Abstract
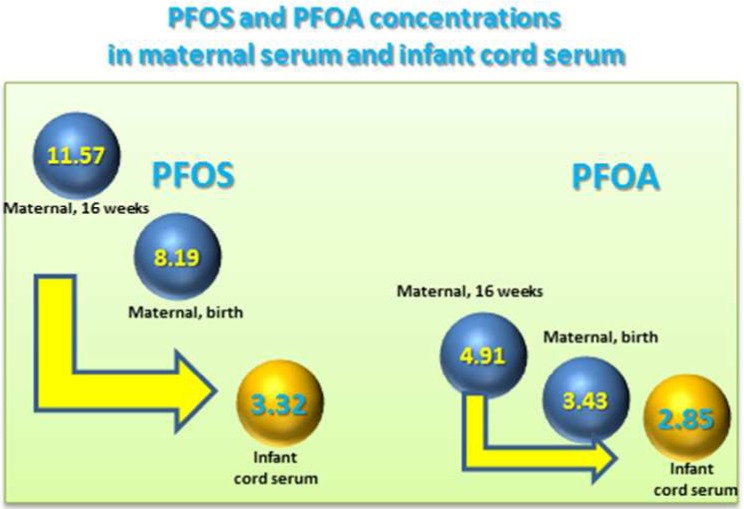
Data on predictors of gestational exposure to poly- and perfluoroalkyl substances (PFASs) in the United States are limited. To fill in this gap, in a multiethnic cohort of Ohio pregnant women recruited in 2003–2006, we measured perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and six additional PFASs in maternal serum at ∼16 weeks gestation (N = 182) and delivery (N = 78), and in umbilical cord serum (N = 202). We used linear regression to examine associations between maternal serum PFASs concentrations and demographic, perinatal, and lifestyle factors. PFASs concentrations in maternal sera and in their infants’ cord sera were highly correlated (Spearman rank correlation coefficients = 0.73–0.95). In 71 maternal-infant dyads, unadjusted geometric mean (GM) concentrations (95% confidence interval) (in μg/L) in maternal serum at delivery of PFOS [8.50 (7.01–9.58)] and PFOA [3.43 (3.01–3.90)] were significantly lower than at 16 weeks gestation [11.57 (9.90–13.53], 4.91 (4.32–5.59), respectively], but higher than in infants’ cord serum [3.32 (2.84–3.89), 2.85 (2.51–3.24), respectively] (P < 0.001). Women who were parous, with a history of previous breastfeeding, black, or in the lowest income category had significantly lower PFOS and PFOA GM concentrations than other women. These data suggest transplacental transfer of PFASs during pregnancy and nursing for the first time in a U.S. birth cohort.
Introduction
Poly- and perfluoroalkyl substances (PFASs) are synthetic fluorinated chemicals that have been used in a wide range of consumer products and industrial applications over six decades.1 Because the remarkable strength of the fluorine–carbon covalent bond confers thermal and chemical stability, some PFASs are highly resistant to both chemical and biological degradation under normal environmental conditions.1
The global occurrence of certain PFASs, persistence in the environment and bioaccumulation in biota have raised concerns about human exposures to PFASs,2 and human biomonitoring has gained importance for exposure assessment of PFASs in the general population of several countries.3−9 While human exposure to PFASs is well recognized, pregnant women, infants, and young children are often poorly represented in general population biomonitoring surveys. Biomonitoring studies among subpopulations susceptible to the effects of potentially harmful environmental chemicals, such as PFASs, are of interest because exposures during these critical windows of susceptibility may impact health later in life.
Pregnant women worldwide are exposed to PFASs10−24—even though data on pregnant women in the United States are limited14,15,25—and several studies have identified previous pregnancies, breastfeeding duration, and diet as important determinants of exposure to PFASs among pregnant women.12,14,15,23,26 Of interest, PFASs can be transported across the placenta; several PFASs have been detected in cord serum.13,17,18,20,21,24,27−43 Furthermore, the transfer of PFASs across the placenta may differ depending on the compound. For example, available scientific evidence suggest that PFOA may pass through the placenta more easily than PFOS.17,20,21,24,28,30,32,34,35,39,41 Data on paired maternal and cord blood PFASs concentrations also exist for populations around the world, but not in the United States.13,17,18,20,21,24,28,30,32,34,35,37,39,41,43
To improve our understanding of in utero exposure to PFASs among U.S. women, we quantified the concentrations of PFASs in maternal serum during pregnancy and at delivery, and in cord sera, and evaluated the trends of PFASs concentrations and their correlations during gestation in a cohort of Ohio women. We also determined the correlations of select PFASs for paired maternal–cord sera, and examined associations between maternal serum PFASs concentrations and demographic and lifestyle factors.
Experimental Section
Study Population
Data were collected from mothers and their children participating in the Health Outcomes and Measures of the Environment (HOME) Study, an ongoing prospective birth cohort in the Cincinnati, Ohio (U.S.A.) metropolitan area, designed to examine low-level exposure to environmental toxicants and the efficacy of injury and lead hazard controls in the home. Between March 2003 and January 2006, pregnant women were recruited from seven prenatal clinics associated with three hospitals. Eligibility criteria for the study included ≤19 weeks of gestation, age ≥18 years, living in a house built before 1978, negative HIV status, and not taking medications for seizure or thyroid disorders. HOME Study staff mailed letters to 5184 women who were ≥18 years of age and living in a house built before 1978. Of the 1263 eligible women, 468 provided informed consent and enrolled in the study. The Institutional review boards of all involved research institutions, hospitals, and laboratories approved the study protocol.
Women provided three serum samples collected at approximately 16 and 26 weeks of gestation and within 24 h of parturition. For this project, we used the concentrations of PFASs in the 16 week samples, collected at the women’s prenatal care appointment visit, to estimate the woman’s gestational exposure to PFASs. Umbilical cord blood was collected at the time of infant delivery. We analyzed a total of 182 (16-week), 78 (delivery), and 202 (cord) serum samples, including 71 maternal-infant dyads with mothers’ PFASs concentrations available at both 16 week of gestation and delivery.
Quantification of PFASs Concentrations in Serum
By using a modification of our analytical method,44 we measured eight PFASs in maternal and umbilical cord sera: 2-(N-methyl-perfluorooctane sulfonamido) acetate (Me-PFOSA-AcOH), 2-(N-ethyl-perfluorooctane sulfonamido) acetate (Et-PFOSA-AcOH), perfluorohexanesulfonate (PFHxS), perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), perfluorononanoate (PFNA), perfluorodecanoate (PFDeA), and perfluorooctane sulfonamide (PFOSA). We used the following isotope-labeled internal standards for quantification: 13C4-PFOS, 18O2-PFHxS, 13C2-PFOA, 13C5-PFNA, 13C2-PFDeA, D3-Me-PFOSA-AcOH, and D5-Et-PFOSA-AcOH, 18O2-PFOSA. To account for potential matrix effects, we spiked the calibration standards into calf serum (Gibco, Grand Island, NY). Briefly, we added 325 μL of 0.1 M formic acid and 25 μL of internal standard solution to 50 μL of serum in a 1.5 mL polypropylene autosampler vial, and the spiked serum was vortex-mixed. The sera vials were placed on a Symbiosis online SPE system (Spark Holland, Plainsboro, NJ) for the preconcentration of the analytes on a Polaris C18 cartridge (7 μm, 10 × 1 mm; Spark Holland). The analytes were transferred onto a Betasil C8 HPLC column (3 × 50 mm, 5 μm; ThermoHypersil Keystone, Bellefonte, PA), separated by HPLC (mobile phase A: 20 mM ammonium acetate in water, pH = 4; mobile phase B: acetonitrile), and detected by negative-ion TurboIonspray-tandem mass spectrometry on an API 4000 mass spectrometer (Applied Biosystems, Foster City, CA). The limits of detection (LODs) were 0.087 μg/L for Me-PFOSA-AcOH, 0.082 μg/L for PFNA, 0.1 μg/L for PFHxS, PFOA, PFDeA, PFOSA, and Et-PFOSA-AcOH, and 0.2 μg/L for PFOS. The reported concentrations are for the sum of linear and branched isomers of PFOS and PFOA. Low-concentration quality control materials (QCs) and high-concentration QCs, prepared from a calf serum pool, were analyzed with the study samples and with reagent and serum blanks to ensure the accuracy and reliability of the data.44 The coefficients of variation of repeated measurements of the QCs within a period of almost one year are around 6% (http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/PFAS_F_Polyfluorinated_Compounds_met.pdf).
Predictors of PFASs Concentrations
We examined the association between maternal serum PFASs concentrations and demographic, perinatal, and environmental variables collected from questionnaires and biological samples. These variables have been used as covariates in PFASs-related epidemiological studies. Demographic factors included maternal age, education, race, and household income. Perinatal and maternal factors included gestational age, parity, history of breastfeeding, prepregnancy body mass index (BMI), and serum cotinine. Serum cotinine concentrations were measured in maternal samples collected at 16 weeks of gestation and within 24 h of birth, as well as in cord serum at delivery.
Statistical Analysis
All analyses were conducted using SAS version 9.1 (SAS Institute, Cary, NC). For statistical analysis, PFAS concentrations were log10 transformed. For concentrations below the LOD, we used LOD divided by the square root of 2.45 We calculated the median concentration for maternal and cord sera, and for the 71 maternal-infant dyads, we used the nonparametric Wilcoxon signed rank test to compare each woman’s medians at different time points over the course of pregnancy and in their infant’s cord sera. Moreover, for the 71 pairs with available maternal serum concentrations at 16 weeks and birth, and cord serum concentrations at delivery, we calculated the unadjusted geometric mean (GM) PFASs concentrations, and used a paired t test to compare the unadjusted GM for each pair of time points. We also determined the Spearman rank correlations among the log10 transformed concentrations of PFASs in maternal and cord sera at birth, and in maternal sera at birth and at 16 weeks gestation. Statistical significance was set at P < 0.05 for all analyses.
We used linear regression to model separately the log10 transformed PFAS concentrations of cord serum or mothers’ serum at 16 weeks gestation for each predictor variable (covariate). We included the following covariates one at a time: gestational age (term [ ≥ 37 weeks], preterm [<37 weeks]), maternal age, parity, prepregnancy BMI (obese [≥30 kg/m2], overweight [25–29.9 kg/m2], normal weight [18.5–24.9 kg/m2], underweight [<18.5 kg/m2]), history of previous breast feeding (yes/no), household income (<$20K, $20K–$40K, $40K–<$80K, >$80K), maternal education, race (non-Hispanic white, non-Hispanic black, other), and serum cotinine [active (>3 μg/L), secondhand (0.015–3 μg/L), or no (<0.015 μg/L) environmental tobacco smoke exposure]. Cotinine in serum was measured using HPLC-tandem mass spectrometry (LOD = 0.015 μg/L) as described before.46 We calculated the GM concentrations and their 95% confidence intervals (CI) by categories of each predictor. Beta coefficients were exponentiated from the regression models to produce the ratio of PFAS GM concentrations between categories of predictor variables. Thus, numeric estimates indicate that the GM concentrations were higher (>1) or lower (<1) for the predictor in that category compared with the reference category.
Results
The frequency of detection and concentrations of 8 PFASs in maternal sera during pregnancy and delivery and in cord sera are shown in Table 1 for the 71 paired maternal/infant samples and in the Supporting Information, SI (Tables S1 and S2). The geometric means of concentration ratios for the 71 dyads are also shown in the SI (Table S3). We detected PFOS, PFOA, PFNA, and PFHxS in more than 90% of the paired samples, and Me-PFOSA-AcOH in about 90%. For the other analytes, we did not perform additional analyses because the frequency of detection was <30% at all of the time points examined (i.e., Et-PFOSA-AcOH, PFOSA) or the median was close to the LOD (i.e., PFDeA). The median concentrations in cord serum were lower than the corresponding paired maternal concentrations (Table 1).
Table 1. Median Concentrations (in μg/L) of PFASs and Frequency of Detection for 71 Paired Maternal–Infant Serum Samples Collected during Gestation and at Deliverya.
| maternal, 16 weeks [frequency of detection, %] | maternal, delivery [frequency of detection, %] | infant’s cord serum [frequency of detection, %] | |
|---|---|---|---|
| Et-PFOSA-AcOH | <LOD [23.9] | <LOD [9.9] | <LOD [11.3] |
| Me-PFOSA-AcOH | 0.50 [100] | 0.20 [88.7] | 0.30 [93.0] |
| PFOSA | <LOD [1.4] | <LOD [0] | <LOD [0] |
| PFHxS | 1.20 [98.6] | 1.20 [93.0] | 0.60 [97.2] |
| PFOS | 12.70 [100] | 8.50 [100] | 3.50 [98.6] |
| PFOA | 4.80 [100] | 3.30 [100] | 3.10 [100] |
| PFNA | 0.82 [100] | 0.66 [100] | 0.41 [98.6] |
| PFDeA | 0.20 [97.2] | 0.20 [90.1] | <LOD [16.9] |
The limits of detection (LODs) were 0.087 μg/L (Me-PFOSA-AcOH), 0.082 μg/L (PFNA), 0.1 μg/L (PFHxS, PFOA, PFDeA, PFOSA, Et-PFOSA-AcOH), and 0.2 μg/L (PFOS).
We determined the Spearman rank correlations between the log10 transformed maternal concentrations of Me-PFOSA-AcOH, PFHxS, PFOS, PFOA, and PFNA at 16 weeks and at delivery and their infants’ cord serum concentrations (Table 2). Correlations between the concentrations in maternal and infants’ samples collected at delivery (Spearman rank correlation coefficient (r) = 0.79–0.92) and between the maternal samples at 16 weeks gestation and the infants’ cord serum (r = 0.73–0.95) were high. The PFAS concentrations in maternal sera at 16 weeks gestation and at delivery were also highly correlated (r = 0.84–0.94).
Table 2. Spearman Rank Correlation Coefficients between Log10 Concentrations of Select PFASs in Maternal Serum at 16 Weeks Gestation, At Birth, And in Infant Cord Serum (n = 71 Mother–Infant Pairs)a,b.
| analyte | maternal serum at 16 weeks and at birth (N = 71) | maternal serum at 16 weeks and infant cord serum (N = 182) | maternal serum at birth and infant cord serum (N = 78) |
|---|---|---|---|
| Me-PFOSA-AcOH | 0.84 | 0.73 | 0.92 |
| PFHxS | 0.92 | 0.95 | 0.89 |
| PFOS | 0.87 | 0.83 | 0.82 |
| PFOA | 0.94 | 0.92 | 0.88 |
| PFNA | 0.88 | 0.77 | 0.79 |
Selected PFASs were those detected in >60% of samples at all time points.
P values for all the comparisons were <0.05.
We observed a statistically significant decrease in the unadjusted GM maternal serum concentrations of most PFASs from 16 weeks gestation to delivery (all paired t test P values <0.001) (Figure 1). Still, maternal concentrations of PFOS, PFOA, PFNA, and PFHxS at birth were significantly higher than cord concentrations (all paired t test P values <0.001), but were lower for Me-PFOSA-AcOH (P = 0.01). Specifically, the maternal geometric mean concentrations of PFOS, PFOA, PFNA, and PFHxS decreased 25–43%, depending on the compound, between 16 weeks gestation and delivery (SI Table S4). The percent change of geometric mean concentrations between maternal and cord sera during gestation and delivery for these five PFASs are shown in the SI (Table S4).
Figure 1.
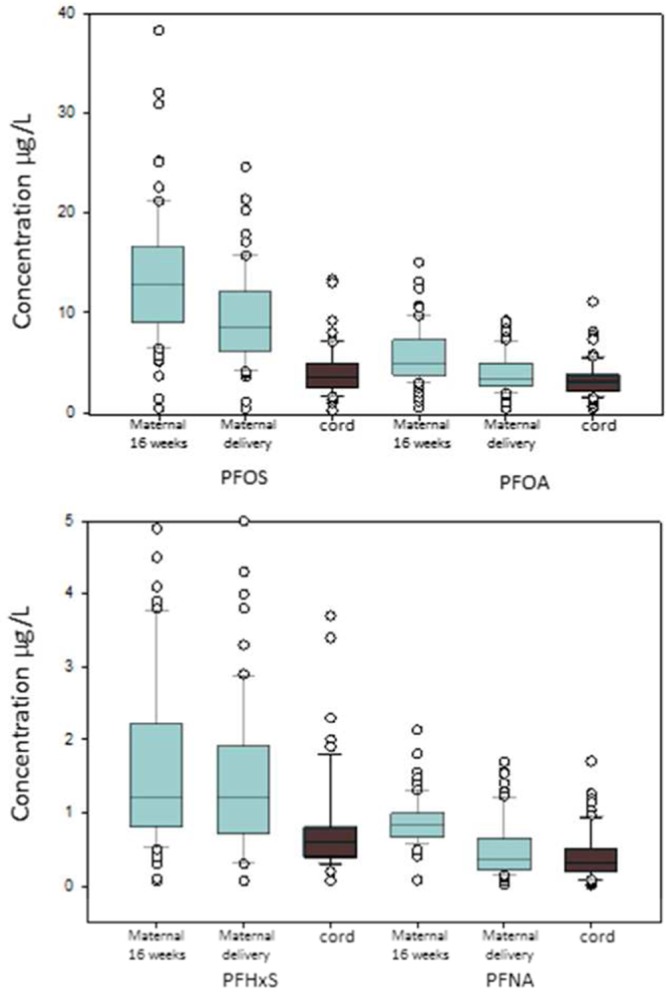
Concentrations (in μg/L) for select PFASs in maternal serum at 16 weeks gestation and delivery, and in cord serum (n = 71 paired samples). Whiskers represent the 5th and 95th percentiles. The top and bottom edges of each box represent the 75th and 25th percentiles, respectively. The horizontal line in each box represents the median. Circles represent observations outside the 10th and 90th percentiles.
The Wilcoxon signed rank test showed the same pattern for the medians: maternal serum median concentrations of PFOS, PFOA, PFNA, and Me-PFOSA-AcOH decreased significantly from 16 weeks gestation to birth (all P values <0.001). Also, maternal median concentrations of PFOS, PFOA, PFNA, and PFHxS at delivery were significantly higher than the infants’ cord serum concentrations (P values <0.001). The maternal median concentrations of Me-PFOSA-AcOH were lower than in cord serum, but they did not reach statistical significance (P = 0.46). Because concentrations of Me-PFOSA-AcOH in the mothers’ serum at birth and their infants’ cord serum were close to the LOD, these data should be interpreted cautiously.
Maternal serum concentrations of PFOS, PFOA, PFNA, and PFHxS at 16 weeks gestation were lower in women younger than 25 years of age compared with older women, but only reached statistical significance for PFOS and PFHxS (Table 3). Non-Hispanic black women and women with the lowest household income had significantly lower concentrations of PFOS, PFOA, PFNA, and PFHxS than non-Hispanic white women and women in the most affluent households, respectively. Obese women had significantly lower PFOS and PFNA concentrations than other women; active smokers had significantly lower PFOS concentrations than nonsmokers or second-hand smokers; women with a high school education or below had significantly lower PFHxS concentrations than women having more than a high school education. Also, a history of previous breastfeeding was inversely and significantly associated with PFOS and PFOA serum concentrations, and nulliparous women had significantly higher concentrations of PFOS and PFOA than parous women (Table 3). Breastfeeding and parity were significantly associated with each other (χ2 test P-value = <0.001), but parous women’s GM concentrations were essentially the same when we included parity and breastfeeding history together (data not shown) or separately (Table 3) in the model. For example, GM concentrations of PFOS were 13.19 μg/L (parity = 1) and 11.3 μg/L (parity >1) compared to 13.06 μg/L and 11.37 μg/L, respectively. Gestational age (preterm vs term) was not significantly associated with the concentrations of any of the PFASs examined. Cord serum concentrations varied similarly by demographic, perinatal, and lifestyle factors (SI Table S5) and will not be discussed further.
Table 3. Geometric Mean Maternal Prenatal (16 weeks) Concentrations of PFOS, PFOA, PFNA, PFHxS, and Me-PFOSA-AcOH (μg/L) According to Demographic, Perinatal and Lifestyle Factorsa.
| variables | PFOS |
PFOA |
PFNA |
PFHxS |
Me-PFOSA-AcOH |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | GM (95% CI) | GM ratio (95% CI) | GM (95% CI) | GM ratio (95% CI) | GM (95% CI) | GM ratio (95% CI) | GM (95% CI) | GM ratio (95% CI) | GM (95% CI) | GM ratio (95% CI) | |
| maternal age (years) | |||||||||||
| <25 | 40 (22) | 10.87 (9.16–12.9) | 0.8 (0.65–0.97)b | 4.89 (4.12–5.81) | 0.94 (0.77–1.14) | 0.73 (0.64–0.83) | 0.91 (0.78–1.05) | 1.02 (0.8–1.29) | 0.63 (0.48–0.83)b | 0.47 (0.38–0.57) | 1.12 (0.88–1.41) |
| >35 | 30 (16) | 14.94 (12.26–18.21) | 1.09 (0.87–1.37) | 6.77 (5.55–8.26) | 1.3 (1.04–1.62)b | 0.93 (0.8–1.07) | 1.15 (0.98–1.36) | 1.65 (1.26–2.16) | 1.02(0.75–1.38) | 0.43 (0.34–0.54) | 1.02 (0.78–1.32) |
| 25–34 | 112 (62) | 13.67 (12.34–15.14) | ref | 5.22 (4.71–5.79) | ref | 0.8 (0.75–0.87) | ref | 1.62 (1.41–1.87) | ref | 0.42 (0.37–0.47) | ref |
| race | |||||||||||
| non-hispanic black | 56 (31.1) | 10.85 (9.4–12.53) | 0.76 (0.64–0.9)b | 4.41 (3.82–5.1) | 0.75 (0.63–0.9)b | 0.73 (0.65–0.81) | 0.86 (0.76–0.98)b | 0.86 (0.72–1.03) | 0.47 (0.38–0.59)b | 0.46 (0.39–0.54) | 1.12 (0.91–1.38) |
| other | 10 (5.6) | 16.07 (11.43–22.59) | 1.12 (0.79–1.6) | 6.05 (4.3–8.53) | 1.03 (0.72–1.47) | 0.98 (0.76–1.26) | 1.17 (0.9–1.52) | 2.43 (1.58–3.74) | 1.34(0.85–2.09) | 0.51 (0.34–0.76) | 1.25 (0.83–1.89) |
| non-hispanic white | 114 (63.3) | 14.3 (12.93–15.82) | ref | 5.87 (5.3–6.5) | ref | 0.84 (0.78–0.9) | ref | 1.82 (1.6–2.07) | ref | 0.41 (0.36–0.46) | ref |
| household income | |||||||||||
| <$20 000 | 29 (16) | 9.44 (7.73–11.51) | 0.63 (0.49–0.81)b | 4.1 (3.35–5.03) | 0.7 (0.54–0.9)b | 0.64 (0.55–0.74) | 0.77 (0.64–0.93)b | 0.84 (0.64–1.1) | 0.49 (0.35–0.69)b | 0.48 (0.38–0.6) | 1.02 (0.76–1.36) |
| $20 000–$40 000 | 37 (20.6) | 13.29 (11.15–15.85) | 0.89 (0.71–1.13) | 5.35 (4.47–6.4) | 0.91 (0.72–1.15) | 0.84 (0.74–0.96) | 1.02 (0.86–1.21) | 1.4 (1.1–1.77) | 0.82 (0.59–1.12) | 0.43 (0.35–0.53) | 0.92 (0.7–1.21) |
| $40 000–$80 000 | 64 (35.6) | 13.98 (12.23–15.98) | 0.94 (0.77–1.15) | 5.69 (4.96–6.52) | 0.97 (0.79–1.19) | 0.86 (0.78–0.95) | 1.04 (0.9–1.21) | 1.72 (1.43–2.06) | 1 (0.76–1.32) | 0.38 (0.33–0.45) | 0.81 (0.64–1.03) |
| >$80 000 | 50 (27.8) | 14.87 (12.78–17.3) | ref | 5.89 (5.05–6.87) | ref | 0.83 (0.74–0.92) | ref | 1.71 (1.39–2.1) | ref | 0.47 (0.39–0.56) | ref |
| BMIc | |||||||||||
| obese (≥30 kg/m2) | 67 (36.8) | 11.45 (9.77–13.43) | 0.81 (0.66–1)b | 4.95 (4.22–5.82) | 0.88 (0.71–1.08) | 0.7 (0.63–0.79) | 0.81 (0.7–0.94)b | 1.22 (0.98–1.52) | 0.84 (0.63–1.11) | 0.44 (0.36–0.52) | 0.93 (0.73–1.17) |
| overweight (25– 29.9 kg/m2) | 47 (25.8) | 13.59 (11.89–15.54) | 0.97 (0.8–1.17) | 5.45 (4.75–6.24) | 0.96 (0.8–1.17) | 0.84 (0.76–0.92) | 0.96 (0.84–1.1) | 1.71 (1.42–2.06) | 1.17 (0.9–1.52) | 0.39 (0.34–0.46) | 0.83 (0.67–1.04) |
| normal (<24.9 kg/m2) | 66 (36.4) | 14.08 (12.32–16.08) | ref | 5.65 (4.93–6.46) | ref | 0.87 (0.79–0.96) | ref | 1.46 (1.21–1.76) | ref | 0.47 (0.4–0.55) | ref |
| breast feeding history | |||||||||||
| prev breast feed | 74 (41) | 11.85 (10.44–13.45) | 0.83 (0.71–0.98)b | 4.47 (3.95–5.06) | 0.73 (0.62–.86)b | 0.77 (0.7–0.85) | 0.93(0.82–1.05) | 1.34 (1.13–1.61) | 0.86 (0.69–1.09) | 0.43 (0.37–0.5) | 0.99 (0.82–1.2) |
| not prev breast feed | 108 (59) | 14.2 (12.78–15.76) | ref | 6.1 (5.5–6.75) | ref | 0.83 (0.77–0.9) | ref | 1.56 (1.35–1.8) | ref | 0.43 (0.38–0.49) | ref |
| education | |||||||||||
| <12 years | 12 (6.7) | 11.08 (8.07–15.23) | 0.81 (0.58–1.13) | 4.99 (3.62–6.88) | 0.92 (0.66–1.28) | 0.69 (0.55–0.87) | 0.84 (0.66–1.07) | 0.94 (0.61–1.45) | 0.59 (0.38–0.93)b | 0.51 (0.36–0.73) | 1.24 (0.85–1.8) |
| 12 years | 25 (13.9) | 11.77 (9.45–14.67) | 0.86 (0.68–1.09) | 5.24 (4.19–6.54) | 0.96 (0.76–1.23) | 0.8 (0.68–0.94) | 0.97 (0.82–1.16) | 1.14 (0.84–1.54) | 0.72 (0.52–1.00)b | 0.5 (0.39–0.64) | 1.2 (0.91–1.58) |
| >12 years | 143 (79.4) | 13.68 (12.48–15) | ref | 5.44 (4.96–5.97) | ref | 0.82 (0.77–0.88) | ref | 1.59 (1.4–1.8) | ref | 0.41 (0.37–0.46) | ref |
| gestational age | |||||||||||
| preterm (<37 weeks) | 14 (7.7) | 11.25 (8.39–15.09) | 0.84 (0.62–1.14) | 5.18 (3.85–6.96) | 0.96 (0.71–1.31) | 0.73 (0.59–0.91) | 0.9 (0.72–1.13) | 1.31 (0.87–1.97) | 0.89 (0.58–1.36) | 0.5 (0.36–0.7) | 1.18 (0.83–1.68) |
| term (≥37 weeks) | 168 (92.3) | 13.37 (12.28–14.55) | ref | 5.39 (4.95–5.87) | ref | 0.81 (0.76–0.86) | ref | 1.48 (1.32–1.67) | ref | 0.43 (0.39–0.47) | ref |
| serum cotinine (μg/L) | |||||||||||
| active smoker (≥3) | 14 (7.7) | 9.04 (6.76–12.07) | 0.68 (0.49–0.94)b | 4.76 (3.55–6.39) | 0.95 (0.68–1.32) | 0.66 (0.53–0.81) | 0.82 (0.64–1.04) | 1.06 (0.7–1.59) | 0.67 (0.42–1.06) | 0.43 (0.31–0.61) | 1.12 (0.76–1.63) |
| secondhand smoker (0.015–3) | 117 (64.3) | 13.76 (12.45–15.21) | 1.04 (0.86–1.24) | 5.61 (5.07–6.22) | 1.12 (0.93–1.34) | 0.83 (0.77–0.89) | 1.03 (0.9–1.18) | 1.48 (1.29–1.7) | 0.94 (0.73–1.22) | 0.45 (0.4–0.51) | 1.16 (0.94–1.43) |
| non smoker (<0.015) | 51 (28) | 13.28 (11.41–15.45) | ref | 5.03 (4.31–5.87) | ref | 0.8 (0.72–0.9) | ref | 1.57 (1.27–1.95) | ref | 0.39 (0.33–0.46) | ref |
| parity | |||||||||||
| >1 | 47 (25.8) | 11.37 (9.7–13.33) | 0.78 (0.64–0.96)b | 4.83 (4.14–5.64) | 0.74 (0.61–0.91)b | 0.75 (0.67–0.85) | 0.87 (0.75–1.01) | 1.31 (1.05–1.64) | 0.79 (0.6–1.05) | 0.52 (0.43–0.62) | 1.08 (0.85–1.36) |
| 1 | 57 (31.3) | 13.06 (11.31–15.08) | 0.9 (0.74–1.09) | 4.53 (3.94–5.21) | 0.7 (0.58–0.84)b | 0.77 (0.7–0.86) | 0.89 (0.78–1.03) | 1.36 (1.11–1.67) | 0.82 (0.63–1.07) | 0.5 (0.42–0.59) | 1.04 (0.84–1.3) |
| 0 | 78 (42.9) | 14.53 (12.85–16.43) | ref | 6.49 (5.76–7.32) | ref | 0.86 (0.79–0.95) | ref | 1.65 (1.39–1.97) | ref | 0.48 (0.42–0.55) | ref |
Ratios are the exponentiated beta coefficients from a linear regression model with the maternal concentrations as the outcome. Ratios represent the multiplicative difference in PFAS concentrations from the reference category. Thus, numeric estimates indicate that GM concentrations were higher (>1) or lower (<1) for the predictor (variable) in that category compared with the reference category. Each predictor was run in a separate model.
P < 0.05.
We excluded two underweight participants with BMI <18 kg/m2.
Discussion
We found that serum concentrations of PFOS, PFOA, PFNA, and PFHxS among a group of U.S. pregnant women in 2003–2006 were within the concentration ranges of these compounds in the U.S. general population.5 These results suggest that PFAS exposure is ubiquitous in U.S. pregnant women. This information is important because, to date, published data on background exposure to PFASs among pregnant women in the United States are limited to 180 pregnant participants of the 2003–2008 National Health and Nutrition Examination Survey (NHANES).14,25
Of interest, the PFOS median concentrations in these Cincinnati women recruited during early gestation in 2003–2006 (12.7 μg/L) are quite close to those reported for NHANES 2003–2004 pregnant participants (12.0 μg/L), while the PFOA median concentrations (4.8 μg/L) are about two times higher (2.6 μg/L).14,25 These findings suggest similar exposure to PFOS but higher exposure to PFOA for this cohort of Cincinnati pregnant women compared to the U.S. general population. Consistent with our findings, previous investigations reported higher serum concentrations of PFOA, but not of other PFASs, in a group of 6–8 year-old Cincinnati girls recruited starting in the spring of 2004 from a school district in northern Kentucky47,48 and in more than 65 000 residents of six water districts in two states near Parkersburg, West Virginia enrolled in 2005–2006.49 The source of the exposure to PFOA in these populations was identified to be drinking water from the Ohio River that had been contaminated with PFOA.50 Therefore, we speculate that the higher than background concentrations of PFOA in our study population may also be related to the consumption of PFOA contaminated drinking water as reported recently for Cincinnati girls recruited around the same time48 as the HOME Study participants.
The observed PFAS concentration sequence PFOS ≫ PFOA > PFNA ∼ PFHxS in maternal and cord sera and the strong correlations between the PFAS serum concentrations between all of the maternal and infant samples are consistent with previous reports.13,17,18,20,21,24,28,30,32,34,35,37,39,41,43 The significantly higher maternal serum concentrations of these PFASs at 16 weeks than both the maternal and infant’s concentrations at birth suggest transplacental transfer of PFASs. Of interest, the ratio of concentrations between maternal and infant’s samples varied depending on the compound. For example, at delivery, the ratio between the median maternal and cord serum concentration was about 1 for PFOA but ∼2 for PFOS. These ratios, which are similar to those previously reported, indicate that there are differences in the transplacental transfer of these compounds.17,20,21,24,28,30,32,34,35,39,41
Our results are consistent with other studies that examined PFAS concentrations in serial maternal blood samples collected during pregnancy, and compared maternal and infant’s concentrations at birth.18,21 Glynn et al. reported a significant decline in mean serum concentrations between the first and third trimester of pregnancy for PFOS, PFOA, and PFNA among 19 primiparous Swedish women.18 Fromme et al. did not observe significant concentration differences of PFOS, PFOA, PFHxS, and PFNA between maternal samples collected at 34–37 weeks of pregnancy and at delivery from 27 German women,21 but the time interval between the two maternal samples was much shorter than the interval Glynn et al. examined. Consistent with our findings, both research groups reported lower concentrations of PFASs in the cord serum compared with maternal samples. Thus, our results, which show strong correlations between the concentrations of all five PFASs in the maternal sera early during pregnancy and at birth, are consistent with these two previous studies,18,21 while also providing information for PFHxS and Me-PFOSA-AcOH, and with a larger sample size.
We found that maternal age was positively associated with PFAS concentrations during pregnancy. PFOS, PFOA, PFNA, and PFHxS concentrations were lower in women younger than 25 years of age compared with older women. In the early 2000s, 3M, the main manufacturer of PFOS worldwide, discontinued the production of PFOS precursors and related compounds (including PFHxS and PFOA) in the United States. This fact may explain why younger women had lower concentrations of these compounds than older women, who may have had experienced increased exposures when production of these PFASs peaked during the 1980s–1990s time period.51 NHANES data during 1999–2008 also suggest that PFOS, PFOA, PFHxS, and PFNA concentrations in females increased with age.52
Non-Hispanic black women and women who reported the lowest household income had lower concentrations of PFOS, PFOA, PFNA, and PFHxS than non-Hispanic white women and women in the more affluent households, respectively. These results are in agreement with findings from the U.S. general population. Specifically, 1999–2008 NHANES data also suggest that non-Hispanic black females had lower concentrations of PFOA and PFHxS than non-Hispanic white females.52 Similarly, in an analysis of 2003–2008 NHANES data, PFOA and PFOS concentrations were higher in more affluent populations.53 The reason for these differences is unknown, but may reflect differences related to lifestyle (e.g., use of PFASs-containing products), diet, physiology (e.g., elimination), or a combination of the above.52
Active smokers had significantly lower PFOS concentrations than nonsmokers or second-hand smokers. Of interest, the associations between smoking and PFASs exposure are inconsistent among studies. In agreement with our findings, current Danish male smokers had lower PFOA and PFOS plasma concentrations than never smokers.54 By contrast, smoking was positively associated with serum concentrations of PFNA and PFOA in 17–39 year old 2003–2008 NHANES female participants.14 In another study, smoking was not associated with concentrations of PFASs in French women’s breast milk.55
Obese women had significantly lower PFOS and PFNA concentrations than other women. Like for smoking, there is no consistency in the literature about the potential associations between exposure to PFASs and BMI. Similar to our findings, plasma concentrations of PFOS and PFOA were inversely associated with BMI in Danish men.54 However, in Korean adolescents and adults,7,56 serum concentrations of several PFASs, including PFHxS, PFOS, PFOA, and PFNA, showed a positive association with BMI. Finally, others have reported no associations between plasma concentrations of PFASs (e.g., PFOS, PFOA, PFHxS, and PFNA) in a group of Vietnamese pregnant women57 or middle-aged Norwegian women.58 Interpreting these results is difficult because of the differences in study populations and inconsistent findings among studies.
Nulliparous women had significantly higher concentrations of PFOS and PFOA than parous women, in agreement with previous studies.12,26,28,40,59,60 We also observed a significant decrease in maternal concentrations of these compounds from 16 weeks gestation to delivery. Together, these findings suggest gestational transfer of PFASs to the fetus.17,20,28,30,32,34,40 However, we cannot rule out that physiologic changes occurring during pregnancy (e.g., increased maternal blood volume, fat and total body water; decreased plasma protein concentrations, especially albumin) may also be related to the observed decrease in maternal PFAS concentrations.61,62
Finally, we observed that a history of previous breastfeeding was inversely associated with PFOS and PFOA serum concentrations, suggesting that breastfeeding may further decrease the mother’s body burden of these compounds and contribute to infants’ exposure to PFASs.14,21,35,37,43,55,63−72 Consistent with our findings, the duration of being breast-fed was also positively associated with serum PFOA concentrations among 6–8 year-old girls in Greater Cincinnati (N = 353) and the San Francisco Bay Area (N = 351).48
Our study has several limitations. We lacked detailed information on some potentially important sources of PFAS exposure including paper/cardboard use, packaged food consumption, or use of fabric or carpet treatment products during pregnancy. Furthermore, we did not examine sources of dietary exposure as dietary variables collected in the larger HOME Study were designed to assess exposures to heavy metals, pesticides and other persistent pollutants rather than PFASs. Lastly, results from this study population may not be generalizable to the general population of U.S. pregnant women because our participants resided in an area in the United States where the nature of the exposure to at least some of the PFASs (i.e., PFOA) may have differed from the rest of the country. Nonetheless, our data support the hypothesis that PFASs transfer from the mother to her child, and suggests that nursing may decrease a woman’s body burden of PFASs. Our findings also show for the first time in a U.S. longitudinal birth cohort that exposure to PFASs among a population of pregnant women is a function of age, race, household income, parity, and previous history of breastfeeding. Future research should build on these results to examine the impact of prenatal and postnatal exposure to these persistent organic pollutants among the children in the HOME Study and other birth cohorts.
Acknowledgments
This research was supported, in part, by an appointment (C.D.) to the Research Participation Program at the National Center for Environmental Health, Division of Laboratory Sciences, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the Centers for Disease Control and Prevention (CDC). We acknowledge T. Jia for technical assistance. The HOME Study was supported by grants from the National Institute of Environmental Health Sciences (NIEHS P01 ES11261, R01 ES014575, and R01 ES020349).
Supporting Information Available
Median concentrations (μg/L) of PFASs and frequency of detection for maternal sera (at each collection point) and cord sera (Table S1); geometric mean serum concentrations (in μg/L) for select PFASs for 71 paired samples (Table S2); geometric means of serum concentration ratios for select PFASs for 71 paired samples (Table S3); percent change of geometric mean serum concentrations for select PFASs for 71 paired samples (Table S4); geometric mean cord serum concentrations of PFOS, PFOA, PFNA, PFHxS, and Me-PFOSA-AcOH (μg/L) according to demographic, perinatal, and lifestyle factors (Table S5). This material is available free of charge via the Internet at http://pubs.acs.org.
The use of trade names is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services or the CDC. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the NIEHS or the CDC.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Lau C.; Anitole K.; Hodes C.; Lai D.; Pfahles-Hutchens A.; Seed J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 2007, 992366–394. [DOI] [PubMed] [Google Scholar]
- Lau C. Perfluoroalkyl acids: Recent activities and research progress. Reprod. Toxicol. 2009, 273–4209–211. [DOI] [PubMed] [Google Scholar]
- Health Canada. Report on Human Biomonitoring of Environmental Chemicals in Canada. Results of the Canadian Health Measures Survey Cycle 1 (2007–2009). [http://www.hc-sc.gc.ca/ewh-semt/pubs/contaminants/chms-ecms/index-eng.php]. 2010. Ottawa, Ontario. 3–19–2014.
- Health Canada. Canadian Health Measures Survey: Cycle 2 Data Tables. 2009 to 2011. Catalogue no. 82–626-X[http://www.statcan.gc.ca/pub/82-626-x/82-626-x2012002-eng.pdf]. 2012. Ottawa, Canada, Statistics Canada. 3–17–2014.
- CDC. Fourth National Report on Human Exposure to Environmental Chemicals. Updated Tables, July 2014. [http://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Jul2014.pdf]. 2013. Atlanta, GA, Centers for Disease Control and Prevention; National Center for Environmental Health; Division of Laboratory Sciences. 7–22–2014.
- Yamaguchi M.; Arisawa K.; Uemura H.; Katsuura-Kamano S.; Takami H.; Sawachika F.; Nakamoto M.; Juta T.; Toda E.; Mori K.; Hasegawa M.; Tanto M.; Shima M.; Sumiyoshi Y.; Morinaga K.; Kodama K.; Suzuki T.; Nagai M.; Satoh H. Consumption of seafood, serum liver enzymes, and blood levels of PFOS and PFOA in the Japanese population. J. Occup. Health. 2013, 553184–194. [DOI] [PubMed] [Google Scholar]
- Ji K.; Kim S.; Kho Y.; Paek D.; Sakong J.; Ha J.; Kim S.; Choi K. Serum concentrations of major perfluorinated compounds among the general population in Korea: Dietary sources and potential impact on thyroid hormones. Env. Int. 2012, 45, 78–85. [DOI] [PubMed] [Google Scholar]
- Zhang T.; Wu Q.; Sun H. W.; Zhang X. Z.; Yun S. H.; Kannan K. Perfluorinated compounds in whole blood samples from infants, children, and adults in China. Environ. Sci. Technol. 2010, 44114341–4347. [DOI] [PubMed] [Google Scholar]
- Wilhelm M.; Angerer J.; Fromme H.; Holzer J. Contribution to the evaluation of reference values for PFOA and PFOS in plasma of children and adults from Germany. Int. J. Hyg. Environ. Health. 2009, 212156–60. [DOI] [PubMed] [Google Scholar]
- Jiang W. W.; Zhang Y. F.; Zhu L. Y.; Deng J. M. Serum levels of perfluoroalkyl acids (PFAAs) with isomer analysis and their associations with medical parameters in Chinese pregnant women. Env. Int. 2014, 64, 40–47. [DOI] [PubMed] [Google Scholar]
- Lyngso J.; Ramlau-Hansen C. H.; Hoyer B. B.; Stovring H.; Bonde J. P.; Jonsson B. A. G.; Lindh C. H.; Pedersen H. S.; Ludwicki J. K.; Zviezdai V.; Toft G. Menstrual cycle characteristics in fertile women from Greenland, Poland and Ukraine exposed to perfluorinated chemicals: a cross-sectional study. Hum. Reprod. 2014, 292359–367. [DOI] [PubMed] [Google Scholar]
- Brantsaeter A. L.; Whitworth K. W.; Ydersbond T. A.; Haug L. S.; Haugen M.; Knutsen H. K.; Thomsen C.; Meltzer H. M.; Becher G.; Sabaredzovic A.; Hoppin J. A.; Eggesbo M.; Longnecker M. P. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Env. Int. 2013, 54, 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen L.; Dudarev A. A.; Huber S.; Odland J. O.; Nieboer E.; Sandanger T. M. Partition of perfluoroalkyl substances (PFASs) in whole blood and plasma, assessed in maternal and umbilical cord samples from inhabitants of arctic Russia and Uzbekistan. Sci. Total Environ. 2013, 447, 430–437. [DOI] [PubMed] [Google Scholar]
- Jain R. B. Effect of pregnancy on the levels of selected perfluoroalkyl compounds for females aged 17–39 years: Data from National Health and Nutrition Examination Survey 2003–2008. J. Toxicol. Environ. Health Part A 2013, 767409–421. [DOI] [PubMed] [Google Scholar]
- Javins B.; Hobbs G.; Ducatman A. M.; Pilkerton C.; Tacker D.; Knox S. S. Circulating maternal perfluoroalkyl substances during pregnancy in the C8 Health Study. Environ. Sci. Technol. 2013, 4731606–1613. [DOI] [PubMed] [Google Scholar]
- Okada E.; Kashino I.; Matsuura H.; Sasaki S.; Miyashita C.; Yamamoto J.; Ikeno T.; Ito Y. M.; Matsumura T.; Tamakoshi A.; Kishi R. Temporal trends of perfluoroalkyl acids in plasma samples of pregnant women in Hokkaido, Japan, 2003–2011. Env. Int. 2013, 60, 89–96. [DOI] [PubMed] [Google Scholar]
- Porpora M. G.; Lucchini R.; Abballe A.; Ingelido A. M.; Valentini S.; Fuggetta E.; Cardi V.; Ticino A.; Marra V.; Fulgenzi A. R.; De Felip E. Placental transfer of persistent organic pollutants: A preliminary study on mother–newborn pairs. Int. J. Environ. Res. Public Health. 2013, 102699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn A.; Berger U.; Bignert A.; Ullah S.; Aune M.; Lignell S.; Darnerud P. O. Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: Serial sampling during pregnancy and nursing, and temporal trends 1996–2010. Environ. Sci. Technol. 2012, 46169071–9079. [DOI] [PubMed] [Google Scholar]
- Christensen K. Y.; Maisonet M.; Rubin C.; Holmes A.; Calafat A. M.; Kato K.; Flanders W. D.; Heron J.; McGeehin M. A.; Marcus M. Exposure to polyfluoroalkyl chemicals during pregnancy is not associated with offspring age at menarche in a contemporary British cohort. Env. Int. 2011, 371129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.; Choi K.; Ji K.; Seo J.; Kho Y.; Park J.; Kim S.; Park S.; Hwang I.; Jeon J.; Yang H.; Giesy J. P. Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environ. Sci. Technol. 2011, 45177465–7472. [DOI] [PubMed] [Google Scholar]
- Fromme H.; Mosch C.; Morovitz M.; ba-Alejandre I.; Boehmer S.; Kiranoglu M.; Faber F.; Hannibal I.; Genzel-Boroviczeny O.; Koletzko B.; Volkel W. Pre- and postnatal exposure to perfluorinated compounds (PFCs). Environ. Sci. Technol. 2010, 44187123–7129. [DOI] [PubMed] [Google Scholar]
- Hamm M. P.; Cherry N.; Chan E.; Martin J. W.; Burstyn I. Maternal exposure to perfluorinated acids and fetal growth. J. Expo. Sci. Environ. Epidemiol. 2010, 207589–597. [DOI] [PubMed] [Google Scholar]
- Halldorsson T. I.; Fei C. Y.; Olsen J.; Lipworth L.; McLaughlin J. K.; Olsen S. F. Dietary predictors of perfluorinated chemicals: A study from the Danish National Birth Cohort. Environ. Sci. Technol. 2008, 42238971–8977. [DOI] [PubMed] [Google Scholar]
- Monroy R.; Morrison K.; Teo K.; Atkinson S.; Kubwabo C.; Stewart B.; Foster W. G. Serum levels of perfluoroalkyl compounds in human maternal and umbilical cord blood samples. Environ. Res. 2008, 108156–62. [DOI] [PubMed] [Google Scholar]
- Woodruff T. J.; Zota A. R.; Schwartz J. M. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ. Health Perspect. 2011, 1196878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ode A.; Rylander L.; Lindh C. H.; Kallen K.; Jonsson B. A. G.; Gustafsson P.; Olofsson P.; Ivarsson S. A.; Rignell-Hydbom A. Determinants of maternal and fetal exposure and temporal trends of perfluorinated compounds. Environ. Sci. Pollut. Res. Int. 2013, 20117970–7978. [DOI] [PubMed] [Google Scholar]
- Arbuckle T. E.; Kubwabo C.; Walker M.; Davis K.; Lalonde K.; Kosarac I.; Wen S. W.; Arnold D. L. Umbilical cord blood levels of perfluoroalkyl acids and polybrominated flame retardants. Int. J. Hyg. Environ. Health. 2013, 2162184–194. [DOI] [PubMed] [Google Scholar]
- Lee Y. J.; Kim M. K.; Bae J.; Yang J. H. Concentrations of perfluoroalkyl compounds in maternal and umbilical cord sera and birth outcomes in Korea. Chemosphere 2013, 9051603–1609. [DOI] [PubMed] [Google Scholar]
- Lien G. W.; Huang C. C.; Wu K. Y.; Chen M. H.; Lin C. Y.; Chen C. Y.; Hsieh W. S.; Chen P. C. Neonatal-maternal factors and perfluoroalkyl substances in cord blood. Chemosphere. 2013, 927843–850. [DOI] [PubMed] [Google Scholar]
- Zhang T.; Sun H. W.; Lin Y.; Qin X. L.; Zhang Y. F.; Geng X.; Kannan K. Distribution of poly- and perfluoroalkyl substances in matched samples from pregnant women and carbon chain length related maternal transfer. Environ. Sci. Technol. 2013, 47147974–7981. [DOI] [PubMed] [Google Scholar]
- Chen M. H.; Ha E. H.; Wen T. W.; Su Y. N.; Lien G. W.; Chen C. Y.; Chen P. C.; Hsieh W. S. Perfluorinated compounds in umbilical cord blood and adverse birth outcomes. Plos One. 2012, 78e42474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzkow K. B.; Haug L. S.; Thomsen C.; Sabaredzovic A.; Becher G.; Brunborg G. Placental transfer of perfluorinated compounds is selective—A Norwegian mother and child sub-cohort study. Int. J. Hyg. Environ. Health. 2012, 2152216–219. [DOI] [PubMed] [Google Scholar]
- Llorca M.; Perez F.; Farre M.; Agramunt S.; Kogevinas M.; Barcelo D. Analysis of perfluoroalkyl substances in cord blood by turbulent flow chromatography coupled to tandem mass spectrometry. Sci. Total Environ. 2012, 433, 151–160. [DOI] [PubMed] [Google Scholar]
- Beesoon S.; Webster G. M.; Shoeib M.; Harner T.; Benskin J. P.; Martin J. W. Isomer profiles of perfluorochemicals in matched maternal, cord, and house dust samples: Manufacturing sources and transplacental transfer. Environ. Health Perspect. 2011, 119111659–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. K.; Lee K. T.; Kang C. S.; Tao L.; Kannan K.; Kim K. R.; Kim C. K.; Lee J. S.; Park P. S.; Yoo Y. W.; Ha J. Y.; Shin Y. S.; Lee J. H. Distribution of perfluorochemicals between sera and milk from the same mothers and implications for prenatal and postnatal exposures. Environ. Pollut. 2011, 1591169–174. [DOI] [PubMed] [Google Scholar]
- Lien G. W.; Wena T. W.; Hsiehb W. S.; Wua K. Y.; Chenc C. Y.; Chena P. C. Analysis of perfluorinated chemicals in umbilical cord blood by ultra-high performance liquid chromatography/tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 8799–10641–646. [DOI] [PubMed] [Google Scholar]
- Liu J. Y.; Li J. G.; Liu Y.; Chan H. M.; Zhao Y. F.; Cai Z. W.; Wu Y. N. Comparison on gestation and lactation exposure of perfluorinated compounds for newborns. Env. Int. 2011, 3771206–1212. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Lin Z. K.; Hu M. Y.; Wang X. D.; Lian Q. Q.; Lin K. F.; Dong Q. X.; Huang C. J. Perfluorinated chemicals in blood of residents in Wenzhou, China. Ecotoxicol. Environ. Saf. 2011, 7461787–1793. [DOI] [PubMed] [Google Scholar]
- Hanssen L.; Rollin H.; Odland J. O.; Moe M. K.; Sandanger T. M. Perfluorinated compounds in maternal serum and cord blood from selected areas of South Africa: Results of a pilot study. J. Environ. Monit. 2010, 1261355–1361. [DOI] [PubMed] [Google Scholar]
- Apelberg B. J.; Goldman L. R.; Calafat A. M.; Herbstman J. B.; Kuklenyik Z.; Heidler J.; Needham L. L.; Halden R. U.; Witter F. R. Determinants of fetal exposure to polyfluoroalkyl compounds in Baltimore, Maryland. Environ. Sci. Technol. 2007, 41113891–3897. [DOI] [PubMed] [Google Scholar]
- Midasch O.; Drexler H.; Hart N.; Beckmann M. W.; Angerer J. Transplacental exposure of neonates to perfluorooctanesulfonate and perfluorooctanoate: A pilot study. Int. Arch. Occup. Environ. Health. 2007, 807643–648. [DOI] [PubMed] [Google Scholar]
- Inoue K.; Okada F.; Ito R.; Kato S.; Sasaki S.; Nakajima S.; Uno A.; Saijo Y.; Sata F.; Yoshimura Y.; Kishi R.; Nakazawa H. Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: Assessment of PFOS exposure in a susceptible population during pregnancy. Environ. Health Perspect. 2004, 112111204–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham L. L.; Grandjean P.; Heinzow B.; Jorgensen P. J.; Nielsen F.; Patterson D. G.; Sjodin A.; Turner W. E.; Weihe P. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ. Sci. Technol. 2011, 4531121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K.; Basden B. J.; Needham L. L.; Calafat A. M. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J. Chromatogr. A 2011, 1218, 2133–2137. [DOI] [PubMed] [Google Scholar]
- Hornung R. W.; Reed L. D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990, 5146–51. [Google Scholar]
- Bernert J. T.; McGuffey J. E.; Morrison M. A.; Pirkle J. L. Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. J. Anal. Toxicol. 2000, 245333–339. [DOI] [PubMed] [Google Scholar]
- Hernick A. D.; Brown M. K.; Pinney S. M.; Biro F. M.; Ball K. M.; Bornschein R. L. Sharing unexpected biomarker results with study participants. Environ. Health Perspect. 2011, 11911–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney S. M.; Biro F. M.; Windham G. C.; Herrick R. L.; Yaghjyan L.; Calafat A. M.; Succop P.; Sucharew H.; Ball K. M.; Kato K.; Kushi L. H.; Bornschein R. Serum biomarkers of polyfluoroalkyl compound exposure in young girls in Greater Cincinnati and the San Francisco Bay Area, U.S.A. Environ. Pollut. 2014, 184, 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbee S. J.; Brooks A. P.; Maher A.; Flensborg P.; Arnold S.; Fletcher T.; Steenland K.; Shankar A.; Knox S. S.; Pollard C.; Halverson J. A.; Vieira V. M.; Jin C. F.; Leyden K. M.; Ducatman A. M. The C8 Health Project: Design, methods, and participants. Environ. Health Perspect. 2009, 117121873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett E. A.; Shofer F. S.; Zhang H.; Freeman D.; Desai C.; Shaw L. M. Community exposure to perfluorooctanoate: Relationships between serum concentrations and exposure sources. J. Occup. Environ. Med. 2006, 488759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevedouros K.; Cousins I. T.; Buck R. C.; Korzeniowski S. H. Sources, fate and transport of perfluorocarboxylates. Environ. Sci. Technol. 2006, 40132–44. [DOI] [PubMed] [Google Scholar]
- Kato K.; Wong L. Y.; Jia L. T.; Kuklenyik Z.; Calafat A. M. Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999–2008. Environ. Sci. Technol. 2011, 45198037–8045. [DOI] [PubMed] [Google Scholar]
- Uhl S. A.; James-Todd T.; Bell M. L. Association of osteoarthritis with perfluorooctanoate and perfluorooctane sulfonate in NHANES 2003–2008. Environ. Health Perspect. 2013, 1214447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen K. T.; Sorensen M.; McLaughlin J. K.; Tjonneland A.; Overvad K.; Raaschou-Nielsen O. Determinants of plasma PFOA and PFOS levels among 652 Danish men. Environ. Sci. Technol. 2011, 45198137–8143. [DOI] [PubMed] [Google Scholar]
- Antignac J. P.; Veyrand B.; Kadar H.; Marchand P.; Oleko A.; Le Bizec B.; Vandentorren S. Occurrence of perfluorinated alkylated substances in breast milk of French women and relation with socio-demographical and clinical parameters: Results of the ELFE pilot study. Chemosphere 2013, 916802–808. [DOI] [PubMed] [Google Scholar]
- Ji K.; Kim S.; Kho Y.; Sakong J.; Paek D.; Choi K. Major perfluoroalkyl acid (PFAA) concentrations and influence of food consumption among the general population of Daegu, Korea. Sci. Total Environ. 2012, 438, 42–48. [DOI] [PubMed] [Google Scholar]
- Rylander C.; Duong T. P.; Odland J. O.; Sandanger T. M. Perfluorinated compounds in delivering women from south central Vietnam. J. Environ. Monit. 2009, 11112002–2008. [DOI] [PubMed] [Google Scholar]
- Rylander C.; Sandanger T. M.; Froyland L.; Lund E. Dietary patterns and plasma concentrations of perfluorinated compounds in 315 Norwegian women: The NOWAC postgenome study. Environ. Sci. Technol. 2010, 44135225–5232. [DOI] [PubMed] [Google Scholar]
- Fei C. Y.; McLaughlin J. K.; Tarone R. E.; Olsen J. Perfluorinated chemicals and fetal growth: A study within the Danish National Birth Cohort. Environ. Health Perspect. 2007, 115111677–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washino N.; Saijo Y.; Sasaki S.; Kato S.; Ban S.; Konishi K.; Ito R.; Nakata A.; Iwasaki Y.; Saito K.; Nakazawa H.; Kishi R. Correlations between prenatal exposure to perfluorinated chemicals and reduced fetal growth. Environ. Health Perspect. 2009, 1174660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantine M. M.Front. Pharmacol. 2014, Apr 3;5:65. DOI: 10.3389/fphar.2014.00065. eCollection 2014. [DOI] [Google Scholar]
- Ouzounian J. G.; Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiol. Clin. 2012, 303317–329. [DOI] [PubMed] [Google Scholar]
- Barbarossa A.; Masetti R.; Gazzotti T.; Zama D.; Astolfi A.; Veyrand B.; Pession A.; Pagliuca G. Perfluoroalkyl substances in human milk: A first survey in Italy. Env. Int. 2013, 51, 27–30. [DOI] [PubMed] [Google Scholar]
- Guerranti C.; Perra G.; Corsolini S.; Focardi S. E. Pilot study on levels of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) in selected foodstuffs and human milk from Italy. Food Chem. 2013, 1401–2197–203. [DOI] [PubMed] [Google Scholar]
- Karrman A.; Lindstrom G. Trends, analytical methods and precision in the determination of perfluoroalkyl acids in human milk. TrAC-Trends Anal. Chem. 2013, 46, 118–128. [Google Scholar]
- Kubwabo C.; Kosarac I.; Lalonde K. Determination of selected perfluorinated compounds and polyfluoroalkyl phosphate surfactants in human milk. Chemosphere. 2013, 916771–777. [DOI] [PubMed] [Google Scholar]
- Croes K.; Colles A.; Koppen G.; Govarts E.; Bruckers L.; Van de Mieroop E.; Nelen V.; Covaci A.; Dirtu A. C.; Thomsen C.; Haug L. S.; Becher G.; Mampaey M.; Schoeters G.; Van Larebeke N.; Baeyens W. Persistent organic pollutants (POPs) in human milk: A biomonitoring study in rural areas of Flanders (Belgium). Chemosphere 2012, 898988–994. [DOI] [PubMed] [Google Scholar]
- Fujii Y.; Yan J. X.; Harada K. H.; Hitomi T.; Yang H.; Wang P. Y.; Koizumi A. Levels and profiles of long-chain perfluorinated carboxylic acids in human breast milk and infant formulas in East Asia. Chemosphere 2012, 863315–321. [DOI] [PubMed] [Google Scholar]
- Thomsen C.; Haug L. S.; Stigum H.; Froshaug M.; Broadwell S. L.; Becher G. Changes in concentrations of perfluorinated compounds, polybrominated biphenyl ethers, and polychlorinated biphenyls in Norwegian breast-milk during twelve months of lactation. Environ. Sci. Technol. 2010, 44249550–9556. [DOI] [PubMed] [Google Scholar]
- Tao L.; Kannan K.; Wong C. M.; Arcaro K. F.; Butenhoff J. L. Perfluorinated compounds in human milk from Massachusetts, U.S.A. Environ. Sci. Technol. 2008, 4283096–3101. [DOI] [PubMed] [Google Scholar]
- Volkel W.; Genzel-Boroviczeny O.; Demmelmair H.; Gebauer C.; Koletzko B.; Twardella D.; Raab U.; Fromme H. Perfluorooctane sulphonate (PFOS) and perfluorooctanoic acid (PFOA) in human breast milk: Results of a pilot study. Int. J. Hyg. Environ. Health. 2008, 2113–4440–446. [DOI] [PubMed] [Google Scholar]
- Karrman A.; Ericson I.; van Bavel B.; Darnerud P. O.; Aune M.; Glynn A.; Lignell S.; Lindstrom G. Exposure of perfluorinated chemicals through lactation: Levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ. Health Perspect. 2007, 1152226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


