Abstract
This exploratory phase II study evaluated the safety and efficacy of belatacept in de novo adult liver transplant recipients. Patients were randomized (N = 260) to one of the following immunosuppressive regimens: (i) basiliximab + belatacept high dose [HD] + mycophenolate mofetil (MMF), (ii) belatacept HD + MMF, (iii) belatacept low dose [LD] + MMF, (iv) tacrolimus + MMF, or (v) tacrolimus alone. All received corticosteroids. Demographic characteristics were similar among groups. The proportion of patients who met the primary end point (composite of acute rejection, graft loss, death by month 6) was higher in the belatacept groups (42–48%) versus tacrolimus groups (15–38%), with the highest number of deaths and grafts losses in the belatacept LD group. By month 12, the proportion surviving with a functioning graft was higher with tacrolimus + MMF (93%) and lower with belatacept LD (67%) versus other groups (90%: basiliximab + belatacept HD; 83%: belatacept HD; 88%: tacrolimus). Mean calculated GFR was 15–34 mL/min higher in belatacept-treated patients at 1 year. Two cases of posttransplant lymphoproliferative disease and one case of progressive multifocal leukoencephalopathy occurred in belatacept-treated patients. Follow-up beyond month 12 revealed an increase in death and graft loss in another belatacept group (belatacept HD), after which the study was terminated.
Keywords: Clinical research/practice; liver transplantation/hepatology; immunosuppressant, calcineurin inhibitor: tacrolimus, glomerular filtration rate (GFR); immunosuppressant, fusion proteins and monoclonal antibodies: belatacept
Introduction
Liver transplantation is a life-saving procedure for patients with end-stage liver disease (ESLD) that improves overall survival and quality of life (1,2). Liver transplant (LT) recipients are at increased risk for cardiovascular disease, and chronic kidney disease (CKD; (3–7)).
Stage 4 or 5 CKD has been reported in ∼18% of LT recipients by 5 years posttransplant (4) and is associated with increased morbidity and mortality (4–7). Data from 1997 to 2008 in the Organ Procurement Transplant Network/United Network for Organ Sharing (OPTN/UNOS) database showed a threefold increased rate of kidney transplantation in patients who previously received LT (8). While the causes of CKD in LT patients are multifactorial, including pretransplant and peritransplant factors like hepatitis C virus (HCV), diabetes and hepatorenal syndrome, calcineurin inhibitors (CNIs) appear to be significant contributing factors (4,7,9–11). In the OPTN/UNOS analysis, ∼50% of LT patients who received a kidney transplant had a diagnosis consistent with CNI toxicity (8). Thus, there is a need for immunosuppressive regimens that provide efficacy while avoiding the nephrotoxic, cardiovascular and metabolic risks of CNIs in LT recipients.
Belatacept, a selective costimulation blocker (12), is designed to provide effective immunosuppression and avoid both renal and nonrenal toxicities associated with CNIs. Results from two phase III clinical trials in kidney transplant recipients found that belatacept-based immunosuppression was associated with similar rates of patient and graft survival, significantly better renal function and an improved cardiovascular/metabolic risk profile versus cyclosporine-based therapy ≤3 years after transplantation (13–17). Higher rates and grades of acute rejection (AR) were observed in belatacept-treated patients receiving standard-criteria donor kidneys (13,15). Posttransplant lymphoproliferative disease (PTLD) with central nervous system (CNS) involvement, primarily in patients seronegative for Epstein–Barr virus (EBV) at the time of transplant and progressive multifocal leukoencephalopathy (PML) were the most serious adverse events (AEs) reported in belatacept-treated patients (13,14,18).
The objective of this exploratory, phase II study was to evaluate the efficacy and safety of belatacept in adult recipients of first LTs from a deceased donor. To identify an optimal immunosuppressive regimen in LT recipients, three belatacept regimens were studied and compared with two tacrolimus regimens. In addition, a follow-up long-term extension (LTE) study (i.e. ≥12 months) was conducted to assess longer-term safety and tolerability. The LTE study was terminated in 2011 (when patients were ∼2 years posttransplant) because of worse patient and graft survival in two of the three belatacept treatment groups. Both the 12-month results and those from the LTE are reported herein.
Materials and Methods
Study design
This was a randomized, partially blinded, active-controlled, parallel group, multicenter, phase II clinical trial in adult recipients of first LTs (ClinicalTrials.gov: NCT00555321). Three belatacept regimens were studied, representing a stepwise decrease in the level of overall immunosuppression, and compared with the approved immunosuppressive regimen (tacrolimus alone) and the most widely used immunosuppressive regimen (tacrolimus + mycophenolate mofetil [MMF]) in LT. This exploratory study intended to assess the comparability of a belatacept-based regimen with a tacrolimus-based regimen in terms of AR, graft loss and death.
Immediately before transplantation, patients were randomized to one of the following treatment groups: (i) basiliximab + belatacept high dose (HD) + MMF; (ii) belatacept HD + MMF; (iii) belatacept low dose (LD) + MMF; (iv) tacrolimus + MMF or (v) tacrolimus alone. Subjects were randomized in a 1:1:1:1:1 ratio using an interactive voice response system with centralized randomization and stratified by HCV status (yes or no) in blocks of five. All patients received corticosteroid therapy for the first 3 months. The trial was fully blinded to patients and study personnel with respect to belatacept dosing regimen (HD or LD) and basiliximab assignment (through the use of placebo infusions), open-label to belatacept or tacrolimus treatment and open-label between the two tacrolimus groups.
The study duration was 12 months with an LTE (study was initiated January 22, 2008 and completed May 2, 2011). An external Data Monitoring Committee (DMC) comprising a chair and four members (specialty physicians and one statistician) reviewed emerging safety and efficacy data on a regular basis. The study was conducted in accordance with the Declaration of Helsinki and was consistent with International Conference on Harmonisation Good Clinical Practice and applicable regulatory requirements. The study protocol and amendments were reviewed and approved by the institutional review board/independent ethics committee for each site before initiation of the study (see Table S1 for enrollment by site).
Patients and interventions
The study population included adults of 18–70 years of age, who were recipients of first LTs from a deceased donor (see Table 1 for additional inclusion and exclusion criteria). Belatacept was administered via intravenous (IV) infusion.
Table 1.
Study inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Adults of 18–70 years of age, who were recipients of first LTs from a deceased donor | Donation after cardiac death |
| Informed consent from all patients | Living-donor recipients |
| Additional inclusion criteria | Split liver recipients |
| Reliable IV access | Recipients of organs |
| ABO compatible donor-recipient pairs | From donors <12 years or >65 years of age |
| Patients with hepatocellular carcinoma meeting Milan criteria (one nodule ≤5 cm in diameter or three or fewer nodules, with none >3 cm in diameter) | With anticipated cold ischemia time >14 h |
| From donors who were positive for HBV or HCV when recipients were negative for HBV or HCV, respectively | |
| From donors with known human immunodeficiency virus infection | |
| Patients receiving dialysis before LT for ≥2 consecutive weeks before enrollment or who were anticipated to have prolonged dialysis posttransplant | |
| Patients with known intrinsic kidney disease (e.g. a urine protein/creatinine ratio >150 mg/g or presence of an abnormal number of red blood cells or granular casts in urine) AND a calculated GFR (cGFR) <40 mL/min/1.73 m2 body surface area (modified MDRD) within 1 month of enrollment | |
| Patients with acute liver failure, hypercoagulable state or malignancy within the previous 5 years (except for nonmelanoma skin cancer cured by local resection or hepatocellular carcinoma as defined above) | |
| Patients who were seronegative for Epstein–Barr virus (subsequent study amendment) |
HBV, hepatitis B virus; HCV, hepatitis C virus; IV, intravenous; LT, liver transplant.
HD regimens (groups 1 and 2)
Belatacept 10 mg/kg was given on days 1 (day of transplant), 3 and 5, and weeks 2, 4, 6, 8, 10, 12, 16, 20 and 24 for the first 6 months and then 5 mg/kg every 4 weeks from months 7–12.
LD regimen (group 3)
Belatacept 10 mg/kg was given on days 1, 3 and 5, and weeks 2, 4, 8 and 12 for the first 3 months and then 5 mg/kg every 4 weeks from months 3–12.
The belatacept doses in the present study were different from those studied in renal transplant recipients in that an additional belatacept dose was administered on day 3 in all belatacept treatment groups, and one additional dose was allowed for excessive bleeding (>3L) or ascites loss (>4L) during the first 2 weeks following LT. Eight patients in the basiliximab + belatacept HD + MMF group, 9 patients in the belatacept HD + MMF group and 10 patients in the belatacept LD + MMF group received an additional dose of belatacept for bleeding or ascites.
Tacrolimus (groups 4 and 5) was administered orally at an initial dose of 0.10–0.15 mg/kg/day after transplantation; doses were adjusted to achieve target trough concentrations between 6 and 12 ng/mL.
Basiliximab induction (20 mg IV) was given to patients in group 1 only (belatacept HD regimen) on days 1 and 5. Patients in groups 1 through 4 received MMF at 2 g/day orally, which was later amended to 1 g/day after a case of PML was reported in a patient receiving belatacept HD + MMF. At that time, all but two had received ≥6 months of study treatment. All patients received tapered corticosteroids (Figure 1).
Figure 1.
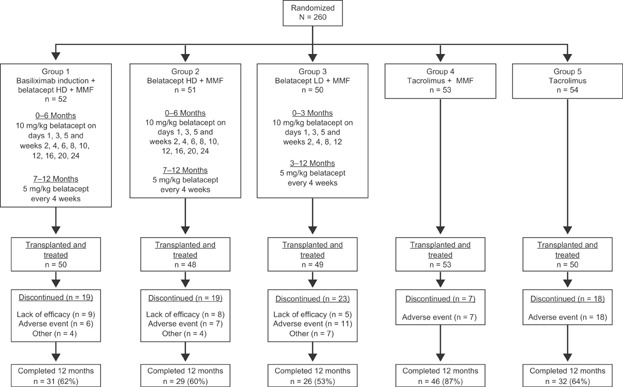
Patient disposition and dosing. All patients received corticosteroids on days 1–5, which was tapered to ≤10 mg/day by day 30 and ≤5 mg/day by day 90. Thereafter, withdrawal of corticosteroids was at the discretion of the investigator. HD, high dose; LD, low dose; MMF, mycophenolate mofetil.
All patients received antiviral prophylaxis for cytomegalovirus (CMV) and herpes simplex virus for ≥3 months posttransplant and for 3 months upon administration of T cell–depleting agents. Additionally, all patients were to receive a 6-month course of prophylaxis for Pneumocystis jiroveci pneumonia.
Outcomes
The primary end point was the composite incidence of AR, graft loss and death at 6 months after transplantation. AR was included in the composite end point if it was clinically suspected and proven via biopsy. All biopsies for suspected AR were assessed by a central histopathologist blinded to treatment assignment using the Banff schema for grading of LT rejection and rejection activity index for staging (19). Graft loss was defined as impairment of liver function that resulted in patient death or re-transplantation.
Secondary end points included the incidence, severity, treatment and outcome of AR by 12 months; graft loss and death by 12 months; change in renal function over time as determined by measured GFR (mGFR) and calculated GFR (cGFR; MDRD methodology); incidence of HCV recurrence by 12 months, defined as histologic confirmation on liver biopsy scored by the Ishak (modified Knodell) system, a score of ≥5 of 18 on modified histological activity index grading, and a fibrosis score of ≥2 of 6 (20). Incidence rates of cardiovascular and metabolic comorbidities (i.e. posttransplant diabetes mellitus, dyslipidemia and hypertension) and the overall safety of the belatacept-based regimens were also evaluated.
Liver allograft biopsies (confirmed by a central pathology laboratory) were obtained at baseline and at 12 months to assess histology. Biopsies were also performed in patients who met protocol-specified criteria for clinical suspicion of AR or HCV recurrence.
Statistical analyses
A statistical analysis plan was prepared prior to unblinding this study. The purpose of the study was to provide initial clinical experience regarding the efficacy and safety of belatacept in LT recipients and identify a belatacept-based regimen with an acceptable composite rate of AR, graft loss and death. Therefore, statistical testing was not prespecified and the study was not powered to demonstrate a difference between treatment groups. A calculated sample size of 50 patients per treatment group was determined to provide initial data regarding safety and efficacy. If the observed primary end point (composite incidence of AR, graft loss and death by 6 months) for a belatacept-based group was 40%, it was estimated that the two-sided 95% confidence interval (CI) of this incidence would extend from 26.4% to 53.6%, which would be within the general range observed in large LT studies that used CNI-based immunosuppression (21,22).
Analyses were performed on the intent-to-treat population (i.e. those who were randomized and received a transplant). Most analyses were descriptive in nature, using point estimates and 95% CIs. mGFR was assessed via iothalamate at months 2 (baseline) and 12. cGFR was assessed pretransplant and at 1, 2, 3, 6 and 12 months.
Results
A total of 260 patients at 39 centers worldwide were randomized, of whom 250 received a transplant and were treated. A total of 164 patients completed 12 months of treatment (Figure 1). Demographic and baseline characteristics were generally similar across treatment groups (Table 2). The mean age was 54 years, and 46% of patients were HCV-positive. The primary reasons for discontinuing treatment, as defined by the study investigators, were AEs and lack of efficacy in the belatacept groups and AEs in the tacrolimus groups.
Table 2.
Demographic characteristics of recipients by treatment group
| Characteristic | Basiliximab + belatacept HD + MMF | Belatacept HD + MMF | Belatacept LD + MMF | Tac + MMF | Tac |
|---|---|---|---|---|---|
| (n = 50) | (n = 48) | (n = 49) | (n = 53) | (n = 50) | |
| Mean age, years | 54.0 | 53.4 | 55.2 | 53.0 | 54.7 |
| Sex, male, % | 78 | 71 | 63 | 87 | 84 |
| Race, % | |||||
| White | 88 | 83 | 94 | 93 | 86 |
| Black | 8 | 6 | 2 | 6 | 4 |
| Cause of ESLD, % | |||||
| Noncholestatic cirrhosis | 76 | 69 | 67 | 74 | 82 |
| HCV-positive, % | 46 | 48 | 43 | 47 | 48 |
| Mean MELD score | 22.6 | 21.1 | 20.6 | 24.3 | 21.6 |
| Hepatorenal syndrome, % | 10 | 10 | 10 | 13 | 6 |
ESLD, end-stage liver disease; HCV, hepatitis C virus; HD, high dose, LD, low dose; MELD, Model for End-Stage Liver Disease; MMF, mycophenolate mofetil; Tac, tacrolimus.
An imbalance in the frequency of deaths and graft losses in the belatacept LD group early in the study was noted by the DMC. Based on the DMC recommendation, randomization to the belatacept LD group was stopped. Study enrollment, however, was nearly complete (247/250 patients), and patients were permitted to remain on the belatacept LD regimen at the discretion of the investigator. Randomization into the other four treatment groups continued.
Outcomes: composite end point, death, graft loss and AR
Composite end point
At the time of the primary analysis at month 6, the incidence rates of the composite end point were higher in the belatacept groups (42–48%) than in the tacrolimus groups (15–38%) (Table 3). This difference was primarily driven by a higher number of AR episodes in the belatacept groups than in the tacrolimus groups. At month 12, patient and graft survival were highest in the tacrolimus + MMF group and lowest in the belatacept LD group. The proportion of patients surviving with a functioning graft was 90%, 83% and 67% in the basiliximab + belatacept HD, belatacept HD, and belatacept LD groups, respectively, and 93% and 88% in the tacrolimus + MMF and tacrolimus groups, respectively.
Table 3.
Outcomes: composite end point (death, graft loss and AR) by 6 months (primary end point) and 12 months and AR up to month 12
| Composite end point | Basiliximab + belatacept HD + MMF | Belatacept HD + MMF | Belatacept LD + MMF | Tac + MMF | Tac (n = 50) |
|---|---|---|---|---|---|
| (n = 50) | (n = 48) | (n = 49) | (n = 53) | ||
| 6 months (primary end point), n (%) | 24 (48.0) | 20 (41.7) | 23 (46.9) | 8 (15.1) | 19 (38.0) |
| Difference from Tac + MMF, % (95% CI) | 32.9 (16.1–49.8) | 26.6 (9.6–43.5) | 31.8 (14.8–48.5) | — | — |
| Difference from Tac, % (95% CI) | 10.0 (−8.7–29.6) | 3.7 (−15.3–23.2) | 8.9 (−9.8–28.4) | — | — |
| AR, n | 20 | 15 | 15 | 5 | 15 |
| Death, n | 4 | 4 | 6 | 1 | 3 |
| Graft loss, n | 2 | 2 | 6 | 4 | 3 |
| Survival with a functioning graft, n (%) | 45 (90.0) | 43 (89.6) | 38 (77.6) | 49 (92.5) | 45 (90.0) |
| (95% CI) | (81.7–98.3) | (80.9–98.2) | (65.9–89.2) | (85.3–99.6) | (81.7–98.3) |
| 12 months, n (%) | 26 (52.0) | 23 (47.9) | 26 (53.1) | 10 (18.9) | 20 (40.0) |
| AR, n | 22 | 16 | 16 | 7 | 15 |
| Death, n | 4 | 7 | 10 | 1 | 4 |
| Graft loss, n | 2 | 2 | 8 | 4 | 4 |
| Survival with a functioning graft, n (%) | 45 (90.0) | 40 (83.3) | 33 (67.3) | 49 (92.5) | 44 (88.0) |
| (95% CI) | (81.7–98.3) | (72.8–93.9) | (54.2–80.5) | (85.3–99.6) | (79.0–97.0) |
| AR (centrally read) up to month 12, n (%) | |||||
| Patients with AR | 22 (44.0) | 16 (33.3) | 16 (32.7) | 7 (13.2) | 15 (30.0) |
| Treated, n/N (%) | 12/22 (54.5) | 12/16 (75.0) | 8/16 (50.0) | 5/7 (71.4) | 12/15 (80.0) |
| Corticosteroids only, n (%) | 12 (24.0) | 7 (14.6) | 8 (16.3) | 4 (7.5) | 10 (20.0) |
| Initial lymphocyte-depleting therapy, n (%) | 0 (0) | 3 (14.6) | 0 (0) | 0 (0) | 0 (0) |
| Grade of AR, n (%) | |||||
| I | 15 (30.0) | 7 (14.6) | 7 (14.3) | 6 (11.3) | 7 (14.0) |
| II | 7 (14.0) | 8 (16.7) | 8 (16.3) | 1 (1.9) | 6 (12.0) |
| III | 0 (0) | 1 (2.1) | 1 (2.0) | 0 (0) | 2 (4.0) |
| Death or graft loss in patients with AR, n | 1 | 1 | 6 | 1 | 1 |
AR, acute rejection; CI, confidence interval; HD, high dose, LD, low dose; MMF, mycophenolate mofetil; Tac, tacrolimus.
Death
At the 6-month primary analysis, a higher number of deaths was observed in patients treated with belatacept (basiliximab + belatacept HD [n = 4], belatacept HD [n = 4], belatacept LD [n = 6]) than in those treated with tacrolimus (tacrolimus + MMF [n = 1], tacrolimus [n = 3]). A similar pattern was also observed at 12 months posttransplant (Table 3). The causes of death reported in more than one patient included multisystem organ failure (n = 7), sepsis (n = 7), gastrointestinal bleeding (n = 2) and myocardial infarction (n = 2). In two cases, the cause of death was unknown (Table 4).
Table 4.
Causes of death
| Treatment group | Age/sex | Reported cause of death | Study day |
|---|---|---|---|
| Basiliximab + belatacept HD + MMF | 57/M | Sepsis | 18 |
| 61/M | Myocardial infarction | 23 | |
| 49/M | Multiple system organ failure | 25 | |
| 56/M | Sepsis | 127 | |
| Belatacept HD + MMF | 43/M | Sepsis | 24 |
| 37/M | Sepsis | 84 | |
| 62/F | Multiple system organ failure | 111 | |
| 47/M | Gastrointestinal bleed | 147 | |
| 62/F | Unknown | 274 | |
| 58/M | Unknown | 278 | |
| 51/M | PML | 322 | |
| Belatacept LD + MMF | 65/M | Multiple system organ failure | 2 |
| 59/F | Colon perforation | 8 | |
| 48/M | Acute hepatic failure | 16 | |
| 63/F | Pulmonary failure | 21 | |
| 53/M | Sepsis | 65 | |
| 50/M | Gunshot injury | 117 | |
| 58/F | Multiple system organ failure | 202 | |
| 55/M | Multiple system organ failure | 208 | |
| 65/F | Multiple system organ failure | 339 | |
| 51/M | PTLD | 364 | |
| Tacrolimus + MMF | 54/M | Sepsis | 168 |
| Tacrolimus | 49/M | Myocardial infraction | 34 |
| 52/M | Multiple system organ failure | 63 | |
| 46/M | Gastrointestinal bleed | 68 | |
| 49/M | Sepsis | 286 |
F, female; HD, high dose, LD, low dose; M, male; MMF, mycophenolate mofetil; PML, progressive multifocal leukoencephalopathy; PTLD, posttransplant lymphoproliferative disease.
Graft loss
By month 6, numerically fewer cases of death-censored graft loss were reported in the belatacept HD groups (n = 1 each) than in the belatacept LD group (n = 5) and tacrolimus groups (tacrolimus + MMF [n = 3]; tacrolimus [n = 2]). At month 12, a similar pattern was observed. Across treatment groups, ∼50% of graft losses occurred within the first month posttransplant, the main causes of which were primary nonfunction (n = 5) and arterial thrombosis (n = 4).
Acute rejection
Overall, AR (centrally read) was more common in the belatacept groups than in the tacrolimus groups (Table 3). At the month 6 primary analysis, the percent of patients with AR in the basiliximab + belatacept HD, belatacept HD and belatacept LD groups was 40%, 31% and 31%, respectively, versus 9% and 30% for the tacrolimus + MMF and tacrolimus groups, respectively. Most episodes of AR (57–94%) occurred early after transplant (i.e. by month 3) and were mild to moderate in severity (Banff grade I or II). Among patients with AR, ∼50% received treatment for AR in the basiliximab + belatacept HD and belatacept LD groups, while 70–80% received treatment in the other three groups. The majority of patients who were treated for AR received corticosteroids only.
Analysis by HCV status
In HCV-positive patients, the percent with AR by month 6 in the belatacept groups ranged from 33% to 39% versus 12% and 38% for the tacrolimus + MMF and tacrolimus groups, respectively. In patients who were HCV-negative, the percent with AR by month 6 in the belatacept groups was 29–41% versus 7% and 23% for the tacrolimus + MMF and tacrolimus groups, respectively.
Safety
Serious AEs, including serious infections and malignancies, occurred with a similar frequency across all treatment groups (Table 5). The most commonly reported serious AEs across all groups included pneumonia, sepsis, biliary strictures, cholangitis, pyrexia and acute renal failure. The most common serious infections resulted from CMV, fungal and mycobacterial pathogens. One fatal case of PML occurred ∼6 months after transplantation in a patient receiving belatacept HD and higher than recommended doses of MMF (3–4 g/day for 7.5 weeks).
Table 5.
Adverse events and events of interest at 12 months
| Events | Patients | ||||
|---|---|---|---|---|---|
| Basiliximab + belatacept HD + MMF | Belatacept HD + MMF | Belatacept LD + MMF | Tac + MMF | Tac | |
| (n = 50) | (n = 48) | (n = 49) | (n = 53) | (n = 50) | |
| HCV at baseline, n | 23 | 23 | 21 | 25 | 24 |
| HCV recurrence,1 n (%) | 14 (60.9) | 7 (30.4) | 6 (28.6) | 13 (52.0) | 9 (37.5) |
| Serious adverse events, n (%) | 28 (56.0) | 29 (60.4) | 37 (75.5) | 40 (75.5) | 35 (70.0) |
| Infections and infestations | 11 (22.0) | 12 (25.0) | 13 (26.5) | 12 (22.6) | 12 (24.0) |
| Malignancies, n | 1 | 0 | 2 | 2 | 2 |
| PTLD | 12 | 0 | 1 | 0 | 0 |
| All infections and infestations, n (%) | 32 (64.0) | 39 (81.3) | 30 (61.2) | 31 (58.5) | 29 (58.0) |
| Bacterial | 5 (10.0) | 11 (22.9) | 11 (22.4) | 6 (11.3) | 13 (26.0) |
| Fungal infections | 6 (12.0) | 9 (18.8) | 14 (28.6) | 6 (11.3) | 5 (10.0) |
| Viral infections3 | 10 (20.0) | 11 (22.9) | 14 (28.6) | 9 (17.0) | 7 (14.0) |
| CMV | 5 (10.0) | 4 (8.3) | 10 (20.4) | 4 (7.5) | 1 (2.0) |
| Herpes | 3 (6.0) | 3 (6.3) | 4 (8.2) | 3 (5.7) | 2 (4.0) |
| Adverse events of nervous system disorders, n (%) | 23 (46.0) | 19 (39.6) | 15 (30.6) | 34 (64.2) | 34 (68.0) |
| Headache | 10 (20.0) | 8 (16.7) | 5 (10.2) | 14 (26.4) | 14 (28.0) |
| Tremor | 2 (4.0) | 2 (4.2) | 4 (8.2) | 17 (32.1) | 13 (26.0) |
| Adverse events of renal and urinary disorders, n (%) | |||||
| Renal failure | 1 (2.0) | 4 (8.3) | 3 (6.1) | 5 (9.4) | 14 (28.0) |
| Acute renal failure | 2 (4.0) | 5 (10.4) | 2 (4.1) | 13 (24.5) | 8 (16.0) |
| Renal impairment | 0 (0) | 1 (2.1) | 0 (0) | 1 (1.9) | 8 (16.0) |
CMV, cytomegalovirus; HAI, histological activity index; HCV, hepatitis C virus; HD, high dose, LD, low dose; MMF, mycophenolate mofetil; PML, progressive multifocal leukoencephalopathy; PTLD, posttransplant lymphoproliferative disease; Tac, tacrolimus.
HCV recurrence confirmed histologically by central pathologist; modified HAI grading score ≥5/18 and fibrosis score ≥2.
PTLD case occurred after month 12.
One case of PML in belatacept HD + MMF.
Malignancies were reported in 2–4% of belatacept patients and in 4% of patients receiving tacrolimus-based treatments. Two cases of PTLD were reported in belatacept-treated patients (one in the belatacept LD group at 11 months posttransplant and one in the basiliximab + belatacept HD group after month 12), both of which involved the liver. Neither case involved the CNS; both patients were EBV-seropositive at the time of transplantation. One patient died as a result of PTLD; the other was treated with chemotherapy and is alive with a functioning graft.
Overall, most infections were mild or moderate in severity (Table 5); urinary tract infections were the most common. Patients in the belatacept LD group had numerically higher rates of viral and fungal infections (Table 5), most of which were nonserious. A similar proportion of patients across all treatment groups experienced at least one AE. AEs related to neurotoxicity (i.e. headache and tremor) occurred less frequently in patients receiving belatacept-based regimens versus tacrolimus-based regimens (Table 5).
HCV recurrence
Approximately 72% (153/212) of patients with a functioning graft at month 12 had biopsies available for evaluation. Recurrence of HCV among patients who were HCV-positive at baseline was higher in the basiliximab + belatacept HD (61%) and tacrolimus + MMF (52%) groups versus the other treatment groups (29–38%) (Table 5).
Renal function
Mean cGFR at baseline was 66–80 mL/min. The differences in cGFR between the belatacept-treated patients and tacrolimus-treated patients were observed as early as month 1 and persisted through month 12 (15–34 mL/min/1.73 m2 higher in each belatacept group vs. the tacrolimus groups at month 12) (Figure 2). By month 12 in the intent-to-treat analysis, mean mGFR was 89–93 mL/min in the belatacept HD groups and 71–75 mL/min in the belatacept LD and tacrolimus groups. An on-treatment analysis revealed similar findings at 12 months (mean cGFR was 88 mL/min for basiliximab + belatacept HD; 100 mL/min for belatacept HD; 94 mL/min for belatacept LD; 67 mL/min for tacrolimus + MMF and 62 mL/min for tacrolimus). The proportion of patients with >10 mL/min improvement in cGFR from baseline to month 12 was 47–65% in the belatacept groups versus 12–27% in the tacrolimus groups.
Figure 2.
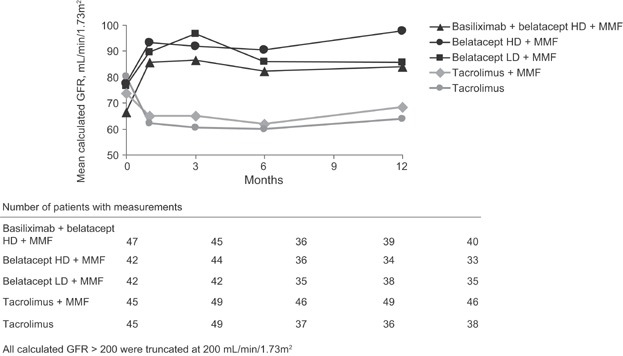
Mean calculated GFR (MDRD methodology) over time (intent-to-treat analysis, as observed data with no imputation for missing values). All calculated GFR >200 were truncated at 200 mL/min. HD, high dose; LD, low dose; MMF, mycophenolate mofetil.
Cardiovascular/metabolic profile at 12 months
Systolic and diastolic blood pressures at 12 months were lower among patients receiving belatacept (121–127 mmHg and 75–77 mmHg, respectively) versus those receiving tacrolimus-based regimens (137–138 mmHg and 80 mmHg, respectively). Serum lipids increased from baseline in all treatment groups, with triglyceride levels increasing more in the tacrolimus groups and LDL levels increasing more in the belatacept groups (data not shown). The incidence of new-onset diabetes mellitus (i.e. ≥30 days of treated diabetes in patients without a diagnosis of diabetes before randomization) was somewhat lower in the belatacept HD and LD groups (16% and 14%, respectively) versus patients receiving basiliximab + belatacept HD (36%), tacrolimus + MMF (24%), and tacrolimus (38%).
Pharmacokinetics
The pharmacokinetics of belatacept in LT recipients was similar to those in kidney transplantation recipients receiving a similar dosing regimen (23). The pattern of minimum belatacept concentration values with the HD and LD regimens in the early posttransplant period were consistent with the differences between the two regimens. Steady-state trough serum concentrations of belatacept in the maintenance phase (during 5-mg/kg dosing) were similar between the HD and LD regimens. Mean serum trough concentrations of belatacept and tacrolimus, and mean daily MMF and steroid doses are shown in Figure 3. Some of the patients who received additional belatacept to compensate for fluid loss went on to experience death or graft loss, but results were mixed and potentially confounded by intra- and postoperative complications. In the overall study population, there was no association between trough belatacept levels and death or graft loss.
Figure 3.
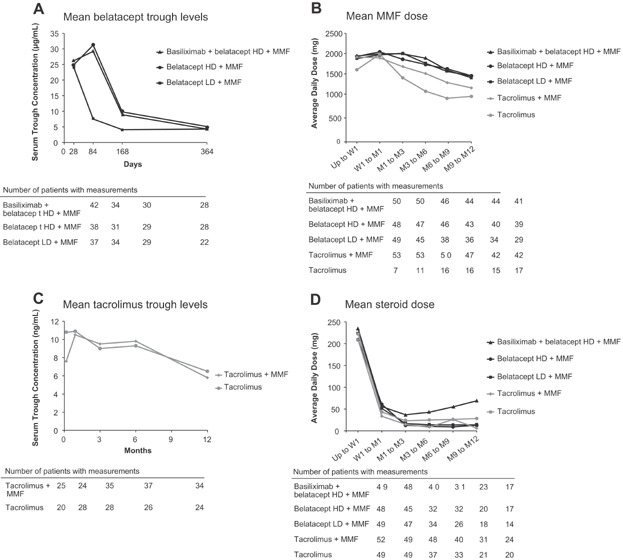
Serum trough levels and mean daily dosing at specified time points. Concentrations of belatacept in human serum were determined using a validated enzyme-linked immunosorbent assay (ELISA) by PPD (Richmond, VA). (A) Mean serum trough levels of belatacept. (B) Mean daily dose of MMF. (C) Mean serum trough levels of tacrolimus. (D) Mean daily dose of steroids. HD, high dose; LD, low dose; M, month; MMF, mycophenolate mofetil; W, week.
Donor-specific antibodies
All treatment groups had patients who tested positive for donor-specific antibodies (DSA) at baseline (Table 6). By 12 months after transplantation, the number of patients with DSA decreased in two belatacept groups (HD and LD), remained the same in two groups (basiliximab + belatacept HD and tacrolimus), and increased in one group (tacrolimus + MMF) (Table 6). There were few cases of de novo DSA in all groups.
Table 6.
DSA: number of patients with detectable antidonor HLA antibodies
| Patients with DSA/patients in analysis, n (%) | Basiliximab + belatacept HD + MMF | Belatacept HD + MMF | Belatacept LD + MMF | Tac + MMF | Tac |
|---|---|---|---|---|---|
| Baseline DSA (pretransplant) | 5/48 (10) | 5/46 (11) | 8/48 (17) | 3/50 (6) | 4/49 (8) |
| Total DSA by month 12 | 5/47 (11) | 3/47 (6) | 4/43 (9) | 8/52 (15) | 4/45 (9) |
| De novo DSA1 by month 12 | 3/47 (6) | 1/47 (2) | 1/43 (2) | 6/52 (12) | 3/45 (7) |
DSA, donor-specific antibodies; HD, high dose, LD, low dose; MMF, mycophenolate mofetil; Tac, tacrolimus.
De novo DSA was defined as appearance of antibody to a new HLA specificity following transplantation.
Long-term extension
Of the 164 patients who completed 1 year of treatment, 145 entered the LTE phase (Figure 4). During the LTE, additional deaths and graft losses were noted in the belatacept HD group (n = 4). Because of the cumulative number of deaths and graft losses in two of the three belatacept groups relative to the tacrolimus groups, the DMC recommended termination of the LTE study. Although a causal relationship to belatacept could not be clearly established, it could also not be rejected. All patients who were currently on belatacept were switched to standard-of-care immunosuppression.
Figure 4.
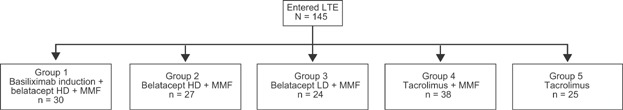
Patient disposition in LTE. HD, high dose; LD, low dose; LTE, long-term extension; MMF, mycophenolate mofetil.
Based on these findings, a comprehensive assessment of the deaths observed in belatacept-treated patients was conducted, and a series of post hoc analyses were performed. Factors evaluated included demographic characteristics, donor age, baseline disease status, mean Model for End-Stage Liver Disease score, and belatacept trough concentrations. Deaths and graft losses were not evenly distributed among the centers. No demographic or disease-related risk factors were predictive of death associated with belatacept, and similarly, no clear explanation for the higher rates of death observed in the belatacept HD and LD groups was identified. Death and graft loss in the subset of patients who were HCV-positive at the time of transplantation were numerically higher in the belatacept groups at month 6 but comparable with the tacrolimus groups at month 12.
Discussion
This phase II, randomized, multicenter study was the first clinical trial to explore the use of belatacept in LT recipients. The primary objective was to evaluate the safety and efficacy of belatacept relative to tacrolimus, as reflected by the incidence of AR, graft loss and death in de novo LT recipients. At the conclusion of this study, two of three belatacept groups had higher rates of death and graft loss relative to the standard-of-care control group tacrolimus + MMF. All three belatacept groups also had higher rates of AR, and there was an increase in viral and fungal infections, the majority of which were not serious. Two cases of PTLD and one case of PML were reported in belatacept-treated patients. The two belatacept HD groups had substantially better mGFR than the remaining three groups, while all three belatacept groups had better cGFR. There was also evidence of fewer neurotoxicity events in the belatacept groups. A high rate of discontinuation was observed in the belatacept treatment groups, such that by the time of the 12-month assessment, ∼40% of patients had discontinued assigned belatacept therapy.
In contrast to the findings in renal transplant recipients, which showed comparable patient and graft survival relative to the CNI cyclosporine (13–17), this exploratory study in LT recipients demonstrated increased rates of death and graft loss in two of three belatacept groups compared with tacrolimus (different CNI comparator). Multiple factors may account for differences in survival outcomes between kidney transplant and LT recipients, including organ-specific aspects of alloimmune responses (24,25).
In the present study, it is notable that basiliximab did not lead to a lower rate of rejection versus placebo in the two belatacept HD groups. There are several hypotheses, including the role of CD28-independent T cells and/or inhibition of regulatory pathways that could explain this observation (26). Further studies are required to understand the mechanism and contribution of these factors to AR in the setting of belatacept therapy.
Important nonimmunologic differences include the degree of illness at the time of transplantation, extent of surgical trauma and massive perioperative fluid shifts. Doses of belatacept in this study were higher than those used in the kidney transplant trials, with the assumption that perioperative fluid shifts and fluid losses would necessitate additional belatacept. There was no association between trough belatacept levels and death or graft loss. This was expected, given that there is no exposure–response relationship in the kidney transplant setting, such that therapeutic drug monitoring is not needed with belatacept treatment.
While preservation of renal function was a key finding in the pivotal phase III clinical trials of belatacept in renal transplant recipients, the magnitude of the renal function difference in the current study highlights the capacity for native kidneys to recover substantial function following LT in the absence of CNI exposure.
The tendency toward more viral and fungal infections observed in belatacept-treated patients raises the possibility of overimmunosuppression in some patients, as observed with regimens that include MMF (27). This finding is somewhat paradoxical, however, given the higher rates of AR in the belatacept groups, which would suggest underimmunosuppression or immunomodulatory effects not yet understood in the context of LT. It is also possible that the management of AR placed patients at risk for subsequent infectious complications. The standard treatment for rejection may have been more intense than was needed in belatacept-treated patients, suggesting that overimmunosuppression may be part of the explanation for the infectious complications. The limited number of patients and events preclude the ability to definitively interpret the relative contributions of overimmunosuppression versus underimmunosuppression.
LT recipients are an inherently complex population, with diverse and serious underlying medical concerns that have the potential to adversely affect posttransplant outcomes. Distinguishing the contribution of belatacept alone or an interaction between belatacept and such factors in the context of this phase II study is not feasible. An additional limitation of the study includes the open-label design (belatacept vs. tacrolimus assignments).
Findings from this phase II study did not allow for the identification of a safe and effective dose or a regimen for further development of belatacept fulfilling the substantial need for nonnephrotoxic immunosuppressive therapy in LT recipients.
Acknowledgments
This study was supported by Bristol-Myers Squibb and is registered with ClinicalTrials.gov (ID: NCT00555321). A list of primary investigators of sites that enrolled patients is provided in Table S1. The authors would like to acknowledge Mamta Agarwal for her valuable contributions to the study and manuscript development. The authors also thank Susan A. Nastasee (an employee of Bristol-Myers Squibb) and CodonMedical for medical writing and editorial assistance (funded by Bristol-Myers Squibb). All authors provided input into the content of this publication and have reviewed every draft.
Glossary
- AE
adverse event
- AR
acute rejection
- cGFR
calculated GFR
- CI
confidence interval
- CKD
chronic kidney disease
- CMV
cytomegalovirus
- CNI
calcineurin inhibitor
- CNS
central nervous system
- DMC
Data Monitoring Committee
- DSA
donor-specific antibodies
- EBV
Epstein–Barr virus
- ESLD
end-stage liver disease
- HAI
histological activity index
- HCV
hepatitis C virus
- HD
high dose
- IV
intravenous
- LD
low dose
- LT
liver transplant
- LTE
long-term extension
- MELD
Model for End-Stage Liver Disease
- mGFR
measured GFR
- MMF
mycophenolate mofetil
- OPTN/UNOS
Organ Procurement Transplant Network/United Network for Organ Sharing
- PML
progressive multifocal leukoencephalopathy
- PTLD
posttransplant lymphoproliferative disease
- Tac
tacrolimus
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. John Lake is a consultant for Novartis, Vital Therapies and Bristol-Myers Squibb. He receives grant support from Vital Therapies, Ocera, Eisai, Salix and Gilead. Lionel Rostaing and Kimberly Brown have received honoraria for participating in Bristol-Myers Squibb advisory boards. Björn Nashan has participated in clinical trials sponsored by Bristol-Myers Squibb, Pfizer, Novartis and Astellas, and has received honoraria and research grants from Bristol-Myers Squibb, Pfizer, Novartis and Astellas. Sandy Feng has participated in clinical trials sponsored by Cumberland, Novartis, Genzyme, LifeCycle, Enzon and ViroPharma. Hugo Vargas receives grant support from BMS, Novartis, Ikaria, Vertex, Gilead, Janssen, Idenix, Eisai and Merck.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Table S1: Enrollment by site.
References
- 1.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 2.Ravaioli M, Grazi GL, Dazzi A, et al. Survival benefit after liver transplantation: A single European center experience. Transplantation. 2009;88:826–834. doi: 10.1097/TP.0b013e3181b26807. [DOI] [PubMed] [Google Scholar]
- 3.Johnston SD, Morris JK, Cramb R, Gunson BK, Neuberger J. Cardiovascular morbidity and mortality after orthotopic liver transplantation. Transplantation. 2002;73:901–906. doi: 10.1097/00007890-200203270-00012. [DOI] [PubMed] [Google Scholar]
- 4.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 5.Gonwa TA, Mai ML, Melton LB, et al. Renal replacement therapy and orthotopic liver transplantation: The role of continuous veno-venous hemodialysis. Transplantation. 2001;71:1424–1428. doi: 10.1097/00007890-200105270-00012. [DOI] [PubMed] [Google Scholar]
- 6.Moreno JM, Cuervas-Mons V, Rubio E, et al. Chronic renal dysfunction after liver transplantation in adult patients: Prevalence, risk factors, and impact on mortality. Transplant Proc. 2003;35:1907–1908. doi: 10.1016/s0041-1345(03)00642-0. [DOI] [PubMed] [Google Scholar]
- 7.Gonwa TA, Mai ML, Melton LB, et al. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: Risk of development and treatment. Transplantation. 2001;72:1934–1939. doi: 10.1097/00007890-200112270-00012. [DOI] [PubMed] [Google Scholar]
- 8.Gonwa TA, McBride MA, Mai ML, Wadei HM. Kidney transplantation after previous liver transplantation: Analysis of the organ procurement transplant network database. Transplantation. 2011;92:31–35. doi: 10.1097/TP.0b013e31821c1e54. [DOI] [PubMed] [Google Scholar]
- 9.Pham PT, Pham PC, Wilkinson AH. Management of renal dysfunction in the liver transplant recipient. Curr Opin Organ Transplant. 2009;14:231–239. doi: 10.1097/MOT.0b013e32832b34a4. [DOI] [PubMed] [Google Scholar]
- 10.Distant DA, Gonwa TA. The kidney in liver transplantation. J Am Soc Nephrol. 1993;4:129–136. doi: 10.1681/ASN.V42129. [DOI] [PubMed] [Google Scholar]
- 11.Lee JP, Heo NJ, Joo KW, et al. Risk factors for consequent kidney impairment and differential impact of liver transplantation on renal function. Nephrol Dial Transplant. 2010;25:2772–2785. doi: 10.1093/ndt/gfq093. [DOI] [PubMed] [Google Scholar]
- 12.Larsen CP, Pearson TC, Adams AB, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5:443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 13.Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) Am J Transplant. 2010;10:535–546. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 14.Durrbach A, Pestana JM, Pearson T, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study) Am J Transplant. 2010;10:547–557. doi: 10.1111/j.1600-6143.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 15.Larsen CP, Grinyó J, Medina-Pestana J, et al. Belatacept-based regimens versus a cyclosporine A-based regimen in kidney transplant recipients: 2-year results from the BENEFIT and BENEFIT-EXT studies. Transplantation. 2010;90:1528–1535. doi: 10.1097/TP.0b013e3181ff87cd. [DOI] [PubMed] [Google Scholar]
- 16.Vincenti F, Larsen CP, Alberu J, et al. Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. Am J Transplant. 2012;12:210–217. doi: 10.1111/j.1600-6143.2011.03785.x. [DOI] [PubMed] [Google Scholar]
- 17.Pestana JO, Grinyo JM, Vanrenterghem Y, et al. Three-year outcomes from BENEFIT-EXT: A phase III study of belatacept versus cyclosporine in recipients of extended criteria donor kidneys. Am J Transplant. 2012;12:630–639. doi: 10.1111/j.1600-6143.2011.03914.x. [DOI] [PubMed] [Google Scholar]
- 18.Grinyó J, Charpentier B, Pestana JM, et al. An integrated safety profile analysis of belatacept in kidney transplant recipients. Transplantation. 2010;90:1521–1527. doi: 10.1097/TP.0b013e3182007b95. [DOI] [PubMed] [Google Scholar]
- 19.Demetris AJ, Batts KP, Dhillon AP, et al. Banff schema for grading liver allograft rejection: An international consensus document. Hepatology. 1997;25:658–663. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 20.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 21.Neuhaus P, Clavien PA, Kittur D, et al. Improved treatment response with basiliximab immunoprophylaxis after liver transplantation: Results from a double-blind randomized placebo-controlled trial. Liver Transpl. 2002;8:132–142. doi: 10.1053/jlts.2002.30302. [DOI] [PubMed] [Google Scholar]
- 22.Wiesner R, Rabkin J, Klintmalm G, et al. A randomized double-blind comparative study of mycophenolate mofetil and azathioprine in combination with cyclosporine and corticosteroids in primary liver transplant recipients. Liver Transpl. 2001;7:442–450. doi: 10.1053/jlts.2001.23356. [DOI] [PubMed] [Google Scholar]
- 23.Shen J, Gelb JS, Townsend RM, et al. Rationale for belatacept less intensive regimen in renal transplant recipients. Am J Transplant. 2011;11:352. (Suppl S2, Abstract #1096) [Google Scholar]
- 24.Sánchez-Fueyo A, Strom TB. Immunologic basis of graft rejection and tolerance following transplantation of liver and other solid organs. Gastroenterology. 2011;140:51–64. doi: 10.1053/j.gastro.2010.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crispe IN. Liver antigen-presenting cells. J Hepatol. 2011;54:357–365. doi: 10.1016/j.jhep.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wekerle T, Grinyó JM. Belatacept: From rational design to clinical application. Transpl Int. 2012;25:139–150. doi: 10.1111/j.1432-2277.2011.01386.x. [DOI] [PubMed] [Google Scholar]
- 27.Moreso F, Serón D, Morales JM, et al. Incidence of leukopenia and cytomegalovirus disease in kidney transplants treated with mycophenolate mofetil combined with low cyclosporine and steroid doses. Clin Transplant. 1998;12:198–205. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Enrollment by site.


