Abstract
Breast cancer is one of the most frequently diagnosed malignancies and a leading cause of cancer death in women. Great advances in the treatment of primary tumors have led to a significant increment in the overall survival rates, however recurrence and metastatic disease, the underlying cause of death, are still a medical challenge. Breast cancer is highly dependent on neovascularization to progress. In the last years several anti-angiogenic drugs have been developed and administered to patients in combination with chemotherapeutic drugs.
Collected preclinical evidence has proposed the endocannabinoid system as a potential target in cancer. The endocannabinoid anandamide has been reported to affect breast cancer growth at multiple levels, by inhibiting proliferation, migration and invasiveness in vitro and in vivo and by directly inhibiting angiogenesis. Aim of the present work is to investigate if anandamide is able to affect the proangiogenic phenotype of the highly invasive and metastatic breast cancer cells MDA-MB-231. We found that following anandamide treatment, MDAMB-231 cells lose their ability to stimulate endothelial cells proliferation in vitro, due to a significant inhibition of all the pro-angiogenic factors produced by these cells. This finding adds another piece of evidence to the anti-tumor efficacy of anandamide in breast cancer.
Keywords: angiogenesis, endocannabinoid system, breast cancer, anandamide
I. INTRODUCTION
Breast cancer is the most common malignancy in women, excluding skin cancer, and is the second for mortality following lung cancer [1]. Great improvements in a comprehensive treatment for breast cancer have led to a significant increment in the overall survival rates, however recurrence and metastatic disease still represent an urgent medical problem. A huge amount of studies have demonstrated that breast cancer, like many solid tumors, depends on vascular growth to progress. Indeed, breast tumor angiogenesis is considered as an independent prognostic factor for breast cancer [2]. Several currently used conventional therapeutic regimens, such as numerous chemotherapeutic agents and endocrine therapies, show an intrinsic anti-angiogenic efficacy. Moreover, specific drugs that target angiogenesis have been developed. Among these, the most famous is the VEGF-neutralizing monoclonal antibody bevacizumab [3]. Unfortunately, numerous clinical trials conducted in breast cancer have revealed that this malignancy is modestly sensitive to bevacizumab, even when combined with standard chemotherapy protocols, showing a lack of survival advantage. These disappointing results led to recently discontinue its FDA approval in breast cancer treatment [4].
The endocannabinoid system, whose complex biological regulatory functions have been disclosed as crucial especially in several patho-physiological conditions, is emerging as a potential new target in cancer, due to the numerous evidence collected also in preclinical studies [5]. Natural and synthetic cannabinoids and compounds belonging to the endocannabinoid system (lipid molecules containing long-chain polyunsaturated fatty acids, amides, esters and ethers, like anandamide and 2-arachidonoylglycerol (2-AG)), have been reported to affect tumor growth at more than one step in several types of malignancies. Indeed, they have been shown to inhibit cell proliferation and induce cell death, mainly through apoptosis and autophagy, but also to prevent tumor progression, metastasis formation and to inhibit oxygen and nutrient supply through a direct effect on tumor neovascularization [5]. As regard to breast cancer, CB1 and CB2 receptors expression was found in several breast cancer cell lines with peculiar oncogenic patterns and different metastatic potential, as well as in human breast tumor tissues [6,7]. Cannabinoids and endocannabinoids-related compounds were able to affect breast cancer cell proliferation, inducing apoptosis, and to inhibit migration and invasiveness [8–11]. The metabolically stable analogue of anandamide, Met-F-AEA, was reported to inhibit the proliferation of the estrogen receptor negative (ER-) MDA-MB-231 breast cancer cells, inducing a S phase cell cycle arrest correlated with DNA damage and Chk1 activation [12,13]. Anandamide inhibited also HMG-CoA reductase activity, thus affecting the pattern of expression of oncogenic prenylated proteins involved in the proliferation and metastatic potential of breast cancer cells, such as Ras and RhoA [14]. Indeed, anandamide was able to reduce the invasiveness of highly metastatic MDA-MB-231 cells, inhibiting their migration through the RhoA signalling pathway [12, 15]. The efficacy of anandamide was maintained in the in vivo setting, since it was able to reduce the number and dimension of metastatic nodes in a mouse model of metastatic spreading [12]. Interestingly, we previously demonstrated that anandamide also directly affects angiogenesis, inhibiting endothelial cell proliferation, tube formation and neovascularization in vivo [16]. Aim of this study is to investigate if anandamide is able to affect the proangiogenic phenotype of breast cancer cells, adding another piece of evidence to its anti-tumor efficacy.
II. METHODOLOGY
Cell lines
The human breast cancer cell line MDA-MB-231was from Interlab cell line collection (IST, Genova) and was grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum. Human umbilical endothelial cells (HUVEC) were isolated from umbilical cords, as described [17] and grown in M199 medium supplemented with 10% fetal bovine serum, aFGF, bFGF, EGF, hydrocortisone and heparin. Cells were cultured at 37°C in a humidified 5% CO2 atmosphere.
Drugs
Met-F-AEA (2-methyl-2’-F-anandamide), a metabolically stable analogue of anandamide, was purchased from Sigma-Aldrich. Met-F-AEA is indicated in the text as anandamide (AEA).
Proliferation assay
Cell proliferation was evaluated, in vitro, by measuring [3H]-thymidine incorporation. In brief, 5×103 cells/ml were seeded into 96-well plates, immediately treated with the drugs, incubated for 24 h at 37°C (5% CO2), then pulsed with 0.5 μCi/well of [3H]-thymidine (Amersham Biosciences Europe, Italy) and harvested 4 h later. Radioactivity was measured in a scintillation counter (Wallac, Turku, Finland).
Human angiogenesis array
Human angiogenesis antibody array on MDA-MB-231 tumor-conditioned medium was performed following manufacturer’s instructions (RayBiotech, Inc.). Culture supernatants corresponding to the final 24 h of incubation with anandamide (10 µM) were collected and assayed for angiogenic factors production. The array membranes were incubated with tumor-conditioned medium, stained using a chemiluminescence system (ECL-Amersham Biosciences) and then exposed to X-ray films (Kodak). Immunoreactive spots were quantified using Quantity One 1-D analysis software (Bio-Rad).
Statistical analysis
Statistical computations were done using the GraphPad prism 6.0 software (San Diego, CA, USA). Data obtained from multiple experiments were calculated as mean±SD and analysed for statistical significance using Student’s T test or 1-way ANOVA for independent groups, with Tukey correction for multiple comparisons. P values less than 0.05 were considered significant.
III. RESULTS
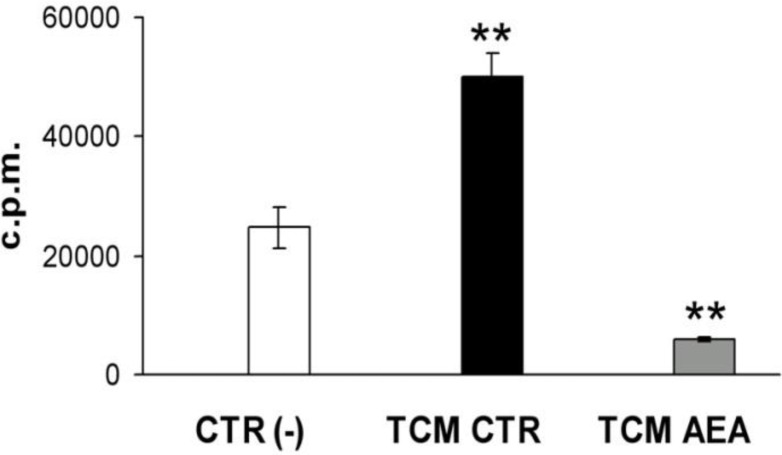
In order to evaluate the effect of anandamide on breast cancer-induced angiogenesis, we performed a simple in vitro angiogenesis assay using as angiogenic stimulus the tumor-conditioned medium (TCM) derived from MDAMB-231 cells. Briefly, MDA-MB-231 cells were treated with anandamide (10 μM, 24 h) and then the TCM was collected and tested on human umbilical vein endothelial cells (HUVEC) proliferation by [3H]-thymidine incorporation into DNA. As expected, TCM significantly induced endothelial basal proliferation vs control. Interestingly, we found that in the presence of TCM from anandamide-treated MDA-MB-231 cells, the proliferation of endothelial cells was strongly reduced (Fig. 1).
Fig. 1.
Tumor conditioned medium from anandamide-treated MDAMB-231 inhibits angiogenesis.
The graph reports the [3H]-thymidine incorporation levels (counts per minute) in endothelial cells expressed as mean ± SD values of three experiments in triplicates. ANOVA, **P<0.01. CTR, control; AEA, anandamide.
This could be the result of an anandamide-switched balance between pro-angiogenic and anti-angiogenic factors produced by breast cancer cells in behalf of angiogenesis inhibitors. The next step was indeed to investigate the nature and levels of angiogenic molecules present in breast cancer TCM and their modulation after anandamide treatment. To this end we used an angiogenesis protein array. We found that, for all tested pro-angiogenic factors, there was a reduction trend following anandamide treatment (Table 1).
TABLE 1.
ANGIOGENIC FACTORS SECRETED BY MDA-MB-231 BREAST CANCER CELLS FOLLOWING ANANDAMIDE TREATMENT
| Angiogenic factors | TCM | TCM+ AEA | % |
|---|---|---|---|
| Angiogenin | 20 ± 0.8 | 19.3 ± 2.3 | 5% |
| ENA-78 | 3.8 ± 1.4 | 2.7 ± 1.5 | 29% |
| bFGF | 10.2 ± 2 | 9.8 ± 3.3 | 4% |
| GRO | 12.5 ± 0.1 | 10.7 ± 1.5 | 15% |
| IFNγ | 1.4 ± 0.07 | 1.1 ± 0.08** | 21% |
| IL6 | 18 ± 0.5 | 14.9 ± 4.6 | 17% |
| Leptin | 2.2 ± 0.2 | 0.9±0.07*** | 58% |
| MCP-1 | 57.1 ± 6.0 | 49.4 ± 9.5 | 13% |
| PDGF-BB | 9.7 ± 1.9 | 6.8 ± 1.1 | 29% |
| PlGF | 10.5 ± 1.8 | 8.3 ± 1.2 | 21% |
| RANTES | 8.6 ± 0.1 | 7.9 ± 0.5 | 7% |
| TGFβ1 | 35.2 ± 3.7 | 26.7 ± 1.8* | 23% |
| TIMP1 | 37.1 ± 2.6 | 30.2 ± 1.8* | 18% |
| TIMP2 | 75.7 ± 10.7 | 50 ± 2.8* | 33% |
| Thrombopoietin | 10.5 ± 1.3 | 6.8 ± 0.6* | 35% |
| VEGF | 42.6 ± 7.6 | 22.4 ± 3.2* | 47% |
| VEGF-D | 10.6 ± 2.3 | 6.8 ± 1.9 | 36% |
Data are presented as mean ± SD of three experiments. % = percentage of inhibition vs TCM.,
P <0.05,
P < 0.01,
P < 0.001; Student’s T test.
In particular, IFNγ, leptin, TGFβ1, TIMP1, TIMP2, thrombopoietin and VEGF levels were significantly inhibited by anandamide in the conditioned medium of MDA-MB-231 breast cancer cells.
IV. DISCUSSION
In the present work we demonstrated for the first time that a metabolically stable anandamide analogue, Met-F-AEA, already known to have an interesting anti-tumor efficacy in several tumor types including breast cancer [12–15], along with a direct anti-angiogenic activity on endothelial cells [16], was able to affect the pro-angiogenic phenotype of the highly metastatic MDA-MB-231 breast cancer cells. Indeed, following anandamide treatment, MDAMB-231 cells lost the capability to stimulate endothelial cells proliferation in vitro, due to a significant inhibition of all the pro-angiogenic factors produced by tumor cells and usually responsible of their highly pro-angiogenic phenotype, allowing tumor cells to get nourish and thus to growth [18].
Our data (Fig. 1 and Table 1) show that the conditioned medium from breast cancer cells contains an amount of potent angiogenic factors and pro-inflammatory cytokines, as detected by an angiogenesis array.
Among the factors secreted in large amounts in the tumor conditioned medium and deeply inhibited after treatment with anandamide in comparison to the control, there is VEGF (47% of inhibition vs control). VEGF is one of the major promoters of angiogenesis, through the binding to its tyrosine kinase receptors EGFR-1/Flt-1 and VEGFR-2/KDR/Flk-1 it is able to regulate more than one step in angiogenesis, from endothelial cell survival and proliferation, to migration and vascular permeability [19]. As a critical factor in the induction of angiogenesis, VEGF has become an attractive target for the development of anti-angiogenic drugs, the most famous of whose is the VEGF-neutralizing monoclonal antibody bevacizumab [3]. Several solid cancers, such as colon and lung cancer, have been successfully treated with bevacizumab, whereas its approval in breast cancer has been recently revoked [20]. Indeed, despite the success of bevacizumab and other anti-angiogenic drugs, it has to be taken into account that even in responsive patients, anti-angiogenic drugs generally prolong survival only in the order of few months and the clinical outcome is associated with the development of resistance and an increased risk of invasion and metastasis [2]. This could be due to the high redundancy of angiogenic molecules that tumors can produce to provide oxygen and nutrients stimulating their own growth and vascularization. Moreover, tumors can also switch between different types of vascularization that don’t necessarily require VEGF and its signalling. Therefore, strategies aimed to target angiogenesis at multiple levels could improve the efficacy and reduce the risk of resistance development. Interestingly, we found that anandamide is able to inhibit multiple angiogenic molecules beyond VEGF, thus significantly affecting the pro-angiogenic phenotype of breast cancer cells.
In our study, we reported a significant reduction in the levels of the adipocytokine leptin, that has been reported to stimulate mammary tumor growth. Furthermore high levels of leptin in women correlate to an increased risk of breast cancer, metastatic tumor phenotype and poor prognosis [21,22]. Leptin stimulates angiogenesis and breast cancer invasiveness through the enhanced neovascularization, so it is a key factor to control breast cancer progression [23].
Noteworthy, most of the other growth factors and chemokines inhibited by anandamide treatment (IL8, GRO, ENA-78) have been reported to play a significant role in mediating neovascularization during tumorigenesis in several tumor types and have been shown to be of great importance in tumor progression [18]. These chemokines are under the regulation of PPARγ pathway and PPARγ ligands have been shown to inhibit tumor-associated angiogenesis by blocking the production of ELR+CXC chemokines [24]. It has been reported that anandamide induces transcriptional activation of PPARγ, binding directly to PPAR-ligand binding domain [25,26]. Therefore this could be the mechanism by which anandamide is able to inhibit the production of these angiogenesis-stimulating molecules, thus inhibiting breast cancer neovascularization.
V. CONCLUSION
In conclusion, our study adds another piece of evidence to the anti-tumor efficacy of the endocannabinoid anandamide in breast cancer.
Acknowledgments
This work was supported by Associazione Educazione e Ricerca Medica Salernitana (ERMES). E.C. and M.C.P. were supported by a fellowship from FIRC.
REFERENCES
- [1].DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- [2].Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest. 2013;123(8):3190–200. doi: 10.1172/JCI70212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3(5):391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- [4].Kerbel RS. Strategies for improving the clinical benefit of antiangiogenic drug based therapies for breast cancer. J Mammary Gland Biol Neoplasia. 2012;17(3–4):229–39. doi: 10.1007/s10911-012-9266-0. [DOI] [PubMed] [Google Scholar]
- [5].Pisanti S, Picardi P, D’Alessandro A, Laezza C, Bifulco M. The endocannabinoid signaling system in cancer. Trends Pharmacol Sci. 2013;34(5):273–82. doi: 10.1016/j.tips.2013.03.003. [DOI] [PubMed] [Google Scholar]
- [6].Caffarel MM, Andradas C, Mira E, Pérez-Gómez E, Cerutti C, Moreno-Bueno G, Flores JM, García-Real I, Palacios J, Mañes S, Guzmán M, Sánchez C. Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition. Mol Cancer. 2010;9:196. doi: 10.1186/1476-4598-9-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Qamri Z, Preet A, Nasser MW, Bass CE, Leone G, Barsky SH, Ganju RK. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol Cancer Ther. 2009;8(11):3117–29. doi: 10.1158/1535-7163.MCT-09-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pisanti S, Bifulco M. Endocannabinoid system modulation in cancer biology and therapy. Pharmacol Res. 2009;60(2):107–16. doi: 10.1016/j.phrs.2009.03.011. [DOI] [PubMed] [Google Scholar]
- [9].De Petrocellis L, Bisogno T, Ligresti A, Bifulco M, Melck D, Di Marzo V. Effect on cancer cell proliferation of palmitoylethanolamide, a fatty acid amide interacting with both the cannabinoid and vanilloid signalling systems. Fundam Clin Pharmacol. 2002;16(4):297–302. doi: 10.1046/j.1472-8206.2002.00094.x. [DOI] [PubMed] [Google Scholar]
- [10].Ligresti A, Moriello AS, Starowicz K, Matias I, Pisanti S, De Petrocellis L, Laezza C, Portella G, Bifulco M, Di Marzo V. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther. 2006;318(3):1375–87. doi: 10.1124/jpet.106.105247. [DOI] [PubMed] [Google Scholar]
- [11].Guindon J, Hohmann AG. The endocannabinoid system and cancer: therapeutic implication. Br J Pharmacol. 2011;163(7):1447–63. doi: 10.1111/j.1476-5381.2011.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Grimaldi C, Pisanti S, Laezza C, Malfitano AM, Santoro A, Vitale M, Caruso MG, Notarnicola M, Iacuzzo I, Portella G, Di Marzo V, Bifulco M. Anandamide inhibits adhesion and migration of breast cancer cells. Exp Cell Res. 2006;312(4):363–73. doi: 10.1016/j.yexcr.2005.10.024. [DOI] [PubMed] [Google Scholar]
- [13].Laezza C, Pisanti S, Crescenzi E, Bifulco M. Anandamide inhibits Cdk2 and activates Chk1 leading to cell cycle arrest in human breast cancer cells. FEBS Lett. 2006;580(26):6076–82. doi: 10.1016/j.febslet.2006.09.074. [DOI] [PubMed] [Google Scholar]
- [14].Laezza C, Malfitano AM, Proto MC, Esposito I, Gazzerro P, Formisano P, Pisanti S, Santoro A, Caruso MG, Bifulco M. Inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity and of Ras farnesylation mediate antitumor effects of anandamide in human breast cancer cells. Endocr Relat Cancer. 2010;17(2):495–503. doi: 10.1677/ERC-10-0009. [DOI] [PubMed] [Google Scholar]
- [15].Laezza C, Pisanti S, Malfitano AM, Bifulco M. The anandamide analog, Met-F-AEA, controls human breast cancer cell migration via the RHOA/RHO kinase signaling pathway. Endocr Relat Cancer. 2008;15(4):965–74. doi: 10.1677/ERC-08-0030. [DOI] [PubMed] [Google Scholar]
- [16].Pisanti S, Borselli C, Oliviero O, Laezza C, Gazzerro P, Bifulco M. Antiangiogenic activity of the endocannabinoid anandamide: correlation to its tumor-suppressor efficacy. J Cell Physiol. 2007;211(2):495–503. doi: 10.1002/jcp.20954. [DOI] [PubMed] [Google Scholar]
- [17].Pisanti S, Picardi P, Prota L, Proto MC, Laezza C, McGuire PG, Morbidelli L, Gazzerro P, Ziche M, Das A, Bifulco M. Genetic and pharmacologic inactivation of cannabinoid CB1 receptor inhibits angiogenesis. Blood. 2011;117(20):5541–50. doi: 10.1182/blood-2010-09-307355. [DOI] [PubMed] [Google Scholar]
- [18].Joimel U, Gest C, Soria J, Pritchard LL, Alexandre J, Laurent M, Blot E, Cazin L, Vannier JP, Varin R, Li H, Soria C. Stimulation of angiogenesis resulting from cooperation between macrophages and MDA-MB-231 breast cancer cells: proposed molecular mechanism and effect of tetrathiomolybdate. BMC Cancer. 2010;10:375. doi: 10.1186/1471-2407-10-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- [20].Amit L, Ben-Aharon I, Vidal L, Leibovici L, Stemmer S. The impact of Bevacizumab (Avastin) on survival in metastatic solid tumors--a meta-analysis and systematic review. PLoS One. 2013;8(1):e51780. doi: 10.1371/journal.pone.0051780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jardé T, Caldefie-Chézet F, Goncalves-Mendes N, Mishellany F, Buechler C, Penault-Llorca F, Vasson MP. Involvement of adiponectin and leptin in breast cancer: clinical and in vitro studies. Endocr Relat Cancer. 2009;16(4):1197–210. doi: 10.1677/ERC-09-0043. [DOI] [PubMed] [Google Scholar]
- [22].Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, Russo A, Sulkowski S, Surmacz E. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006;12(5):1447–53. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- [23].Dubois V, Delort L, Billard H, Vasson MP, Caldefie-Chezet F. Breast cancer and obesity: in vitro interferences between adipokines and proangiogenic features and/or antitumor therapies? PLoS One. 2013;8(3):e58541. doi: 10.1371/journal.pone.0058541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Keshamouni VG, Arenberg DA, Reddy RC, Newstead MJ, Anthwal S, Standiford TJ. PPAR-gamma activation inhibits angiogenesis by blocking ELR+CXC chemokine production in non-small cell lung cancer. Neoplasia. 2005;7(3):294–301. doi: 10.1593/neo.04601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bouaboula M, Hilairet S, Marchand J, Fajas L, Le Fur G, Casellas P. Anandamide induced PPARgamma transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur J Pharmacol. 2005;517(3):174–81. doi: 10.1016/j.ejphar.2005.05.032. [DOI] [PubMed] [Google Scholar]
- [26].O’Sullivan SE, Kendall DA. Cannabinoid activation of peroxisome proliferator-activated receptors: potential for modulation of inflammatory disease. Immunobiology. 2010;215(8):611–6. doi: 10.1016/j.imbio.2009.09.007. [DOI] [PubMed] [Google Scholar]