Abstract
Purpose
Pivoting neuromuscular control and proprioceptive acuity may play an important role in ACL injuries. The goal of this study was to investigate whether pivoting neuromuscular training on an offaxis elliptical trainer (POINT) could improve pivoting neuromuscular control, proprioceptive acuity, and functional performance.
Methods
Among 41 subjects, 21 subjects participated in 18 sessions of POINT (3 sessions/week for 6 weeks), and 20 subjects served as controls who did their regular workout. Both groups received pre-, mid-, and post-intervention evaluations. Propensity score analysis with multivariable regression adjustment was used to investigate the effect of training on pivoting neuromuscular control (pivoting instability, leg pivoting stiffness, maximum internal and external pivoting angles), proprioceptive acuity, and functional performance in both groups.
Results
Compared to the control group, the training group significantly improved pivoting neuromuscular control as reduced pivoting instability, reduced maximum internal and external pivoting angles, increased leg pivoting stiffness, and decreased entropy of time to peak EMG in the gluteus maximus and lateral gastrocnemius under pivoting perturbations. Furthermore, the training group enhanced weight-bearing proprioceptive acuity and improved the single leg hop distance.
Conclusion
Improvement of pivoting neuromuscular control in functional weight-bearing activities and task performances following POINT may help develop lower limb injury prevention and rehabilitation methods to reduce ACL and other musculoskeletal injuries associated with pivoting sports.
Keywords: POINT, ACL injuries, proprioceptive acuity, injury prevention, functional performances
INTRODUCTION
Anterior cruciate ligament (ACL) injury prevention is important, given the increased incidence, short- and long-term consequences, and economic burden associated with this injury (17, 29). Each year, an estimated 80,000 to 250,000 ACL injuries occur in the United States, with over $1 billion spent on ACL repair and related procedures (17). Clinically, individuals with ACL injuries may be at greater risks of secondary pathologies, such as meniscus tears and early development of osteoarthritis regardless of treatment methods that individuals received following ACL injuries (29). Nearly 70% of ACL injuries are non-contact in nature occurring due to individuals’ own movements during pivoting movements at foot-ground-contact with a sudden deceleration or directional change, such as sidestep cutting, pivoting, or planting a foot involving multi-joint movements in axial and frontal planes (6, 24, 34). Unfavorable lower extremity alignment and laxity, notch morphology, inadequate muscle protection, or poor neuromuscular control during these risky movements have been attributed to non-contact ACL injuries (17). Among these factors, inadequate neuromuscular control resulting in functional instability during cutting/pivoting movements is likely modifiable and deserves attention in the effort to decrease ACL injuries (22).
A number of non-contact ACL injury prevention programs, focusing on improving neuromuscular control through a combination of flexibility, proprioception, agility, plyometrics, and muscle strength training, have been developed and implemented during pre-season regimes (17). These programs showed positive changes in movement risk factors, such as decreased knee valgus moments, reduced peak vertical ground reaction force during landing tasks, increased hip and knee flexion angles, and improved dynamic balance (17). When compared to the control groups, several neuromuscular control training studies reported ACL injury rate reductions (17).
Despite the positive results from previous studies and the popularity of these programs, the number of annual ACL injury incidents during sports has not decreased (5, 21). This discordance could be partially attributed to incorrect or incomplete implementations of these training programs. In addition, given that the aforementioned neuromuscular training methods were mostly conducted in controlled predictable conditions within a group setting (20), it is possible that such training methods may not completely address the primary underlying injury mechanism of inadequate neuromuscular control during pivoting/twisting with different foot-ground-contact conditions and may not fully target individual’s deficiencies. To our knowledge, individualized and targeted training methods for improving the ability to control pivoting movements, leg pivoting neuromuscular control, under external perturbation or slippery walkway are scarce. Training under these conditions may provide individuals more opportunities to learn necessary motor skills to avoid risky postures in real-life situations (5).
Neuromuscular control requires complex interactions between the nervous and musculoskeletal systems to produce a desired body movement (14, 27). Especially, pivoting neuromuscular control can be investigated in terms of instability, maximum internal or external pivoting angles, and muscle activations including measures of nonlinear dynamics such as entropy of time to peak EMG, which might be related to potential ACL injury risk factors (27, 35). Furthermore, the threshold of detecting passive movement, proprioceptive acuity, is an important factor to contribute to neuromuscular control and prevent injuries (2, 35). Therefore, comprehensive evaluations including both proprioceptive acuity and pivoting neuromuscular control may help us to understand potential benefits of the neuromuscular training, which may lead to reduction of ACL injuries (27, 35).
The goal of this study was to investigate effects of 6 week pivoting neuromuscular training (POINT) (35) on proprioceptive acuity, pivoting neuromuscular control, and functional performance. Specifically, we hypothesized that, after 6-week POINT, compared to the control group, the training group would 1) improve pivoting proprioceptive acuity under weight-bearing. After 6-week POINT, compared to the control group, the training group would improve pivoting neuromuscular control at each stepping task under pivoting perturbations in terms of 2) reduced pivoting instability, 3) increased pivoting stiffness, 4) reduced maximum internal or external pivoting angles, 5) reduced entropy of time to peak EMG in each muscle. Furthermore, compared to the control group, the training group would 6) improve functional performance in 10-meter walk speed, 12-meter hop time, and single leg hop distance.
MATERIALS AND METHODS
Subjects
Forty-one healthy subjects without any lower limb musculoskeletal injury participated in the study. All subjects gave written informed consent approved by the Institute of Review Board at Northwestern University. Group assignment into either the training group or the control group was not randomized due to subjects’ availability to participate in the three study sessions per week over a 6 week period. Among them, 21 subjects in the study group (10 males, 11 females) participated in 18 sessions of Pivoting Off-axis Intensity adjustable Neuromuscular control Training (POINT) (3 times/week for six weeks consisting of 15 training sessions and 3 evaluation sessions) and the remaining 20 subjects (10 males, 10 Females) served as the control group who did their regular workout. Both groups received -pre, -mid, and -post evaluations at the 1st, 9th, and 18th sessions. Exclusion criteria included any known history of orthopedic injury or surgery, lower limb pain, or neurological injury, cardiac arrhythmia and hypertension. At the first evaluation, Q angle was measured with a goniometer as an angle between the line connecting the Anterior-Superior-Iliac-Crest (ASIS) to the center of patella and the line connecting the center of patellar to the tibial tubercle in a supine and fully extended knee position (1), then averaged Q angle from the left and right leg was reported. A simple survey (16) was given to subjects to assess physical activity level among 6 categories as “0 points for “did not do regular physical activities”, 1 points for “did once a week recreational sport”, 2 points for “did once a week strenuous/competitive sport”, 3 points for “did twice a week strenuous/competitive sports”, 4 points for “did three to four times a week strenuous/competitive sports”, and 5 points for “I am a semi-professional sportsman.”
Instrumentation
The primary equipment used in this study was the off-axis elliptical trainer (ET) (Fig. 1). Details about the trainer can be found in (16, 35). Briefly, the off-axis ET allowed transverse-plane pivoting and frontal plane sliding movements and measured biomechanical data using torque, force, and position sensors at each footplate. The stepping cycle was measured using a potentiometer (35). The off-axis ET was connected to a display monitor for showing real-time audio-visual biofeedback and to a control computer for user interface adjusting torque, stiffness values, and adjusting different modes that generated various sensory stimuli (e.g. external perturbation, low or high friction) to each footplate via servomotor controls. Through the servo-controlled off-axis mechanisms, various pivoting/sliding training protocols can be implemented with real-time audio-visual biofeedback of task performance, such as pivoting angle and frontal lower-limb alignment (35). Because the purpose of the study was to focus on improving pivoting neuromuscular control, sliding parts were locked. Activities of eight muscles of the right leg were recorded including the biceps femoris (BF), semitendinosus (ST), medial gastrocnemius (MG), lateral gastrocnemius (LG), vastus medialis obliquus (VMO), vastus lateralis (VL), gluteus maximus (Glmax) and gluteus medius (Glmed) using a Bagnoli-8 EMG system (Delsys Inc, Boston, MA). The pivoting angles, torques, stepping cycles, and EMG signals were sampled at 1000Hz (National Instruments™, Austin, TX).
Figure 1.
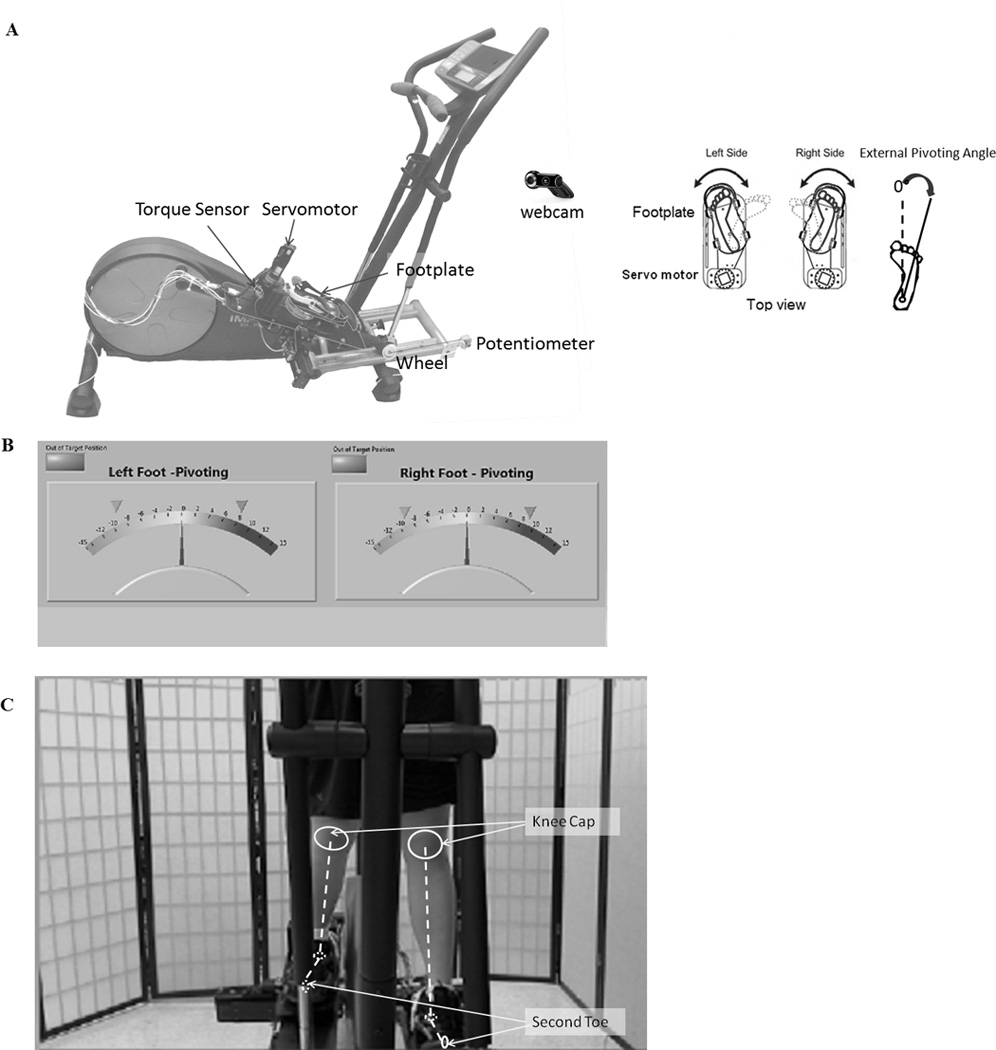
A: The offaxis elliptical trainer allowing pivoting movements in left and right legs simultaneously or individually with various sensory stimuli and torque perturbations to the feet under servomotor control that included footplates, servomotors, cable driven mechanisms, torque sensors, and encoders. Real-time visual feedback consists of (B) pivoting angle and (C) lower limb alignment during each training session with the middle target position. Virtual lines were drawn to indicate knee caps and second toes for a clear explanation. During evaluation, visual feedback of lower limb alignment through the web camera was not shown (35).
Experimental Protocol
The training group participated in POINT three times a week for six weeks, consisting of total of 18 sessions including 3 evaluation sessions. The control group came to our lab for 3 evaluation sessions following the same timeline as the evaluation sessions for the training group. The duration of each training session was half an hour. The subjects wore a safety harness and stood on the footplates of the ET with each tibial long axis aligned with the center of the pivoting axis on each footplate. Each shoe was strapped to the footplate with toe and heel straps so that the foot and the footplate rotated together without heel lifts to simulate foot-ground-contact situations (35).
Training Protocol
The unique part of POINT in this study was rotatable footplates allowing pivoting movements, real-time audiovisual feedback, and servomotor controls delivering various perturbations to the footplates. During POINT, subjects were asked to maintain the second toe pointing forward (the middle target in Fig. 1B) and align the lower limbs in the frontal plane to improve leg pivoting neuromuscular control. They received real-time audio-visual feedback (Figs. 1B and C) from a large screen displaying their lower limb performance indicated as pivoting angle and real-time visual feedback of their frontal limbs via high-resolution webcam (960×720 pixels). If subjects’ foot positions were out of the specified range as ±30°, they would hear a beeping sound. The beeping sounds occurred with mean ± SD as 0.89 ± 2.49 % (2.13 ± 5.97 sec) in the external pivoting direction during a challenging task, the motor external perturbation task (4 minutes). During other tasks, the beeping sounds rarely occurred.
Overall, the training program included three different training modes representing different foot-ground-contact conditions, which may help the subjects to acquire motor skills to be away from potential injury scenarios (5, 17). In the first mode, the footplates were free to pivot (free pivoting task, the FPT). During the FPT, subjects felt that they were walking on ice due to minimum friction of footplates. In the second mode, the footplates were pushed from both sides with assistive spring torque (assistive spring torque task, the ASTT). During the ASTT, subjects did not have any difficulties to maintain their target position because the restoring torque from the virtual springs helped subjects to stay at the target position (35). In the third mode, the footplates were perturbed in sinusoidal pivoting torques with an adjustable intensity in internal or external pivoting (motor internal perturbation task, the MIPT or motor external perturbation task, the MEPT) with torque limit of 10Nm. These tasks in the third mode were designed for subjects to maximize their abilities to control pivoting movements by gradually increasing amplitudes of their resistant torque over multiple sessions of training, so that they would potentially acquire motor skills to coordinate their lower limbs during real life complex circumstances. At each training session, subjects were first asked to do a 2-minute warm-up of regular stepping task (RST) when each pivoting components of the footplate was fixed. Then, they performed the FPT, ASTT, MIPT, MEPT, ASTT, FPT, each for 4 minutes, and ended the session with a 2-miunte cool-down of the RST.
Outcome Evaluation
The outcomes of POINT were assessed by both the proprioceptive acuity in pivoting, pivoting neuromuscular control, and functional performances. At the first, second, and third evaluation sessions, proprioceptive acuity as threshold to detection angle in internal and external pivoting directions using the off-axis ET. Pivoting instability, pivoting stiffness, endpoint kinematics as maximum internal and external pivoting angles, and entropy of time to peak EMG from the aforementioned eight muscles of the right leg were assessed using the off-axis ET.
Proprioception tasks tested how quickly subjects could detect the subtle passive pivoting movement (1°/s) (35). At the lowest position of the right footplate on the ET, subjects put all their weight on the right leg with a full knee extension, and minimally bore their weight on the left leg due to the mechanical structure of the ET. The initial pivoting position was at the second toe forward position. At that posture, subjects were asked to press a handheld button immediately and report the direction when they felt a subtle movement in four possible pivoting directions, namely left and right internal pivoting, and left and right external pivoting directions (Fig. 2A). After each task, the footplate returned to the initial position for the next task. All subjects had a familiarization period to ensure that they were well aware about the tasks prior to the actual proprioception tasks. A total of 16 sequential pseudo-randomized trials were performed so that the trials of each four direction were evenly distributed and subjects could not guess what would be the next trial (10). To be considered as a successful trial, subjects needed to correctly identify the direction and the leg moved. The proprioception tasks were performed at a quiet environment and subjects were asked to close their eyes during the tasks to exclude visual feedback.
Figure 2.
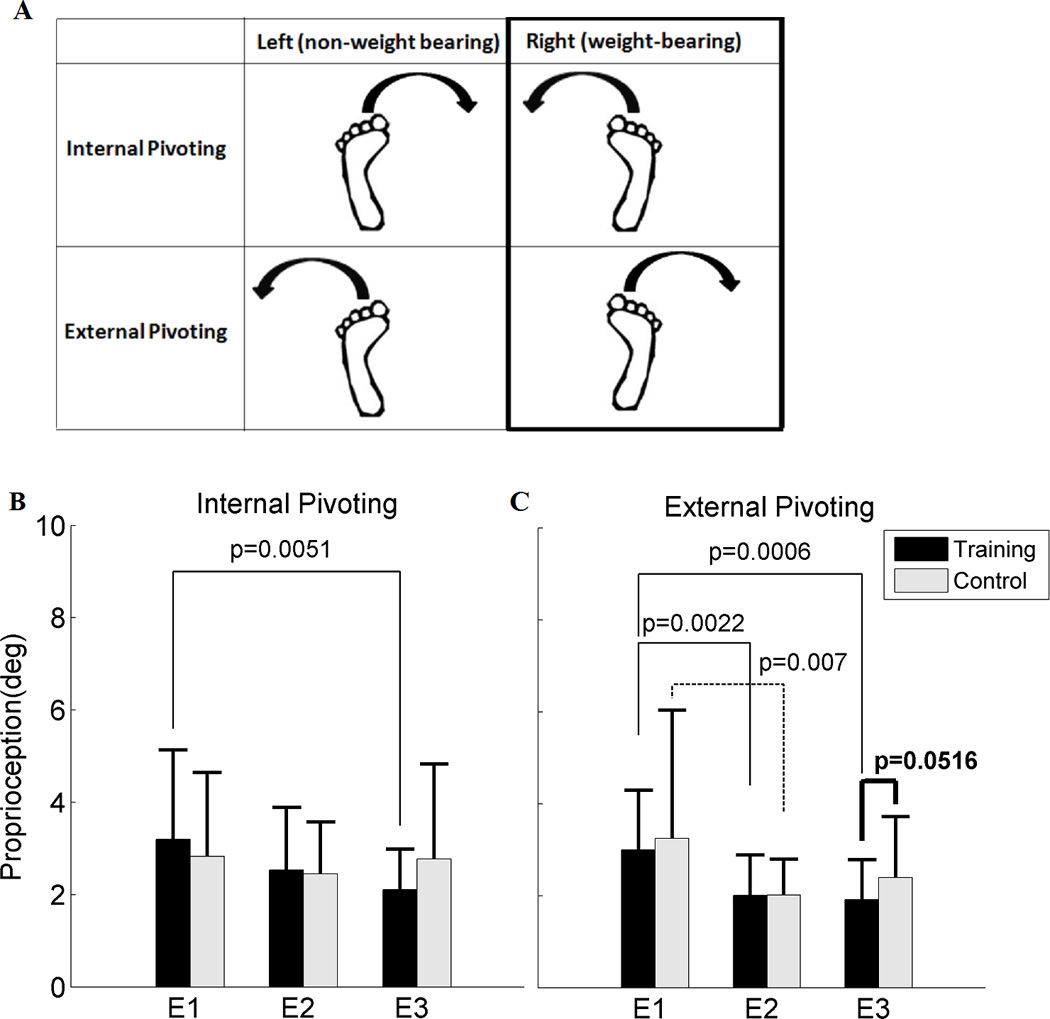
A: Four possible pivoting passive motions in proprioception tasks. The weight-bearing conditions as indicated as the black bold line box were reported for B and C. B: Proprioception (deg) in internal pivoting, and C: Proprioception (deg) in external pivoting under weight-bearing between the training and the control groups. Each bar indicates mean with 1SD between the training group (black color) and the control group (gray color) at each evaluation (N=20 in the training group (T), N=20 the control group (C) at E1, N=20 (T), N=20 (C) at E2, and N=21 (T), N=19 (C) at E3). E1, E2 and E3 denote the first, second and third evaluations, respectively. The bold black line indicates the p value between the training group and the control group at each evaluation. The black line indicates the p value between evaluations (E1 and E2, or E1 and E3) within the training group and the black dotted line indicates the p value between the evaluations within the control group.
Pivoting instability, pivoting stiffness, maximum internal or external pivoting angles, and entropy of time to peak EMG in lower limb muscles were assessed on the off-axis ET during the following two stepping tasks with the external torque pivoting perturbations. Each task lasted a minute at subjects’ comfortable speed. The first task was a motor internal perturbation task (MIPT) in which subjects were asked to maintain the target position (Fig. 1B) during sagittal stepping while a 5Nm internal pivoting offset torque superimposed with 1.8Nm 2Hz peak sinusoidal pivoting torque was applied to each footplate. The second task was a motor external perturbation task (MEPT), similar to the MIPT except the perturbation was in the external pivoting direction. Between each task, 1–2 minutes of rest was given for subjects to minimize fatigue. Subjects were instructed to lightly hold on handlebars of the ET to help maintain stability and minimize the influence of upper limb biomechanics on the lower limb performances.
During each evaluation session, real-time audio-visual feedback from the large screen was provided for subjects to maintain the target position similarly to the training sessions, but there was no feedback of their frontal lower limb alignment through the web-camera, which was provided during training sessions. Muscle activities of the eight muscles on the right leg were recorded throughout all the tasks on the ET.
To investigate whether POINT improved functional performances, 10-m walk time, 12-m single leg hopping time, single leg hop for distance were recorded at the first and the third evaluation sessions. Specifically, subjects did the 10m walk test at their comfortable speed for three times. The subjects hopped on one leg for 12m at their fastest speed without losing balance then repeated on the other leg, and hopped as far as they could without losing balance on one leg then repeated on the other leg (33).
Data Analysis
Proprioceptive acuity was analyzed as threshold to detection angle when subjects pressed the hand-held button immediately when they felt the subtle passive pivoting movements (35). Trials of each internal and external pivoting under weight-bearing were averaged for statistical analysis.
Pivoting instability was quantified as standard deviation of pivoting angles as normalized task performances to account for subject-to-subject variation due to natural stepping patterns when pivoting movements were allowed (27, 35) during the MIPT and MEPT. Higher pivoting instability indicates worse task performance. Pivoting stiffness was quantified using a non-parametric system identification method based on the relationships between the perturbed torque, measured torque, and measured angle (9, 23). Briefly, pivoting stiffness frequency response function (FRF) was determined using Welch’s periodogram method to estimate the power spectral density of the applied 2Hz perturbed torque, measured pivoting torque, and measured pivoting angle (4, 9, 23). Then, leg pivoting stiffness was estimated as the magnitude of the pivoting stiffness FRF at 2Hz (4, 9, 23). The reliability of leg pivoting stiffness estimation was validated based on coherence of the perturbed torque and the measured angle (close to 1) and coherence of the perturbed torque and the measured torque (close to 1) with low phase angle (close to 0°) of the FRF at 2Hz (4, 9, 23). Maximum internal and external pivoting angles across all the stepping cycles were also computed per each task from each evaluation at each person.
Entropy quantifies the variations in signals using the concept of information theory based on probability distribution of the observed events; higher value indicates greater variation (25, 27, 36). In assessing neuromuscular control, higher entropy suggests uncertainty of the neuromuscular system in selecting muscle activities or joint motions for a given task (8, 25, 27). Specifically, entropy of time to peak EMG in the aforementioned muscles was investigated, since time to peak EMGs in lower limb muscles may be closely related to lower limb coordination and potential injury risk factors (20, 27). To compute entropy of time to peak EMG in measured muscles, raw EMGs in each measured muscle were rectified and linear envelopes (LEs) were obtained using zero delay 6th order Butterworth low-pass filter with a cut-off frequency of 7Hz. The EMG LEs were segmented into individual stepping cycles based on the time intervals between the successive times when subjects positioned the same footplate at the most anterior position, similar to gait analysis (26, 27). Then, the segmented data were re-sampled and expressed in terms of stepping cycle (i.e. 0–100%) (26, 27). Time to peak EMG was determined at all cycles, then a number of discrete states were expressed as bins of time to peak EMG (5% of percent of stepping cycle) (25, 27). The bin widths were determined from previous studies to investigate functionally meaningful discrete states during muscle activities on a conventional elliptical trainer (7, 27). Then, the probability of each bin was computed and entropy of time to peak EMG was computed as follows.
| (1) |
where Pd is the discrete probability of time to peak EMG at each bin d. A lower entropy value indicates greater certainty of selecting specific time to peak EMG during a task.
Statistical Analysis
Subject characteristics between the training and control groups were compared with two-tailed independent t test and Fisher exact test. The effect of training was examined by propensity score analysis with multivariable regression adjustment to account for potential bias that could be present due to non-randomization of the group assignment between the training and control groups (12, 18). First, propensity scores (PS) were obtained by fitting a multivariable logistic regression on baseline-measured covariates (gender, weight, height, age, and physical activity level) using SAS software (SAS Institute Inc., 2011) to predict the individual probability to receive POINT conditionally (12, 18). Then, the effects of treatment on the outcome variables were computed based on multivariable regression adjustment with the aforementioned covariates and PS using the SAS software (12). The outcome variables were functional performances including mean 10-m walk time from three trials, mean 12-m single leg hop time of both legs, mean single leg hop for distance of both legs, proprioceptive acuity as threshold to detection angle in internal and external pivoting direction, pivoting instability, pivoting stiffness, maximum internal and external pivoting angles, and entropy of time to peak EMG per muscle at each task. The within group factor was the three evaluations including the first, second and third evaluations (E1, E2, and E3 respectively) for all measurements except functional activities (only for E1 and E3), and the between group factor was the groups as the control group or training group. All p value was set to 0.05 for statistical significance. All results were presented as mean ± 1SD.
RESULTS
Subject Characteristics
There were no statistical differences in age, weight, height, BMI, Q angle, and physical activity level between the training group (Age: 24.7±3.7 yr, Weight: 67.9±10.1 kg, Height: 172.4 ±11.3 cm, BMI: 22.8 ±2.0 kg/m2, Q angle: 12.1 ±3.4°, Physical activity level: 3.0 ±1.4 point), and the control group (Age: 25.0±3.4 yr, Weight: 70.0±16.4 kg, Height: 171.7 ±9.4 cm, BMI: 23.5 ±3.8 kg/m2, Q angle: 10.2 ±2.8°, Physical activity level: 2.5±1.8 point).
Proprioceptive Acuity
Subjects reported successful trials in almost all cases (99.0% of the total trials) as they identified the correct direction and the leg of the movement. None of the subjects reported consistent unsuccessful trials across three evaluations. The training group showed marginally significant improvement of proprioceptive acuity in terms of lower proprioception angle in external pivoting direction compared to the control group at E3 (p=0.0516), and significant differences were not found between the training and control groups at E1 and E2 in both internal and external pivoting directions (Fig. 2B and C).
Task Performance: Pivoting Instability, Pivoting Stiffness, and Endpoint Kinematics
Following POINT, the training group improved pivoting neuromuscular control evidenced by reduced pivoting instability, increased pivoting stiffness, and reduced maximum external and internal pivoting angles. Specifically, when compared to the controls, the training group significantly reduced pivoting instability at E2 during the MIPT and MEPT, (p=0.0391, p=0.0031, respectively) (Fig. 3A), and at E3 during the MEPT (p=0.0117) (Fig. 3A). Compared to the control group, the training group increased pivoting stiffness at E3 during the MIPT (p<0.0001) (Fig. 3B). Within the training group, pivoting stiffness was significantly increased between E1 and E2 (p=0.0018), and E1 and E3 (p<0.0001) during the MIPT, and between E1 and E2 (p=0.0427) during the MEPT. The results were obtained with high coherences (close to unity) and low phase angles (close to 0°) (see Table 1). Following POINT, the training group reduced their maximum external and internal pivoting angles during the MIPT and MEPT (Figs. 3C and D). Specifically, the training group, compared to the control group, showed significantly lower maximum external pivoting angle at E2 (p=0.003) during the MEPT (Fig. 3C). Compared to the controls, the training group significantly reduced maximum internal pivoting angle at E3 during the MIPT (p=0.0002, Fig. 3D) and at E2 and E3 during the MEPT (p=0.0052, p=0.0078 respectively).
Figure 3.
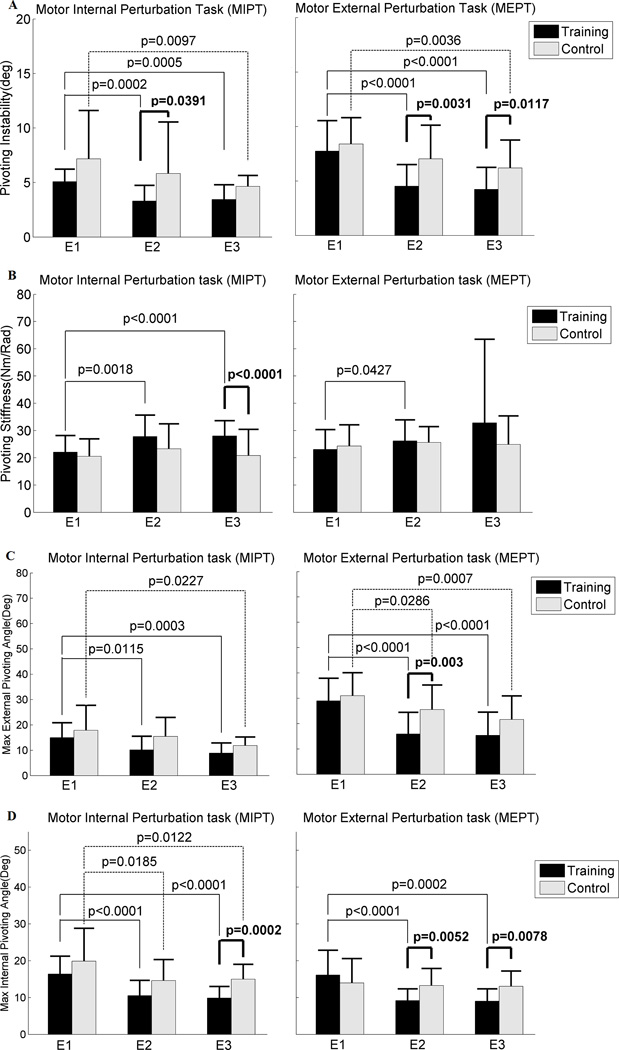
A: Pivoting instability (deg), B: Pivoting stiffness (Nm/Rad), C: Max external pivoting angle (deg), and D: Max internal pivoting angle (deg) between the training and the control groups at each evaluation. Each bar indicates mean with 1SD between the training group (black color) and the control group (gray color) at each evaluation at each task, namely the motor internal perturbation task (MIPT) and motor external perturbation task (MEPT) (N=20 in the training group (T), N=20 in the control group (C) at E1, N=21 (T), N=20 (C) at E2, and N=21 (T), N=19 (C) at E3). E1, E2 and E3 denote the first, second and third evaluations, respectively. The bold black line indicates the p value between the training group and the control group at each evaluation. The black line indicates the p value between evaluations (E1 and E2, or E1 and E3) within the training group and the black dotted line indicates the p value between the evaluations within the control group.
Table 1.
High coherence and low phase angle at 2Hz.
| E1 |
E2 |
E3 |
||||
|---|---|---|---|---|---|---|
| Training (N= 20) |
Control (N=20) |
Training (N=21) |
Control (N=19) |
Training (N=21) |
Control (N=19) |
|
| MIPT | ||||||
| Coherence 1 | 0.96(0.02) | 0.94(0.07) | 0.96(0.04) | 0.96(0.05) | 0.97(0.04) | 0.96(0.03) |
| Coherence 2 | 0.90(0.07) | 0.85(0.12) | 0.92(0.09) | 0.89(0.13) | 0.88(0.21) | 0.88(0.12) |
| Phase(°) | 0.65(0.27) | 0.70(0.30) | 0.85(0.40) | 0.63(0.24) | 0.97(0.44) | 0.62(0.32) |
| MEPT | ||||||
| Coherence 1 | 0.95(0.03) | 0.94(0.03) | 0.94(0.11) | 0.95(0.04) | 0.96(0.03) | 0.96(0.03) |
| Coherence 2 | 0.89(0.11) | 0.90(0.04) | 0.92(0.08) | 0.88(0.18) | 0.87(0.20) | 0.92(0.05) |
| Phase (°) | 0.52(0.24) | 0.38(0.73) | 0.75(0.31) | 0.47(0.30) | 0.87(0.47) | 0.38(0.71) |
Coherence between the perturbed torque and the measured angle at 2Hz (Coherence 1), coherence between the perturbed torque and the measured torque at 2Hz (Coherence 2), phase angle (°) of the pivoting stiffness frequency response function at 2Hz in the training and control groups at each evaluation (i.e. E1, E2, and E3). Values are means (1SD).
Entropy of Time to Peak EMG
The training group significantly reduced the entropy of time to peak EMG in the LG and Glmax during the MIPT and MEPT. Specifically, when compared to the controls, the training group significantly reduced the entropy of time to peak EMG in the LG and Glmax at E2 and E3 (LG: p=0.0121, p=0.0286, and Glmax: p=0.0099, p=0.0211 respectively), and in the Glmed at E2 (p=0.0076) during the MIPT (Table 2). The training group, compared to the control group, lowered entropy of time to peak EMG in the BF, VMO and VL at E3 (p=0.0434, p=0.0227, p= 0.0157 respectively), in the LG at E2 (p=0.0476), and in the Glmax at E2 and E3 (p=0.0269, p=0.0024 respectively) during the MEPT (Table 2).
Table 2.
Entropy of time to peak EMGs between the training and control groups.
| E1 |
E2 |
E3 |
||||
|---|---|---|---|---|---|---|
| Training (N= 20) |
Control (N=20) |
Training (N=21) |
Control (N=19) |
Training (N=21) |
Control (N=19) |
|
| MIPT | ||||||
| BF | 2.77(0.40) | 2.99(0.44) | 2.68(0.53) | 2.92(0.45) | 2.56(0.59) | 2.77(0.54) |
| MG | 2.70(0.65) | 2.77(0.71) | 2.41(0.77) | 2.76(0.71) | 2.33(0.95) | 2.69(0.50) |
| ST | 2.81(0.48) | 2.92(0.50) | 2.76(0.61) | 2.75(0.41) | 2.74(0.65) | 2.68(0.45) |
| LG | 2.85(0.50) | 2.82(0.55) | 2.45(0.66)*+ | 2.92(0.67) | 2.27(0.72)*++ | 2.78(0.67) |
| VMO | 2.46(0.46) | 2.56(0.48) | 2.55(0.60) | 2.41(0.60) | 2.39(0.54) | 2.41(0.49) |
| VL | 2.60(0.43) | 2.71(0.40) | 2.76(0.45) | 2.63(0.49) | 2.51(0.50) | 2.54(0.40) |
| Glmax | 2.63(0.31) | 2.78(0.30) | 2.52(0.38)**+ | 2.79(0.34) | 2.41(0.35)* | 2.69(0.42) |
| Glmed | 2.70(0.45) | 2.76(0.49) | 2.53(0.42)** | 2.90(0.59) | 2.47(0.58) | 2.66(0.50) |
| MEPT | ||||||
| BF | 2.89(0.42) | 2.87(0.42) | 2.70(0.53) | 2.82(0.45) | 2.58(0.54)* | 2.84(0.49) |
| MG | 2.99(0.63) | 2.91(0.60) | 3.00(0.80) | 3.00(0.55) | 2.89(0.71) | 3.10(0.60) |
| ST | 2.66(0.36) | 2.74(0.47) | 2.75(0.53) | 2.78(0.35) | 2.57(0.59) | 2.78(0.39) |
| LG | 2.85(0.67) | 3.02(0.58) | 2.63(0.84)* | 2.94(0.54) | 2.54(0.66) | 2.93(0.62) |
| VMO | 2.68(0.48) | 2.70(0.48) | 2.50(0.66) | 2.68(0.46) | 2.46(0.61)* | 2.71(0.44) |
| VL | 2.80(0.50) | 2.92(0.36) | 2.61(0.49) | 2.78(0.41) | 2.62(0.40)* | 2.88(0.43) |
| Glmax | 2.70(0.63) | 2.76(0.42) | 2.71(0.53)* | 2.95(0.45) | 2.55(0.44)** | 2.94(0.43) |
| Glmed | 2.65(0.43) | 2.92(0.52) | 2.78(0.41) | 2.73(0.27) | 2.63(0.51) | 2.80(0.36) |
Values are means (1SD) at each evaluation (i.e. E1, E2, and E3) during the motor internal perturbation task (MIPT) and the motor external perturbation task (MEPT) per muscle, namely the BF, MG, ST, LG, VMO, VL, Glmax, and Glmed.
indicates p<0.05 between the training and the control groupsat each evaluation,
indicates p<0.01 between the training and the control groupsat each evaluation.
indicates p<0.05,
indicates p<0.01 between E1 and either E2 or E3 within the group.
Functional Performances
There was a significant improvement in single-leg hop for distance (cm) between the training and control groups at E3 (p=0.021, Table 3). Although the training group showed a tendency of hopping faster, there were no statistically significant differences in 10-m walk time and 12-m hop time between the these two groups at E3 (Table 3). No significant baseline differences between the training and control groups at E1 were found in 10-m walk time, 12-m hop time, and single leg hop for distance.
Table 3.
Functional activities between the training and the control groups.
| E1 |
E3 |
|||
|---|---|---|---|---|
| Training (N=20) |
Control (N=19) |
Training (N=20) |
Control (N=18) |
|
| 10m walk time (sec) | 7.3(1.0) | 7.0(0.9) | 7.3(1.0) | 7.2(0.9) |
| 12m hop time (sec) | 5.6(1.4) | 6.1(3.2) | 5.5(1.6) | 6.3(3.2) |
| Single leg hop for distance (cm) | 140.1(33.8) | 130.7(39.7) | 153.0(41.8) | 125.9(35.5)* |
Values are means (1SD) at the first (E1) and the third (E3) evaluation.
indicates p<0.05 between the training and the control groups at each evaluation.
DISCUSSION
Developing an effective neuromuscular training program for knee injury prevention and rehabilitation has been one of the research priorities in musculoskeletal medicine. To the best of our knowledge, this is the first study investigating the effects of a novel pivoting neuromuscular training on proprioceptive acuity under weight-bearing and on pivoting neuromuscular control under injury-relevant scenarios during foot-ground-contact with pivoting perturbation. We found that pivoting neuromuscular training on an off-axis elliptical trainer (POINT) improved pivoting neuromuscular control, including decreased pivoting instability and maximum internal pivoting angle, reduced entropy of time to peak EMG in the gluteus maximus and lateral gastrocnemius muscles, and increased leg stiffness under pivoting perturbations. POINT also enhanced weight-bearing proprioceptive acuity in external pivoting and improved functional performance of single leg hop distance.
Proprioceptive Acuity
POINT marginally improved weight-bearing proprioceptive acuity evidenced by lower threshold to detection angle in external pivoting (Fig. 2). Training-induced improvement in proprioceptive acuity may be a result of heightened sensitivity of peripheral muscle spindles coupled with central facilitation of neural information, selective attention to tasks, and increased somatosensory field of proprioception in the sensory cortex (2, 38). The improvement may require multi-sessions of training, since the between-group difference approached statistical significance in external pivoting at E3, not at E2 (Fig. 2). Similar to studies investigating knee proprioceptive acuity in non-weight-bearing following multi-week neuromuscular training (11, 13), our study reported training-induced improvement. Our findings are unique and functionally relevant because proprioceptive acuity was measured under weight-bearing, where the somatosensory inputs from the hip, knee, and ankle were integrated and processed as a whole (28, 37). Thus, the findings may suggest that individuals following POINT may better sense risky pivoting positions at foot-ground-contact and potentially reduce injury (24).
Task and Functional Performance: Pivoting Instability, Pivoting Stiffness, Maximum Pivoting Angle, and Single Leg Hop Distance
POINT reduced pivoting instability and maximum internal and external pivoting angle as well as increased pivoting stiffness (Fig. 3), suggesting improved neuromuscular control in response to pivoting perturbations simulating risky situations in sports activities. Functionally, the training group single-leg-hopped farther without losing balance, implying gains in balance and knee strength during physical activities (20). Training methods emphasizing task performance improvement, rather than strength or flexibility changes, are more likely to facilitate participants’ cooperation and compliance (20). Performance measures, such as single-leg hop for distance, speed, strength, and single-limb stability, are commonly used outcomes in neuromuscular training studies (17), although these tasks mainly challenge the sagittal plane lower limb movement control. A strength of this study is that both clinically relevant performance outcomes and injury-related lower limb pivoting neuromuscular control were measured. POINT not only improved pivoting task-specific performance, but also translated into better functional performance of single leg hop for distance, indicating enhanced agility and balance. Therefore, improved pivoting neuromuscular control and single leg hop task performance following POINT may transfer to quicker and more effective control and response during real-life twisting/pivoting maneuvers.
Entropy of Time to Peak EMG
Reduced entropy of time to peak EMG in certain muscle groups might suggest improved certainty in selective muscle activation patterns during tasks. Extending the sensorimotor system’s performance beyond its prior limits (19, 39), motor skill acquisition is hallmarked by reduction of endpoint errors (35) and progressively more specific and efficient motor commands over the course of training/learning (3, 31). Reduction of entropy of time to peak EMG coupled with better accuracy in maintaining lower limb positioning during pivoting perturbations may suggest higher specificity and efficiency in motor commands. Reduced entropy of time to peak EMG of gluteus maximus (Glmax) and lateral gastrocnemius (LG) under pivoting perturbations (Table 2) may signify better neuromuscular coordination in controlling and avoiding potential injurious lower limb positions. With a large cross-sectional area and three-dimensional orientation, Glmax is a potent hip extensor and external rotator, acting concentrically or eccentrically to generate power, absorb impact, and maintain optimal lower limb positions (16). Crossing the both knee and ankle, the gastrocnemius plays a critical role in lower limb dynamic stability (15).
The positive outcomes following POINT might be achieved through multi-modal feedback provided during training, including real-time audio-visual feedback of subjects’ pivoting angle (reflecting the ability to control pivoting movements), lower limb frontal-plane alignment (via the web camera), and sensory stimuli (e.g. spring or slippery surface, external perturbation) to the feet. Similar to previous ACL injury prevention training utilizing verbal and visual biofeedback (32) and instructional videos to facilitate awareness and maintenance of proper body positions and movements (30) during running, jumping, or landing, our study subjects were instructed to maintain proper lower limb positions by pointing the second toe forward through real-time biofeedback during stepping, while experiencing pivoting perturbations and slippery walkaway mimicking potential ACL injury related situations at foot-ground-contact.
It is unclear whether the positive training outcomes in the current study predicts future ACL injury rate reduction; future larger cohort studies are needed to investigate injury rates following POINT. We were not able to randomly assign subjects into the training or control group due to subject availability for 6-week multiple training sessions. To reduce potential bias from sample non-randomization, we used propensity score analysis with multivariable regression adjustment, a common method used in clinical and epidemiological studies when randomization is challenging (12, 18), to compare the outcomes between groups. Considering that some studies lacked a control group and did not use randomization (17), our results are valuable in providing a basis for further investigation, such as prospective cohort studies and randomized clinical trials, to validate the effectiveness of POINT on injury prevention and rehabilitation. Task-level measures in our study may not specifically address deficits at individual joints and the relationship between task-level measures (contributed by multi-joints). However, for injury prevention, task-level neuromechanical responses might allow us to focus on improving task-level performance under injury-related situations.
CONCLUSION
Six-week Pivoting Off-axis Intensity adjustable Neuromuscular control Training (POINT) improved proprioceptive acuity, pivoting neuromuscular control, and functional task performance in healthy individuals. The learned motor skills in pivoting neuromuscular control under potentially injury-causing conditions may translate to better lower limb control during unstable circumstances involving perturbations or slippery walkway. The findings of this study may provide a basis for developing ACL injury prevention and rehabilitation strategies addressing the primary underlying mechanism of inadequate neuromuscular control during pivoting movements at foot-ground-contact.
ACKNOWLEDGEMENT
The authors would like to thank the National Institutes of Health, the National Science Foundation, and National Institute on Disability and Rehabilitation Research for grant supports as well as all subjects who participated in the current study, and Dr. Jungwha Lee who provided advice on the statistical methods. The results of the present study do not constitute endorsement by ACSM.
Footnotes
CONFLICTS OF INTEREST
Li-Qun Zhang and Yupeng Ren hold equity positions in Rehabtek LLC, which is involved in developing the off-axis elliptical trainer in this study. Song Joo Lee, Alison H. Chang, and François Geiger do not have conflicts of interest.
REFERNCES
- 1.Aglietti P, Insall JN, Cerulli G. Patellar pain and incongruence. I: Measurements of incongruence. Clin. Orthop. Relat. Res. 1983;(176):217–224. [PubMed] [Google Scholar]
- 2.Ashton-Miller JA, Wojtys EM, Huston LJ, Fry-Welch D. Can proprioception really be improved by exercises? Knee Surg. Sports Traumatol. Arthrosc. 2001;9:128–136. doi: 10.1007/s001670100208. [DOI] [PubMed] [Google Scholar]
- 3.Basmajian J, De Luca CJ. Muscle Alive-Their functions revealed by electromyography. 5th ed. Baltimore: Williams and Wilkins; 1985. pp. 1–561. [Google Scholar]
- 4.Bendat JS, Piersol AG. Random Data: Analysis & Measurement Procedures. 4th ed. Hoboken, NJ: John Wiley & Sons; 2010. pp. 1–530. [Google Scholar]
- 5.Benjaminse A, Otten E. ACL injury prevention, more effective with a different way of motor learning? Knee Surg. Sports Traumatol. Arthrosc. 2011;19(4):622–627. doi: 10.1007/s00167-010-1313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boden BP, Dean GS, Feagin JA, Jr, Garrett WE., Jr Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- 7.Burnfield JM, Shu Y, Buster T, Taylor A. Similarity of joint kinematics and muscle demands between elliptical training and walking: implications for practice. Phys. Ther. 2010;90(2):289–305. doi: 10.2522/ptj.20090033. [DOI] [PubMed] [Google Scholar]
- 8.Cavanaugh JT, Guskiewicz KM, Stergiou N. A nonlinear dynamic approach for evaluating postural control: new directions for the management of sport-related cerebral concussion. Sports Med. 2005;35(11):935–950. doi: 10.2165/00007256-200535110-00002. [DOI] [PubMed] [Google Scholar]
- 9.Chang PH, Kang SH. Stochastic estimation of human arm impedance under nonlinear friction in robot joints: a model study. J. Neurosci. Methods. 2010;189(1):97–112. doi: 10.1016/j.jneumeth.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Clark FJ, Burgess RC, Chapin JW, Lipscomb WT. Role of intramuscular receptors in the awareness of limb position. J. Neurophysiol. 1985;54(6):1529–1540. doi: 10.1152/jn.1985.54.6.1529. [DOI] [PubMed] [Google Scholar]
- 11.Cug M, Ak E, Ozdemir RA, Korkusuz F, Behm DG. The effect of instability training on knee joint proprioception and core strength. J. Sports Sci. Med. 2012;11:468–474. [PMC free article] [PubMed] [Google Scholar]
- 12.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 1998;17(19):2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 13.Eils E, Schroter R, Schroder M, Gerss J, Rosenbaum D. Multistation proprioceptive exercise program prevents ankle injuries in basketball. Med. Sci. Sports Exerc. 2010;42(11):2098–2105. doi: 10.1249/MSS.0b013e3181e03667. [DOI] [PubMed] [Google Scholar]
- 14.Enoka RM, editor. Neuromechanics of Human Movement. 3rd ed. Boulder, CO: Human Kinetics; 2002. pp. 249–300. [Google Scholar]
- 15.Fleming BC, Renstrom PA, Ohlen Gl, et al. The gastrocnemius muscle is an antagonist of the anterior cruciate ligament. J. Orthop. Res. 2001;19(6):1178–1184. doi: 10.1016/S0736-0266(01)00057-2. [DOI] [PubMed] [Google Scholar]
- 16.Geiger F. Training for knee injury prevention and rehabilitation using a pivoting elliptical exercise machine [Master's thesis] Zurich: ETH Zurich; 2008. pp. 1–150. [Google Scholar]
- 17.Griffin LY, Albohm MJ, Arendt EA, et al. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am. J. Sports Med. 2006;34(9):1512–1532. doi: 10.1177/0363546506286866. [DOI] [PubMed] [Google Scholar]
- 18.Guo S, Fraser MW. Propensity Score Analysis: Statistical Methods and Applications. Los Angeles ; London: SAGE Publications; 2009. pp. 1–210. [Google Scholar]
- 19.Hallett M, Pascual-Leone A, Topka H. The Acquisition of Motor Behavior in Vertebrates. Cambridge, MA: MIT press; 1996. pp. 287–430. [Google Scholar]
- 20.Hewett TE, Shultz SJ, Griffin LY American Orthopaedic Society for Sports Medicine. Understanding and preventing noncontact ACL injuries. Champaign, IL: Human Kinetics; 2007. pp. 1–182. [Google Scholar]
- 21.Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J. Athl. Train. 2007;42(2):311–319. [PMC free article] [PubMed] [Google Scholar]
- 22.Hubscher M, Zech A, Pfeifer K, Hansel F, Vogt L, Banzer W. Neuromuscular training for sports injury prevention: a systematic review. Med. Sci. Sports Exerc. 2010;42(3):413–421. doi: 10.1249/MSS.0b013e3181b88d37. [DOI] [PubMed] [Google Scholar]
- 23.Kearney RE, Hunter IW. System identification of human joint dynamics. Crit. Rev. Biomed. Eng. 1990;18(1):55–87. [PubMed] [Google Scholar]
- 24.Kobayashi H, Kanamura T, Koshida S, et al. Mechanisms of the anterior cruicate ligament injury in sports activities: a twenty-year clinical research of 1700 athletes. J. Sports Sci. Med. 2010;9:669–675. [PMC free article] [PubMed] [Google Scholar]
- 25.Kurz MJ, Stergiou N. The aging human neuromuscular system expresses less certainty for selecting joint kinematics during gait. Neurosci. Lett. 2003;348(3):155–158. doi: 10.1016/s0304-3940(03)00736-5. [DOI] [PubMed] [Google Scholar]
- 26.Lee SJ, Hidler J. Biomechanics of overground vs. treadmill walking in healthy individuals. J. Appl. Physiol. 2008;104(3):747–755. doi: 10.1152/japplphysiol.01380.2006. [DOI] [PubMed] [Google Scholar]
- 27.Lee SJ, Ren Y, Geiger F, Zhang L-Q. Gender differences in offaxis neuromuscular control during stepping under a slippery condition. Eur. J. Appl. Physiol. 2013;113(11):2857–2866. doi: 10.1007/s00421-013-2727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lephart SM, Pincivero DM, Rozzi SL. Proprioception of the ankle and knee. Sports Med. 1998;25(3):149–155. doi: 10.2165/00007256-199825030-00002. [DOI] [PubMed] [Google Scholar]
- 29.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am. J. Sports Med. 2007;35(10):1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 30.Mandelbaum BR, Silvers HJ, Watanabe DS, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am. J. Sports Med. 2005;33(7):1003–1010. doi: 10.1177/0363546504272261. [DOI] [PubMed] [Google Scholar]
- 31.Moore SP, Marteniuk RG. Kinematic and electromyographic changes that occur as a function of learning a time-constrained aiming task. J. Mot. Behav. 1986;18(4):397–426. doi: 10.1080/00222895.1986.10735388. [DOI] [PubMed] [Google Scholar]
- 32.Myklebust G, Engebretsen L, Braekken IH, Skjolberg A, Olsen OE, Bahr R. Prevention of anterior cruciate ligament injuries in female team handball players: a prospective intervention study over three seasons. Clin. J. Sport Med. 2003;13(2):71–78. doi: 10.1097/00042752-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am. J. Sports Med. 1991;19:513–518. doi: 10.1177/036354659101900518. [DOI] [PubMed] [Google Scholar]
- 34.Quatman CE, Quatman-Yates CC, Hewett TE. A 'plane' explanation of anterior cruciate ligament injury mechanisms: a systematic review. Sports Med. 2010;40(9):729–746. doi: 10.2165/11534950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Ren Y, Lee SJ, Park HS, Zhang LQ. A Pivoting Elliptical Training System for Improving Pivoting Neuromuscular Control and Rehabilitating Musculoskeletal Injuries. IEEE Trans. Neural Syst. Rehabil. Eng. 2013;21(5):860–868. doi: 10.1109/TNSRE.2013.2273874. [DOI] [PubMed] [Google Scholar]
- 36.Rieke F, Warland D, de Ruyter van Steveninck R, Bialek W. Spikes: Exploring the neural code. Cambridge, MA: MIT press; 1999. pp. 102–113. [Google Scholar]
- 37.Riemann BL, Lephart SM. The Sensorimotor System, Part II: The Role of Proprioception in Motor Control and Functional Joint Stability. J. Athl. Train. 2002;37(1):80–84. [PMC free article] [PubMed] [Google Scholar]
- 38.Roos EM, Herzog W, Block JA, Bennell KL. Muscle weakness, afferent sensory dysfunction and exercise in knee osteoarthritis. Nat. Rev. Rheumatol. 2011;7(1):57–63. doi: 10.1038/nrrheum.2010.195. [DOI] [PubMed] [Google Scholar]
- 39.Shadmehr R, Wise SP. The Computational Neurobiology of Reaching and Pointing. Cambridge: MIT press; 2005. pp. 46–51. [Google Scholar]


