Abstract
Sequestering semi-polar compounds can be difficult with low-density polyethylene (LDPE), but those pollutants may be more efficiently absorbed using silicone. In this work, optimized methods for cleaning, infusing reference standards, and polymer extraction are reported along with field comparisons of several silicone materials for polycyclic aromatic hydrocarbons (PAHs) and pesticides. In a final field demonstration, the most optimal silicone material is coupled with LDPE in a large-scale study to examine PAHs in addition to oxygenated-PAHs (OPAHs) at a Superfund site. OPAHs exemplify a sensitive range of chemical properties to compare polymers (log Kow 0.2–5.3), and transformation products of commonly studied parent PAHs. On average, while polymer concentrations differed nearly 7-fold, water-calculated values were more similar (about 3.5-fold or less) for both PAHs (17) and OPAHs (7). Individual water concentrations of OPAHs differed dramatically between silicone and LDPE, highlighting the advantages of choosing appropriate polymers and optimized methods for pollutant monitoring.
Keywords: Polyethylene, silicone, passive sampling, PAHs, OPAHs, oxygenated-PAHs, pesticides
INTRODUCTION
Many benefits of passive sampling are practical, whether it is cost, ease of use over grab samples, concentrated extracts over diffuse matrices, or time-weighted averages over the deployment period (Namieśnik et al., 2005; Vrana et al., 2005; Zabiegala et al., 2010; Seethapathy and Gorecki, 2012). Another important benefit is that passive sampling concentrations represent bioavailable contaminants in the sample media (Anderson and Hillwalker, 2008). One challenge with passive sampling is choosing a receiving phase among the many diverse options that exist. For example, at least 22 different types of materials, sorbents, or solvents are reported as receiving phases for passive sampling in a recent review (Vrana et al., 2005). Some PSDs specialize in targeting polar or non-polar compounds, and some materials can be used in tandem with others to broaden the total range of sequestered compounds (Petty et al., 2004; Harman et al., 2012; Allan et al., 2013). In addition to compound selectivity, considerations for using PSDs derive from previous development of uptake kinetics and published laboratory methods (Vrana et al., 2005; Rusina et al., 2007). One of the most commonly used passive samplers is low-density polyethylene (LDPE) due to the low cost of the material, hydrophobic properties for targeting many persistent organic pollutants (POPs), and available partitioning and sampling rate estimates (Booij et al., 2002; Anderson et al., 2008; Choi et al., 2013).
However, LDPE does not sequester lower log Kow compounds (especially below 4) as well as another polymer, silicone (Rusina et al., 2007; Allan et al., 2013). Silicone has become an increasingly popular passive sampler in the past 10 years, and has been compared with LDPE to see differences in sequestration of target chemicals (Allan et al., 2010; Allan et al., 2013). Although quantitative comparisons between aqueous concentrations were similar (< 2 to 3-fold among PAHs and PCBs), a dramatic increase of absorbed analytes was seen using silicone over LDPE for compounds with log Kow values lower than 6 (Allan et al., 2013). More efficient absorption of analytes into the polymer can result in several advantages, including greater flexibility in deployment times, lower detection limits, and applications to bioassays due to greater concentrations in samples as long as extracts or silicone polymer background may be sufficiently reduced. Explaining sequestration differences between LDPE and silicone begin with the structural make-up of each polymer. Silicone is made of a silicon-oxygen backbone with various functional groups bonded to silicon such as methyl, phenyl, vinyl, or fluoro constituents (Rusina et al., 2007; Seethapathy and Gorecki, 2012). In contrast, LDPE consists of carbon and hydrogen (Rusina et al., 2007), which gives this polymer a more hydrophobic property. Differences in chemical structures of polymers influence intermolecular forces acting between the target compounds and the polymers in the sampled media. Along with compound diffusivity, the resulting fugacity of compounds out of the aqueous phase into the polymers can be modeled using first order kinetics. Ultimately, both polymers yield more accurate data than other passive sampling devices (Allan et al., 2010), so advantages of using one or the other depend on the choice of targeted compounds. Unlike previous comparisons of silicone and LDPE which focused on POPs (Allan et al., 2010; Allan et al., 2013), this research includes compounds that are transformation products of pollutants. One class of compounds that is well suited for a comparison between silicone and LDPE is oxygenated-PAHs (OPAHs). OPAHs are degradation products of PAHs (Lundstedt et al., 2007), and are emerging contaminants of interest that have log Kow values less than 6 for 22 previously studied OPAHs (O’Connell et al., 2013). Other classes of pollutants, such as pesticides, span a wide range of chemical properties that are also beneficial for polymer comparisons. By addressing data gaps through monitoring emerging compounds of interest using passive samplers, this research can highlight differences in silicone and LDPE and ultimately assess a greater range of contamination. Considering the chemical structure of the silicone polymer and previous evidence illustrating polymer differences in sequestration, silicone should sequester higher concentrations and a greater range of OPAH compounds than LDPE.
Therefore, our objective focused on three aims. First, laboratory methods were employed to optimize silicone pre-deployment cleaning to reduce background chromatographic interferences, and for infusing silicone with labeled internal standards for uptake rates and water concentration estimates. Second, we compared five silicone polymers in a field application at a Portland Harbor Superfund site with a history of POP (including PAH and pesticide) contamination (Sethajintanin and Anderson, 2006; Sower and Anderson, 2008). Sequestration data and operational logistics were used to select silicone polymers best suited for co-deployment studies with LDPE. Finally, the optimal silicone was compared with LDPE for PAHs and OPAHs. By optimizing silicone passive samplers to be used in tandem with LDPE, this work provides a field validated method for quantification of a wide range of contaminants including PAHs, OPAHs, and pesticides.
METHODS
Analyte information
OPAH, PAH, and single pesticide standards were bought from Sigma Aldrich (St. Louis, MO), Chiron (Trondheim, Norway), and Fluka (part of Sigma-Aldrich). Pesticide and PAH mixes were purchased from Accustandard (New Haven, CT). Labeled standards used as performance reference compounds (PRCs), laboratory surrogates, or instrument internal standards were obtained from either CDN Isotopes (Pointe-Claire, Quebec, Canada), or Cambridge Isotope Laboratories (Tewksbury, MA). A complete list of all quantitative analytes including surrogates, PRCs, and internal standards is given in the Supporting Information (Table S1). All solvents were Optima-grade or equivalent (Fisher Scientific, Pittsburgh, PA), and all laboratory water used for infusions or post-deployment cleaning was filtered through a Barnstead D7389 purifier (Dubuque, IA).
Polymer construction
A total of five silicone polymers were purchased from three companies: Stockwell Elastomerics Inc. (Philadelphia, PA), Altec Products Limited (Bude, Cornwall, UK), and CS Hyde Company (Lake Villa, IL) (Table 1). Silicone was purchased in square-yard rolls, and strips were cut from the sheet using a table cutter/trimmer (Fletcher-Terry Company, Farmington, CT). The AteSil™ silicone was purchased as talc-free silicone in 30 × 30 cm sheets. Three AteSil™ strips were cut and extracted together to obtain approximate dimensions of the other polymer strips. All deployed strips were of similar dimensions, approximately 91 cm × 2.6 cm, although thickness differed between materials (Table 1). Random subsets of strips were weighed during construction to determine variability, and averages were used to normalize absorption data (Table 1). Polyethylene strips were cut from pre-sized layflat tubing (width approximately 2.7 cm) at 110 cm, and loops were formed on each end for deployment after heat-sealing each end. Total length of LDPE strips were approximately 100 cm.
Table 1.
Silicone and LDPE polymers by manufacturer, abbreviation, and physical information
| Supplier | PSD Material (Abbreviation) | Depiction | Strip Surface Areas (cm2) | Strip Volume (cm3) | Strip Weight (n = 5, g) |
|---|---|---|---|---|---|
| Stockwell Elastomerics | Silicone Sponge (SS) |

|
~480 | ~18 | 15.5 ± 1.4 |
| Stockwell Elastomerics | Thin Translucent Sheet (ST) |

|
~480 | ~7.2 | 7.87 ± 0.34 |
| CS Hyde | Commercial-Grade Sponge (CS) |

|
~480 | ~18 | 17.6 ± 0.10 |
| CS Hyde | Translucent Sheet (CT) |

|
~480 | ~18 | 27.9 ± 0.26 |
| Altec Products Limited | AlteSil™ (AA) |

|
~470 | ~11 | 15.4 ± 0.33 |
| Brentwood Plastic, Inc. | Low density polyethylene (LDPE) |

|
~540 | ~5.1 | 4.82 ± 0.08 |
Laboratory optimization: pre-cleaning, infusion, post-deployment cleaning, and extraction
Before deployment, silicone was cleaned with solvents to remove chromatographic interferences from the curing process of the polymer (Rusina et al., 2007). Initially, silicone extraction and pre-cleaning experiments used ethyl acetate since it does not severely impact the integrity of the silicone itself (Rusina et al., 2007), and ethyl acetate is a solvent with both polar and non-polar properties that might be conducive to OPAHs. In addition, to exploit inherent differences in each polymer, ethyl acetate was used as the primary solvent for silicone, while the more non-polar hexane was used for LDPE cleaning as previously described (Anderson et al., 2008). Pre-cleaning experiments for silicones were adapted from several studies (Booij et al., 2002; Rusina et al., 2007; Yates et al., 2007; Allan et al., 2009; Schafer et al., 2010). Ultimately, sufficient reduction of siloxane background was only achieved with a combination of three extraction periods of 1:1, hexane:ethyl acetate, followed by two more periods of 1:1 methanol:ethyl acetate. Roughly 65 g of silicone was placed into an amber jar (1L) before the mixed solvents were added to fill each container. Each extraction period was at least 2 hours, but no more than 14 hours (overnight). Samples were shaken at approximately 60 rotations per minute (rpm) in a water bath at 40 °C (New Brunswick Scientific, Edison, NJ). Once an adequate pre-cleaning method was finalized, a secondary experiment evaluated the effectiveness of using recycled solvents to reduce waste. Silicone strips were cleaned with a portion of solvents re-used from a previous exchange (see Figure S1 for more details). All polymers were dried under filtered vacuum in either sealed glassware or stainless steel kegs (AEB Kegs, Delebio SO, Italy).
Deployed polymers for the final comparison were spiked with non-target deuterated compounds, (also known as performance reference compounds, or PRCs) used to estimate in-situ sampling rates in order to calculate water concentrations (Booij et al., 1998; Booij et al., 2002; Huckins et al., 2002). Infusion solutions were modified to a 50% mixture of methanol/water rather than 80% used in a previous study (Booij et al., 2002). Increasing the water content increases the fugacity of the PRC compounds into the silicone, and reduces the total amount of compound needed for the infusion process. Briefly, 5–6 strips (or 60–90 g of silicone) were placed into a 1 L amber glass jar and filled with 750 mL of methanol/water (1:1, v:v). PRC compounds were spiked into the solution and allowed to equilibrate with the silicone for 3 days at 60 rpm and 40°C. Fluorene-d10, benzo[b]fluorenthene-d12, p,p-DDE-d4, and 9,10-anthraquinone-d8 were used for both silicone and LDPE, and spiking concentrations were adjusted for differences in partitioning, polymer mass, and length of deployment (Booij et al., 2002). Polyethylene was infused with PRCs at 4 to 100 μg per strip directly spiked within the tubing before sealing the other end of the strip.
After deployment, each polymer was cleaned with ambient waters to remove any surface sediment or biological material on the polymer (Figure 1). Once in the laboratory, silicone was rinsed further with filtered water and isopropanol, while LDPE was rinsed with water, dilute hydrochloric acid, and isopropanol based on previous work (Allan et al., 2012). Post deployment-cleaned strips were stored at −20 °C until extraction. Laboratory surrogates (Table S1) were spiked into amber jars at 500 ng/mL before extraction. Individual silicone strips were extracted with two sequential rounds of 100 mL of ethyl acetate on an orbital shaker set at 60 rpm (ambient temperature), and the total extraction time was 18 hours. LDPE was extracted with hexanes in a similar fashion (Anderson et al., 2008). All extracts were quantitatively concentrated to 1 mL using closed cell evaporators (TurboVaps®, Biotage, Charlotte, NC), and transferred to chromatography vials. Extracts were stored at 4 °C until analysis.
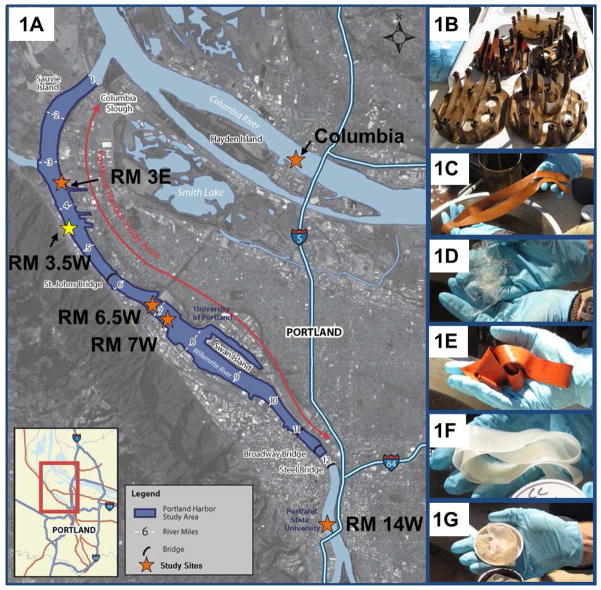
Figure 1.
Field deployment of multiple polymers in Portland Harbor Superfund, OR: 1A) stars represent field sites in and outside of the Superfund in 2011, and the yellow star (RM 3.5W) was a deployment site in 2010 and 2011; 1B) silicone polymers immediately after deployment in 2010; 1C–G) polymers after deployment before storage in amber jars: SS, ST, CS, CT, AA, respectively, see table 1 for abbreviations. Map of Portland Harbor Superfund courtesy of U.S. Environmental Protection Agency.
Site characterization
Portland Harbor Superfund is located in downtown Portland, OR, and stretches approximately nine miles along the Willamette River. Contaminants of concern at this site include PCBs, dioxins, PAHs, pesticides, and heavy metals (USEPA, 2013). Site names in this study use rough river mile (RM) designations and are not strict distances, but the latitude and longitude of each study site can be found in SI-Table 2. In 2010, five silicone polymers were deployed at RM 3.5 west (W), while in 2011, just three silicones were deployed along with LDPE (Figure 2). Both 2010 and 2011 deployments included RM 3.5W (Figure 2, yellow star). Deployment took place from September 2–30, 2010 (28 days), and September 1–22, 2011 (22 days). Water cages were purchased from Environmental Sampling Technologies, Inc. (St. Joseph, MO) with all polymers co-deployed within the same cage, and multiple cages deployed at each site in both sampling years. The deployment system consisted of an anchor, steel cable, water cages, and two buoys: one for buoyancy, and another on top for retrieval (Sethajintanin and Anderson, 2006). Each cage was approximately 2.5 meters off the bottom of the river.
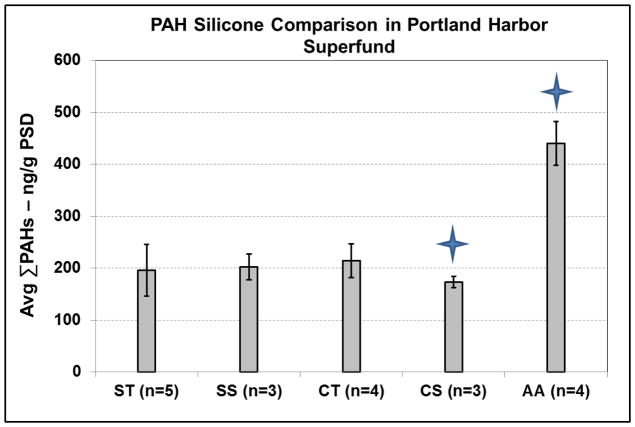
Figure 2.
Average summation of 25 PAHs from all silicone in 2010 at RM 3.5W. Concentrations were normalized to polymer mass to highlight differences between silicone polymers. Blue stars indicate severe degradation of polymer during extraction.
Analytical methods
Specific method details can be found for PAH (Allan et al., 2012), OPAH (O’Connell et al., 2013), and pesticide (Anderson et al., 2014) analyses published previously. Internal standards for each method were spiked into extract aliquots just prior to instrumental analyses. A gas chromatograph (GC) with an Agilent DB-5 column (30 m length, 0.25 mm inner diameter, 0.25 μm film thickness) was used to analyze OPAHs and PAHs (2010 deployment), while an Agilent DB-XLB (30m, 0.25mm, 0.25 μm) and a DB-17MS (30m, 0.25mm, 0.25 μm) was used to analyze pesticides with dual column confirmation (Anderson et al., 2014). An Agilent Select PAH column (30m, 0.25mm, 0.15 μm) was used for PAHs in the 2011 deployment. The OPAH and PAH methods used mass spectrometry (MS) detection (model 5975B, Agilent), while the pesticide method utilized dual electron capture detection (model 6890N, Agilent). All compounds were calibrated with calibration curves of five points or more, and had correlations of 0.99 or better. Contaminant screening for additional compounds was performed with GC/MS retention time locking Automated Mass Deconvolution Identification Software (AMDIS) in conjunction with created and purchased libraries totaling 1,180 unique compounds. Identification and confirmation criteria has been described previously (O’Connell et al., 2014), but each compound had at least a 60% spectral match before additional confirmation criteria were used for each qualitative determination. Any compounds indentified in blank samplers were removed from data described below or presented in the Supporting Information.
Quality Control
Including field, laboratory, cleaning, and instrumental blanks, over 40% of the analyzed samples were for quality control (QC) purposes. During polymer construction, at least two strips were analyzed from each batch to assess adequate removal of chromatographic interferences. If the highest background peak had an area less than 15 fold of a 500 ng/mL spiked internal standard, then that background level was considered adequate. Both strips had to pass this criterion to allow a polymer batch to be used. During PRC infusion, five strips of each polymer type were set aside to determine an average initial concentration of each PRC prior to deployment. Each trip to Portland Harbor included field blanks to monitor contamination from travel or field processing. During post-deployment cleaning, non-deployed strips were used to monitor any contamination prior to freezer storage (−20 °C). When samples were extracted, laboratory reagent blanks accompanied each batch or day of extraction. The final type of QC samples was a verification standard, which included all target compounds for the appropriate method. Compounds were verified +/− 20% of the true value for at least 90% of the target list before samples were analyzed. The reporting limit was set as the average of all blank samples from field and laboratory plus three times the standard deviation. Concentrations below the reporting limits were not included in results.
Calculated Water Concentrations
For the final comparison, water concentrations were derived using empirical models with the most optimal silicone and LDPE for PAHs and OPAHs. Typically for LDPE and silicone, sampling rates (Rs) are determined through in-situ calibration with PRCs (Booij et al., 2002; Huckins et al., 2002). Partition coefficients (Ksw) for PAHs were obtained from the literature for LDPE (Choi et al., 2013) and for the final silicone that was the most similar in density and thickness to the silicone used in previous work, “Silastic A” (Smedes et al., 2009). Because there is not a compound specific model for estimating OPAH partition coefficients, PAH models based on Kow were used since that parameter would be more sensitive to OPAH compounds than molecular weight (ex: log Kows: OPAHs −0.2 to 5.3; PAHs −3.3 to 7.3, MW: OPAHs −108 to 282; PAHs −128 to 302). All partition coefficient models have above 0.88 r2 correlations whether Kow or molecular weight chemical parameters were used (Smedes et al., 2009; Choi et al., 2013). For LDPE sampling rate (Rs) estimates, we used an empirical uptake theory with compound-specific adjustments for target compounds (Huckins et al., 2006). Compound-specific adjustments for target compounds from PRC sampling rates were based on a model using over 400 data points from several compound classes and various deployments to give an averaged adjustment to each analyte (see SI-Equation 3–4). However, this model was originally based on LDPE filled with triolein (called semi-permeable membrane devices, or SPMDs) and despite previous work showing little difference in sampling rates between SPMDs and LDPE (Booij et al., 2003), it is acknowledged that future adjustments might be improved by repeating this model with LDPE once enough published data is available. Silicone Rs values were estimated from an empirical model as well (Rusina et al., 2009). This model uses a constant (β) derived from PRC dissipation over the sampling period that is then applied for each target analyte coupled with Ksw values to result in unique Rs estimates (see SI-Equation 5). Final water concentrations for either polymer were determined making no assumptions about stages of uptake at time of retrieval and used the same final equation (SI-Equation 6, Huckins et al., 2006). Additional details concerning equations and models are given in the Supporting Information.
RESULTS AND DISCUSSION
Laboratory Optimization of Silicone
Silicone background was reduced to similar levels as in LDPE, but the process was iterative (see Supporting Information for more details). Although polymer cleaning results are rarely reported, this methodology compares well with others that rely on solvent exchanges (Booij et al., 2002; Rusina et al., 2007; Schafer et al., 2010), and it is faster (≤ 48h) than soxhlet extraction methods (90h) (Yates et al., 2007; Monteyne et al., 2013). Moreover, solvents could be effectively recycled (both hexane/ethyl acetate and methanol/ethyl acetate mixtures) between batches of silicone (SI-Figure 1B). By using this recycled solvent, the total solvent use is reduced by 20% (SI-Figure 2). In addition, the background of SS silicone utilizing the recycled solvent method was similar to LDPE (SI-Figure 1B – green and black chromatograms, respectively). An additional benefit is that the optimal background is achieved without relying on post-extraction silica cleanup used in other silicone work (Booij et al., 2002; Allan et al., 2009; Monteyne et al., 2013), and the silicone extract can be easily integrated into zebrafish bioassays (manuscript in preparation).
The PRC infusion process resulted in excellent precision across the five different strips set aside to determine initial PRC concentrations, and were extracted over two different days. The average relative standard deviations (RSDs) of these samples were <13% for all four PRCs. The repeatability compares well with other published infusion RSDs of 10% or less (Booij et al., 2002). The efficiency of the infusion after modifying the methanol/water ratio was calculated by dividing the average amount in the silicone by the amount in the initial infusion solution. Infusion efficiencies of PRCs ranged from 20 ± 7 % (9,10-anthraquinone-d8) to 111 ± 11% (p,p′-DDE-d4), indicating that the infusion process was successful transferring most, if not all, of the compounds into silicone strips. Extraction efficiencies were not measured in this study, but assumed to be adequate and similar to the 96% extraction efficiency reported from our group using other silicone with the same methodology for PAHs (O’Connell et al., 2014).
Initial Field Comparison of Five Silicones
During field retrieval, all silicone polymers had minimal biofouling after a few seconds of physical agitation with ambient water (Figure 1C–G). In total, 25 PAHs were identified among all polymers (Figure 2). Polymers were first compared using PAHs due to analytical methods available at that time, and since PAHs are still contaminants of concern in Portland Harbor (Sower and Anderson, 2008). Concentrations were normalized for each silicone by mass so differences in volume between polymers would not confound comparisons. AA sequestered roughly 2 fold more ΣPAHs than other silicone polymers (Figure 2). However, both AA and CT silicone were heavily degraded during the extraction process, leaving behind silicone residue in both glassware and instrumentation. The leftover residue likely resulted in the very low PAH surrogate recoveries seen for both AA (2–11%) and CT (2–43%), which contrasts with the higher recoveries seen with ST (70–130%), SS (62–138%), and CS (73–130%). Despite the common use of AA as a silicone PSD (Rusina et al., 2007; Yates et al., 2007; Rusina et al., 2009; Smedes et al., 2009), the other types of silicone were substantially easier to extract, and resulted in better recoveries and precision of analytes (Figure 2). The siloxane background of AA and CT also interfered with full scan analyses. Therefore, only ST, SS, and CS were further evaluated for qualitative sensitivity of low Kow compounds which can be seen in the Supporting Information. Overall, 30 compounds were identified between polymers (Table S3), and LDPE did not sequester any compounds below a log Kow value of 4.9, which is similar to previous field data (Allan et al., 2010; Allan et al., 2013). Because sequestration was similar between the three silicones and advantages among them were not immediately apparent, ST, SS, and CS were deployed along with LDPE the following year.
Quantitative Surrogate Recoveries and Ideal Silicone Polymer Selection
Six different field sites were sampled using LDPE, SS, ST, and CS polymers to further assess and compare silicones to LDPE. Samples were analyzed using quantitative methods for PAHs, OPAHs, and pesticides (see Table S1). Laboratory surrogate recoveries after extraction varied widely among each method. For silicones, pesticide recoveries ranged from 13–113%, averaging 60%. PAH recoveries ranged from 35–185%, and averaged 94%. OPAH recoveries performed well, ranging from 72–140% with an average of 105%. Recoveries of surrogates in LDPE extractions were similar for pesticides (34–86%) and PAHs (30–98%), and like silicone, the best recoveries were for OPAHs, which ranged from 69–110% with an average of 88%. Lower recoveries (outside of ± 30% of the true value) were almost always associated with more volatile surrogates. For instance, most low recoveries for PAHs were for naphthalene-d8, and for pesticides, all low recoveries were attributed to tetrachloro-m-xylene. Additional variability may be due to the study size (> 70 samples) and multiple weeks of extractions. However, most recoveries were within 30% of the true value, and for comparison purposes, recoveries were similar for each method across polymers, so concentrations were assumed to be affected similarly across the values reported below.
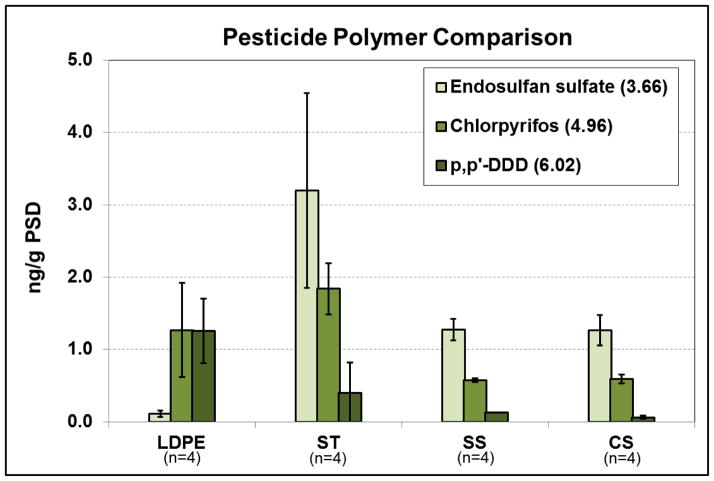
Concentrations of three pesticides identified in Portland Harbor predictably differed between LDPE and silicone based on log Kow, and were consistent with earlier AMDIS results. Specifically, Figure 3 illustrates all silicones having greater amounts sequestered (ng/g PSD) of endosulfan sulfate (log Kow 3.7) compared with LDPE. This is in contrast with p,p′-DDD, which was greater in LDPE and more hydrophobic (log Kow 6.0). Chlorpyrifos was more variable among the polymers (Figure 3), with a log Kow (4.96) value between that of the other compounds. One goal of this research was to develop samplers for co-deployment, and the pesticide and AMDIS data suggests that the methodology successfully exploited inherent differences in polymers initially reported in other work (Rusina et al., 2007; Allan et al., 2013). While ST had the highest amount of endosulfan sulfate sequestered in the polymer, it was more difficult to use in the field and laboratory due to a tendency to adhere to metal and glass surfaces when dry, and had higher variability within a complementary range of Kow sequestration (Figure 3). Therefore, SS silicone was chosen as the best silicone coupled with LDPE since it had the highest precision across all field testing and chemical compound classes. However, it is acknowledged that there is little difference between either silicone sponge material overall (SS or CS).
Figure 3.
Average concentrations of three pesticides found in Portland Harbor RM 14W, 2011. Concentrations are normalized per mass of each PSD to highlight sequestration differences. Numbers in parentheses after compound names represent log Kow values.
Final Polymer Comparisons for PAHs using Normalized and Water Calculated Data
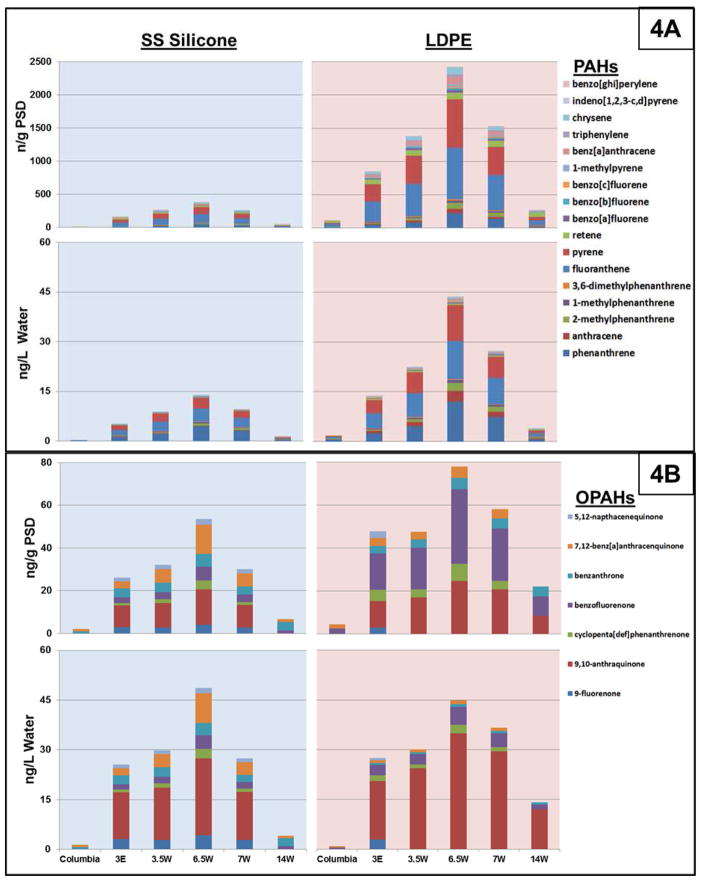
In the last comparison using SS silicone and LDPE, PAH data was evaluated to see if differences in absorption or extraction methodology would be reconciled after calculations to water concentrations. In Figure 4A, ΣPAH concentrations in the SS silicone polymer (ng/g) are about 7 fold lower than in LDPE. While acknowledging differences in solvents which could impact extraction efficiency, this was surprising considering previous evidence showing much higher concentrations of PAHs in silicone over LDPE (Allan et al., 2013). Regardless, overall differences were reconciled to average 3.5-fold or less (individual or ΣPAH) once both polymer extracts were calculated to water concentrations in ng/L (Figure 4A). Moreover, this nominal 3-fold difference is consistent with other PAH silicone and LDPE data from aqueous field deployments (Allan et al., 2013). LDPE is clearly the better polymer for PAHs using this methodology, as it sequesters PAHs at higher concentrations and likely has more accurate results than silicone given the polymer-specific partition coefficients for polyethylene. Although silicone partitioning coefficients were shown to vary little (on a log scale) in a multi-silicone comparison (Smedes et al., 2009), small differences in these estimates could explain the gap in quantitation for this work. Future work would be improved by empirically determining Ksws for SS silicone. Overall, both polymers consistently sequestered 17 PAHs of varied molecular size and physicochemistry (log Kow range: phenanthrene −4.56 to indeno(1,2,3-c,d)pyrene −6.58). The ratio of individual analytes was conserved between polymers, with phenanthrene, fluoranthene, and pyrene comprising the majority of ΣPAH amounts (56 to 84% in silicone, 67 to 81% in LDPE). Although LDPE sequesters more PAHs, both polymers in this field study would have resulted in similar descriptions of field sites if used solely for Portland Harbor characterization. Both polymers showed elevated levels of PAHs within the Superfund (sites RM 3E to 7W) as compared to outside the area (Columbia and RM 14W sites, Figure 4A). Results of replication are also similar (SI-Figure 3A), with RSDs averaging 7% for LDPE and 11% for SS silicone across field sites.
Figure 4.
Average (n=3) individual PAH and OPAH concentrations before (ng/g PSD) and after deriving freely dissolved water calculations (ng/L) for SS Silicone (blue background) and LDPE (light red background) for six Portland Harbor sites in 2011. 4A) PAH concentrations are consistently higher in LDPE before and after back-calculation although individual contributions are similar. 4B) OPAH concentrations are more similar than PAHs before and especially after back-calculation between polymers, although individual contributions are more disparate. Fluorenone and 5,12-napthacenequinone are below the reporting limit for LDPE at all sites except RM 3E.
Final Polymer Comparisons with Emerging Oxygenated-PAHs
OPAHs are an emerging concern in PAH contaminated areas (Lundstedt et al., 2007), and represent a good example of the physicochemistry range that might be sensitive to differences between silicone and LDPE (log Kow 0.2 – 5.3). The data in Figure 4B represents some of the first aqueous concentrations of OPAHs at a Superfund site using passive samplers, and includes evidence showing OPAHs having similar magnitudes to PAHs in Superfund waters (Figure 4). Comparisons between ΣPAH and ΣOPAHs have been shown to be similar among several other matrices (Layshock et al., 2010), and in a very recent publication, concentrations of 9-fluorenone and 9,10-anthraquinone were found to be higher than corresponding PAH homologues in waste, river, and effluent waters (Qiao et al., 2014). Overall, contrary to our original hypothesis that LDPE would not sequester OPAHs similarly to silicone, ΣOPAHs are similar with4 out of 6 sites not significantly differing between polymers (p ≥ 0.44 from t-tests, see SI-Figure 3B). In contrast to PAHs, the amount of OPAHs sequestered in each polymer is similar despite the log Kow range from 3.4 (9,10-anthraquinone) to 4.8 (5,12-naphthacenequinone) (Figure 4B). While it has long been demonstrated that Kow alone cannot account for uptake differences observed in model passive samplers (Huckins et al., 1999; Luellen and Shea, 2002; Huckins et al., 2004), the use of additional parameters to more accurately and precisely model uptake has been elusive. As an example, out of several physiochemical parameters (molecular weight, polar surface area, Van der Waals volume, C:H ratio), a regression using Kow to predict OPAH sequestration has a model coefficient (R2) of just 0.08, while one using a ratio of Van der Waals volume over the polar surface area is a slightly better predictor of partitioning (R2 = 0.20, see SI-Figure 4). In other work, larger molecular size was found to possibly contribute to differences in compound diffusivity between LDPE and silicone (Allan et al., 2013). Clearly, more work is needed to predict absorption between polymers, but future studies might benefit from using these or other physiochemical parameters.
Perhaps the most interesting OPAH results are differences observed between polymers for individual OPAH concentrations. Specifically, benzofluorenone and 7,12-benz[a]anthracenequinone (Figure 4B) differed dramatically between polymers by 50% and 109%, respectively. Differences persist even after calculating water concentrations, but determining specific partitioning coefficients might rectify some of these discrepancies. Like the ΣPAHs, the ΣOPAHs from either polymer suggest higher concentrations within the Superfund site than outside of it. However, individual contributions to these ΣOPAHs would indicate that 9,10-anthraquinone and benzofluorenone would be the primary components based on LDPE results (averaging 83% of the total), while no individual OPAH comprised more than 25% of ΣOPAHs in SS silicone. Four to five individual OPAHs are needed to achieve 83% or more of ΣOPAHs in the silicone at any site. In this respect, the original hypothesis is partially supported because silicone sequesters more individual OPAHs than LDPE. In fact, fluorenone and 5,12-napthacenequinone are below the reporting limit for LDPE at all sites except RM 3E. The difference between polymers is critical, because early evidence suggests that there are large differences between individual OPAH toxicities (Knecht et al., 2013). If remediation or toxicity concerns are important at a contaminated area, then methods that capture a large range of individual OPAHs will have additional value. Ultimately, both PAHs and OPAHs were quantitated using both polymers, but silicone appears more appropriate for OPAHs given the greater sensitivity for individual compounds (especially 9-fluorenone, benzanthrone, and 5,12-naphtacenequinone). This work advances methods for using silicone passive samplers alone or in conjunction with LDPE, provides information on analytical criteria for passive sampling choices, and provides valuable real world OPAH information for this emerging compound class.
Supplementary Material
HIGHLIGHTS.
Silicone passive sampler membranes are best cleaned with a combination of non-polar and semi-polar solvents
Silicone membranes sequester pesticides and other pollutants more consistently with lower log Kow values than LDPE
PAHs and OPAHs are present in similar concentrations at a Superfund site
More individual OPAHs are consistently sequestered with silicone than with LDPE passive samplers
Acknowledgments
This project was supported in part by award number P42 ES016465 and the associated Analytical Chemistry Facility Core, P30 ES000210 and R21 ES020120 from the National Institute of Environmental Health Sciences and the OSU Food Safety and Environmental Stewardship Program. Steven O’Connell was supported in part by NIEHS Training Grant Fellowship T32ES007060-32 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or the National Institutes of Health. The authors would like to thank the boat captain, Vaughn Tidwell, as well as Jorge Padilla and Ricky Scott for their help during laboratory processing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Steven G. O’Connell, Email: oconnels@onid.oregonstate.edu.
Melissa A. McCartney, Email: melissa.mccartney@oregonstate.edu.
L. Blair Paulik, Email: paulikl@onid.oregonstate.edu.
Sarah E. Allan, Email: sarah.allan@noaa.gov.
Lane G. Tidwell, Email: tidwelll@onid.oregonstate.edu.
Glenn Wilson, Email: glenn.wilson@oregonstate.edu.
References
- Allan I, Booij K, Paschke A, Vrana B, Mills G, Greenwood R. Field performance of seven passive sampling devices for monitoring of hydrophobic substances. Environ Sci Technol. 2009;43:5383–5390. doi: 10.1021/es900608w. [DOI] [PubMed] [Google Scholar]
- Allan IJ, Harman C, Kringstad A, Bratsberg E. Effect of sampler material on the uptake of PAHs into passive sampling devices. Chemosphere. 2010;79:470–475. doi: 10.1016/j.chemosphere.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Allan IJ, Harman C, Ranneklev SB, Thomas KV, Grung M. Passive sampling for target and non-target analyses of moderately polar and nonpolar substances in water. Environ Toxicol Chem. 2013;32:1718–1726. doi: 10.1002/etc.2260. [DOI] [PubMed] [Google Scholar]
- Allan SE, Smith BW, Anderson KA. Impact of the Deepwater Horizon Oil Spill on Bioavailable Polycyclic Aromatic Hydrocarbons in Gulf of Mexico Coastal Waters. Environ Sci Technol. 2012;46:2033–2039. doi: 10.1021/es202942q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KA, Hillwalker WE. Bioavailability. In: Jorgensen SE, Fath BD, editors. Encyclopedia of Ecology. Elsevier; Oxford: 2008. pp. 348–357. [Google Scholar]
- Anderson KA, Seck D, Hobbie KA, Traore AN, McCartney MA, Ndaye A, Forsberg ND, Haigh TA, Sower GJ. Passive sampling devices enable capacity building and characterization of bioavailable pesticide along the Niger, Senegal and Bani Rivers of Africa. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369 doi: 10.1098/rstb.2013.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KA, Sethajintanin D, Sower G, Quarles L. Field Trial and Modeling of Uptake Rates of In Situ Lipid-Free Polyethylene Membrane Passive Sampler. Environ Sci Technol. 2008;42:4486–4493. doi: 10.1021/es702657n. [DOI] [PubMed] [Google Scholar]
- Booij K, Hofmans HE, Fischer CV, Van Weerlee EM. Temperature-dependent uptake rates of nonpolar organic compounds by semipermeable membrane devices and low-density polyethylene membranes. Environ Sci Technol. 2003;37:361–366. doi: 10.1021/es025739i. [DOI] [PubMed] [Google Scholar]
- Booij K, Sleiderink HM, Smedes F. Calibrating the uptake kinetics of semipermeable membrane devices using exposure standards. Environ Toxicol Chem. 1998;17:1236–1245. [Google Scholar]
- Booij K, Smedes F, van Weerlee EM. Spiking of performance reference compounds in low density polyethylene and silicone passive water samplers. Chemosphere. 2002;46:1157–1161. doi: 10.1016/s0045-6535(01)00200-4. [DOI] [PubMed] [Google Scholar]
- Choi Y, Cho YM, Luthy RG. Polyethylene-Water Partitioning Coefficients for Parent- and Alkylated-Polycyclic Aromatic Hydrocarbons and Polychlorinated Biphenyls. Environ Sci Technol. 2013;47:6943–6950. doi: 10.1021/es304566v. [DOI] [PubMed] [Google Scholar]
- Harman C, Allan IJ, Vermeirssen ELM. Calibration and use of the polar organic chemical integrative sampler-a critical review. Environ Toxicol Chem. 2012;31:2724–2738. doi: 10.1002/etc.2011. [DOI] [PubMed] [Google Scholar]
- Huckins JN, Petty JD, Booij K. Monitors of Organic Chemicals in the Environment - Semipermeable Membrane Devices. Springer; New York: 2006. [Google Scholar]
- Huckins JN, Petty JD, Lebo JA, Almeida FV, Booij K, Alvarez DA, Clark RC, Mogensen BB. Development of the permeability/performance reference compound approach for in situ calibration of semipermeable membrane devices. Environ Sci Technol. 2002;36:85–91. doi: 10.1021/es010991w. [DOI] [PubMed] [Google Scholar]
- Huckins JN, Petty JD, Orazio CE, Lebo JA, Clark RC, Gibson VL, Gala WR, Echols KR. Determination of uptake kinetics (Sampling rates) by lipid-containing semipermeable membrane devices (SPMDs) for polycyclic aromatic hydrocarbons (PAHs) in water. Environ Sci Technol. 1999;33:3918–3923. [Google Scholar]
- Huckins JN, Prest HF, Petty JD, Lebo JA, Hodgins MM, Clark RC, Alvarez DA, Gala WR, Steen A, Gale R, Ingersoll CG. Overview and comparison of lipid-containing semipermeable membrane devices and oysters (Crassostrea gigas) for assessing organic chemical exposure. Environ Toxicol Chem. 2004;23:1617–1628. doi: 10.1897/03-366. [DOI] [PubMed] [Google Scholar]
- Knecht AL, Goodale BC, Truong L, Simonich MT, Swanson AJ, Matzke MM, Anderson KA, Waters KM, Tanguay RL. Comparative developmental toxicity of environmentally relevant oxygenated PAHs. Toxicol Appl Pharmacol. 2013;271:266–275. doi: 10.1016/j.taap.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layshock JA, Wilson G, Anderson KA. Ketone and Quinone-Substitued Polycyclic Aromatic Hydrocarbons in Mussel Tissue, Sediment, Urban Dust, and Diesel Particulate Matrices. Environ Toxicol Chem. 2010;29:2450–2460. doi: 10.1002/etc.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luellen DR, Shea D. Calibration and field verification of semipermeable membrane devices for measuring polycyclic aromatic hydrocarbons in water. Environ Sci Technol. 2002;36:1791–1797. doi: 10.1021/es0113504. [DOI] [PubMed] [Google Scholar]
- Lundstedt S, White PA, Lemieux CL, Lynes KD, Lambert LB, Oberg L, Haglund P, Tysklind M. Sources, fate, and toxic hazards of oxygenated polycyclic aromatic hydrocarbons (PAHs) at PAH-contaminated sites. Ambio. 2007;36:475–485. doi: 10.1579/0044-7447(2007)36[475:sfatho]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Monteyne E, Roose P, Janssen CR. Application of a silicone rubber passive sampling technique for monitoring PAHs and PCBs at three Belgian coastal harbours. Chemosphere. 2013;91:390–398. doi: 10.1016/j.chemosphere.2012.11.074. [DOI] [PubMed] [Google Scholar]
- Namieśnik J, Zabiegała B, Kot-Wasik A, Partyka M, Wasik A. Passive sampling and/or extraction techniques in environmental analysis: a review. Anal Bioanal Chem. 2005;381:279–301. doi: 10.1007/s00216-004-2830-8. [DOI] [PubMed] [Google Scholar]
- O’Connell SG, Haigh T, Wilson G, Anderson KA. An Analytical Investigation of 24 Oxygenated-PAHs (OPAHs) using Liquid and Gas Chromatography-Mass Spectroscopy. Anal Bioanal Chem. 2013;405:8885–8896. doi: 10.1007/s00216-013-7319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell SG, Kincl LD, Anderson KA. Silicone Wristbands as Personal Passive Samplers. Environ Sci Technol. 2014;48:3327–3335. doi: 10.1021/es405022f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty JD, Huckins JN, Alvarez DA, Brumbaugh WG, Cranor WL, Gale RW, Rastall AC, Jones-Lepp TL, Leiker TJ, Rostad CE, Furlong ET. A holistic passive integrative sampling approach for assessing the presence and potential impacts of waterborne environmental contaminants. Chemosphere. 2004;54:695–705. doi: 10.1016/j.chemosphere.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Qiao M, Qi W, Liu H, Qu J. Oxygenated, nitrated, methyl and parent polycyclic aromatic hydrocarbons in rivers of Haihe River System, China: Occurrence, possible formation, and source and fate in a water-shortage area. Sci Total Environ. 2014;481:178–185. doi: 10.1016/j.scitotenv.2014.02.050. [DOI] [PubMed] [Google Scholar]
- Rusina TP, Smedes F, Klanova J, Booij K, Holoubek I. Polymer selection for passive sampling: A comparison of critical properties. Chemosphere. 2007;68:1344–1351. doi: 10.1016/j.chemosphere.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Rusina TP, Smedes F, Koblizkova M, Klanova J. Calibration of Silicone Rubber Passive Samplers: Experimental and Modeled Relations between Sampling Rate and Compound Properties. Environ Sci Technol. 2009 doi: 10.1021/es900938r. [DOI] [PubMed] [Google Scholar]
- Schafer RB, Hearn L, Kefford BJ, Mueller JF, Nugegoda D. Using silicone passive samplers to detect polycyclic aromatic hydrocarbons from wildfires in streams and potential acute effects for invertebrate communities. Water Res. 2010;44:4590–4600. doi: 10.1016/j.watres.2010.05.044. [DOI] [PubMed] [Google Scholar]
- Seethapathy S, Gorecki T. Applications of polydimethylsiloxane in analytical chemistry: A review. Anal Chim Acta. 2012;750:48–62. doi: 10.1016/j.aca.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Sethajintanin D, Anderson KA. Temporal Bioavailability of Organochlorine Pesticides and PCBs. Environ Sci Technol. 2006;40:3689–3695. doi: 10.1021/es052427h. [DOI] [PubMed] [Google Scholar]
- Smedes F, Geertsma RW, van der Zande T, Booij K. Polymer-Water Partition Coefficients of Hydrophobic Compounds for Passive Sampling: Application of Cosolvent Models for Validation. Environ Sci Technol. 2009;43:7047–7054. doi: 10.1021/es9009376. [DOI] [PubMed] [Google Scholar]
- Sower GJ, Anderson KA. Spatial and Temporal Variation of Freely Dissolved Polycyclic Aromatic Hydrocarbons in an Urban River Undergoing Superfund Remediation. Environ Sci Technol. 2008;42:9065–9071. doi: 10.1021/es801286z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA; Lower Willamete Group, editor. Portland Harbor Final Remedial Investigation Report - Appendix F: Baseline Human Health Risk Assessment. Kennedy Jenks Consultants; Portland, OR: 2013. p. 141. [Google Scholar]
- Vrana B, Allan IJ, Greenwood R, Mills GA, Dominiak E, Svensson K, Knutsson J, Morrison G. Passive sampling techniques for monitoring pollutants in water. TrAC Trends in Analytical Chemistry. 2005;24:845–868. [Google Scholar]
- Yates K, Davies I, Webster L, Pollard P, Lawton L, Moffat C. Passive sampling: partition coefficients for a silicone rubber reference phase. J Environ Monit. 2007;9:1116–1121. doi: 10.1039/b706716j. [DOI] [PubMed] [Google Scholar]
- Zabiegala B, Kot-Wasik A, Urbanowicz M, Namiesnik J. Passive sampling as a tool for obtaining reliable analytical information in environmental quality monitoring. Anal Bioanal Chem. 2010;396:273–296. doi: 10.1007/s00216-009-3244-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.