Abstract
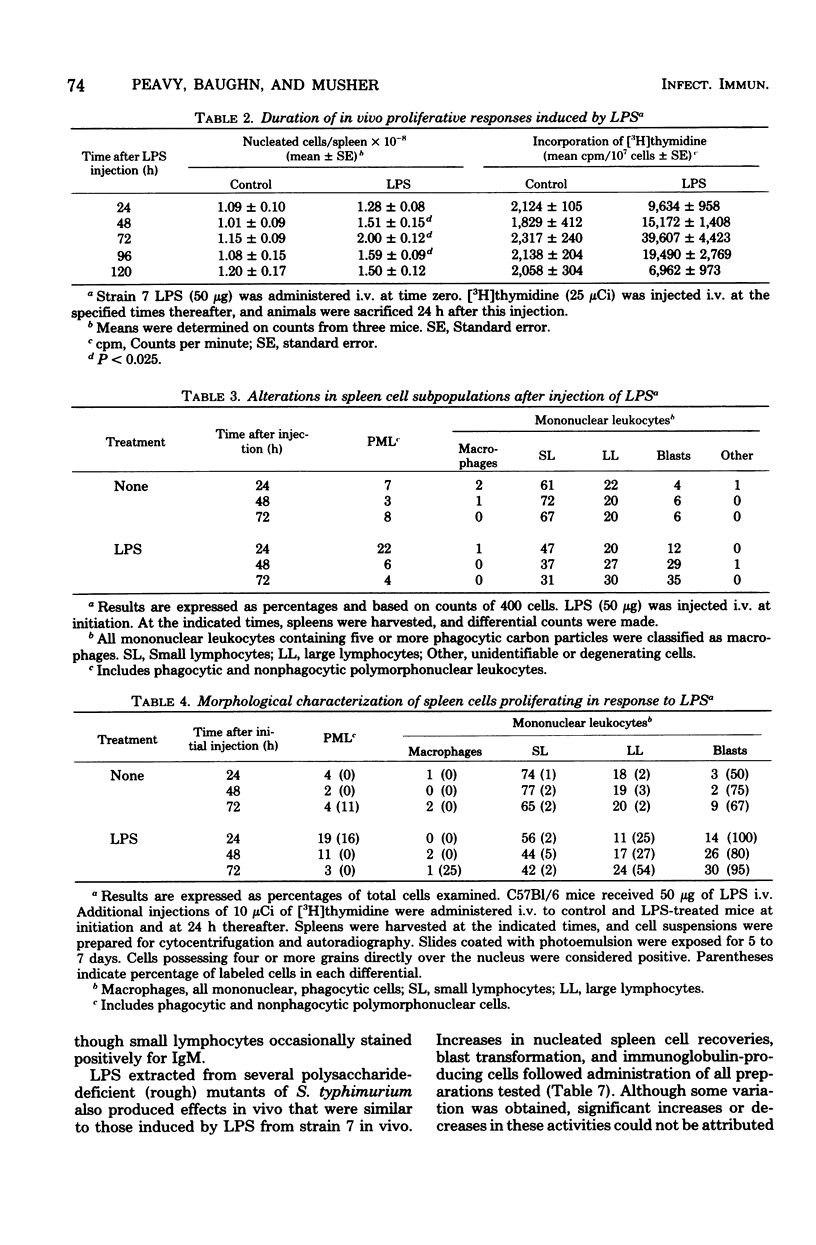
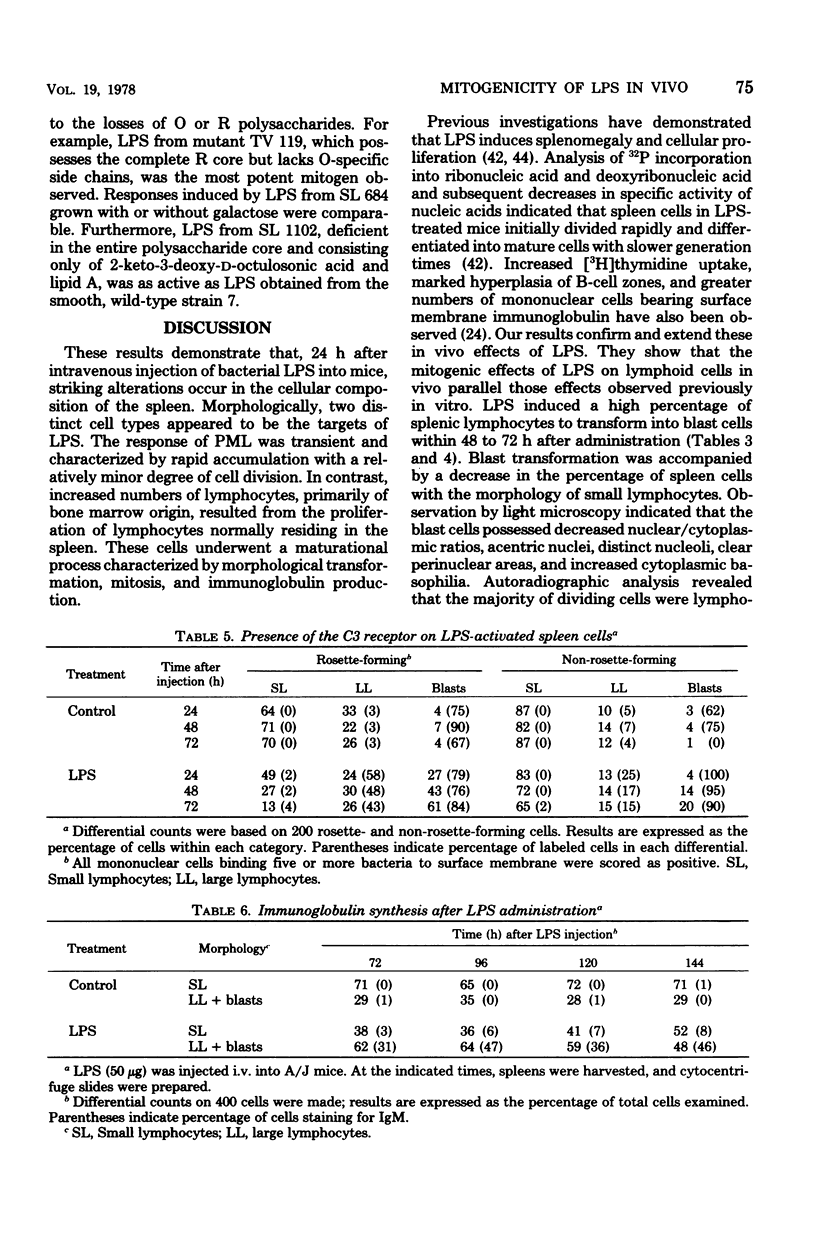
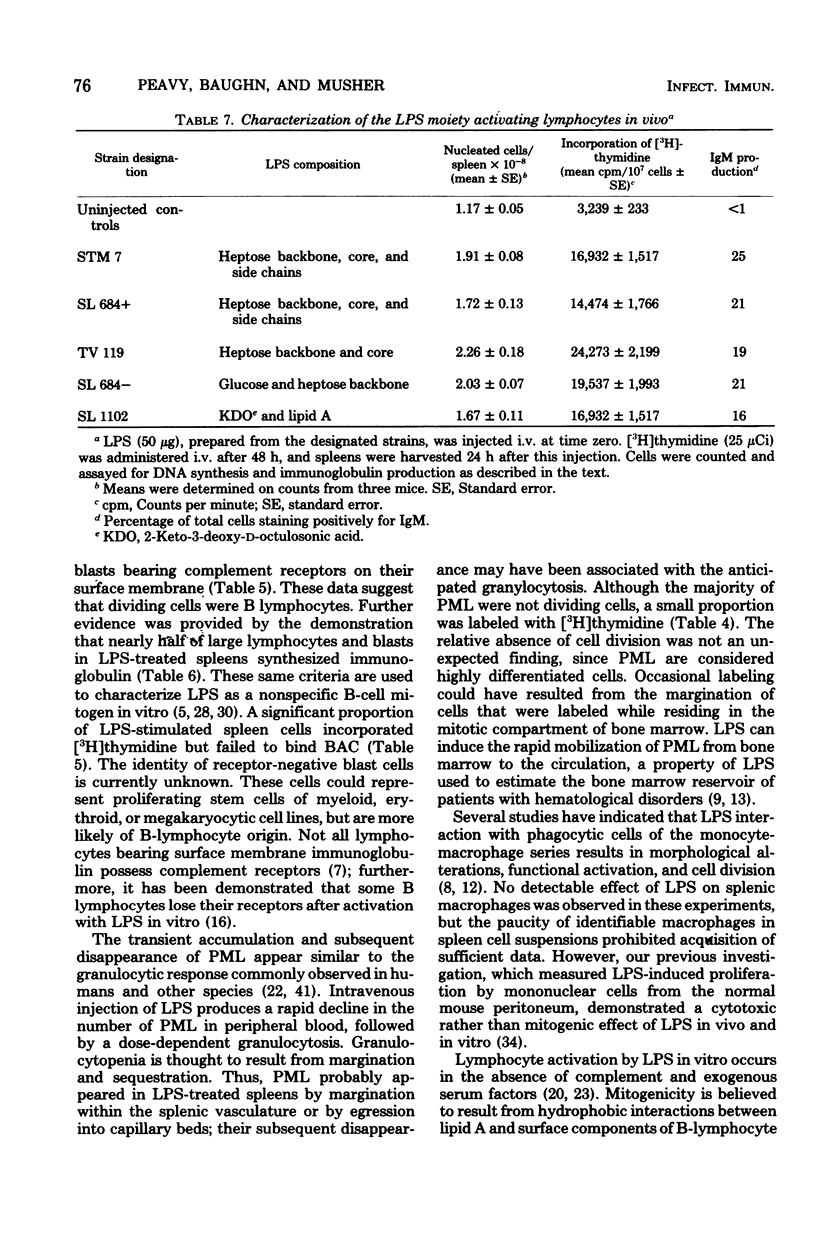
The in vivo effect of bacterial lipopolysaccharides (LPS) on mouse spleen cell subpopulations was investigated. Intravenous administration of LPS resulted in marked enlargement of the spleen, accompanied by increased cellular proliferation and enhanced nucleated cell recoveries. At least two morphologically distinct cell types appeared to be targets for LPS. Polymorphonuclear leukocytes accumulated rapidly with a relatively minor degree of cell division. In contrast, a substantial proportion of splenic lymphocytes transformed into large lymphocytes and blast cells which actively incorporated [3H]thymidine. Proliferating cells were identified as bone marrow-derived (B) lymphocytes by their ability to form C3-dependent rosettes and to synthesize immunoglobulin. These cellular responses were not antigenically induced, since LPS derived from mutants lacking the polysaccharide moiety gave similar results. Thus, splenic B lymphocytes appear to interact and respond to LPS in vivo in the same manner as observed in vitro. These data suggest that the capacity of LPS to directly activate B lymphocytes, initiate cellular proliferation, and induce immunoglobulin production by bone marrow-derived cells in vivo may contribute to its adjuvant activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler W. H., Takiguchi T., Marsh B., Smith R. T. Cellular recognition by mouse lymphocytes in vitro. I. Definition of a new technique and results of stimulation by phytohemagglutinin and specific antigens. J Exp Med. 1970 Jun 1;131(6):1049–1078. doi: 10.1084/jem.131.6.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. C., Davies A. J. Requirement of thymus-dependent lymphocytes for potentiation by adjuvants of antibody formation. Nature. 1971 Oct 1;233(5318):330–332. doi: 10.1038/233330a0. [DOI] [PubMed] [Google Scholar]
- Andersson J., Melchers F., Galanos C., Lüderitz O. The mitogenic effect of lipopolysaccharide on bone marrow-derived mouse lymphocytes. Lipid A as the mitogenic part of the molecule. J Exp Med. 1973 Apr 1;137(4):943–953. doi: 10.1084/jem.137.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Möller G., Sjöberg O. Selective induction of DNA synthesis in T and B lymphocytes. Cell Immunol. 1972 Aug;4(4):381–393. doi: 10.1016/0008-8749(72)90040-8. [DOI] [PubMed] [Google Scholar]
- Andersson J., Sjöberg O., Möller G. Induction of immunoglobulin and antibody synthesis in vitro by lipopolysaccharides. Eur J Immunol. 1972 Aug;2(4):349–353. doi: 10.1002/eji.1830020410. [DOI] [PubMed] [Google Scholar]
- André-Schwartz J., Rubenstein H. S., Coons A. H. Electron microscopy of cellular responses following immunization with endotoxin. Am J Pathol. 1968 Sep;53(3):331–353. [PMC free article] [PubMed] [Google Scholar]
- BIOZZI G., BENACERRAF B., HALPERN B. N. The effect of Salm. typhi and its endotoxin on the phagocytic activity of the reticuloendothelial system in mice. Br J Exp Pathol. 1955 Jun;36(3):226–235. [PMC free article] [PubMed] [Google Scholar]
- BRAUDE A. I., CAREY F. J., ZALESKY M. Studies with radioactive endotoxin. II. Correlation of physiologic effects with distribution of radioactivity in rabbits injected with radioactive sodium chromate. J Clin Invest. 1955 Jun;34(6):858–866. doi: 10.1172/JCI103141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs D. R., Marsh J. C., Chervenick P. A., Cartwright G. E., Wintrobe M. M. Neutrophil releasing activity in plasma of normal human subjects injected with endotoxin. Proc Soc Exp Biol Med. 1968 Mar;127(3):689–693. doi: 10.3181/00379727-127-32774. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRADDOCK C. G., Jr, PERRY S., VENTZKE L. E., LAWRENCE J. S. Evaluation of marrow granulocytic reserves in normal and disease states. Blood. 1960 Jun;15:840–855. [PubMed] [Google Scholar]
- Chiller J. M., Skidmore B. J., Morrison D. C., Weigle W. O. Relationship of the structure of bacterial lipopolysaccharides to its function in mitogenesis and adjuvanticity. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2129–2133. doi: 10.1073/pnas.70.7.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORBES I. J. INDUCTION OF MITOSIS IN MACROPHAGES BY ENDOTOXIN. J Immunol. 1965 Jan;94:37–39. [PubMed] [Google Scholar]
- Gery I., Krüger J., Spiesel S. Z. Stimulation of B-lymphocytes by endotoxin. Reactions of thymus-deprived mice and karyotypic analysis of dividing cells in mice bearing T 6 T 6 thymus grafts. J Immunol. 1972 Apr;108(4):1088–1091. [PubMed] [Google Scholar]
- Gormus B. J., Crandall R. B., Shands J. W., Jr Endotoxin-stimulated spleen cells: mitogenesis, the occurrence of the C3 receptor, and the production of immunoglobulin. J Immunol. 1974 Feb;112(2):770–775. [PubMed] [Google Scholar]
- HERRING W. B., HERION J. C., WALKER R. I., PALMER J. G. Distribution and clearance of circulating endotoxin. J Clin Invest. 1963 Jan;42:79–87. doi: 10.1172/JCI104698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaoka T., Katz D. H. Cellular site of action of various adjuvants in antibody responses to hapten-carrier conjugates. J Immunol. 1973 Nov;111(5):1554–1563. [PubMed] [Google Scholar]
- Henry C., Jerne N. K. Competition of 19S and 7S antigen receptors in the regulation of the primary immune response. J Exp Med. 1968 Jul 1;128(1):133–152. doi: 10.1084/jem.128.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Humphrey J. H., Pepys M. B., Greaves M. F. Complement independence of stimulation of mouse splenic B lymphocytes by mitogens. Nat New Biol. 1973 Sep 26;245(143):108–112. doi: 10.1038/newbio245108a0. [DOI] [PubMed] [Google Scholar]
- MECHANIC R. C., FREI E., 3rd, LANDY M., SMITH W. W. Quantitative studies of human leukocytic and febrile response to single and repeated doses of purified bacterial endotoxin. J Clin Invest. 1962 Jan;41:162–172. doi: 10.1172/JCI104459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLLER G., WIGZELL H. ANTIBODY SYNTHESIS AT THE CELLULAR LEVEL. ANTIBODY-INDUCED SUPPRESSION OF 19S AND 7S ANTIBODY RESPONSE. J Exp Med. 1965 Jun 1;121:969–989. doi: 10.1084/jem.121.6.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers F., Andersson J. Synthesis, surface deposition and secretion of immunoglobulin M in bone marrow-derived lymphocytes before and after mitogenic stimulation. Transplant Rev. 1973;14:76–130. doi: 10.1111/j.1600-065x.1973.tb00103.x. [DOI] [PubMed] [Google Scholar]
- Moatamed F., Karnovsky M. J., Unanue E. R. Early cellular responses to mitogens and adjuvants in the mouse spleen. Lab Invest. 1975 Mar;32(3):303–312. [PubMed] [Google Scholar]
- NOSSAL G. J., ADA G. L., AUSTIN C. M. ANTIGENS IN IMMUNITY. IV. CELLULAR LOCALIZATION OF 125-I- AND 131-I-LABELLED FLAGELLA IN LYMPH NODES. Aust J Exp Biol Med Sci. 1964 Jun;42:311–330. [PubMed] [Google Scholar]
- Peavy D. L., Adler W. H., Shands J. W., Smith R. T. Selective effects of mitogens on subpopulations of mouse lymphoid cells. Cell Immunol. 1974 Mar 30;11(1-3):86–98. doi: 10.1016/0008-8749(74)90009-4. [DOI] [PubMed] [Google Scholar]
- Peavy D. L., Adler W. H., Smith R. T. The mitogenic effects of endotoxin and staphylococcal enterotoxin B on mouse spleen cells and human peripheral lymphocytes. J Immunol. 1970 Dec;105(6):1453–1458. [PubMed] [Google Scholar]
- Peavy D. L., Shands J. W., Jr, Adler W. H., Smith R. T. Mitogenicity of bacterial endotoxins: characterization of the mitogenic principle. J Immunol. 1973 Aug;111(2):352–357. [PubMed] [Google Scholar]
- Pierce C. W. Immune responses in vitro. I. Cellular requirements for the immune response by nonprimed and primed spleen cells in vitro. J Exp Med. 1969 Aug 1;130(2):345–364. doi: 10.1084/jem.130.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D., TURNER K. J. INCREASE IN MACROGLOBULIN ANTIBODIES OF MOUSE AND PIG FOLLOWING INJECTION OF BACTERIAL LIPOPOLYSACCHARIDE. Immunology. 1964 Jul;7:394–402. [PMC free article] [PubMed] [Google Scholar]
- Rutenburg S., Skarnes R., Palmerio C., Fine J. Detoxification of endotoxin by perfusion of liver and spleen. Proc Soc Exp Biol Med. 1967 Jun;125(2):455–459. doi: 10.3181/00379727-125-32118. [DOI] [PubMed] [Google Scholar]
- SMITH W. W., ALDERMAN I. M., GILLESPIE R. E. Increased survival in irradiated animals treated with bacterial endotoxins. Am J Physiol. 1957 Oct;191(1):124–130. doi: 10.1152/ajplegacy.1957.191.1.124. [DOI] [PubMed] [Google Scholar]
- Shands J. W., Jr, Peavy D. L., Gormus B. J., McGraw J. In vitro and in vivo effects of endotoxin on mouse peritoneal cells. Infect Immun. 1974 Jan;9(1):106–112. doi: 10.1128/iai.9.1.106-112.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shands J. W., Jr, Peavy D. L., Smith R. T. Differential morphology of mouse spleen cells stimulated in vitro by endotoxin, phytohemagglutinin, pokeweed mitogen and staphylococcal enterotoxin B. Am J Pathol. 1973 Jan;70(1):1–24. [PMC free article] [PubMed] [Google Scholar]
- Skidmore B. J., Chiller J. M., Weigle W. O., Riblet R., Watson J. Immunologic properties of bacterial lipopolysaccharide (LPS). III. Genetic linkage between the in vitro mitogenic and in vivo adjuvant properties of LPS. J Exp Med. 1976 Jan 1;143(1):143–150. doi: 10.1084/jem.143.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. W., Brecher G., Fred S., Budd R. A. Effect of endotoxin on the kinetics of hemopoietic colony-forming cells in irradiated mice. Radiat Res. 1966 Apr;27(4):710–717. [PubMed] [Google Scholar]
- Spitznagel J. K., Allison A. C. Mode of action of adjuvants: effects on antibody responses to macrophage-associated bovine serum albumin. J Immunol. 1970 Jan;104(1):128–139. [PubMed] [Google Scholar]
- Sultzer B. M. Genetic control of leucocyte responses to endotoxin. Nature. 1968 Sep 21;219(5160):1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- THORBECKE G. J., ASOFSKY R. M., HOCHWALD G. M., SISKIND G. W. Gamma globulin and antibody formation in vitro. III. Induction of secondary response at different intervals after the primary; the role of secondary nodules in the preparation for the secondary response. J Exp Med. 1962 Sep 1;116:295–310. doi: 10.1084/jem.116.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Mizuno D. Dynamic state of the spleen cells of mice after administration of the endotoxin of Proteus vulgaris. I. Cellular proliferation after administration of the endotoxin. Jpn J Exp Med. 1968 Jun;38(3):171–183. [PubMed] [Google Scholar]
- Twentyman P. R. The effects of repeated doses of bacterial endotoxin on erythropoiesis in the normal and splenectomized mouse. Br J Haematol. 1972 Feb;22(2):169–177. doi: 10.1111/j.1365-2141.1972.tb08798.x. [DOI] [PubMed] [Google Scholar]
- WARD P. A., JOHNSON A. G., ABELL M. R. Studies on the adjuvant action of bacterial endotoxins on antibody formation. III. Histologic response of the rabbit spleen to a single injection of a purified protein antigen. J Exp Med. 1959 May 1;109(5):463–474. doi: 10.1084/jem.109.5.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. I. Evidence for a single gene that influences mitogenic and immunogenic respones to lipopolysaccharides. J Exp Med. 1974 Nov 1;140(5):1147–1161. doi: 10.1084/jem.140.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Trenkner E., Cohn M. The use of bacterial lipopolysaccharides to show that two signals are required for the induction of antibody synthesis. J Exp Med. 1973 Sep 1;138(3):699–714. doi: 10.1084/jem.138.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]


