Abstract
Four highly purified salivary glycoproteins were used to study salivary-bacterial interactions. One pair of glycoproteins was mucin-like in composition, whereas the second pair was not. By an agglutination assay, it was found that only the mucin-glycoproteins agglutinated Streptococcus sanguis and S. mutans. Removal of sialic acid from these molecules resulted in a loss of agglutination of S. sanguis but not of S. mutans. The agglutination phenomenon was shown to require a salivary macromolecule of at least 150,000 daltons.
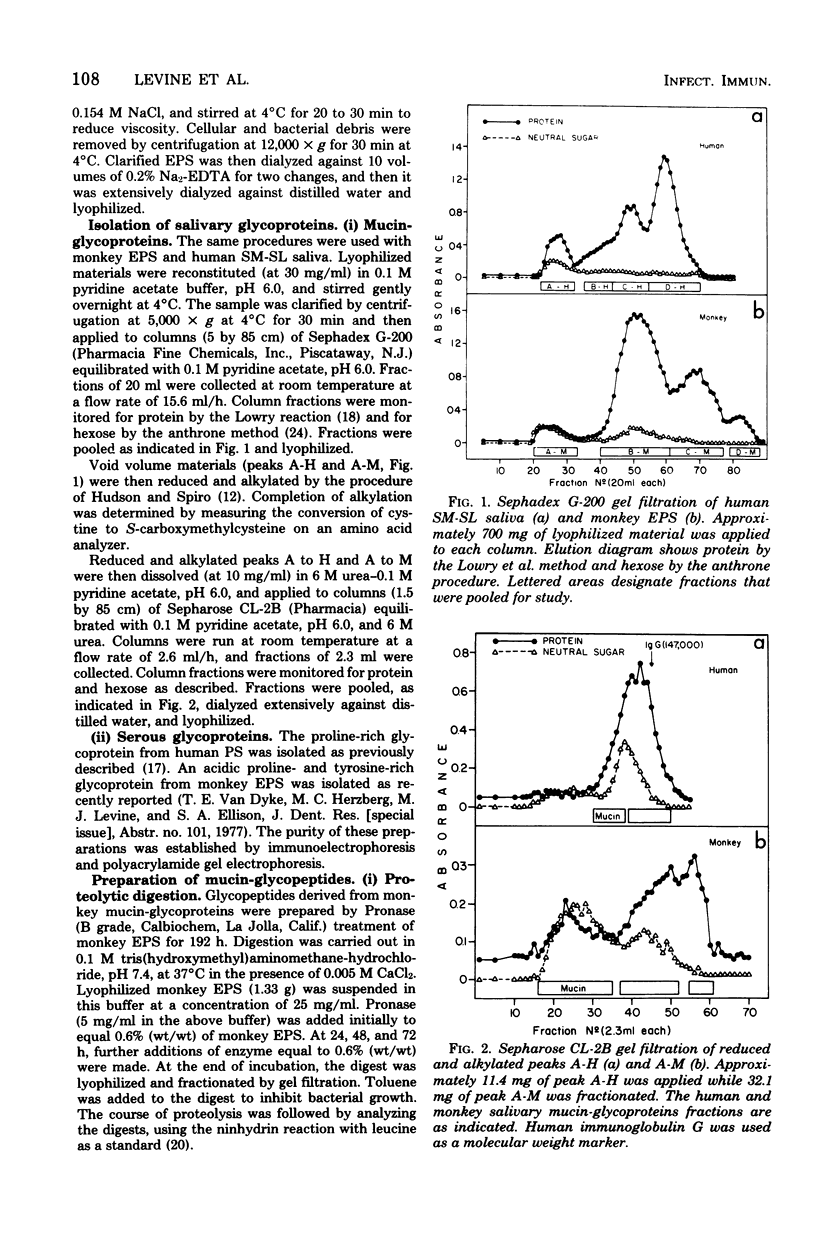
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arneberg P., Helgeland K., Tjörnhom T. Proline-rich proteins in membranes and contents of monkey (Macaca irus and Cercopithecus aethiops) parotid zymogen granules. Arch Oral Biol. 1976;21(6):379–383. doi: 10.1016/s0003-9969(76)80006-4. [DOI] [PubMed] [Google Scholar]
- Baig M. M., Winzler R. J., Rennert W. M. Isolation of mucin from human submaxillary secretions. J Immunol. 1973 Dec;111(6):1826–1833. [PubMed] [Google Scholar]
- CURBY W. A. Device for collection of human parotid saliva. J Lab Clin Med. 1953 Mar;41(3):493–496. [PubMed] [Google Scholar]
- Cassidy J. T., Jourdian G. W., Roseman S. The sialic acids. VI. Purification and properties of sialidase from Clostridium perfringens. J Biol Chem. 1965 Sep;240(9):3501–3506. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Hay D. I., Gibbons R. J., Spinell D. M. Characteristics of some high molecular weight constituents with bacterial aggregating activity from whole saliva and dental plaque. Caries Res. 1971;5(2):111–123. doi: 10.1159/000259739. [DOI] [PubMed] [Google Scholar]
- Hudson B. G., Spiro R. G. Fractionation of glycoprotein components of the reduced alkylated renal glomerular basement membrane. J Biol Chem. 1972 Jul 10;247(13):4239–4247. [PubMed] [Google Scholar]
- Jacobsen N., Arneberg P. Basic, proline-rich glycoproteins in the submandibular gland secretion of the Cercopithecus aethiops. Comp Biochem Physiol B. 1976;54(3):423–425. doi: 10.1016/0305-0491(76)90269-8. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. The structure of a phytohemagglutinin receptor site from human erythrocytes. J Biol Chem. 1970 May 25;245(10):2536–2545. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee Y. C., Johnson G. S., White B., Scocca J. An accelerated system for analysis of neutral sugars in complex carbohydrates. Anal Biochem. 1971 Oct;43(2):640–643. doi: 10.1016/0003-2697(71)90301-0. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Ellison S. A., Bahl O. P. The isolation from human parotid saliva and partial characterization of the protein core of a major parotid glycoprotein. Arch Oral Biol. 1973 Jul;18(7):827–837. doi: 10.1016/0003-9969(73)90053-8. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Ellison S. A. Immuno-electrophoretic and chemical analyses of human parotid saliva. Arch Oral Biol. 1973 Jul;18(7):839–853. doi: 10.1016/0003-9969(73)90054-x. [DOI] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem. 1954 Dec;211(2):907–913. [PubMed] [Google Scholar]
- Oemrawsingh I., Roukema P. A. Isolation, purification and chemical characterization of mucins from human submandibular glands. Arch Oral Biol. 1974 Aug;19(8):615–626. doi: 10.1016/0003-9969(74)90129-0. [DOI] [PubMed] [Google Scholar]
- PUSZTAI A., MORGAN W. T. Studies in immunochemistry. 20. The action of papain and ficin on blood-group-specific substances. Biochem J. 1961 Dec;81:639–647. doi: 10.1042/bj0810639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payza N., Robert M., Herp A. The molecular weight of bovine and porcine submaxillary mucins. Int J Protein Res. 1970;2(2):109–115. doi: 10.1111/j.1399-3011.1970.tb01665.x. [DOI] [PubMed] [Google Scholar]
- ROE J. H. The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem. 1955 Jan;212(1):335–343. [PubMed] [Google Scholar]
- SPENCER B. The ultramicro determination of inorganic sulphte. Biochem J. 1960 Jun;75:435–440. doi: 10.1042/bj0750435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENNERHOLM E., SVENNERHOLM L. Quantitative paper partition chromatography of sialic acids. Nature. 1958 Apr 19;181(4616):1154–1155. doi: 10.1038/1811154a0. [DOI] [PubMed] [Google Scholar]
- Spiro M. J. Subunit heterogeneity of thyroglobulin. J Biol Chem. 1973 Jun 25;248(12):4446–4460. [PubMed] [Google Scholar]
- Sönju T., Christensen T. B., Kornstad L., Rölla G. Electron microscopy, carbohydrate analyses and biological activities of the proteins adsorbed in two hours to tooth surfaces in vivo. Caries Res. 1974;8(2):113–122. doi: 10.1159/000260099. [DOI] [PubMed] [Google Scholar]
- Sönju T., Rölla G. Chemical analysis of the acquired pellicle formed in two hours on cleaned human teeth in vivo. Rate of formation and amino acid analysis. Caries Res. 1973;7(1):30–38. doi: 10.1159/000259822. [DOI] [PubMed] [Google Scholar]
- Sönju T., Rölla G. Distribution of sulphated macromolecules in the salivary secretions of macaca irus. Arch Oral Biol. 1974 Oct;19(10):897–902. doi: 10.1016/0003-9969(74)90052-1. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972 Aug 25;177(4050):697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]




