Abstract
Health-related quality of life (HRQoL) is a multidimensional concept that includes subjective reports of symptoms, side effects, functioning in multiple life domains, and general perceptions of life satisfaction and quality. Rather than estimating it from external observations, interview, or clinical assessment, it is best measured by direct query. Due to a perception that respondents may not be reliable or credible, there has been some reluctance to use self-report outcomes in psychiatry. More recently, and increasingly, HRQoL assessment through direct patient query has become common when evaluating a range of psychiatric, psychological, and social therapies. With few exceptions, psychiatric patients are credible and reliable reporters of this information. This article summarizes studies that highlight the development, validation, and application of HRQoL measures in psychiatry. Thoughtful application of these tools in psychiatric research can provide a much-needed patient perspective in the future of comparative effectiveness research, patient-centered outcomes research, and clinical care.
Keywords: health-related quality of life, quality of life, psychiatric outcome, psychiatric symptom, patient-reported outcome
Abstract
La calidad de vida relacionada con la salud (CdVRS) es un concepto multidimensional que incluye la percepcíon subjetiva de los síntomas, los efectos secundarios, el funcíonamiento en múltiples aspectos de la vida y la percepción general de satisfacción y calidad de vida. Más que estimarla a partir de observaciones externas, la entrevista o la evaluación clínica, es mejor cuantificarla mediante preguntas directas. Ya que se ha considerado que los encuestados pueden no ser confiables o creíbles, en psiquiatría ha habido cierta reticencia a los resultados de auto-reporte. Más recientemente y en forma crecíente la determinación de la CdVRS mediante preguntas directas al paciente se ha hecho común en la evaluación de una serie de terapias psiquiátricas, psicológicas y sociales. Los pacientes psiquiátricos excepcionalmente son creíbles y confiables al entregar esta información. Este artículo resume estudios psiquiátricos que destacan el desarrollo, validación y aplicación de las mediciones de la CdVRS. La aplicación cuidadosa de estas herramientas en la investigación psiquiátrica puede proporcionar una perspectiva muy necesaria del paciente para el futuro de la investigación sobre comparación de eficacia, de la investigación acerca de los resultados centrados en el paciente y de los cuidados clínicos.
Abstract
La qualité de vie liée à la santé (QdVLS), concept multi-dimensionnel, prend en compte des éléments subjectifs des symptômes, des effets secondaires, du fonctionnement dans différents domaines de la vie et de la perception générale de la satisfaction et de la qualité de vie. Son estimation par questions directes est préférable à celle d'observations externes, d'entretien ou d'évaluation clinique. En psychiatrie, l'utilisation de résultats auto déclarés a fait l'objet d'une certaine réticence en raison du sentiment d'un manque de fiabilité ou de crédibilité des personnes interrogées. L'évaluation de la QdVLS par interrogation directe du patient est de plus en plus utilisée ces derniers temps, pour évaluer une série de thérapies psychiatriques, psychologiques et sociales. À de rares exceptions près, les patients psychiatriques sont crédibles et fiables dans leurs réponses à ces évaluations. Cet article résume des études qui soulignent le développement, la validation et la mise en œuvre des mesures de la QdVLS en psychiatrie. Dans l'avenir, l'emploi judicieux de ces outils en recherche psychiatrique nous donnera le point de vue essentiel du patient en ce qui concerne la recherche d'efficacité comparative, la recherche des résultats centrés sur le patient et les soins cliniques.
Introduction
This selective review focuses on the use of patient-reported outcomes in major mental disorders, including schizophrenia, bipolar disorder, major depressive disorder, and generalized anxiety disorder. In psychiatry, key outcomes of interest include patient symptoms, functional status, and medication side effects. Traditionally, these outcomes have been estimated by health care providers (including psychiatrists, psychologists, and case workers) or by family members or other proxies, and not the patient directly.
Treatment effects upon the presence or severity of symptoms can only be truly known by the patient, for example the presence of auditory or visual hallucinations or paranoid ideation.1-3 The majority of psychiatric symptoms are not overtly visible, so the best reporter of them should ideally be the patient. A prevailing perspective is that psychiatric patients are not reliable symptom reporters. However, psychiatric patients are indeed able to report on symptoms that are not readily apparent to even the most skilled observers. Patients are also able to report on and evaluate the personal meaningfulness of treatment effects, such as how a medication makes them feel. Psychiatric therapies often reduce psychotic or mood symptoms but may induce other symptoms such as lethargy or fatigue, best reported by the patient. Although there are issues with psychiatric patients as reporters, the use of psychiatric patients to self-report does reduce any issues of inter-rater reliability that might occur if observer raters change between visits.1 In many respects, the trend toward increasing use of patient self-reports in psychiatry parallels changes in the approach to psychiatric treatments themselves, beginning with deinstitutionalization occurring in the United States in the 1960s and 1970s and moving into increased patient autonomy.
There have been efforts to estimate the quality of life of people receiving treatment for psychiatric disorders, by using expert clinical raters. For example, the Quality of Life Scale (QLS) has been used widely in clinical trials in schizophrenia.4-8 The QLS was developed to assess the deficit syndrome in patients with schizophrenia.9 The QLS includes items covering work and occupational behavior, commonplace activities and objects, interpersonal relationships, and emotional and psychological functioning (ie, anhedonia, motivation, sense of purpose). The QLS is administered by a trained clinician as part of a semistructured interview. With proper training, inter-rater reliability has been excellent (0.84-0.97) and test-retest reliability has been more variable (range=0.50-0.90). However, by design, this approach fails to capture the direct reporting of patient experience.
During the last three decades there has been increasing attention in psychiatry given to measurement approaches that include the patient perspective.2,3-10 With the onset of deinstitutionalization in the 1960s, more attention began to be paid to psychiatric patients' personal perspective of their lives, especially as more community-based treatment programs were implemented. Community treatment programs frequently do much more than deliver medications and/or psychotherapy. These programs focus on maintaining the patient in the community and address many basic needs such as a stable residence, ability to interact with others, personal and job skills, and a steady income. Along with this new approach to treatment came a need for novel approaches to evaluation that moved beyond clinician assessment of symptoms. Many of the newer approaches to evaluation have fallen under the concept broadly known as health-related quality of life (HRQoL).2
Definition of HRQoL
Health-related quality of life (HRQoL) is a fairly broad, multidimensional concept that includes symptoms of disease or health condition, treatment side effects, and functional status across physical, social, and mental health life domains. In addition to its multidimensional nature, a core component of the definition is the emphasis on the subjective, patient perspective as paramount. HRQoL outcomes are currently a large subset of an even broader set of outcomes that have come to be referred to as patient-reported outcome (PRO) measures. PROs are defined as any report of the status of a patient's health condition or other clinical outcome that comes directly from the patient without interpretation from a clinician or anyone else.11 HRQoL measures provide a useful adjunct in psychiatry as a method of assessing patient's perceptions of symptoms, treatment side effects, and other dimensions such as physical, social, and mental functioning and well-being. Patients are typically considered the most valid reporters of their own symptoms, although as discussed below, the reliability of the psychiatric patient as a respondent can be a concern.
Definitions of HRQoL used in psychiatry
HRQoL measures represent a common type of PRO used in psychiatric research.2,3 There are numerous approaches to conceptualizing and measuring HRQoL in psychiatry. HRQoL measures used in psychiatry differ somewhat from traditional measures used in physical illnesses. In its broadest sense, HRQoL is a multi-dimensional construct defined as the subjective assessment of the impact of disease and treatment on domains of physical, psychological, and social functioning. As with approaches used in evaluating HRQoL in physical illness, models of HRQoL in psychiatry may include physical, psychological, and social functioning. However, they also often add occupational function, living situation, degree of independence, life satisfaction, treatment adherence, and treatment tolerability. Some psychiatric HRQoL developers adhere to the formal definition of HRQoL, including its subjectivity; for example the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q)12 was developed to measure the degree of enjoyment and satisfaction experienced by patients in various areas of daily function. In contrast, the Lehman Quality of Life Interview13 includes both “objective” and “subjective” sections for each dimension, although the reporter remains the respondent. Awad and Vorungati2 recommend that the more objective dimensions of psychiatric HRQoL measures be considered more of a quality of living construct.
Along with variable definitions of HRQoL in psychiatry, there is a also a lack of conceptual models often resulting in overlap across measures purporting to measure different constructs.2,3 In Awad and Vorunganti's2 article focusing on quality of life in patients with schizophrenia, they note that there are many competing conceptual frameworks of HRQoL, but very few instruments developed on the basis of those conceptual frameworks.
Considerations when using HRQoL measures in psychiatry
Several issues are associated with the assessment of HRQoL in patients with psychiatric disorders, including: (i) reliability of the respondent; (ii) conceptual models guiding instrument development; (iii) impact of psychotic, mood, and cognitive symptoms on life satisfaction and global HRQoL; and (iv) modern measurement theory and instrument development.
Reliability of the respondent
One of the major issues in psychiatry is the reliability (or credibility) of the respondent. Some researchers have questioned the ability of patients to accurately report on their subjective HRQoL and life satisfaction.14 Severe mental illness is associated with psychotic, cognitive, and mood features which may impact patient reporting. Critics counter that patients with schizophrenia and other severe mental disorders are unable to provide valid and reliable subjective assessments of their HRQoL.14 However, research suggests that patients can provide reliable and valid self-reports of their psychological well-being and health status.6,15-19 Global HRQoL and self-evaluation of functioning is correlated with more granular symptoms of the patients' condition, demonstrating that more impaired quality of life is associated with higher levels of symptom severity.3
Conceptual models guiding instrument development
As pointed out by Awad and Voruganti2 in their review on assessing HRQoL in schizophrenia, there is a paucity of conceptual models and instruments developed based on these conceptual models. More research is needed on theoretical and conceptual models of HRQoL in psychiatric disorders. For example, Wilson and Cleary20 developed a conceptual model for linking clinical variables with health-related quality of life Figure 1. Their model provides a framework for understanding the main concepts and relationships among individual and environmental variables, symptoms, functioning, general health perceptions, and global quality of life. Most of these concepts, represented by Wilson and Cleary as a linear chain from biological variables through to global perceptions of life quality, fall into the broad, inclusive concept of health-related quality of life (Figure 1, blue box added for emphasis). Similar models are lacking in psychiatry, although such a model and others used in physical illness can readily be adapted.
Figure 1. Conceptual model for health-related quality of life. Adapted from ref 20: Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273:59-65. Copyright © American Medical Association 1995.
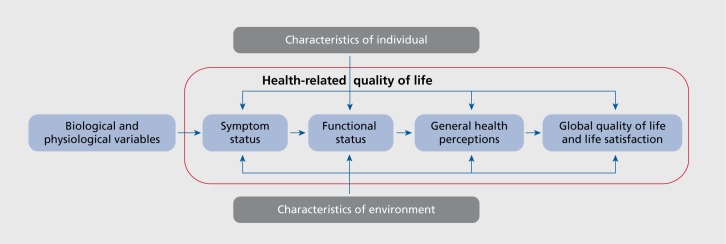
In addition, conceptual models need to be developed to provide the content map for developing HRQoL instruments for use in psychiatry. Without such models, instruments will continue to be developed based on whatever perspective the developer takes. This practice has resulted in a number of measures labeled as measuring HRQoL but covering sometimes very different concepts.
Impact of psychotic, mood, and cognitive symptoms on HRQoL
Several studies, across different psychiatric disorders, have found that greater severity of psychopathologic symptoms are associated with more impaired HRQoL.12,21-30 However, Priebe and colleagues,31 based on a pooled analysis of 16 studies, showed that symptom severity was less strongly correlated with global HRQoL in schizophrenia than in other psychiatric disorders. Similarly, in a study examining the relationship between changes in psychopathologic symptoms and more general HRQoL, Priebe and colleagues32 found an explained variance on only 5.5%.
There are concerns that cognitive deficits may impact the reliability of patient-reported HRQoL. However, the evidence to date on this relationship is inconsistent. Several studies have found that improved cognitive performance was associated with more impaired HRQoL.22,33-37 Other studies have found relationships between deficits in executive functioning, attention, motor skills, and memory with impaired HRQoL.38,39 A more recent study evaluated bias in self-report item reports of cognitive and psychiatric symptoms.19 The researchers found no evidence for bias related to level of cognitive function for any item, but did find evidence of bias for symptom severity. However, the conclusions from the study were that any response bias attributed to psychiatric symptoms or cognitive function, if present, was small and not likely to be clinically important.19
Modern measurement theory and instrument development
Over the past 20 years, the application of modern measurement theory (ie, item response theory) has revolutionized the development of PRO measures. A recent and good example of the use of item response theory analyses in the development and evaluation of PRO measures is the NIH sponsored Patient-Reported Outcome Measurement Information System (PROMIS).40,41 Few of the HRQoL measures or symptom measures used in psychiatric research were developed or evaluated using the methods of modern measurement theory.42,43 These methods can be used to develop item banks, brief short-form scales, and accommodate computerized adaptive testing for HRQoL and other psychiatric instruments. The modern measurement theory approaches may handle some of the disadvantages of the existing HRQoL measures, such as floor and ceiling effects, incomplete coverage of the range of measurement concepts, and adaptivity to a range of levels in psychiatric severity.2 The item response theory methods also provide a comment metric for measurement of various concepts, such as psychotic symptoms, depression, anxiety, anger, social participation, and fatigue.
Examples of PROs used in psychiatry
In this section we review a number of different self-report measures of different aspects of HRQoL in psychiatry. The instruments have been selected based on their use with different psychiatric conditions, as well as having differing conceptual frameworks. This is not meant to be a systematic or exhaustive review of instruments available for use in psychiatry. It is intended to be a selective review illustrating some useful options to consider.
Quality of Life Interview
The Quality of Life Interview (QOLI) was developed in the late 1980s in response to the need for evaluation tools for use in community outpatient treatment for the seriously mentally ill.13 The QOLI is based on a conceptual model that incorporates personal characteristics and objective and subjective quality of life indicators all leading to a sense of global well-being. Questions are asked first about objective HRQoL, and then, using a 7-point Likert-type scale, subjective HRQoL. There are eight core domains: living situation, daily activities and functioning, family relations, social relations, finances, work and school, legal and safety issues, and health.10 The QOLI has demonstrated good reliability and validity10 and consists of the original long form and a short form.
Quality of Life Enjoyment and Satisfaction Questionnaire
The Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q), in both the long and short form (Q-LES-Q-SF), is a widely used instrument for measuring HRQoL and satisfaction. Originally developed for use in clinical trials and among trial participants with a wide variety of mental and medical diseases or disorders.12 The Q-LES-Q was developed to measure the degree of enjoyment and satisfaction experienced by respondents in daily life. It includes items assessing physical health, subjective feelings, work, household duties, school/coursework, leisure time activities, social relationships and general activities. A short form is available. Summary scores demonstrate good psychometric properties.12 The Q-LES-Q has demonstrated good internal consistency reliability, stability (reproducibility), and validity in patients with schizophrenia, bipolar disorder, depression, generalized anxiety disorder, and post-traumatic stress disorder studies.12,27,28-44-48 The Q-LES-Q is an HRQoL measure that has the potential to extend and complement clinical efficacy end points. Since the development of the Q-LES-Q, this measure has been incorporated into multiple clinical trials across a range of psychiatric disorders.
Generic patient-reported outcome measures
For the past 20 years, generic measures of health status, functioning and HRQoL have been incorporated into clinical trials and epidemiologic studies. The most frequently used generic measure of functioning and well-being is the Short-Form 36 Health Survey (SF-36). The SF-36 was developed for the Medical Outcomes Study.49-51 The SF-36 includes eight domain scores, include physical function, bodily pain, role limitations-physical, vitality, social function, general health perceptions, role limitations-emotional, and mental health. Two component scores are generated, the Physical Component Summary and the Mental Component Summary. For version 2 of the SF-36, domain and summary scores are transformed to a normative scale with a mean of 50 and standard deviation (SD) of 10, with higher scores indicating better physical function or well-being.51
Patient-Reported Outcome Measurement Information System (PROMIS)
Ten years ago, the National Institutes of Health sponsored a large, interdisciplinary instrument development and evaluation project measure important health domains and that can be applied across diseases. The PROMIS project developed multiple item banks to assess physical, mental, and social functioning and wellbeing.40,41 PROMIS provides item banks that offer PRO measurement that is more efficient (minimizes item number without compromising reliability) flexible (enables optional use of interchangeable items), and precise (minimal error in the score estimation) measurement than existing PRO measures (www.nihpromis.org). The domain framework for PROMIS includes Physical Health, including physical function, fatigue, pain intensity, interference, behavior and quality, sleep function, and sexual function. Mental Health includes depression, anxiety, anger, positive psychological function, and cognitive function, and Social Health includes satisfaction with participation in social roles, satisfaction with social roles and activities, ability to participate in social roles and activities, social support, social isolation, and companionship. For each domain a multi-item bank was developed which can be used to generate a wide range of fixed short-form scales, or for computerized adaptive testing (CAT). In a CAT, items are selected using a dynamic measurement process which tailors the scale to the individual respondent, thus allowing for more efficient and precise individual assessment. PROMIS measures have been included in studies in major depressive disorder,52 and have been adopted by the American Psychiatric Association as measures of crosscutting function for DSM-5. PROMIS measures should be considered for future research in psychiatry.
Neuro-QoL (Quality of life in Neurological Disorders)
Another recently developed measurement system which may have applicability for psychiatric research is Neuro-QoL.53,54 Like PROMIS, Neuro-QoL is a dynamic, flexible, and psychometrically-sound HRQoL measurement system applicable for use with a broad range of neurological disorders (www.neuroqol.org). Neuro-QoL is generic across selected conditions to allow for cross-condition comparison, and yet the system was designed to be flexible enough to capture condition-specific HRQoL issues for use in neurology clinical trials and clinical practice. Neuro-QoL consists of thirteen adult and eight pediatric item banks and four calibrated scales. These banks and scales assess aspects of Emotional Health (Anxiety, Depression, Anger, Emotional and Behavioral Dyscontrol, Positive Affect and Weil-Being, Stigma); Physical Function/Health (Upper Extremity Function - Fine Motor, ADL, Lower Extremity Function - Mobility); Physical Symptoms (Fatigue, Sleep Disturbance, Pain); Cognitive Health (Applied Cognition - Executive Function, Applied Cognition General Concerns, Communication); and Social Health (Ability to Participate in Social Roles and Activities, Satisfaction with Social Roles and Activities, Social Relations - Interaction with Peers, Social Relations Interaction with Adults) that are relevant to common neurological conditions.
Short-form scales and CATs can be derived from the different item banks. Also like PROMIS, Neuro-QoL scores are expressed on a T distribution, standardized to a mean of 50 and standard deviation (SD) of 10 points. The Neuro-QoL short forms were validated in five major neurological adult conditions (stroke, Parkinson's disease, multiple sclerosis, adult epilepsy, amyotrophic lateral sclerosis) and two pediatric conditions (pediatric epilepsy and muscular dystrophy).53,54 Clearly, many of these domains may be relevant for various psychiatric disorders, including schizophrenia, bipolar disorder, major depressive disorder, and anxiety disorders.
Summary and discussion
A large number of patient-reported outcome measures have been used to evaluate HRQoL and other outcomes in patients with psychiatric disorders. In general, the same challenges in health outcome assessment for individuals with chronic medical illness are also seen for those with psychiatric disorders. The only real difference relates to cognitive impairment and psychopathology in patients with severe psychiatric disorders, however, research to date has indicated that the majority of patients with schizophrenia and bipolar disorder can accurately report on their HRQoL. Note that there are similar problems associated with self-reporting among patients with dementia and Alzheimer's disease.
Several issues are apparent based on reviews of research in HRQoL instruments in psychiatric disorders. First, although there are a number of instruments developed to assess outcomes in psychiatry, most of these measures have little theoretical or conceptual basis. This has resulted in overlapping and disparate concepts included in different instruments developed to assess HRQoL. Researchers need to closely examine the content of these HRQoL instruments as may have little conceptual overlap, and may measure different outcomes. Further work is needed to develop theoretical models of relevant outcomes for psychiatric disorders. Some of these outcomes may overlap with existing conceptual frameworks, for example, PROMIS.40,41 Concepts, such as psychosis, mania, and negative symptoms, stigma, etc. may be more salient in psychiatric disorders relative to medical conditions. Other reviewers have noted the absence of a conceptual basis for many HRQoL measures developed for use in psychiatry.2,3 Future research needs to focus on developing this conception and theoretical basis as a foundation for developing new HRQoL assessments.
Given the increased focus on comparative effectiveness research,55 we can expect greater utilization of HRQoL instruments in clinical practice and institutional settings. To date, there has been little application of the existing HRQoL measures in clinical settings, with most applications in clinical research. Reasons for the lack of clinical applications may relate to the length of existing HRQoL instruments, and to the absence of clear guidance as to useful severity thresholds or guidelines for clinical action. Attention needs to be focused on developing practical solutions to these barriers. Developing item banks, and short-form scales and computerized adaptive tests based on these item banks may be one way forward, by shortening length and enabling selection of clinical treatment targets to be assessed. Collecting HRQoL data alone is insufficient. There must also be systems developed to disseminate the relevant information on outcomes to clinicians caring for the patients. Future research is needed on developing practical and clearly interpretable systems of outcomes assessment for practice setting.
In conclusion, PRO and HRQoL assessments are important for understanding the broad impact psychiatric disorders have on patient's lives. Given the association between the adverse effects of some treatments on weight gain, hyperlipidemia, and the metabolic syndrome, it is important to assess both physical functioning and well-being and psychiatric symptoms and psychological well-being. Comprehensive, yet brief PRO assessments are needed for the evaluation of treatments and community-based interventions that get at the range of impacts on HRQoL in psychiatric disorders. In the end, psychiatric HRQoL instruments, and their conceptual underpinnings, are not all that different from HRQoL instruments used in physical illnesses. Given recent efforts to encompass a wide range of physical and psychiatric illnesses into broad HRQoL frameworks such as those of PROMIS and Neuro-QoL, these could be quite promising avenues for promoting further development of HRQoL assessment in research and clinical practice. Applying modern item response theory methods of test construction and validation can help advance the development and meaningful use of comprehensive HRQoL measures. Once developed, these assessments can be applied in research, clinical, and community settings to enable patients and their families, clinicians, and health policymakers to determine which interventions work best at maintain and improving the HRQoL of patients with psychiatric disorders. HRQoL instruments, wisely applied, can be cornerstones of comparative effectiveness research, patient-centered outcomes research, and patient care itself.
Contributor Information
Dennis A. Revicki, Evidera, Bethesda, Maryland, USA.
Leah Kleinman, Evidera, Seattle, Washington, USA.
David Cella, Department of Medical Social Sciences, Northwestern University, Chicago, Illinois, USA.
REFERENCES
- 1.McCabe R., Saidi M., Priebe S. Patient-reported outcomes in schizophrenia. Br J Psychiatry Suppl. 2007;50:s21–s28. doi: 10.1192/bjp.191.50.s21. [DOI] [PubMed] [Google Scholar]
- 2.Awad AG., Voruganti LN. Measuring quality of life in patients with schizophrenia: an update. Pharmacoeconomics. 2012;30:183–195. doi: 10.2165/11594470-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Reininghaus U., Priebe S. Measuring patient-reported outcomes in psychosis: conceptual and methodological review. Br J Psychiatry. 2012;201:262–267. doi: 10.1192/bjp.bp.111.107615. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter WT., Jr, Hanlon TE., Heinrichs DW., et al Continuous versus targeted medication in schizophrenic outpatients: outcome results. Am J Psychiatry. 1990;147:1138–1148. doi: 10.1176/ajp.147.9.1138. [DOI] [PubMed] [Google Scholar]
- 5.Meltzer HY., Burnett S., Bastani B., Ramirez LF. Effects of six months of clozapine treatment on the quality of life of chronic schizophrenic patients. Hosp Community Psychiatry. 1990;41:892–897. doi: 10.1176/ps.41.8.892. [DOI] [PubMed] [Google Scholar]
- 6.Revicki DA., Genduso LA., Hamilton SH., Ganoczy D., Beasley CM., Jr Olanzapine versus haloperidol in the treatment of schizophrenia and other psychotic disorders: quality of life and clinical outcomes of a randomized clinical trial. Qual Life Res. 1999;8:417–426. doi: 10.1023/a:1008958925848. [DOI] [PubMed] [Google Scholar]
- 7.Swartz MS., Perkins DO., Stroup TS., et al Effects of antipsychotic medications on psychosocial functioning in patients with chronic schizophrenia: findings from the NIMH CATIE study. Am J Psychiatry. 2007;164:428–436. doi: 10.1176/ajp.2007.164.3.428. [DOI] [PubMed] [Google Scholar]
- 8.Fervaha G., Foussias G., Siddiqui I., Agid O., Remington G. Abbreviated quality of life scales for schizophrenia: Comparison and utility of two brief community functioning measures. Schizophr Res. 2014;154:89–92. doi: 10.1016/j.schres.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Heinrichs DW., Hanlon TE., Carpenter WT., Jr The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10:388–398. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- 10.Lehman AF. Measures of quality of life among persons with severe and persistent mental disorders. Soc Psychiatry Psychiatr Epidemiol. 1996;31:78–88. doi: 10.1007/BF00801903. [DOI] [PubMed] [Google Scholar]
- 11.Food and Drug Administration. Guidance for industry on patient-reported outcome measures: use in medical product development to support labeling claims. Federal Register. 2009;74:65132–65133. [Google Scholar]
- 12.Endicott J., IMee J., Harrison W., Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacoi Bull. 1993;29:321–326. [PubMed] [Google Scholar]
- 13.Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plan. 1988;11:51–61. [Google Scholar]
- 14.Atkinson M., Zibin S., Chuang H. Characterizing quality of life among patients with chronic mental illness: a critical examination of the self-report methodology. Am J Psychiatry. 1997;154:99–105. doi: 10.1176/ajp.154.1.99. [DOI] [PubMed] [Google Scholar]
- 15.Glazer W., Sholomskas D., Williams D., Weissman M. Chronic schizophrenics in the community: are they able to report their social adjustment? Am J Orthopsychiatry. 1982;52:166–171. doi: 10.1111/j.1939-0025.1982.tb02677.x. [DOI] [PubMed] [Google Scholar]
- 16.Awad AG., Hogan TP., Voruganti LN., Heslegrave RJ. Patients' subjective experiences on antipsychotic medications: implications for outcome and quality of life. Int Clin Psychopharmacol. 1995;10 (suppl 3):123–132. doi: 10.1097/00004850-199509000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Dickey B., Wagenaar H., Stewart A. Using health status measures with the seriously mentally ill in health services research. Med Care. 1996;34:112–116. doi: 10.1097/00005650-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Russo J., Trujillo CA., Wingerson D., et al The MOS 36-Item Short Form Health Survey: reliability, validity, and preliminary findings in schizophrenic outpatients. Med Care. 1998;36:752–756. doi: 10.1097/00005650-199805000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Reininghaus U., McCabe R., Burns T., Croudace T., Priebe S. The validity of subjective quality of life measures in psychotic patients with severe psychopathology and cognitive deficits: an item response model analysis. Qual Life Res. 2012;21:237–246. doi: 10.1007/s11136-011-9936-1. [DOI] [PubMed] [Google Scholar]
- 20.Wilson IB., Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273:59–65. [PubMed] [Google Scholar]
- 21.Mechanic D., McAlpine D., Rosenfield S., Davis D. Effects of illness attribution and depression on the quality of life among persons with serious mental illness. Soc Sci Med. 1994;39:155–164. doi: 10.1016/0277-9536(94)90324-7. [DOI] [PubMed] [Google Scholar]
- 22.Corrigan PW., Buican B. The construct validity of subjective quality of life for the severely mentally ill. J Nerv Ment Dis. 1995;183:281–285. doi: 10.1097/00005053-199505000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson G., Hesdon B., Wild D., et al Self-report quality of life measure for people with schizophrenia: the SQLS. Br J Psychiatry. 2000;177:42–46. doi: 10.1192/bjp.177.1.42. [DOI] [PubMed] [Google Scholar]
- 24.Pukrop R., Schlaak V., Moller-Leimkuhler AM., et al Reliability and validity of Quality of Life assessed by the Short-Form 36 and the Modular System for Quality of Life in patients with schizophrenia and patients with depression. Psychiatry Research. 2003;119:63–79. doi: 10.1016/s0165-1781(03)00110-0. [DOI] [PubMed] [Google Scholar]
- 25.Sim K., Mahendran R., Siris SG., Heckers S., Chong SA. Subjective quality of life in first episode schizophrenia spectrum disorders with comorbid depression. Psychiatry Res. 15. 2004;129:141–147. doi: 10.1016/j.psychres.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Orsel S., Akdemir A., Dag I. The sensitivity of quality-of-life scale WHOQOL-100 to psychopathological measures in schizophrenia. Compr Psychiatry. 2004;45:57–61. doi: 10.1016/j.comppsych.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Ritsner M., Kurs R., Ratner Y., Gibel A. Condensed version of the Quality of Life Scale for schizophrenia for use in outcome studies. Psychiatry Res. 2005;135:65–75. doi: 10.1016/j.psychres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Ritsner M., Kurs R., Gibel A., Ratner Y., Endicott J. Validity of an abbreviated quality of life enjoyment and satisfaction questionnaire (Q-LES-Q-18) for schizophrenia, schizoaffective, and mood disorder patients. Qual Life Res. 2005;14:1693–1703. doi: 10.1007/s11136-005-2816-9. [DOI] [PubMed] [Google Scholar]
- 29.Eack SM., Newhill CE. Psychiatric symptoms and quality of life in schizophrenia: a meta-analysis. Schizophr Bull. 2007;33:1225–1237. doi: 10.1093/schbul/sbl071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vatne S., Bjorkly S. Empirical evidence for using subjective quality of life as an outcome variable in clinical studies A meta-analysis of correlates and predictors in persons with a major mental disorder living in the community. Clin Psychol Rev. 2008;28:869–889. doi: 10.1016/j.cpr.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Priebe S., Reininghaus U., McCabe R., et al Factors influencing subjective quality of life in patients with schizophrenia and other mental disorders: a pooled analysis. Schizophr Res. 2010;121:251–258. doi: 10.1016/j.schres.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 32.Priebe S., McCabe R., Junghan U., et al Association between symptoms and quality of life in patients with schizophrenia: a pooled analysis of changes over time. Schizophr Res. 2011;133:17–21. doi: 10.1016/j.schres.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 33.Skantze K., Malm U., Dencker SJ., PR, Corrigan P. Comparison of quality of life with standard of living in schizophrenic out-patients. Br J Psychiatry. 1992;161:797–801. doi: 10.1192/bjp.161.6.797. [DOI] [PubMed] [Google Scholar]
- 34.Brekke JS., Kohrt B., Green MF. Neuropsychological functioning as a moderator of the relationship between psychosocial functioning and the subjective experience of self and life in schizophrenia. Schizophr Bull. 2001;27:697–708. doi: 10.1093/oxfordjournals.schbul.a006908. [DOI] [PubMed] [Google Scholar]
- 35.Green MF., Kern RS., Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Fujii DE., Wylie AM., Nathan JH. Neurocognition and long-term prediction of quality of life in outpatients with severe and persistent mental illness. Schizophr Res. 2004;69:67–73. doi: 10.1016/S0920-9964(03)00122-1. [DOI] [PubMed] [Google Scholar]
- 37.Wegener S., Redoblado-Hodge MA., Lucas S., Fitzgerald D., Harris A., Brennan J. Relative contributions of psychiatric symptoms and neuropsychological functioning to quality of life in first-episode psychosis. Aust NZ J Psychiatry. 2005;39:487–492. doi: 10.1080/j.1440-1614.2005.01608.x. [DOI] [PubMed] [Google Scholar]
- 38.Galletly CA., Clark CR., McFarlane AC., Weber DL. Relationships between changes in symptom ratings, neurophysiological test performance and quality of life in schizophrenic patients treated with clozapine. Psychiatry Res. 1997;72:161–166. doi: 10.1016/s0165-1781(97)00062-0. [DOI] [PubMed] [Google Scholar]
- 39.Ritsner M. Comparison of instruments for measuring the quality of life impairment syndrome in severe mental disorders. In: Ritsner M, Awad AG, eds. Quality of Life Impairment in Schizophrenia, Mood and Anxiety Disorders: New Perspectives on Research and Treatment. New York, NY: Springer. 2007:133–142. [Google Scholar]
- 40.Cella D., Yount S., Rothrock N., et al The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45 (5 suppl 1):S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cella D., Riley W., Stone A., et al The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uher R., Farmer A., Maier W., et al Measuring depression: comparison and integration of three scales in the GENDEP study. Psychol Med. 2008;38:289–300. doi: 10.1017/S0033291707001730. [DOI] [PubMed] [Google Scholar]
- 43.Revicki DA., Chen WH., Frank L., Feltner D., Morlock R. Development and analysis of item response theory based short form depression severity scales based on the HDRS and MADRS. Health Outcomes Research in Medicine. 2010:e111–e112. [Google Scholar]
- 44.Schechter D., Endicott J., Nee J. Quality of life of' normal' controls: association with lifetime history of mental illness. Psychiatry Res. 2007;152:45–54. doi: 10.1016/j.psychres.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mick E., Faraone SV., Spencer T., Zhang HF., Biederman J. Assessing the validity of the Quality of Life Enjoyment and Satisfaction Questionnaire Short Form in adults with ADHD. J Atten Disord. 2008;11:504–509. doi: 10.1177/1087054707308468. [DOI] [PubMed] [Google Scholar]
- 46.Revicki DA., Brandenburg N., Matza L., Hornbrook MC., Feeny D. Health-related quality of life and utilities in primary-care patients with generalized anxiety disorder. Qual Life Res. 2008;17:1285–1294. doi: 10.1007/s11136-008-9406-6. [DOI] [PubMed] [Google Scholar]
- 47.Demyttenaere K., Andersen HF., Reines EH. Impact of escitalopram treatment on Quality of Life Enjoyment and Satisfaction Questionnaire scores in major depressive disorder and generalized anxiety disorder. Int Clin Psychopharmacol. 2008;23:276–286. doi: 10.1097/YIC.0b013e328303ac5f. [DOI] [PubMed] [Google Scholar]
- 48.Wyrwich KW., Harnam N., Locklear JC., Svedsater H., Revicki DA. Understanding the relationships between health outcomes in generalized anxiety disorder clinical trials. Qual Life Res. 2011;20:255–262. doi: 10.1007/s11136-010-9734-1. [DOI] [PubMed] [Google Scholar]
- 49.Stewart AL., Ware JE. Measuring Functioning and Well-Being: The Medical Outcomes Study Approach. Durham, NC: Duke University Press; 1992 [Google Scholar]
- 50.Ware JE., Snow KK., Kosinski M., Gandek B. SF-36. Health Survey: Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center. 1993 [Google Scholar]
- 51.Ware JE., Kosinski M., Dewey JE. How to Score Version Two of the SF-36 Health Survey. Lincoln, Rl: QualityMetric, Inc. 2000 [Google Scholar]
- 52.Pilkonis PA., Choi SW., Reise SP., et al Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): depression, anxiety, and anger. Assessment. 2011;18:263–283. doi: 10.1177/1073191111411667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cella D., Lai JS., Nowinski CJ., et al Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology. 2012;78:1860–1867. doi: 10.1212/WNL.0b013e318258f744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gershon RC., Lai JS., Bode R., et al Neuro-QOL: quality of life item banks for adults with neurological disorders: item development and calibrations based upon clinical and general population testing. Qual Life Res. 2012;21:475–486. doi: 10.1007/s11136-011-9958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahmed S., Berzon RA., Revicki DA., et al The use of patient-reported outcomes (PRO) within comparative effectiveness research: implications for clinical practice and health care policy. Med Care. 2012;50:1060–1070. doi: 10.1097/MLR.0b013e318268aaff. [DOI] [PubMed] [Google Scholar]


