Abstract
Cold transport of epididymides from genetically modified mice is an efficient alternative to the shipment of live animals between research facilities. Mouse sperm from epididymides cold-stored for short periods can maintain viability. We previously reported that cold storage of mouse epididymides in Lifor® perfusion medium prolonged sperm motility and fertilization potential and that the sperm efficiently fertilized oocytes when reduced glutathione was added to the fertilization medium. Cryopreservation usually results in decreased sperm viability; an optimized protocol for cold storage of epididymides plus sperm cryopreservation has yet to be established. Here, we examined the motility and fertilization potential of cryopreserved, thawed (frozen-thawed) sperm from previously cold-stored mouse epididymides. We also examined the protective effect of sphingosine-1-phosphate (S1P) on sperm viability when S1P was added to the preservation medium during cold storage. We assessed viability of frozen-thawed sperm from mouse epididymides that had been cold-transported domestically or internationally and investigated whether embryos fertilized in vitro with these sperm developed normally when implanted in pseudo-pregnant mice. Our results indicate that frozen-thawed sperm from epididymides cold-stored for up to 48 hours maintained high fertilization potential. Fertilization potential was reduced after cold storage for 72 hours, but not if S1P was included in the cold storage medium. Live pups were born normally to recipients after in vitro fertilization using frozen-thawed sperm from cold-transported epididymides. In summary, we demonstrate an improved protocol for cold-storage of epididymides that can facilitate transport of genetically engineered-mice and preserve sperm viability after cryopreservation.
Keywords: in vitro fertilization, cold storage, cold transport, sperm, epididymides, motility, sphingosine-1-phosphate
Introduction
Genetically engineered mice are frequently used in the fields of basic and life sciences. To facilitate mouse genome research, a global network of mouse resource repositories has been established to produce, archive, and distribute genetically modified mice for sharing in the scientific community [4; 5]. As a result, large numbers of genetically engineered mice are regularly transported between mouse banks and various research institutions.
Mouse transport efficiency is an important issue in experimental animal research. Transporting live animals is generally considered undesirable due to the possibility of the spread of infection or animal death/escape during transit, as well as animal welfare concerns [23]. In fact, some major air-cargo carriers have recently adopted policies that restrict the transport of live laboratory animals [38].
The cold transport of fresh mouse embryos or cauda epididymides is often done among research facilities [11; 12; 13; 15; 32; 33; 36]. It is typically a low-cost and low-risk alternative to live animal transport. Previously, we reported that sperm from mouse epididymides cold-stored for 96 hours in Lifor® perfusion solution (Lifor) maintained normal in vitro fertilization (IVF) rates when reduced glutathione (GSH) was added to the fertilization medium [36]. Additionally, viable embryos were obtained by IVF using these sperm, and the embryos developed normally into pups when implanted into recipients.
Establishment of a successful process for mouse sperm cryopreservation after cold transport of epididymides can contribute to a simple and efficient system of transportation, production, and preservation of genetically engineered mice. Following cold transport/storage of mouse epididymides, epididymal sperm can be cryopreserved and later thawed (frozen-thawed) for use in IVF. The use of frozen-thawed sperm for IVF can increase the convenience and efficiency of animal production; however, low fertilization success of frozen-thawed sperm derived from cold-stored mouse epididymides is a major obstacle in IVF. It is essential to optimize the cold storage conditions to maintain high sperm motility and viability prior to cryopreservation.
Sphingosine-1-phosphate (S1P) is phosphorylated sphingosine, which is derived from ceramide formed from cell membrane sphingomyelin [26; 27]. S1P is a bioactive sphingolipid metabolite that regulates cell growth and suppresses programmed cell death [3; 25]. There have been numerous reports on S1P’s protective effects against stress-induced apoptosis in male germ cells [22; 29; 30]; we hypothesized that S1P could exert these effects in mouse epididymides during cold storage.
In this study, we assessed motility and fertilization potential of frozen-thawed sperm collected from cold-stored epididymides of C57BL/6 mice both with and without the addition of S1P to the cold storage medium. Additionally, using the cold storage system and a preservation medium containing S1P, we transported mouse epididymides both domestically (from Asahikawa Medical University to our laboratory) and internationally (from the University of California-Davis [UC Davis] to our laboratory) and assessed the viability of the cold-transported frozen-thawed sperm.
Materials and Methods
Animals
C57BL/6J male and female mice were purchased from CLEA Japan Inc. (Tokyo, Japan) and from the Jackson laboratory (Bar Harbor, ME, United States) for use as sperm and oocyte donors. Sperm was obtained from mice aged 12–16 weeks, and oocytes were obtained from mice aged 8–10 weeks. Pseudo-pregnant ICR mice (8–16 weeks old, CLEA Japan Inc.) served as recipients for the transfer of two-cell embryos. All animals were kept under a 12-hour dark-light cycle (lights on 07:00 to 19:00) at a constant temperature of 22±1 °C with free access to food and water. All animal experiments were approved by the Animal Care and Use Committee at Kumamoto University (Japan) and by the Institutional Animal Care and Use Committee at UC Davis (United States).
Media
Cauda epididymides were collected from male mice and stored in Lifor (Lifeblood Medical Inc., Freehold, NJ, USA) with or without S1P. For sperm cryopreservation, a modified 18% raffinose pentahydrate and 3% skim milk solution (Difco , Beckton Dickinson and Co., Franklin Lakes, NJ, USA) containing 100 mM L-glutamine (mR18S3 solution) was prepared as previously described [34]. A modified Krebs-Ringer bicarbonate solution (TYH medium) containing 1.0 mg/mL polyvinyl alcohol and 0.75 mM methyl-β-cyclodextrin (MBCD, Sigma-Aldrich Co.) was used for sperm preincubation [2; 31]. Human tubal fluid medium (HTF) modified to contain 1.0 mM GSH (mHTF) was used for IVF [18; 35]. Potassium simplex optimization medium (KSOM) was used to culture two-cell embryos to the blastocyst stage [7].
Cold storage and transport of mouse epididymides
Cold storage and transport of mouse epididymides were performed as previously described [36]. For the cold storage experiments, male mice were euthanized via cervical dislocation, and cauda epididymides were collected and transferred to 0.2-mL plastic tubes (three epididymides/tube) each containing 0.2 mL Lifor. The tubes were placed underneath a sheet of cotton wool in a cardboard box with a tracking monitor that also functions as a digital thermometer and data logger (Thermochron® iButton; Maxim Integrated, Inc., San Jose, CA, USA). To minimize any effects of external temperature fluctuations, the box was placed in a vacuum bottle (JMK-500, Thermos LLC, Schaumburg, IL, USA) with two cold packs (60×180 mm each) that had been pre-cooled in a refrigerator at 4 °C for overnight. The bottle was placed in a styrofoam box (205×125×130 mm) and covered with four cold packs (140×250 mm each). The packed styrofoam box was then stored in a refrigerator at 4 °C for 0, 24, 48, or 72 h. To investigate the effects of the addition of S1P to the cold storage medium, the above procedure was repeated with the following exception: some of the collected epididymides were placed in tubes containing 0.2 mL Lifor plus 10μM S1P.
For the domestic transport experiment, a packed styrofoam box of three 0.2-mL tubes containing epididymides (in Lifor plus S1P) was prepared as described above and transported from Asahikawa Medical University to our laboratory (Kumamoto University). The box arrived within 72 hours via parcel delivery. The temperature in the box during transport was maintained at 4–8°C. For the international transport experiment, four 0.2-mL tubes containing epididymides (in Lifor plus S1P) were packaged in a box within a vacuum bottle as described above and placed in a cold reserving box (410×370×420 mm) obtained from Office Oota and Tanpopo Co. Ltd (Osaka, Japan) along with five cold packs precooled at 4°C (140×250 mm each) and four hard bottle-type ice packs precooled at −20°C (140×250 mm each). The box was shipped from UC Davis to our laboratory under temperature-controlled conditions and arrived within 72 h. The temperature in the box during transport was maintained at 4–10 °C. The internal temperature of the box can be kept at 4–8°C for 48 hours under the ambient temperature at 22°C.
Sperm cryopreservation
A previously-described modified sperm cryopreservation procedure was used [17; 34]. A 180-μL aliquot of mR18S3 solution (60 μL/epididymis) was placed in a 35-mm culture dish and covered with paraffin oil. After the cold storage or transport of cauda epididymides, the epididymides were removed from the storage medium and gently wiped with filter paper. The collected epididymides were cleaned of all fat and blood under a microscope before transfer to the culture dish (3 epididymides/dish) where each was cut into five or six pieces with microspring scissors. The dish was maintained at 37°C for 3 min and gently shaken each minute to separate the sperm from the tissue. During sperm preparation, 15 freezing straws (0.25-mL plastic straw; IMV Technologies, Paris, France) were loaded with 100 μL HTF and 15 mm air using a 1-mL syringe. The sperm suspension was then divided into 15 aliquots (10 μL each) on the culture dish. Each aliquot of sperm suspension, along with 15 mm air, was loaded into each of the 15 freezing straws and both ends of the straw were heat-sealed. The sealed straws were transferred to a freezing canister made from a 50-mL syringe and cooled in the gas layer in the neck of a liquid nitrogen tank (MVE XC 47/11-10, CHART Industries, Garfield Heights, OH, USA) for 10 min. Once equilibrium was achieved, the freezing canister containing the straws was plunged directly into liquid nitrogen, and the straws were transferred from the canister to a pre-cooled triangular cassette in the liquid nitrogen tank and stored for 1–3 months until use. The level of liquid nitrogen was checked before sperm cryopreservation and maintained at 25 to 30 cm from the bottom of the tank.
Prior to sperm motility assessments and IVF, the straws were removed from the liquid nitrogen and thawed in a water bath at 37°C for 10 min.
Assessment of sperm motility
Motility of the frozen-thawed sperm collected from cold-stored epididymides (4°C) for 0, 24, 48, or 72 hours in Lifor was determined using an IVOS Sperm Analyzer (Hamilton-Thorne Research, Beverly, MA, USA). A 10-μL aliquot of sperm suspension was transferred and cultured in a 90-μL drop of HTF for 30 minutes. Incubated sperm was applied to a cell counting chamber (Conception Technologies, San Diego, CA, USA). This experiment was independently performed 4 times. The average number of cells counted per sample was approximately 300. The motility of cold-stored sperm in Lifor with or without S1P was examined by the same procedure described above.
In vitro fertilization (IVF)
IVF was performed as described previously [35]. Mature female mice were superovulated via intraperitoneal injections of 7.5 IU equine chorionic gonadotropin (ASKA Pharmaceutical Co. Ltd, Tokyo, Japan) and 7.5 IU human chorionic gonadotropin (ASKA Pharmaceutical Co. Ltd) 48 hours apart. Mice were sacrificed 15–17 hours later via cervical dislocation, and oviducts were quickly removed and transferred into a fertilization dish with paraffin oil. Four to six cumulus-oocyte complexes (COCs) were obtained from the ampullae of the fallopian tubes of two to three females, respectively, using a needle and forceps under a dissecting microscope. The COCs were transferred to a dish containing a 90-μL drop of mHTF medium covered with paraffin oil.
To enhance capacitation a 10-μL aliquot of frozen-thawed sperm was added to a 90-μL drop of TYH medium containing 1.0 mg/mL polyvinyl alcohol and 0.75 mM MBCD, and sperm were pre-incubated with 5% carbon dioxide (CO2) at 37 °C for 30 min. After pre-incubation, an aliquot of sperm suspension was carefully collected from the edge of the sperm pre-incubation drop, transferred to the IVF drop containing mHTF medium and COCs (final motile sperm concentration: 500–1000/μL), and incubated at 37 °C with 5% CO2. Three hours later, the inseminated oocytes were washed 3 times in 80-μL drops of mHTF, covered with paraffin oil, and cultured at 37°C with 5% CO2. At this time, pronuclear formation was observed by phase-contrast microscopy. Twenty-four hours after insemination, fertilization rates were calculated as the total number of two-cell embryos divided by the total number of inseminated oocytes × 100. Sperm from mouse epididymides cold-stored both with and without S1P and for 72-hour cold storage durations were assessed.
Embryo culture and transfer
Two-cell embryos were either cultured in KSOM to the blastocyst stage or transferred into the oviducts (10 embryos/oviduct) of pseudo-pregnant ICR female mice on the day a vaginal plug was found (day 1 of pseudopregnancy) [16]. After 19 d, the number of offspring was recorded at birth. For the embryos that has been cultured in KSOM, the numbers of four-cell embryos, morulae, and blastocysts were recorded also after 19 d. Blastocyst developmental rate (number of blastocysts per number of two-cell embryos×100) as well as birth rate (number of live pups per number of transferred two-cell embryos×100) were calculated.
Statistical analysis
Statistical analysis was performed using Prism v. 5.0 (GraphPad Software Inc., San Diego, CA, USA). Results are expressed as means ± standard deviations (SDs). The means for each treatment were compared by analysis of variance after arcsine transformation of the percentages. Differences between mean values were considered significant at p < 0.05.
Results
Effects of mouse epididymides cold storage on sperm motility and IVF
After cold storage of cauda epididymides for 0, 24, 48, or 72 h, both overall and progressive sperm motility decreased as a function of increasing cold storage duration (Figure 1) and were lower (p< 0.05) after 72 hours of cold storage compared to 0 hours. The IVF rate was high after 24 hours of cold storage (84.3±9.8%), but decreased with increasing cold storage duration. The decreases at 48 and 72 hours were statistically significant (p < 0.05) compared with 0 hours (Table 1).
Figure 1. Effects of cold storage of mouse epididymides on sperm motility.
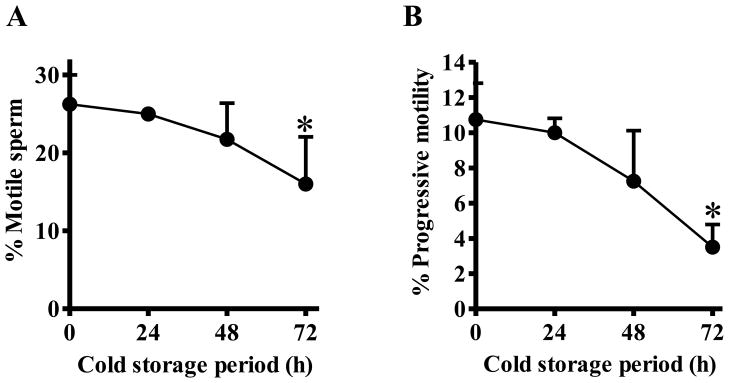
One side of cauda epididymides collected from 3 males were stored in Lifor at 4 °C for 0, 24, 48, or 72 h, cryopreserved, and then thawed. Motility of the frozen-thawed sperm was assessed using an IVOS Sperm Analyzer; an average of 300 sperm per sample were assessed. Four sperm samples were assessed for each of the 4 cold storage treatments. Graph A shows overall motility (i.e., percentage of sperm moving their tails, but not necessarily advancing forward) and graph B shows progressive motility (i.e., percentage of sperm advancing from one place to another in a relatively straight line). Percentages are means, and error bars represent on SD (n=4). Asterisk denotes p<0.05 compared with 0 h.
Table 1.
Results of IVF using frozen-thawed sperm from cold-stored mouse epididymides
| Cold storage period (h) | No. of inseminated oocytes | No. of 2-cell embryos (%) |
|---|---|---|
| 0 | 444 | 426 (95.9±3.8) |
| 24 | 626 | 528 (84.3±9.8) |
| 48 | 357 | 251 (70.3±17.2)* |
| 72 | 318 | 122 (38.4±18.4)* |
Percentages are means±SDs. The number of sperm samples tested per cold storage duration (n) was 5–8 (15–24 males per group). Three to five females were used for oocyte donor for each experiment. Asterisks denote p < 0.05 compared with 0 hours.
Effects of addition of S1P to the mouse epididymides cold storage medium on sperm motility and IVF
To determine whether addition of S1P to Lifor helps to maintain sperm viability during cold storage of cauda epididymides, motility and IVF assessments were performed using frozen-thawed sperm after cold storage of the epididymides in Lifor with or without S1P. Both overall and progressive sperm motility were unaffected by the addition of S1P to the cold storage medium (Figure 2). On the other hand, for the 72-hour cold storage period, Lifor plus S1P significantly improved fertilization rate compared with Lifor alone (Table 2).
Figure 2. Effects of cold storage of mouse epididymides in medium containing S1P on sperm motility.
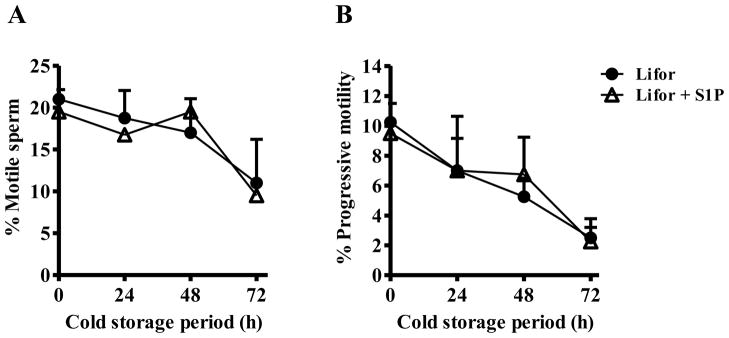
Three pairs of cauda epididymides collected from 3 males were divided and transfer into Lifor with or without 10 μM S1P. The cauda epididymides were cold-stored at 4 °C for 0, 24, 48, or 72 h. Following cold storage, sperm were collected, cryopreserved, and later thawed. Motility of the frozen-thawed sperm was assessed using an IVOS Sperm Analyzer. An average of 300 sperm per sample were assessed. Graph A shows overall motility (i.e., the percentage of sperm moving their tails, but not necessarily advancing forward) and graph B shows progressive motility (i.e., the percentage of sperm advancing from one place to another in a relatively straight line). Percentages are means, and error bars represent one SD (n=4).
Table 2.
Results of IVF using frozen-thawed sperm from mouse epididymides that were cold-stored for 72 hours either with or without 10μM S1P.
| Cold storage medium | No. of inseminated oocytes | No. of 2-cell embryos (%) |
|---|---|---|
| Lifor® | 526 | 197 (37.5±8.9) |
| Lifor® + S1P | 581 | 491 (84.5±4.9)* |
One side of 3 epididymides collected from 3 males were transferred into a tube containing Lifor and another side of 3 epididymides were transferred into a tube containing Lifor + S1P (24 males were used in both groups). Oocytes collected from three females were transferred into the drop of mHTF containing GHS (24 females and 8 drops per group). Percentages of two-cell embryos are means±SDs (n=8). Asterisk denotes p < 0.05.
In vivo and in vitro embryo development following IVF using frozen-thawed sperm from cold-transported mouse epididymides
Mouse epididymides stored in Lifor plus S1P were transported from Asahikawa Medical University to our laboratory (domestic transport within Japan) or from UC Davis to our laboratory (international transport from USA to Japan). When samples arrived (within 72 hours in both cases), sperm were collected from the received epididymides, cryopreserved, and later thawed prior to experiments. In vitro fertilization was carried out using the frozen-thawed sperm, followed by in vitro embryo culture or embryo transfer to pseudo-pregnant mice. Sperm from both domestically- and internationally-transported epididymides had comparable fertilization rates, percentages of blastocysts, and percentages of embryos that developed normally into pups (Table 3).
Table 3.
Fertilization and developmental ability of frozen-thawed sperm from cold-transported mouse epididymides
| Shipping facility | No. of inseminated | No. of 2-cell embryos (%) |
|
|||
|---|---|---|---|---|---|---|
|
in vitro
|
in vivo
|
|||||
| No. of cultured 2-cell embryos | No. of blastocysts (%) | No. of transferred 2-cell | No. of live pups (%) | |||
| Asahikawa Med. Univ. | 301 | 254 (84.4±16.3) | 134 | 92 (68.7±20.9) | 120 | 39 (32.5±4.3) |
| UC Davis | 536 | 447 (83.4±10.5) | 287 | 225 (78.4±14.2) | 160 | 54 (33.8±13.0) |
IVF (No. of droplet for IVF; Asahikawa Med. Univ: 6, UC Davis: 8) was performed using frozen-thawed sperm from different batches of transported epididymides in tubes (three tubes [three epididymides/tube] for the domestic transport and four tubes [three epididymides/tube] for the international transport). The cold storage medium in these experiments consisted of Lifor plus S1P. Twenty embryos were transferred into the oviducts (No. of transferred embryos: 10 embryos/oviduct) of each pseudo-pregnant mouse (No of recipient mice: Asahikawa Med Univ; 6, UC Davis; 8). Percentages are means±SDs.
Discussion
In the present study, we show that cold storage of mouse epididymides for 24 hours had no significant effect on frozen-thawed epididymal sperm IVF rates. Cold storage of epididymides for more than 48 hours, however, resulted in markedly decreased IVF rates compared with 0 hours of cold storage. The addition of S1P to the cold storage medium significantly increased fertilization rate of frozen-thawed sperm derived from cold-stored epididymides for 72 hours. Using a cold-storage system, we have demonstrated that sperm cryopreserved after cold-storage and transportation can be used for IVF. Further, IVF-generated embryos develop normally to live pups. There were no significant differences in fertilization or birth rates between the laboratories in Japan and the United States collaborating on this project.
Worldwide transportation of genetically engineered mice has increased over the years. Live animal transport, however, can be problematic because of concerns about animal welfare, infection control, animal escape or death, and cost. Transportation of cauda epididymides from genetically engineered mice represents an alternative with many advantages. Sperm from transported epididymides can be cryopreserved and stored in liquid nitrogen until needed. The cryopreserved sperm can be used for IVF to quickly produce many embryos and serve as a readily available back-up of the mice.
We previously reported on an IVF process using frozen-thawed or cold-stored C57BL/6 mouse sperm pre-incubated with MBCD and treatment of GSH to oocytes which achieved high fertilization rates [35; 36]. MBCD facilitated cholesterol efflux from the plasma membranes of frozen-thawed C57BL/6 mouse sperm, enhancing fertilization capability [31]. Reduced glutathione broke disulfide bonds of the zona pellucida, loosening its structure and facilitating sperm penetration [35]. Bath (2010) also reported that incubation with GSH increased IVF of frozen-thawed mouse sperm, and that reactive oxygen species (ROS) generated by some incompetent frozen-thawed sperm during fertilization may impair fertilization by other capable mouse sperm [1]. Recently, Gray et al. (2013) showed that enhancing cholesterol efflux with MBCD restored capacitation-dependent function of frozen-thawed mouse sperm and that GSH decreased oxidative stress caused by mitochondrial hydrogen peroxide production and improved IVF rates by reducing peroxidative acrosomal damage [6]. In our present study, with the exception of frozen-thawed sperm from epididymides that had been cold-stored for 48 or 72 hours, IVF rates for cold-stored/cold-transported frozen-thawed sperm were high (>83%) with the addition of MBCD and GSH during IVF. These results suggest that MBCD-induced cholesterol efflux in sperm, ROS scavenging by GSH in sperm, and disulfide bond breakage in the zona pellucida by GSH contribute to the maintenance of high IVF rates using cold-stored, frozen-thawed sperm.
Numerous studies have reported that sperm storage (without loss of viability) is only possible for short periods (e.g., 48 hours) at ambient or refrigeration temperatures [9; 10; 12; 15; 24]. These reports document the difficulties associated with maintaining normal mouse sperm motility and viability in vitro for more than 48 hours. We previously reported that sperm from epididymides cold-stored in Lifor for 96 hours maintained high IVF rates; however, sperm frozen-thawed following >48 hours of cold storage had greatly decreased IVF rates in spite of the addition of MBCD and GSH [36]. In the present study, the addition of S1P to the cold storage medium clearly improved the IVF rates of frozen-thawed sperm from the cold-stored epididymides. S1P is a bioactive sphingolipid metabolite involved in various biological processes including calcium mobilization, cytoskeletal organization, and cell growth, differentiation, survival, and motility [26; 27]. One of the most prominent roles of S1P is the suppression of apoptosis [3]. In male germ cells, S1P suppressed apoptosis initiated by radiation-induced stress [22; 29]. The DNA-binding activity of nuclear factor κB (NF-κB) and expression of phosphorylated Akt which are regulating apoptosis were suppressed by S1P in germ cells. Sphingosine kinase-1, which catalyzes sphingosine phosphorylation to form S1P, is localized in the acrosome region of mature mouse sperm; additionally, the presence of S1P receptors was confirmed in sperm [14]. This suggests that S1P may be involved in the sperm acrosome reaction, a key element of fertilization that facilitates egg penetration [28]. During cold storage of epididymides, S1P may exert protective effects on sperm via binding to various S1P receptors on the sperm cell surface.
The acrosome reaction appears essential for sperm penetration of the zona pellucida and fusion with the oocyte. Jin et al. (2011) reported that most fertilizing sperm underwent the acrosome reaction prior to contact with the zona pellucida [8]. The addition of S1P to the medium for cold storage of epididymides may facilitate the induction of the acrosome reaction in sperm from cold-stored epididymides. In our experiment, pre-incubation with MBCD greatly enhanced IVF rates of sperm from cold-stored epididymides (data not shown). Treatment of sperm with MBCD efficiently induces capacitation by promoting cholesterol efflux from the plasma membrane [2; 37]. In addition, MBCD-treated sperm underwent the acrosome reaction at high frequencies, with rearranging of plasma membrane lipid rafts and release of glycosylphosphatidylinositol-anchored proteins in the sperm head [39]. These findings suggest that MBCD plays a role in induction of the acrosome reaction and therefore can potentially facilitate IVF. Likewise, the effects of S1P on the acrosome reaction indicate that S1P can enhance IVF by sperm from cold-stored epididymides. However, further studies are needed in order to elucidate the mechanisms of S1P in affecting the viability of cold-stored and frozen-thawed sperm.
After cold-storage of epididymides, sperm motility decreased time-dependently. Ogonuki et al. reported live pups were obtained derived from non-motile sperm derived from transport of frozen mouse epididymides [20; 21]. In case of disappearing sperm motility after cold transport of epididymides, intracytoplasmic sperm injection (ICSI) may be useful to produce embryos using the sperm collected from epididymides.
Cold storage of mouse epididymides can enable easy transport of genetically modified mice between research institutes. In the present study, addition of S1P to the cold storage medium Lifor improved IVF by frozen-thawed sperm after cold storage of epididymides for 72 hours. In addition, live pups were born after IVF using frozen-thawed sperm from mouse epididymides that had been cold-transported either domestically or internationally. Moreover, we recently established an IVF process for cryopreserved mouse oocytes and fresh, cold-stored, or cryopreserved sperm [19]. It is important to explore alternatives to the transport of live mice. Our system for cold transport of epididymides and IVF using frozen-thawed epididymal sperm is one such alternative. It enables sharing of genetically engineered mice in the scientific community, and can foster collaboration and facilitate mouse genome research.
Acknowledgments
We are indebted to H. Matsunaga, M. Nishimura, T. Umeno, S. Takeuji, Y. Ishizuka for their great technical support and helpful discussion on this study.
Funding: This study was supported by a Grant-In-Aid for the Fundamental Technologies Upgrading Program, part of the National BioResource Project (NBRP) of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. This study was also supported by the Institutional Program for Young Researcher Overseas Visits of the Japan Society for the Promotion of Science. This study was also supported in part by the Mutant Mouse Regional Resource Center (MMRRC) at UC Davis (NIH Grant No. U42 OD012210) and by the UC Davis Mouse Biology Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bath ML. Inhibition of in vitro fertilizing capacity of cryopreserved mouse sperm by factors released by damaged sperm, and stimulation by glutathione. PLoS One. 2010;5:e9387. doi: 10.1371/journal.pone.0009387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi YH, Toyoda Y. Cyclodextrin removes cholesterol from mouse sperm and induces capacitation in a protein-free medium. Biol Reprod. 1998;59:1328–33. doi: 10.1095/biolreprod59.6.1328. [DOI] [PubMed] [Google Scholar]
- 3.Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–3. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 4.Donahue LR, Hrabe de Angelis M, Hagn M, Franklin C, Lloyd KC, Magnuson T, McKerlie C, Nakagata N, Obata Y, Read S, Wurst W, Horlein A, Davisson MT. Centralized mouse repositories. Mamm Genome. 2012;23:559–71. doi: 10.1007/s00335-012-9420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eppig JT, Strivens M. Finding a mouse: the International Mouse Strain Resource (IMSR) Trends Genet. 1999;15:81–2. doi: 10.1016/s0168-9525(98)01665-5. [DOI] [PubMed] [Google Scholar]
- 6.Gray JE, Starmer J, Lin VS, Dickinson BC, Magnuson T. Mitochondrial Hydrogen Peroxide and Defective Cholesterol Efflux Prevent In Vitro Fertilization by Cryopreserved Inbred Mouse Sperm. Biol Reprod. 2013 doi: 10.1095/biolreprod.113.109157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho Y, Wigglesworth K, Eppig JJ, Schultz RM. Preimplantation development of mouse embryos in KSOM: augmentation by amino acids and analysis of gene expression. Mol Reprod Dev. 1995;41:232–8. doi: 10.1002/mrd.1080410214. [DOI] [PubMed] [Google Scholar]
- 8.Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci U S A. 2011;108:4892–6. doi: 10.1073/pnas.1018202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jishage KSH. Maintenance of the Fertilizing Ability in Capacitated. Mouse. Spermatozoa. Journal of Reproduction and Development. 1993;39:363–367. [Google Scholar]
- 10.Jishage KUO, Suzuki H. Fertility of mouse spermatozoa from cauda epididymis preserved in paraffin oil at 4 °C. Mammalian Ova Research. 1997;14:45–48. [Google Scholar]
- 11.Kamimura E, Nakashima T, Ogawa M, Ohwada K, Nakagata N. Study of low-temperature (4 degrees C) transport of mouse two-cell embryos enclosed in oviducts. Comp Med. 2003;53:393–6. [PubMed] [Google Scholar]
- 12.Kaneko T, Fukumoto K, Haruguchi Y, Kondo T, Machida H, Koga M, Nakagawa Y, Tsuchiyama S, Saiki K, Noshiba S, Nakagata N. Fertilization of C57BL/6 mouse sperm collected from cauda epididymides after preservation or transportation at 4 degrees C using laser-microdissected oocytes. Cryobiology. 2009;59:59–62. doi: 10.1016/j.cryobiol.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Kelley KA. Transport of mouse lines by shipment of live embryos. Methods Enzymol. 2010;476:25–36. doi: 10.1016/S0076-6879(10)76002-X. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto K, Banno Y, Murate T, Akao Y, Nozawa Y. Localization of sphingosine kinase-1 in mouse sperm acrosomes. J Histochem Cytochem. 2005;53:243–7. doi: 10.1369/jhc.4B6507.2005. [DOI] [PubMed] [Google Scholar]
- 15.Mochida K, Ohkawa M, Inoue K, Valdez DM, Jr, Kasai M, Ogura A. Birth of mice after in vitro fertilization using C57BL/6 sperm transported within epididymides at refrigerated temperatures. Theriogenology. 2005;64:135–43. doi: 10.1016/j.theriogenology.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Nakagata N. Embryo transfer through the wall of the fallopian tube in mice. Jikken Dobutsu. 1992;41:387–8. doi: 10.1538/expanim1978.41.3_387. [DOI] [PubMed] [Google Scholar]
- 17.Nakagata N. Cryopreservation of mouse spermatozoa. Mamm Genome. 2000;11:572–6. doi: 10.1007/s003350010109. [DOI] [PubMed] [Google Scholar]
- 18.Nakagata N. Cryopreservation of mouse spermatozoa and in vitro fertilization. Methods Mol Biol. 2011;693:57–73. doi: 10.1007/978-1-60761-974-1_4. [DOI] [PubMed] [Google Scholar]
- 19.Nakagata N, Takeo T, Fukumoto K, Kondo T, Haruguchi Y, Takeshita Y, Nakamuta Y, Matsunaga H, Tsuchiyama S, Ishizuka Y, Araki K. Applications of cryopreserved unfertilized mouse oocytes for in vitro fertilization. Cryobiology. 2013 doi: 10.1016/j.cryobiol.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Ogonuki N, Mochida K, Miki H, Inoue K, Fray M, Iwaki T, Moriwaki K, Obata Y, Morozumi K, Yanagimachi R, Ogura A. Spermatozoa and spermatids retrieved from frozen reproductive organs or frozen whole bodies of male mice can produce normal offspring. Proc Natl Acad Sci U S A. 2006;103:13098–103. doi: 10.1073/pnas.0605755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogonuki N, Mochida K, Shinmen A, Ohkawa M, Miki H, Inoue K, Fray M, Moriwaki K, Obata Y, Ogura A. Microinsemination using male germ cells from epididynudes and testes stored in freezers without cryoprotectant, Reproduction. Fertility and Development. 2006;18:286. [Google Scholar]
- 22.Otala M, Suomalainen L, Pentikainen MO, Kovanen P, Tenhunen M, Erkkila K, Toppari J, Dunkel L. Protection from radiation-induced male germ cell loss by sphingosine-1-phosphate. Biol Reprod. 2004;70:759–67. doi: 10.1095/biolreprod.103.021840. [DOI] [PubMed] [Google Scholar]
- 23.Quigley C. Lost in transit - a forgotten rodent welfare issue. Altern Lab Anim. 2013;41:P8–9. doi: 10.1177/026119291304100118. [DOI] [PubMed] [Google Scholar]
- 24.Sankai T, Tsuchiya H, Ogonuki N. Short-term nonfrozen storage of mouse epididymal spermatozoa. Theriogenology. 2001;55:1759–68. doi: 10.1016/s0093-691x(01)00518-0. [DOI] [PubMed] [Google Scholar]
- 25.Spiegel S, Merrill AH., Jr Sphingolipid metabolism and cell growth regulation. FASEB J. 1996;10:1388–97. doi: 10.1096/fasebj.10.12.8903509. [DOI] [PubMed] [Google Scholar]
- 26.Spiegel S, Milstien S. Sphingosine-1-phosphate: signaling inside and out. FEBS Lett. 2000;476:55–7. doi: 10.1016/s0014-5793(00)01670-7. [DOI] [PubMed] [Google Scholar]
- 27.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 28.Suhaiman L, De Blas GA, Obeid LM, Darszon A, Mayorga LS, Belmonte SA. Sphingosine 1-phosphate and sphingosine kinase are involved in a novel signaling pathway leading to acrosomal exocytosis. J Biol Chem. 2010;285:16302–14. doi: 10.1074/jbc.M109.072439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suomalainen L, Hakala JK, Pentikainen V, Otala M, Erkkila K, Pentikainen MO, Dunkel L. Sphingosine-1-phosphate in inhibition of male germ cell apoptosis in the human testis. J Clin Endocrinol Metab. 2003;88:5572–9. doi: 10.1210/jc.2003-030776. [DOI] [PubMed] [Google Scholar]
- 30.Suomalainen L, Pentikainen V, Dunkel L. Sphingosine-1-phosphate inhibits nuclear factor kappaB activation and germ cell apoptosis in the human testis independently of its receptors. Am J Pathol. 2005;166:773–81. doi: 10.1016/s0002-9440(10)62298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeo T, Hoshii T, Kondo Y, Toyodome H, Arima H, Yamamura K, Irie T, Nakagata N. Methyl-beta-cyclodextrin improves fertilizing ability of C57BL/6 mouse sperm after freezing and thawing by facilitating cholesterol efflux from the cells. Biol Reprod. 2008;78:546–51. doi: 10.1095/biolreprod.107.065359. [DOI] [PubMed] [Google Scholar]
- 32.Takeo T, Kaneko T, Haruguchi Y, Fukumoto K, Machida H, Koga M, Nakagawa Y, Takeshita Y, Matsuguma T, Tsuchiyama S, Shimizu N, Hasegawa T, Goto M, Miyachi H, Anzai M, Nakatsukasa E, Nomaru K, Nakagata N. Birth of mice from vitrified/warmed 2-cell embryos transported at a cold temperature. Cryobiology. 2009;58:196–202. doi: 10.1016/j.cryobiol.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Takeo T, Kondo T, Haruguchi Y, Fukumoto K, Nakagawa Y, Takeshita Y, Nakamuta Y, Tsuchiyama S, Shimizu N, Hasegawa T, Goto M, Miyachi H, Anzai M, Fujikawa R, Nomaru K, Kaneko T, Itagaki Y, Nakagata N. Short-term storage and transport at cold temperatures of 2-cell mouse embryos produced by cryopreserved sperm. J Am Assoc Lab Anim Sci. 2010;49:415–9. [PMC free article] [PubMed] [Google Scholar]
- 34.Takeo T, Nakagata N. Combination medium of cryoprotective agents containing L-glutamine and methyl-{beta}-cyclodextrin in a preincubation medium yields a high fertilization rate for cryopreserved C57BL/6J mouse sperm. Lab Anim. 2010;44:132–7. doi: 10.1258/la.2009.009074. [DOI] [PubMed] [Google Scholar]
- 35.Takeo T, Nakagata N. Reduced glutathione enhances fertility of frozen/thawed C57BL/6 mouse sperm after exposure to methyl-beta-cyclodextrin. Biol Reprod. 2011;85:1066–72. doi: 10.1095/biolreprod.111.092536. [DOI] [PubMed] [Google Scholar]
- 36.Takeo T, Tsutsumi A, Omaru T, Fukumoto K, Haruguchi Y, Kondo T, Nakamuta Y, Takeshita Y, Matsunaga H, Tsuchiyama S, Sakoh K, Nakao S, Yoshimoto H, Shimizu N, Nakagata N. Establishment of a transport system for mouse epididymal sperm at refrigerated temperatures. Cryobiology. 2012;65:163–8. doi: 10.1016/j.cryobiol.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Visconti PE, Galantino-Homer H, Ning X, Moore GD, Valenzuela JP, Jorgez CJ, Alvarez JG, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm. beta-cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J Biol Chem. 1999;274:3235–42. doi: 10.1074/jbc.274.5.3235. [DOI] [PubMed] [Google Scholar]
- 38.Wadman M. Lab-animal flights squeezed. Nature. 2012;489:344–5. doi: 10.1038/489344a. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe H, Kondoh G. Mouse sperm undergo GPI-anchored protein release associated with lipid raft reorganization and acrosome reaction to acquire fertility. J Cell Sci. 2011;124:2573–81. doi: 10.1242/jcs.086967. [DOI] [PubMed] [Google Scholar]


