. [Google Scholar]
- 39.Zabaniotou A, Kassidi E. Life cycle assessment applied to egg packaging made from polystyrene and recycled paper. J Cleaner Production. 2003;11(5):549–559. [Google Scholar]
- 40.Kwon E, Castaldi MJ. Investigation of mechanisms of polycyclic aromatic hydrocarbons (PAHs) initiated from the thermal degradation of styrene butadiene rubber (SBR) in N2 atmosphere. Environ Sci Technol. 2008;42:2175–2180. doi: 10.1021/es7026532. [DOI] [PubMed] [Google Scholar]
- 41.Wheatley L, Levendis YA, Vouros P. Exploratory study on the combustion and PAH emissions of selected municipal waste plastics. Environ Sci Technol. 1993;27:2885–2895. [Google Scholar]
- 42.Muller JF, Manomanii K, Mortimer MR, McLachlan MS. Partitioning of polycyclic aromatic hydrocarbons in the polyethylene/water system. Fresenius J Anal Chem. 2001;371:816–822. doi: 10.1007/s002160101025. [DOI] [PubMed] [Google Scholar]
- 43.Pascall MA, Zabik ME, Zabik MJ, Hernandez RJ. Uptake of polychlorinated biphenyls (PCBs) from an aqueous medium by polyethylene, polyvinyl chloride, and polystyrene films. J Agr Food Chem. 2005;53:164–169. doi: 10.1021/jf048978t. [DOI] [PubMed] [Google Scholar]
- 44.Kirk RE, Othmer DF, Mark HF. Encyclopedia of chemical technology. Vol. 6 Interscience Publishers; 1965. [Google Scholar]
- 45.Lo MT, Sandi E. Residue reviews. Springer; New York: 1978. Polycyclic aromatic hydrocarbons (polynuclears) in foods; pp. 35–86. [DOI] [PubMed] [Google Scholar]
- 46.Huck CW, Bonn GK. Recent developments in polymer-based sorbents for solid-phase extraction. J Chromatog A. 2000;885(1):51–72. doi: 10.1016/s0021-9673(00)00333-2. [DOI] [PubMed] [Google Scholar]
- 47.Penner NA, Nesterenko PN, Ilyin MM, Tsyurupa MP, Davankov VA. Investigation of the properties of hypercrosslinked polystyrene as a stationary phase for high-performance liquid chromatography. Chromatographia. 1999;50(9-10):611–620. [Google Scholar]
- 48.Kartar S, Abou-Seedo F, Sainsbury M. Polystyrene spherules in the Severn estuary—a progress report. Mar Pollut Bull. 1976;7:52. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 shows GC×GC/ToF-MS optimized parameters, Table S2 shows the list of PAHs targeted by GC×GC/ToF-MS, Table S3a and S3b shows the percent distribution of PAHs on PS at Harbor Excursion and Shelter Island, Figure S1 shows a map of our study sites in SD Bay, Figure S2 shows time trends for MPAHs, OPAHs and SPAHs over time, Figure S3 shows PPAHs over time and Figure S4 shows total PPAHs over time for each of the 6 major polymer types.