Abstract
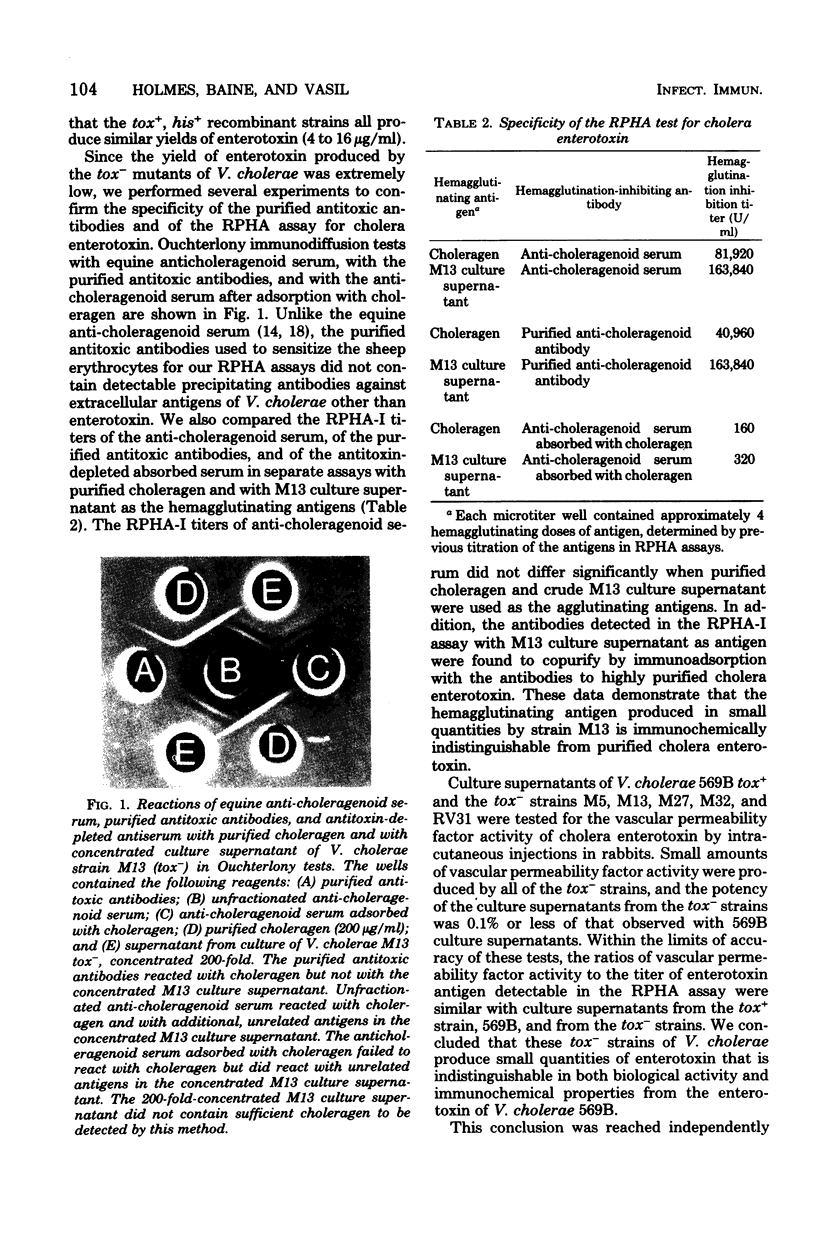
A sensitive and specific reversed passive hemagglutination (RPHA) assay for cholera enterotoxin has been developed. Equine anti-choleragenoid antibodies purified by immunoadsorption were covalently coupled to formalinized sheep erythrocytes, using bis-diazotized benzidine, and the antitoxin-sensitized erythrocytes were shown to agglutinate specifically in the presence of cholera enterotoxin. In a microtiter RPHA assay system, the smallest quantity of enterotoxin that caused hemagglutination was approximately 20 pg. A sensitive assay for antibodies to enterotoxin was also developed, based on inhibition of RPHA. Using such assays, we demonstrated that several nontoxinogenic (tox-) strains of Vibrio cholerae produced small but detectable yields of enterotoxin, 4 to 16 ng/ml, under conditions where the highly toxinogenic strain 569B Inaba produced approximately 16 microgram of enterotoxin per ml. The enterotoxin produced in small quantities by these tox- strains was found to be identical to the enterotoxin from V. cholerae 569B Inaba iv its immunological and biological activities. Strains of V. cholerae that produce intermediate yields of enterotoxin have been obtained by two techniques: (i) as less toxinogenic mutants derived from highly toxinogenic strains and (ii) as more toxinogenic mutants derived from tox- strains. Thus, the yield of enterotoxin in cultures of V. cholerae grown under standardized conditions is is a genetically controlled trait that can be altered by mutation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER W. T. HEMAGGLUTINATION STUDIES WITH FORMALINIZED ERYTHROCYTES. EFFECT OF BIS-DIAZO-BENZIDINE AND TANNIC ACID TREATMENT ON SENSITIZATION BY SOLUBLE ANTIGEN. J Immunol. 1963 May;90:663–671. [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donta S. T., King M., Sloper K. Induction of steroidogenesis in tissue culture by cholera enterotoxin. Nat New Biol. 1973 Jun 20;243(129):246–247. doi: 10.1038/newbio243246a0. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Fujita K., LoSpalluto J. J. Procholeragenoid: an aggregated intermediate in the formation of choleragenoid. J Immunol. 1971 Oct;107(4):1043–1051. [PubMed] [Google Scholar]
- Finkelstein R. A. Immunology of cholera. Curr Top Microbiol Immunol. 1975;69:138–196. [PubMed] [Google Scholar]
- Finkelstein R. A., LaRue M. K., LoSpalluto J. J. Properties of the cholera exo-enterotoxin: effects of dispersing agents and reducing agents in gel filtration and electrophoresis. Infect Immun. 1972 Dec;6(6):934–944. doi: 10.1128/iai.6.6.934-944.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A. Monospecific equine antiserum against cholera exo-enterotoxin. Infect Immun. 1970 Dec;2(6):691–697. doi: 10.1128/iai.2.6.691-697.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Peterson J. W. In vitro detection of antibody to cholera enterotoxin in cholera patients and laboratory animals. Infect Immun. 1970 Jan;1(1):21–29. doi: 10.1128/iai.1.1.21-29.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Vasil M. L., Holmes R. K. Studies on toxinogenesis in Vibrio cholerae. I. Isolation of mutants with altered toxinogenicity. J Infect Dis. 1974 Feb;129(2):117–123. doi: 10.1093/infdis/129.2.117. [DOI] [PubMed] [Google Scholar]
- Guerrant R. L., Brunton L. L., Schnaitman T. C., Rebhun L. I., Gilman A. G. Cyclic adenosine monophosphate and alteration of Chinese hamster ovary cell morphology: a rapid, sensitive in vitro assay for the enterotoxins of Vibrio cholerae and Escherichia coli. Infect Immun. 1974 Aug;10(2):320–327. doi: 10.1128/iai.10.2.320-327.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. K., Vasil M. L., Finkelstein R. A. Studies on toxinogenesis in Vibrio cholerae. III. Characterization of nontoxinogenic mutants in vitro and in experimental animals. J Clin Invest. 1975 Mar;55(3):551–560. doi: 10.1172/JCI107962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M. Enzyme-linked immunosorbent assays for cholera serology. Infect Immun. 1973 May;7(5):759–763. doi: 10.1128/iai.7.5.759-763.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Manganiello V. C., Vaughan M. Hydrolysis of nicotinamide adenine dinucleotide by choleragen and its A protomer: possible role in the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4424–4427. doi: 10.1073/pnas.73.12.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Mechanism of action of choleragen. Evidence for ADP-ribosyltransferase activity with arginine as an acceptor. J Biol Chem. 1977 Apr 10;252(7):2455–2457. [PubMed] [Google Scholar]
- STAVITSKY A. B., ARQUILLA E. R. Micromethods for the study of proteins and antibodies. III. Procedures and applications of hemagglutination and hemagglutination-inhibition reactions with bis-diazotized benzidine and protein-conjugated red blood cells. J Immunol. 1955 Apr;74(4):306–312. [PubMed] [Google Scholar]
- Vasil M. L., Holmes R. K., Finkelstein R. A. Conjugal transfer of a chromosomal gene determining production of enterotoxin in vibrio cholerae. Science. 1975 Mar 7;187(4179):849–850. doi: 10.1126/science.1114331. [DOI] [PubMed] [Google Scholar]
- Vasil M. L., Holmes R. K., Finkelstein R. A. Studies on toxinogenesis in Vibrio cholerae. II. An vitro test for enterotoxin production. Infect Immun. 1974 Jan;9(1):195–197. doi: 10.1128/iai.9.1.195-197.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]




