Abstract
How are excitatory (glutamatergic) and inhibitory (GABAergic) synapses established? Do distinct molecular mechanisms direct differentiation of glutamatergic and GABAergic synapses? In the brain, glutamatergic and GABAergic synaptic connections are formed with specific patterns. To establish such precise synaptic patterns, neurons pass through multiple checkpoints during development, such as the cell fate determination, cell migration and localization, axonal guidance and target recognition, and synapse formation. Each stage offers key molecules for neurons/synapses to obtain glutamatergic or GABAergic specificity. Some mechanisms are based on intrinsic systems to induce gene expression, while others are based on extrinsic systems mediated by cell-cell or axon-target interactions. Recent studies indicate that specific formation of glutamatergic and GABAergic synapses are controlled by the expression or activation of different sets of molecules during development. In this review, we outline stages critical to the determination of glutamatergic or GABAergic specificity, and describe molecules which act as determinants of specificities in each stage, with a particular focus on the synapse formation stage. We also discuss possible mechanisms underlying glutamatergic and GABAergic synapse formation via synapse-type specific synaptic organizers.
Keywords: neuronal development, glutamatergic, GABAergic, synapse formation, FGFs, Neuroligins
Introduction
Precise synaptic connections are one of the most remarkable features of the brain. Brain function is controlled by the specific patterns of synaptic connectivity formed by various types of neurons. Two major types of neurons, glutamatergic and GABAergic, send excitatory and inhibitory outputs to their target neurons, respectively. The balance between excitatory and inhibitory inputs is critical for the proper functioning of the brain, and their imbalance may lead to various neurological disorders such as autism, schizophrenia, Tourette syndrome and epilepsy (Mohler 2006; Rubenstein and Merzenich 2003; Singer and Minzer 2003; Wassef and others 2003). Thus, formation of appropriate glutamatergic and GABAergic synapses is important for proper functioning of the brain.
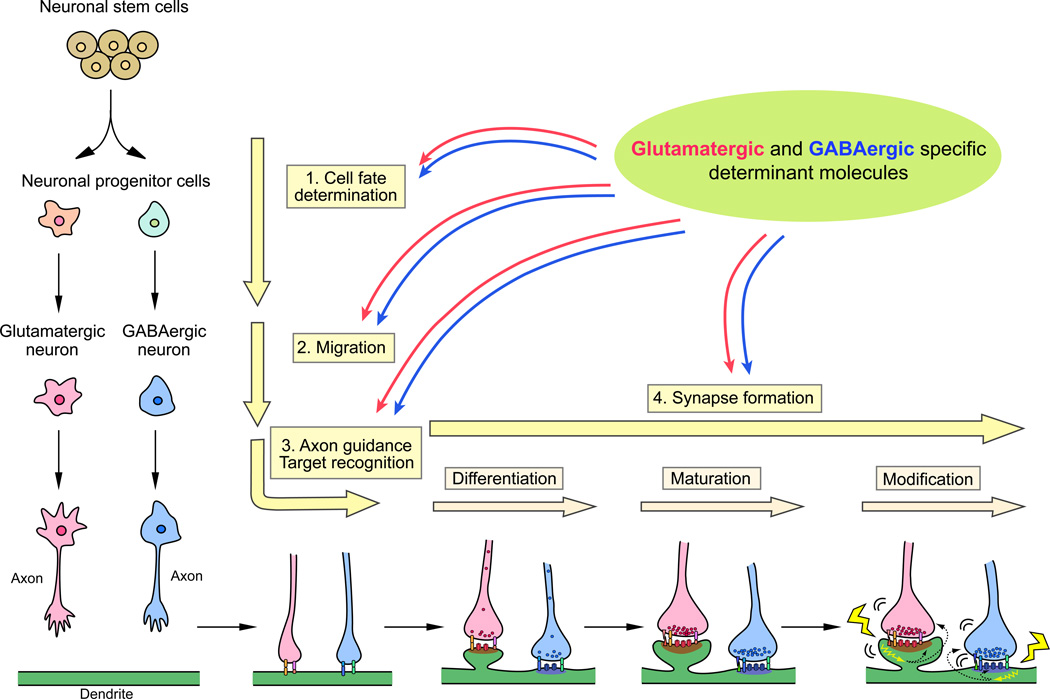
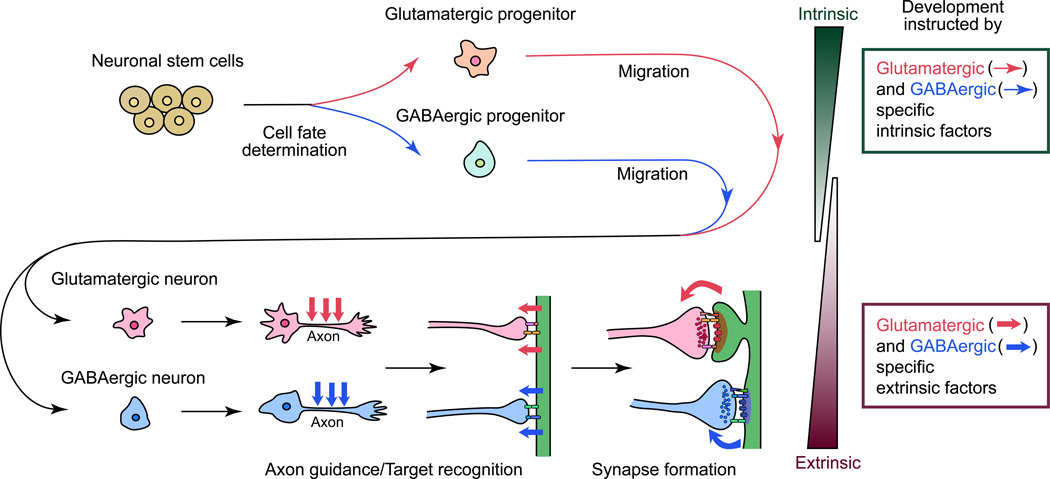
In order to establish glutamatergic and GABAergic synapses, neurons progress through at least four critical developmental processes (Sanes and Yamagata 2009) (Fig. 1). 1) Cell fate determination: Progenitor cells differentiate into glutamatergic or GABAergic neurons. 2) Migration: Glutamatergic and GABAergic neurons migrate to appropriate regions in the brain. 3) Axon guidance/target recognition: Axons of these neurons are guided to their targets and recognize them. 4) Synapse formation: Contact sites between the axons and targets differentiate into synapses. Both glutamatergic and GABAergic synapses are established essentially by following these processes; however, glutamatergic and GABAergic synapses are quite different in terms of their synaptic structure and components. What mechanisms are involved in deciding the fate to become glutamatergic versus GABAergic neurons? What molecules guide their migration and axon growth? What signals are required for recruiting distinct synaptic components and establishing different synaptic structures at glutamatergic and GABAergic synapses? These questions have attracted a lot of research, trying to understand mechanisms of glutamatergic and GABAergic synapse formation. As a result, it is now shown that multiple mechanisms and molecules coordinate in each developmental stage for the determination of neuronal cell fate and for the establishment of specific synaptic types, with distinct sets of molecules for different types of neurons/synapses. Specific differentiation is not only dependent on innate programming in the differentiating neurons, but also on synaptic activity and extrinsic actions from synaptic partners at the contact sites, especially at later stages of synapse development.
Figure 1. Developmental processes to establish glutamatergic and GABAergic neurons and synapses.
Glutamatergic and GABAergic specification requires at least four sequential stages: cell fate determination, migration, axon guidance and target recognition, and synapse formation. The synapse formation stage includes synaptic differentiation, synaptic maturation, and synaptic modification (refinement, plasticity, etc.). A variety of mechanisms coordinate in each stage, with distinct sets of molecules in the establishment of glutamatergic and GABAergic synapses.
In this review, we describe how glutamatergic and GABAergic specificities are determined at the cell and synapse level. We also describe recent findings on molecular determinants of such specificities at each developmental stage, i.e., cell fate determination, cell migration, axon guidance and target recognition, and synapse formation (Fig. 1).
Determination of glutamatergic or GABAergic cell fate
In order to properly generate excitatory and inhibitory circuits, glutamatergic and GABAergic neurons must be specified in germinal zones. Neuronal fate is mostly specified in the progenitor stage. The mechanisms underlying the determination of neuronal fate involve intrinsic properties of the cell such as the expression of specific transcription factors and extrinsic factors such as morphogens and growth factors that activate signaling pathways in the cell (Guillemot 2007; Schuurmans and Guillemot 2002). Recent studies have shown that intrinsic gene expression regulated by a selected set of transcription factors largely contributes to the cell fate determination of glutamatergic versus GABAergic neurons. Extrinsic factors seem to be involved in brain patterning rather than the determination of neuronal types (Guillemot 2007; Rallu and others 2002; Schuurmans and Guillemot 2002; Xu and others 2010), although transplantation studies have shown that neuronal progenitor cells could adopt appropriate cell types in the new environment (Fishell 1995; Magrassi and others 1998).
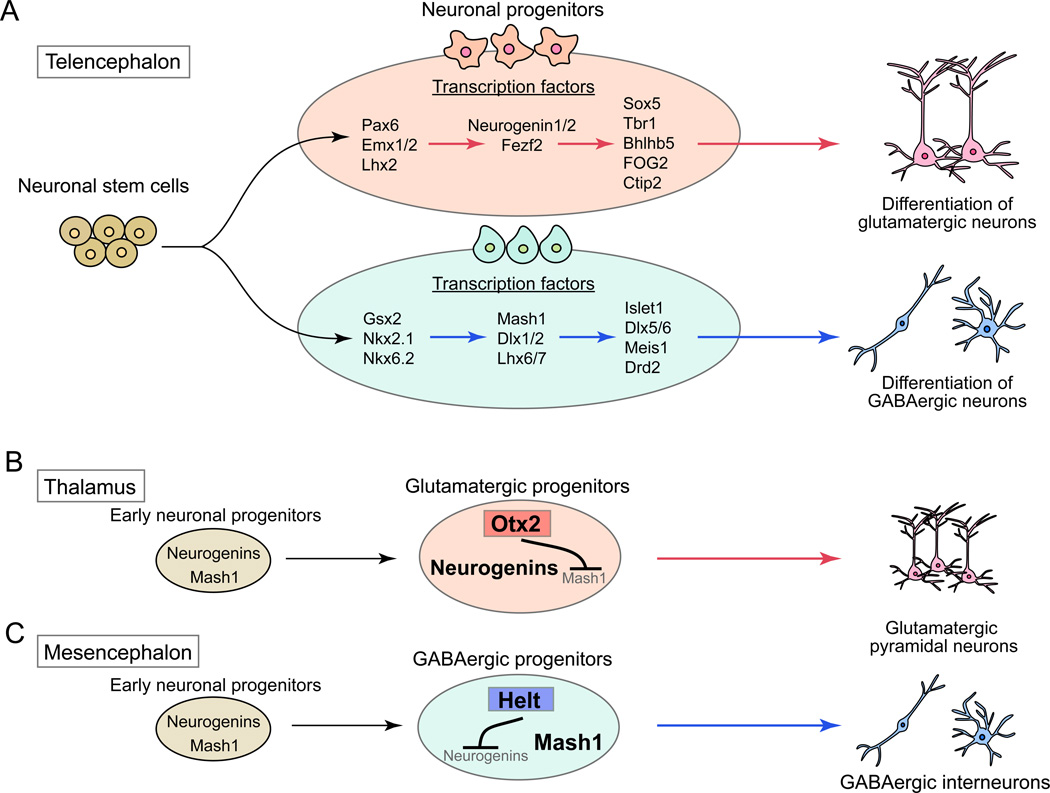
Various transcription factors in neuronal progenitor cells have been identified as determinants of glutamatergic and GABAergic neuronal fates (Fig. 2A). In addition, transcriptional repressors, such as Otx2 in the thalamus and Helt in the mesencephalon, control neuronal fate by silencing the expression of GABAergic or glutamatergic transcription factors to abolish alternative cell fate determination programs (Nakatani and others 2007; Puelles and others 2006) (Fig. 2B and C). These results support the notion that specific transcription factors in early stages of neuronal progenitors instruct them to become glutamatergic or GABAergic neurons. Moreover, recent finding by Rouaux and Arlotta showed that an intrinsic transcription factor could control the neuronal fate irrespective of the extrinsic environment (Rouaux and Arlotta 2010). Exogenous expression of the transcription factor Fezf2, which specifies glutamatergic cortical projection neurons (Fig. 2A), in neural progenitors destined to be GABAergic medium spiny neurons of the striatum resulted in their differentiation into glutamatergic cortical neurons. Taken together, these findings indicate that intrinsic signals, not extrinsic environmental factors, are critical to determine the type of neurons.
Figure 2. Transcription factors in neuronal progenitor cells as determinants of glutamatergic or GABAergic neuronal fate.
(A) Transcription factor determinants for glutamatergic and GABAergic neurons in telencephalon. Glutamatergic and GABAergic neuronal progenitor cells express distinct sets of transcription factors, which determine specific fate of the neurons.
(B and C) Regulation of neuronal cell fate by transcriptional control. In thalamus and mesencephalon, early stages of neuronal progenitors express two types transcription factors, neurogenins and Mash1, which are determinants of glutamatergic and GABAergic neuronal cell fate, respectively. However, one of them is suppressed by a cell-type specific transcription factor to generate glutamatergic or GABAergic neurons. In thalamus, a transcription factor, Otx2, suppresses Mash1 expression, resulting in the generation of glutamatergic neurons (B). In mesencephalon, another transcription factor, Helt, suppresses neurogenins expression to generate GABAergic neurons (C).
Migration of neurons
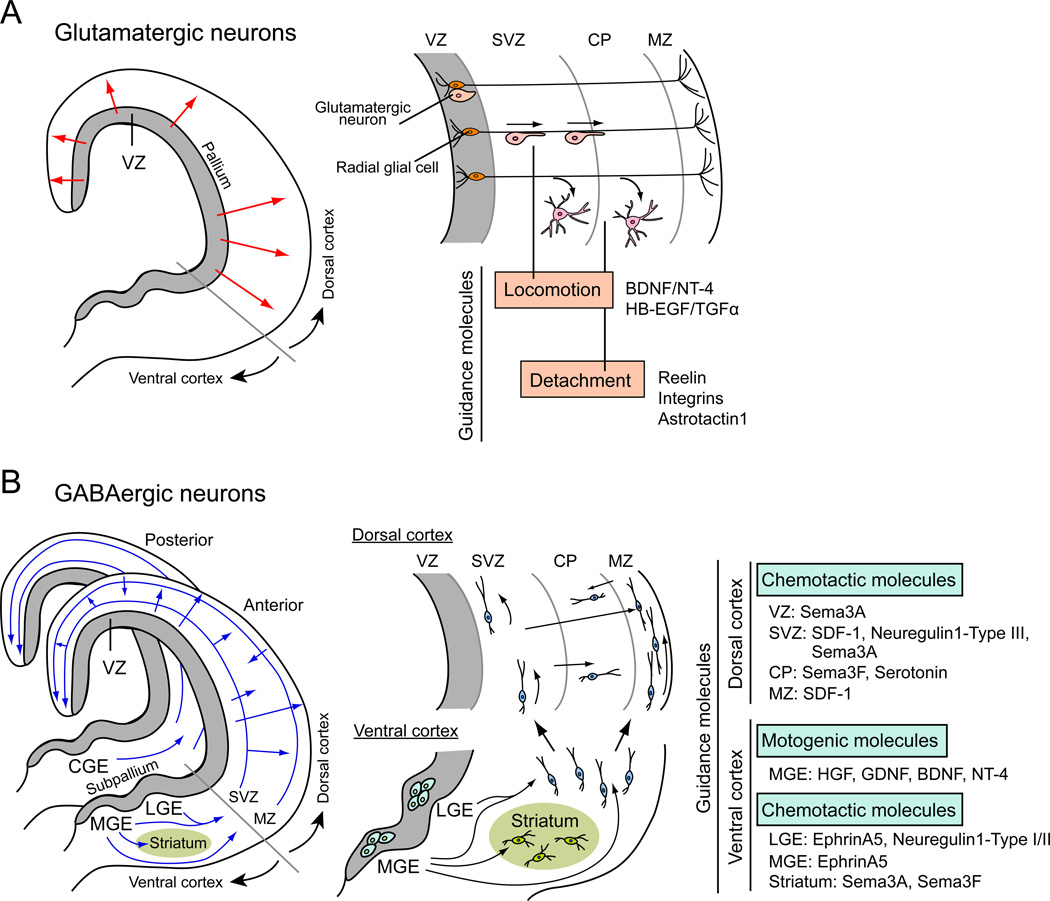
Once neuronal fate is specified, glutamatergic and GABAergic neurons must migrate to appropriate regions of the brain. Migration of specified neurons during brain development is precisely regulated, which is important for the final brain architecture. Migration of glutamatergic pyramidal neurons and GABAergic interneurons has been well studied in the cerebral cortex. Cortical neurons are born in the ventricular zone (VZ) and adopt two types of migration, radial and tangential (Marin and Rubenstein 2003) (Fig. 3). Interestingly, different types of neurons are generated in distinct regions of the VZ and use different migration modes (Marin and others 2010). Pyramidal neurons are generated in the embryonic pallium and move to their final location by radial migration (Fig. 3A). On the other hand, GABAergic interneurons are generated in the embryonic subpallium and adopt tangential migration to reach the cortex, which then migrate with radial movement to their final destination in the cortex (Danglot and others 2006; Hernandez-Miranda and others 2010) (Fig. 3B). In addition, the migration of glutamatergic pyramidal neurons and GABAergic interneurons are based on different cellular mechanisms. For the migration of glutamatergic pyramidal neurons, radial glial cells in developing cortex act as guidance cells. Cell bodies of radial glial cells are located in the VZ, with their long processes extending to the cortical surface (Campbell and Gotz 2002) (Fig. 3A). Migrating glutamatergic pyramidal neurons attach and move along the processes of radial glial cells. Various adhesion molecules and guidance molecules are involved in radial migration movement to their final destinations (Marin and Rubenstein 2003). These molecules contribute to the adhesion to radial glial processes, laminar-specific neuronal migration, detachment of neurons from radial glial cells, and translocation of neurons after the detachment (Fig. 3A) (Bock and Herz 2003; Burrows and others 1997; Caric and others 2001; Cooper 2008; Dulabon and others 2000; Kornblum and others 1997).
Figure 3. Distinct mechanisms and molecules for the migration of glutamatergic and GABAergic neurons.
(A) Migration of glutamatergic neurons in the cortex. Glutamatergic neurons are generated in the ventricular zone (VZ) of the embryonic pallium and move to their final location by radial migration (red arrows). For this, glutamatergic neurons attach to the processes of radial glial cells and move along them to the destination area. There, they detach from radial glia cells and migrate to their final location. The locomotion along radial glial cell processes and detachment from them are regulated by various guidance molecules.
(B) Migration of GABAergic neurons in the cortex. GABAergic interneurons are generated in the VZ of the embryonic subpallium and adopt tangential migration to reach the dorsal cortex (blue arrows). In the dorsal cortex, they also migrate by radial movement within the cortical wall (blue arrows). For GABAergic neuronal migration, guidance molecules play important roles; guidance cells like radial glial cells have not been identified. Motogenic and chemotactic molecules involved in the migration of GABAergic neurons are listed. VZ: ventricular zone, SVZ: subventricular zone, CP: cortical plate, MZ: marginal zone, LGE: lateral ganglionic eminence, MGE: medial ganglionic eminence, CGE: caudal ganglionic eminence, BDNF: brain-derived neurotrophic factor, NT-4: neurotrophin 4, HB-EGF: heparin-binding epidermal growth factor, TGFα: transforming growth factor alpha, SDF-1: stromal cell derived factor 1, HGF: hepatocyte growth factor, GDNF: glial cell line-derived neurotrophic factor.
For the migration of GABAergic interneurons, they do not seem to associate with guidance cells like radial glial cells. Rather, they appear to just follow guidance molecules, which serve as chemoattractants or chemorepellents to regulate the migration of GABAergic interneurons (Gant and others 2009; Ghashghaei and others 2006; Hernandez-Miranda and others 2010; Lopez-Bendito and others 2008; Marin and others 2001; Rudolph and others 2010; Zimmer and others 2008) (Fig. 3B). In addition, several growth factors act as motogenic factors and stimulate the migration of interneurons (Behar and others 1997; Hernandez-Miranda and others 2010; Polleux and others 2002; Powell and others 2001; Pozas and Ibanez 2005) (Fig. 3B).
Axon guidance and target recognition
After cell migration, glutamatergic pyramidal neurons and GABAergic interneurons elongate their axons to the synaptic targets. Glutamatergic pyramidal neurons form long axons in order to reach their targets, whereas GABAergic neurons form short axons (Danglot and others 2006; Huang and others 2007). Nevertheless, axon guidance appears to require various guidance cues for both types of neurons.
Many molecules have been identified as axon guidance molecules for glutamatergic pyramidal neurons. Environmental cues, which serve as chemoattractants or chemorepellents for axons, include both membrane and secreted proteins. Growing axons interact with these molecules to decide their directions. For example, pathfinding of hippocampal pyramidal axons is guided by secreted proteins, netrin1 (attractant), class 3 semaphorins and Slit2 (repellent) (Skutella and Nitsch 2001). Membrane proteins such as ephrin/Eph and semaphorin/plexin are involved in topographical segregation of glutamatergic axons and provide layer specific axon pathfinding (Holt and Harris 1998; Stein and others 1999; Suto and others 2007). Netrin/DCC, slit/Robo, semaphorin/semaphorin receptor (plexin, neuropilin, etc.), and ephrin/Eph are also guidance cues in other areas in the brain (Dickson 2002; Huber and others 2003; Skutella and Nitsch 2001). Activation of these molecules in growing axons alters actin filaments and microtubules and modifies their shape and growth direction. Signaling pathways involved in cytoskeletal dynamics are described in other reviews (e.g., Dent and others 2010).
Axons of GABAergic interneurons are relatively short, but have various patterns of arborization and innervation (Ascoli and others 2008), and the morphology of axon arbors as well as the expression of calcium-binding proteins are used for the classification of GABAergic neurons (Danglot and others 2006). With histological and physiological studies, subclass specific localizations and innervation patterns of GABAergic interneurons in the cerebellar cortex and hippocampus are being characterized (Danglot and others 2006; Huang and others 2007; Karube and others 2004). The morphology of the axon arbors depend on the layers in which the GABAergic interneurons are located, as well as the layers that they innervate (Karube and others 2004; Tamas and others 2000). Thus, axon growth is not random, but is cell-type and environment specific. Cellular and molecular mechanisms of GABAergic neuronal axon growth are still not clear; however, recent studies showed that Close Homologue of L1 (CHL1) expressed by fibers of Bergmann glial cells in the cerebellar cortex play a role to guide axons of stellate interneurons toward Purkinje cells (Ango and others 2008).
Axon guidance brings axons in the proximity of their actual target areas. There, however, multiple types of neurons are located. Thus, mechanisms to recognize correct targets are necessary to form specific axon-target connections. Specific target recognition is required by both glutamatergic pyramidal neurons and GABAergic interneurons (Danglot and others 2006; Klausberger and Somogyi 2008), so recognition molecules are likely specific to individual types of synaptic contact and not common to glutamatergic and GABAergic neurons. Target recognition mechanisms can be separated into three phases: recognition by laminar specificity, cellular specificity, and subcellular specificity. It is thought that extracellular molecular cues such as cell adhesion molecules are the key for such specificities (Sanes and Yamagata 2009). Not many molecules have been identified as such recognition molecules so far, but a role for semaphorins has been recently suggested. For example, sema6A and its receptor plexinA4 control lamina-specific targeting of processes from retinal ganglion cells, amacrine cells, and bipolar cells in the mouse retinal inner plexiform layer (IPL) (Matsuoka and others 2011). In the spinal cord, sema3E in a specific subset of motor neurons and its receptor plexinD1 in sensory neurons are required for proper sensory-motor connections (Pecho-Vrieseling and others 2009). In addition, other molecules such as sidekicks and Dscams are shown to determine sublamina-specific targeting of amacrine cells and bipolar cells in the chick IPL via their homophilic interactions (Yamagata and Sanes 2008). Moreover, neurofascin expressed by cerebellar Purkinje cells controls basket cell axon targeting and subcellular localization at Purkinje axon initial segment (Ango and others 2004). Thus, various site-specific molecules appear to regulate specific target recognition.
Glutamatergic and GABAergic synapse formation
After identifying the appropriate synaptic partners, axons and dendrites form synapses at their contact sites by assembling synaptic components, such as synaptic vesicles, active zones, neurotransmitter receptors, and postsynaptic scaffold proteins. Recruitment of synaptic components specifically occurs at the axon-target contact sites (Fox and Umemori 2006). This indicates that molecules are exchanged between the contacting partners and act as synaptic organizers.
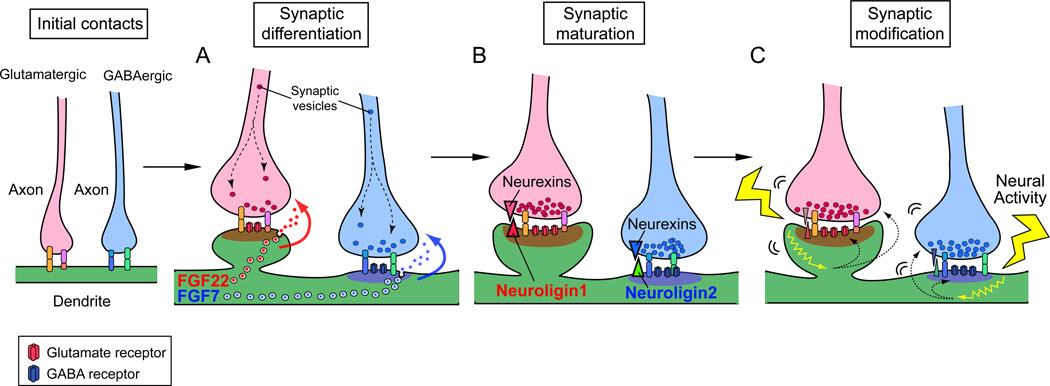
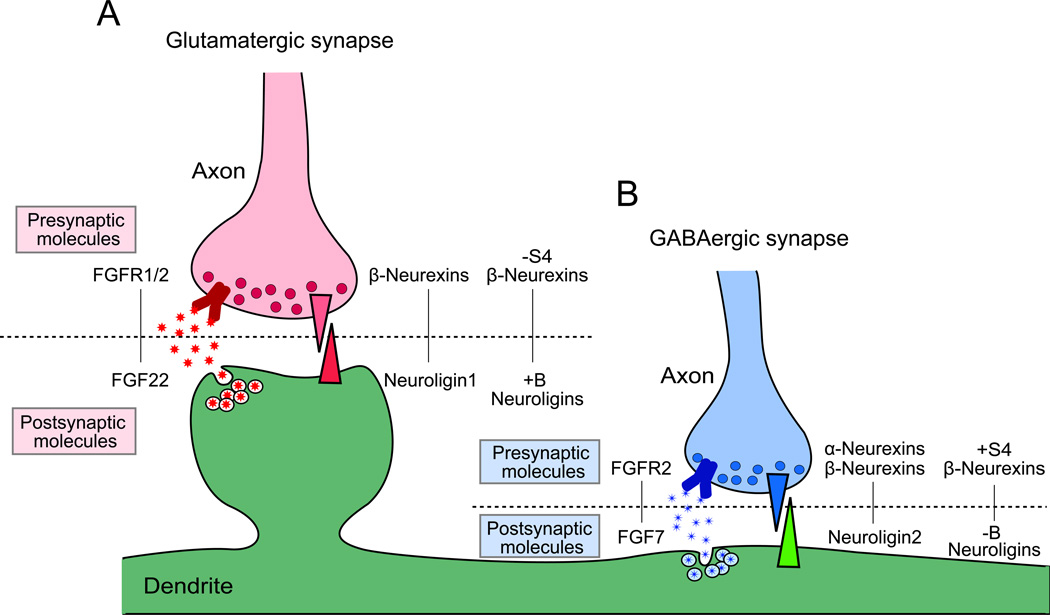
Glutamatergic and GABAergic synapses have different synaptic components and cytoskeletal structures. For examples, glutamatergic synapses contain clear active zones and synaptic vesicles with vesicular glutamate transporters in the presynaptic terminal, and thick postsynaptic densities (PSDs) with glutamate receptors and a scaffolding protein PSD95 in the postsynaptic terminal. Glutamatergic synapses are often found onto spines. In contrast, GABAergic synapses contain less obvious active zones and synaptic vesicles with vesicular GABA transporters in the presynaptic terminal, and thinner PSDs with GABA receptors and a scaffolding protein gephyrin in the postsynaptic terminal. These differences suggest that formation of glutamatergic and GABAergic synapses involve distinct mechanisms. In fact, recent studies revealed glutamatergic or GABAergic synapse specific organizers. It appears that multiple molecules are involved in the formation of glutamatergic and GABAergic synapses (Waites and others 2005). To date, many molecules including cell adhesion and secreted molecules have been identified as synaptic organizers for glutamatergic synapses (e.g., fibroblast growth factors, neuroligins, ephrins/Ephs, netrin-G ligands, LRRTMs, SynCAMs, and Wnts) and a few molecules for GABAergic synapses (e.g., fibroblast growth factors and neuroligins) (for reviews, see Dalva and others 2007; Fox and Umemori 2006; Johnson-Venkatesh and Umemori 2010; Siddiqui and Craig 2010). Here, we focus on two interesting families of synaptic organizers, fibroblast growth factors (FGFs) and neuroligins (NLGNs). FGFs and NLGNs are implicated in synaptic differentiation and maturation, respectively (Fig. 5). Interestingly, they have two closely related proteins that play distinct rather than overlapping roles to selectively promote either excitatory or inhibitory synapse formation.
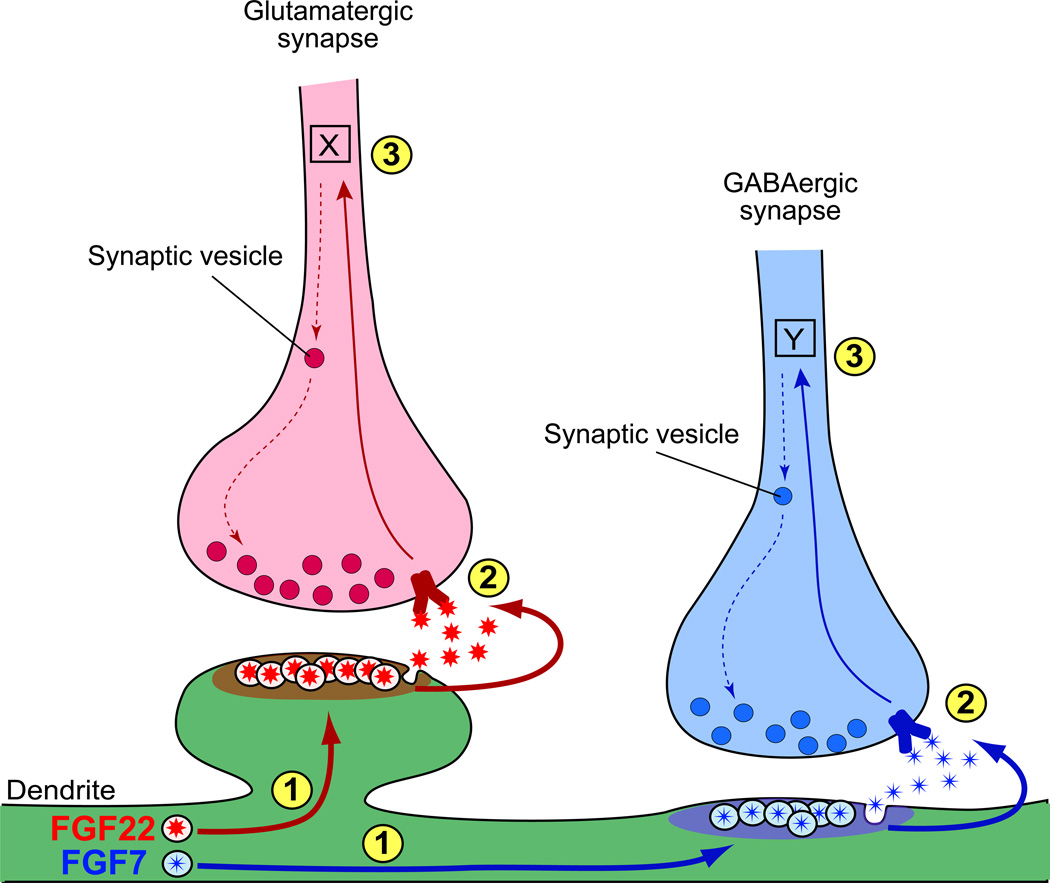
Figure 5. Two closely related members of FGF and neuroligin protein families selectively organize glutamatergic and GABAergic synapse formation.
Steps of synapse formation include (A) Synaptic differentiation: FGF22 and FGF7 specifically released from glutamatergic or GABAergic postsynaptic sites promote accumulation of corresponding synaptic vesicles to the presynaptic terminal. (B) Synaptic maturation: neuroligin1 at glutamatergic postsynaptic terminals and neuroligin2 at GABAergic postsynaptic terminals maturate each synapse through their receptor neurexins. (C) Synaptic modification: neural activity-dependent mechanisms are involved in synapse refinement and plasticity.
Synaptic differentiation of glutamatergic and GABAergic synapses by FGF22 and FGF7
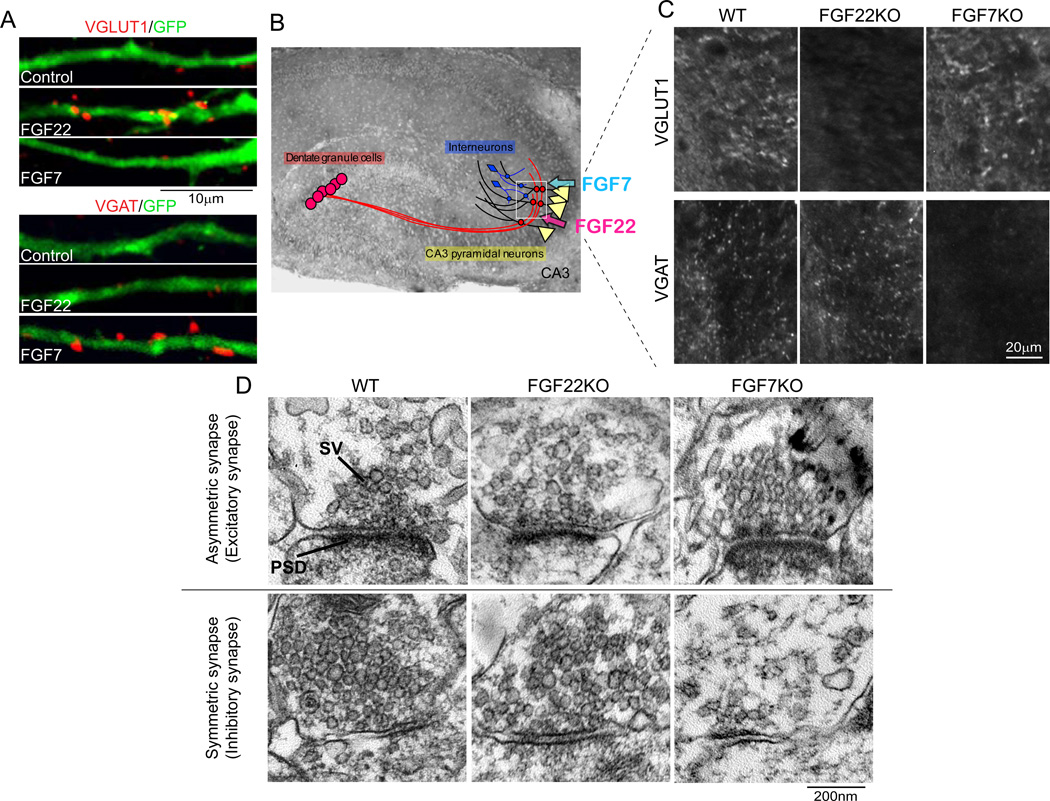
FGFs are a family of intercellular signaling molecules involved in cell proliferation, migration, differentiation, wound healing, and metabolism (Itoh and Ornitz 2010; Thisse and Thisse 2005; Umemori 2009). We have identified FGF22 as a presynaptic organizer that promotes the clustering of synaptic vesicles in cultured neurons by performing biochemical purification from developing mouse brain (Umemori and others 2004). In addition, we found that FGF7 and FGF10, two FGFs in the same subfamily as FGF22, can also induce synaptic vesicle clustering. Using FGF22-knockout (KO) and FGF7KO mice, we found that FGF22 and FGF7 are synapse-type specific presynaptic organizers (Terauchi and others 2010). mRNAs of FGF22 and FGF7 were strongly expressed by CA3 pyramidal neurons in the hippocampus when synaptogenesis starts. When transfected into cultured hippocampal neurons, FGF22 and FGF7 selectively promoted clustering of vesicular glutamate transporter 1 (VGLUT1) and vesicular GABA transporter (VGAT), respectively (Fig. 4A). Remarkably, the clustering of VGLUT1 was significantly decreased in CA3 of FGF22KO but not in FGF7KO mice, whereas that of VGAT was dramatically decreased in CA3 of FGF7KO but not in FGF22KO mice (Fig. 4B and C). These results suggest that FGF22 is specifically involved in glutamatergic presynaptic differentiation, while FGF7 is selectively involved in GABAergic presynaptic differentiation. Results from electron microscopic and electrophysiological studies also supported this notion: decreased excitatory synaptic vesicle clustering and mEPSC frequency in FGF22KO mice and diminished inhibitory synaptic vesicle clustering and mIPSC frequency in FGF7KO mice (Fig. 4D). Finally, postsynaptic expression of FGF22 and FGF7 rescued defects in glutamatergic or GABAergic synaptic vesicle clustering in corresponding FGFKO cultures, indicating that these FGFs are target-derived presynaptic organizers. Taken together, these results demonstrate that FGF22 and FGF7, released from postsynaptic target neurons, promote glutamatergic and GABAergic presynaptic differentiation, respectively (Fig. 5A). Studies identifying of molecules involved in glutamatergic and GABAergic synapse differentiation in other areas of the brain, and understanding the role of FGF10 are underway.
Figure 4. FGF22 and FGF7 promote glutamatergic and GABAergic synaptic differentiation, respectively.
(A) Overexpression of FGF22 and FGF7 selectively promotes VGLUT1 or VGAT clustering on the FGF-expressing hippocampal neurons (labeled with GFP) in culture. (B) CA3 pyramidal neurons, by which FGF22 and FGF7 are expressed, receive excitatory inputs from dentate granule cells (and CA3 and entorhinal cortical neurons) and inhibitory inputs from GABAergic interneurons in CA3. (C) Impaired glutamatergic and GABAergic presynaptic differentiation in CA3 of FGF22KO and FGF7KO mice, respectively. Glutamatergic and GABAergic presynaptic differentiation was assessed by measuring the clustering of VGLUT1 or VGAT. Pictured areas correspond to the boxed area in (B). (D) Representative electron microscopic pictures of asymmetric (excitatory) and symmetric (inhibitory) synapses in CA3 of wild-type (WT), FGF22KO and FGF7KO mice. Relative to WT synapses, excitatory synaptic vesicles are more diffusely distributed in FGF22KO synapses, while less inhibitory synaptic vesicles are observed in FGF7KO synapses. Adapted from Terauchi and others (2010). SV: synaptic vesicle, PSD: postsynaptic density.
Maturation of glutamatergic and GABAergic synapses by neuroligins
NLGNs are type I membrane proteins localized at postsynaptic membrane. Extracellular domains of NLGNs bind to extracellular domains of α- and β-neurexins (NRXNs), another type I membrane proteins at the presynaptic membrane (Lise and El-Husseini 2006). NLGNs have been identified as presynaptic organizers using co-cultures between NLGN-expressing nonneuronal cells and neurons (Scheiffele and others 2000). In addition, overexpression of NLGNs in cultured neurons increased the density of synapses (Chih and others 2005; Graf and others 2004; Levinson and others 2005; Nam and Chen 2005; Prange and others 2004).
Interestingly, two different NLGNs, NLGN1 and NLGN2, are localized at different types of synapses: NLGN1 at glutamatergic and NLGN2 at GABAergic synapses (Graf and others 2004; Song and others 1999; Varoqueaux and others 2004). Overexpression of NLGN1 preferentially increased the number of glutamatergic synapses, while overexpression of NLGN2 increased the number of GABAergic synapses (Chih and others 2005; Chubykin and others 2007; Prange and others 2004). Expression of distinct NLGNs modulates the balance between glutamatergic and GABAergic synapses (Levinson and others 2005). In addition, NLGN1KO mice exhibit a decrease in NMDAR-dependent EPSC amplitudes, while NLGN2KO mice selectively show depressed IPSC amplitudes (Chubykin and others 2007). However, histological analyses performed with NLGNKO mice demonstrated that the density and morphology of synapses as well as the distribution of synaptic components are normal in NLGNsKO mice (Varoqueaux and others 2006). Therefore, NLGNs do not appear to be essential for the initial formation of synapses, but rather are required for synapse maturation and function. Interestingly, NLGN1 function seems to be dependent on synaptic activity via NMDAR, while NLGN2 function is dependent on general synaptic activity (Chubykin and others 2007). An attractive model based on these data is that NLGN1 and NLGN2 are presynaptic organizers for glutamatergic and GABAergic synapses, respectively, that regulate synapse maturation in an activity-dependent manner (Fig. 5B).
Activity dependent synapse modification
The number and strength of glutamatergic and GABAergic synapses dynamically change in response to neural activity. During synapse development, neural activity-dependent signals regulate the number of functional synaptic inputs. Interestingly, the number of glutamatergic synapses appears to be controlled by relative activity among neurons, but the number of GABAergic synapses seems to be dependent on general activity (Burrone and others 2002; Hartman and others 2006). Molecular mechanisms underlying such activity-dependent modifications are still unclear, but the activity-dependent transcription factor Npas4 is implicated in the regulation of the number of inhibitory synapses through the induction of brain-derived neurotrophic factor (BDNF) (Lin and others 2008).
In mature neurons, synaptic plasticity in the form of Hebbian or homeostatic plasticity regulates the strength of synapses in response to changes in neural activity (Bi and Poo 2001; Davis 2006; Luscher and others 2000; Micheva and Beaulieu 1997; Pozo and Goda 2010; Rao and Craig 1997; Turrigiano 2007; Turrigiano 2008).
Mechanisms underlying specific effects by glutamatergic and GABAergic presynaptic organizers
We described that glutamatergic and GABAergic synapse formation is differentially regulated by distinct molecules in the same protein families; e.g., FGF and NLGN. What are the mechanisms by which distinct presynaptic organizers establish specific types of synapses? There are at least three possible mechanisms: 1) each presynaptic organizer is selectively transported to an appropriate type of synapse, 2) each molecule binds to a different set of receptors on the presynaptic terminal, and/or 3) each molecule sends specific signals downstream in the axon to organize glutamatergic or GABAergic synapse formation (Fig. 6). We describe recent views regarding these possible mechanisms underlying glutamatergic and GABAergic synapse formation, focusing on the first two - specific targeting of presynaptic organizers and their specific receptors. These mechanisms are not mutually exclusive and most likely a combination of them plays a role for formation of specific presynaptic terminals.
Figure 6. Possible mechanisms underlying synapse type-specific presynaptic differentiation mediated by FGF22 and FGF7.
Three mechanisms are shown: 1) Specific synaptic targeting of each FGF - FGFs might be differentially transported to their appropriate postsynaptic sites. 2) Distinct presynaptic receptors - FGFs might bind different sets of receptors on the presynaptic terminal. 3) Distinct signaling pathways in the presynaptic neuron - FGFs might activate different signaling cascades.
Trafficking of presynaptic organizers to glutamatergic or GABAergic synaptic sites
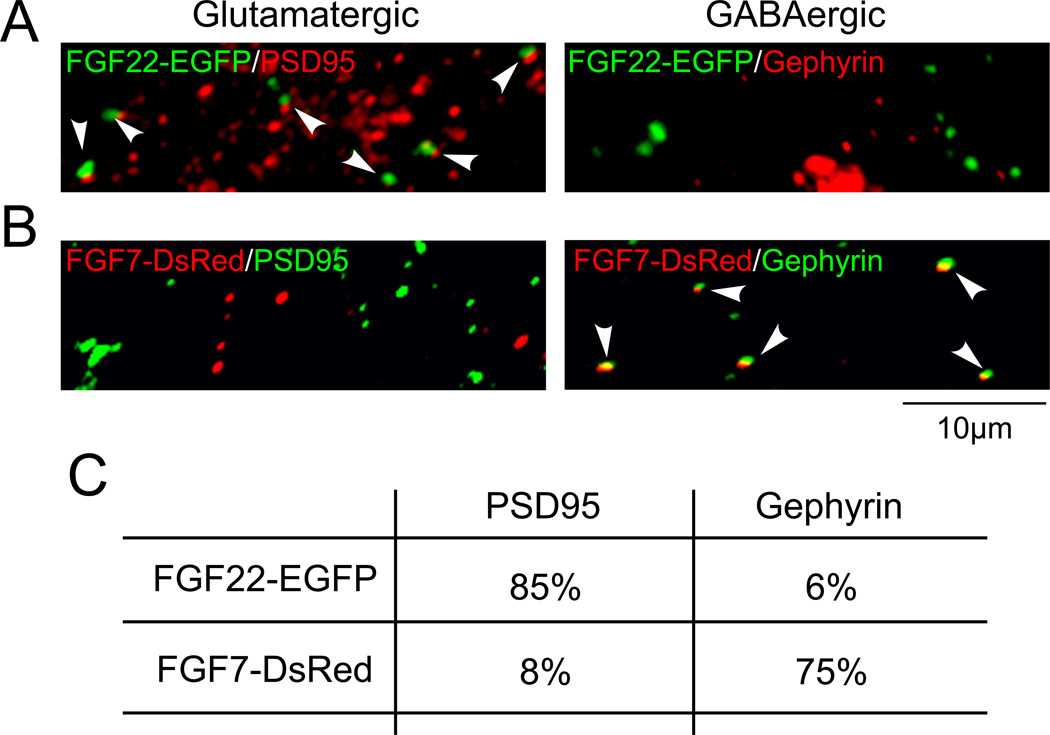
Specific targeting of synaptic organizers is critical to locally organize specific types of synapses. In fact, we found that FGF22 localizes to glutamatergic postsynaptic terminals, whereas FGF7 localizes to GABAergic postsynaptic terminals (Terauchi and others 2010) (Fig. 7). Likewise, NLGN1 localizes to glutamatergic synapses and NLGN2 localizes to GABAergic synapses (Graf and others 2004; Song and others 1999; Varoqueaux and others 2004).
Figure 7. FGF22 and FGF7 localize to glutamatergic and GABAergic synapses, respectively.
(A and B) Co-localization of transfected FGF22-EGFP and FGF7-DsRed with scaffolding proteins at glutamatergic (PSD95) or GABAergic (gephyrin) synapses (arrowheads). Percentages of co-localization are shown in (C). Adapted from Terauchi and others (2010).
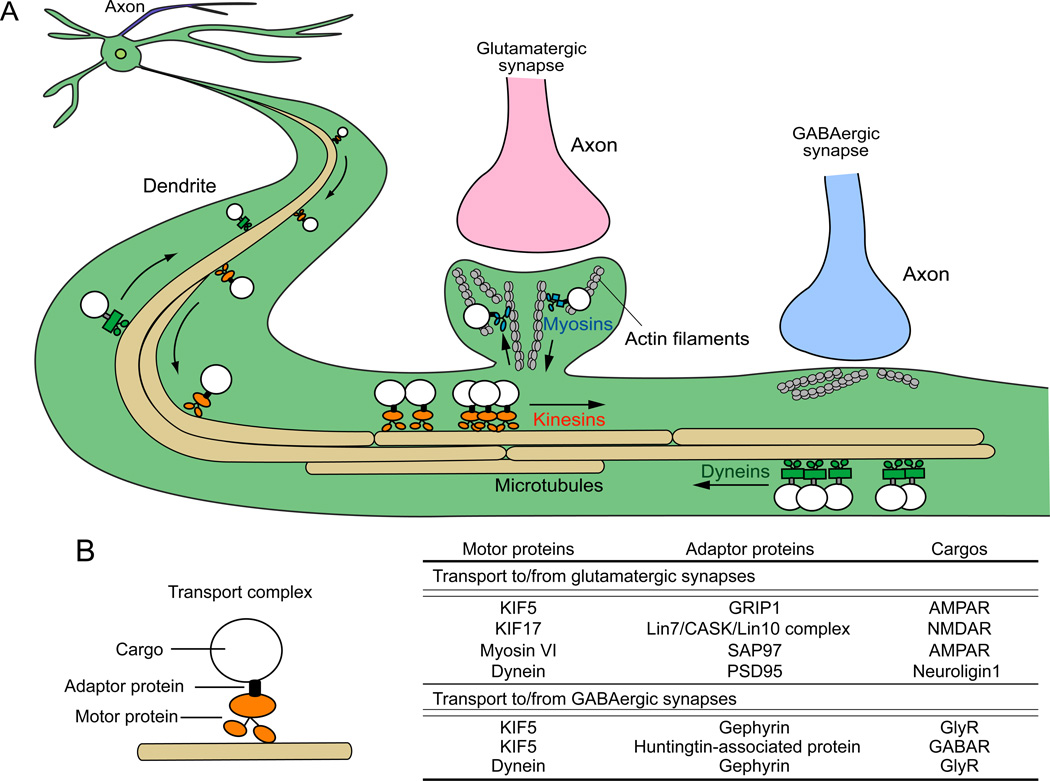
Recently, trafficking mechanisms of glutamatergic and GABAergic synapse specific molecules have been revealed. Intracellular transport of secreted and membrane proteins is operated by molecular motors. They are capable of long-distance transport of membranous organelles and macromolecular complexes along microtubules in axons and dendrites (Hirokawa and others 2010) (Fig. 8A). In dendrites, several postsynaptic scaffolding proteins for glutamatergic and GABAergic synapses act as adaptor proteins between molecular motors and cargos containing synaptic components (Fig. 8B) (Dumoulin and others 2009; Kneussel 2005; Mok and others 2002; Schapitz and others 2010). For example, GRIP, Mint1 (Lin10) complex, SAP97, and CASK, which are scaffolding proteins at glutamatergic synapses, act as adaptor proteins and are involved in the transport of AMPA receptors and NMDA receptors to glutamatergic synapses (Jeyifous and others 2009; Setou and others 2000) (Fig. 8B). On the other hand, gephyrin and huntingtin-associated protein 1, which are scaffolding proteins at glycinergic/GABAergic synapses, act as adaptor proteins for the trafficking of glycine receptors and GABAA receptors (Dumoulin and others 2009; Twelvetrees and others 2010) (Fig. 8B). Synaptic targeting of presynaptic organizers also seems to be controlled by the interaction with glutamatergic and GABAergic synapse specific scaffolding proteins. Recent studies showed that NLGN1 interacts with PSD95, a scaffolding protein at glutamatergic synapses, which is recognized by the dynein motor complex for retrograde transport of NLGN1 (Schapitz and others 2010); however, anterograde transport and trafficking in the spine appear to be independent of NLGN1's binding to PSD95 (Dresbach and others 2004; Rosales and others 2005). Mechanisms of NLGN2 transport to GABAergic postsynaptic terminals are not yet known, but a recent study showed that NLGN2, but not NLGN1 or NLGN3, binds gephyrin-collybistin complexes (Poulopoulos and others 2009). Thus, gephyrin-collybistin complexes might play a role in the transport of NLGN2 to GABAergic postsynaptic sites.
Figure 8. Dendritic transport of postsynaptic components by motor proteins along microtubules and actin filaments.
(A) Transport vesicles containing synaptic components are carried by motor proteins, which walk along microtubules/actin filaments to or from specific synaptic sites. Kinesins (orange) are involved in minus-to-plus end transport, and dyneins (green) are involved in plus-to-minus end transport along microtubules. Myosins (blue) are involved in transport at local synaptic sites along actin filaments. (B) A transport complex contains a cargo with synaptic components, adaptor protein(s), and a motor protein. Scaffolding proteins for glutamatergic and GABAergic synapses may serve as adaptor proteins as shown in the table. Adaptor proteins recognize cargos containing specific synaptic components and interact with appropriate motor proteins, which transport them to glutamatergic or GABAergic synapses.
Secreted synaptic proteins, such as neurotrophins, including BDNF, are also transported from trans-Golgi network to synaptic sites by microtubule/actin-based transport (Lessmann and Brigadski 2009). For example, BDNF binds to phosphorylated form of huntingtin and is carried by KIF5 to synaptic terminals (Colin and others 2008). It is possible that FGF22 and FGF7 are also transported to glutamatergic or GABAergic postsynaptic site via two distinct transport molecules. Which motor molecules are involved to transport cargos containing each FGF? How are the cargos recognized by the motor proteins? Further studies are needed to answer these questions. In addition to proteins, mRNAs are also transported to synaptic sites by microtubule-based transport, which are then translated locally (Bramham and Wells 2007; Hanus and Ehlers 2008). It would be interesting to test whether and how mRNA for glutamatergic and GABAergic synaptic proteins is transported. Finally, it has been reported that neuronal activity regulates cargo transport by modifying microtubules (Hirokawa and Hasezaki 2010; Maas and others 2009). Regulation of trafficking by neural activity is another important question to be addressed.
Specific receptors of presynaptic organizers for glutamatergic and GABAergic synaptic differentiation
Distinct usage of presynaptic receptors by synapse-type specific presynaptic organizers also provides a mechanism for precise glutamatergic and GABAergic synapse formation (Fig. 9). For example, NRXN-1α binds to NLGN2 but not to the major variant of NLGN1 containing a B insert (Boucard and others 2005), and α-NRXNs expressed in non-neuronal cells induce gephyrin and NLGN2 clustering in co-cultured neurons, but not PSD95 or NLGN1, 3, and 4 clustering (Chih and others 2006; Kang and others 2008) (Fig. 9B). β-NRXNs are expressed at both glutamatergic and GABAergic presynaptic sites and are able to bind to NLGN1 and NLGN2 (Fig. 9A and B). However, recent studies suggested that different splice variants of NLGNs and β-NRXNs are involved in synaptic formation at glutamatergic and GABAergic synapse. Two splice sites, site 4 in β-NRXNs and site B in NLGNs, are involved in this regulation. Site 4 lacking β-NRXNs strongly bind to site B containing NLGNs, while site 4 containing β-NRXNs strongly bind to site B lacking NLGNs. In neurons, site B containing NLGN1 enhanced VGLUT1 clustering, whereas site B lacking NLGN1 enhanced VGAT clustering (Craig and Kang 2007). In addition, site 4 containing β-NRXNs induce NLGN2 and gephyrin clustering. These results suggest that site 4 lacking β-NRXNs together with site B containing NLGNs promote glutamatergic synapse formation, and site 4 containing β-NRXNs together with site B lacking NLGNs are involved in GABAergic synapse formation (Fig. 9 A and B).
Figure 9. Differential presynaptic receptors for glutamatergic and GABAergic presynaptic organizers.
Each presynaptic organizer binds specific sets of receptors for proper presynaptic differentiation. Binding partners for FGFs and neuroligins at glutamatergic and GABAergic synapses are shown.
As for FGF22 and FGF7, our recent data suggest that they activate different signaling pathways to promote glutamatergic and GABAergic presynaptic differentiation. We found that even when they are bath applied into hippocampal cell cultures, FGF22 preferentially increased the number and size of VGLUT1-positive puncta, while FGF7 selectively increased the number and size of VGAT-positive puncta (Terauchi and others 2010). These results indicate that FGF22 and FGF7 activate different signaling pathways via their specific receptors (Fig. 9A and B). In vitro assay showed that FGF22 can activate FGFR2b and FGFR1b whereas FGF7 selectively activates FGFR2b (Zhang and others 2006). In FGFR2-knockout mice, VGAT clustering was more significantly decreased compared to a decrease in VGLUT1 clustering (Terauchi and others 2010). These results suggest that a different set of FGF receptors are activated by FGF22 and FGF7 to promote glutamatergic and GABAergic presynaptic differentiation, respectively (Fig. 9A and B).
Conclusions and future directions
As described here, two major types of synapses in the brain, excitatory and inhibitory, are established by a cooperation of various synapse type-specific molecular pathways. Glutamatergic and GABAergic synapses are formed through four sequential steps: cell fate determination, migration, axon guidance and target recognition, and synapse formation. In each step, different sets of molecules are involved and play key roles to instruct glutamatergic or GABAergic specification. Such molecules include intrinsic factors and extrinsic factors. Usage of these factors shifts from intrinsic factors to extrinsic factors as the differentiation steps proceed (Fig. 10). Cell fate determination and migration of glutamatergic and GABAergic neurons are mostly programmed in the cell, and synapse type-specific intrinsic factors such as transcription factors are largely involved in these steps. Migration of glutamatergic and GABAergic neurons is also assisted by glutamatergic and GABAergic specific guidance molecules and cells; thus extrinsic cues are involved in this stage as well. After these steps, extrinsic factors instruct to form glutamatergic or GABAergic synapses. Axon guidance is led by environmental cues. Target recognition and synapse formation are achieved by exchanging locally-restricted synapse type-specific extrinsic factors between connecting partners, i.e., specific adhesion molecules for target recognition and specific synaptic organizers for synapse formation. Selective synaptic targeting of these molecules and specific receptors/signaling contribute to establish synapse specificity. Together, these mechanisms ensure precise formation of glutamatergic and GABAergic synapses to obtain balanced excitatory and inhibitory inputs in a neuronal network.
Figure 10. Intrinsic and extrinsic factors cooperate to specify glutamatergic and GABAergic neurons and synapses.
A variety of synapse type-specific molecules play key roles to instruct glutamatergic or GABAergic specification in every step of differentiation. Intrinsic factors play important roles in specifying glutamatergic and GABAergic neurons in initial steps, and in later steps, extrinsic factors guide these neurons to establish appropriate glutamatergic and GABAergic synapses.
Neuronal disorders such as autism, schizophrenia, and epilepsy are associated with unbalanced excitatory and inhibitory inputs in the neuronal network. Further identification and characterization of molecules that control excitatory and inhibitory synapse formation should lead to novel treatments for these devastating disorders.
Acknowledgements
We thank Michael A. Fox, Erin M. Johnson-Venkatesh, and Adam Iliff for thoughtful comments and discussion.
Grant support (HU): the Ester A. & Joseph Klingenstein Fund, the Edward Mallinckrodt Jr. Foundation, the March of Dimes Foundation, the Whitehall Foundation, and the NIH (R01 MH091429 and NS070005)
References
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–272. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Ango F, Wu C, Van der Want JJ, Wu P, Schachner M, Huang ZJ. Bergmann glia and the recognition molecule CHL1 organize GABAergic axons and direct innervation of Purkinje cell dendrites. PLoS Biol. 2008;6:e103. doi: 10.1371/journal.pbio.0060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Dugich-Djordjevic MM, Li YX, Ma W, Somogyi R, Wen X, et al. Neurotrophins stimulate chemotaxis of embryonic cortical neurons. Eur J Neurosci. 1997;9:2561–2570. doi: 10.1111/j.1460-9568.1997.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Bock HH, Herz J. Reelin activates SRC family tyrosine kinases in neurons. Curr Biol. 2003;13:18–26. doi: 10.1016/s0960-9822(02)01403-3. [DOI] [PubMed] [Google Scholar]
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Sudhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48:229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Wells DG. Dendritic mRNA: transport, translation and function. Nat Rev Neurosci. 2007;8:776–789. doi: 10.1038/nrn2150. [DOI] [PubMed] [Google Scholar]
- Burrone J, O'Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- Burrows RC, Wancio D, Levitt P, Lillien L. Response diversity and the timing of progenitor cell maturation are regulated by developmental changes in EGFR expression in the cortex. Neuron. 1997;19:251–267. doi: 10.1016/s0896-6273(00)80937-x. [DOI] [PubMed] [Google Scholar]
- Campbell K, Gotz M. Radial glia: multi-purpose cells for vertebrate brain development. Trends Neurosci. 2002;25:235–238. doi: 10.1016/s0166-2236(02)02156-2. [DOI] [PubMed] [Google Scholar]
- Caric D, Raphael H, Viti J, Feathers A, Wancio D, Lillien L. EGFRs mediate chemotactic migration in the developing telencephalon. Development. 2001;128:4203–4216. doi: 10.1242/dev.128.21.4203. [DOI] [PubMed] [Google Scholar]
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Chih B, Gollan L, Scheiffele P. Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron. 2006;51:171–178. doi: 10.1016/j.neuron.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, et al. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin E, Zala D, Liot G, Rangone H, Borrell-Pages M, Li XJ, et al. Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. EMBO J. 2008;27:2124–2134. doi: 10.1038/emboj.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA. A mechanism for inside-out lamination in the neocortex. Trends Neurosci. 2008;31:113–119. doi: 10.1016/j.tins.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8:206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danglot L, Triller A, Marty S. The development of hippocampal interneurons in rodents. Hippocampus. 2006;16:1032–1060. doi: 10.1002/hipo.20225. [DOI] [PubMed] [Google Scholar]
- Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- Dent EW, Gupton SL, Gertler FB. The Growth Cone Cytoskeleton in Axon Outgrowth and Guidance. Cold Spring Harb Perspect Biol. 2010 doi: 10.1101/cshperspect.a001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Dresbach T, Neeb A, Meyer G, Gundelfinger ED, Brose N. Synaptic targeting of neuroligin is independent of neurexin and SAP90/PSD95 binding. Mol Cell Neurosci. 2004;27:227–235. doi: 10.1016/j.mcn.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Dulabon L, Olson EC, Taglienti MG, Eisenhuth S, McGrath B, Walsh CA, et al. Reelin binds alpha3beta1 integrin and inhibits neuronal migration. Neuron. 2000;27:33–44. doi: 10.1016/s0896-6273(00)00007-6. [DOI] [PubMed] [Google Scholar]
- Dumoulin A, Triller A, Kneussel M. Cellular transport and membrane dynamics of the glycine receptor. Front Mol Neurosci. 2009;2:28. doi: 10.3389/neuro.02.028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G. Striatal precursors adopt cortical identities in response to local cues. Development. 1995;121:803–812. doi: 10.1242/dev.121.3.803. [DOI] [PubMed] [Google Scholar]
- Fox MA, Umemori H. Seeking long-term relationship: axon and target communicate to organize synaptic differentiation. J Neurochem. 2006;97:1215–1231. doi: 10.1111/j.1471-4159.2006.03834.x. [DOI] [PubMed] [Google Scholar]
- Gant JC, Thibault O, Blalock EM, Yang J, Bachstetter A, Kotick J, et al. Decreased number of interneurons and increased seizures in neuropilin 2 deficient mice: implications for autism and epilepsy. Epilepsia. 2009;50:629–645. doi: 10.1111/j.1528-1167.2008.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Weber J, Pevny L, Schmid R, Schwab MH, Lloyd KC, et al. The role of neuregulin-ErbB4 interactions on the proliferation and organization of cells in the subventricular zone. Proc Natl Acad Sci U S A. 2006;103:1930–1935. doi: 10.1073/pnas.0510410103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot F. Cell fate specification in the mammalian telencephalon. Prog Neurobiol. 2007;83:37–52. doi: 10.1016/j.pneurobio.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Hanus C, Ehlers MD. Secretory outposts for the local processing of membrane cargo in neuronal dendrites. Traffic. 2008;9:1437–1445. doi: 10.1111/j.1600-0854.2008.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman KN, Pal SK, Burrone J, Murthy VN. Activity-dependent regulation of inhibitory synaptic transmission in hippocampal neurons. Nat Neurosci. 2006;9:642–649. doi: 10.1038/nn1677. [DOI] [PubMed] [Google Scholar]
- Hernandez-Miranda LR, Parnavelas JG, Chiara F. Molecules and mechanisms involved in the generation and migration of cortical interneurons. ASN Neuro. 2010;2:e00031. doi: 10.1042/AN20090053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- Hirokawa S, Hasezaki H. Model analysis to investigate the contribution of ground substance to ligament stiffening. Med Eng Phys. 2010;32:610–616. doi: 10.1016/j.medengphy.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Holt CE, Harris WA. Target selection: invasion, mapping and cell choice. Curr Opin Neurobiol. 1998;8:98–105. doi: 10.1016/s0959-4388(98)80013-5. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Di Cristo G, Ango F. Development of GABA innervation in the cerebral and cerebellar cortices. Nat Rev Neurosci. 2007;8:673–686. doi: 10.1038/nrn2188. [DOI] [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem. 2010 doi: 10.1093/jb/mvq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyifous O, Waites CL, Specht CG, Fujisawa S, Schubert M, Lin EI, et al. SAP97 and CASK mediate sorting of NMDA receptors through a previously unknown secretory pathway. Nat Neurosci. 2009;12:1011–1019. doi: 10.1038/nn.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Venkatesh EM, Umemori H. Secreted factors as synaptic organizers. Eur J Neurosci. 2010;32:181–190. doi: 10.1111/j.1460-9568.2010.07338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Zhang X, Dobie F, Wu H, Craig AM. Induction of GABAergic postsynaptic differentiation by alpha-neurexins. J Biol Chem. 2008;283:2323–2334. doi: 10.1074/jbc.M703957200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karube F, Kubota Y, Kawaguchi Y. Axon branching and synaptic bouton phenotypes in GABAergic nonpyramidal cell subtypes. J Neurosci. 2004;24:2853–2865. doi: 10.1523/JNEUROSCI.4814-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M. Postsynaptic scaffold proteins at non-synaptic sites. The role of postsynaptic scaffold proteins in motor-protein-receptor complexes. EMBO Rep. 2005;6:22–27. doi: 10.1038/sj.embor.7400319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblum HI, Hussain RJ, Bronstein JM, Gall CM, Lee DC, Seroogy KB. Prenatal ontogeny of the epidermal growth factor receptor and its ligand, transforming growth factor alpha, in the rat brain. J Comp Neurol. 1997;380:243–261. doi: 10.1002/(sici)1096-9861(19970407)380:2<243::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Brigadski T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: an update. Neurosci Res. 2009;65:11–22. doi: 10.1016/j.neures.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Levinson JN, Chery N, Huang K, Wong TP, Gerrow K, Kang R, et al. Neuroligins mediate excitatory and inhibitory synapse formation: involvement of PSD-95 and neurexin-1beta in neuroligin-induced synaptic specificity. J Biol Chem. 2005;280:17312–17319. doi: 10.1074/jbc.M413812200. [DOI] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, et al. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lise MF, El-Husseini A. The neuroligin and neurexin families: from structure to function at the synapse. Cell Mol Life Sci. 2006;63:1833–1849. doi: 10.1007/s00018-006-6061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bendito G, Sanchez-Alcaniz JA, Pla R, Borrell V, Pico E, Valdeolmillos M, et al. Chemokine signaling controls intracortical migration and final distribution of GABAergic interneurons. J Neurosci. 2008;28:1613–1624. doi: 10.1523/JNEUROSCI.4651-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Nicoll RA, Malenka RC, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci. 2000;3:545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- Maas C, Belgardt D, Lee HK, Heisler FF, Lappe-Siefke C, Magiera MM, et al. Synaptic activation modifies microtubules underlying transport of postsynaptic cargo. Proc Natl Acad Sci U S A. 2009;106:8731–8736. doi: 10.1073/pnas.0812391106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrassi L, Ehrlich ME, Butti G, Pezzotta S, Govoni S, Cattaneo E. Basal ganglia precursors found in aggregates following embryonic transplantation adopt a striatal phenotype in heterotopic locations. Development. 1998;125:2847–2855. doi: 10.1242/dev.125.15.2847. [DOI] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Marin O, Valiente M, Ge X, Tsai LH. Guiding neuronal cell migrations. Cold Spring Harb Perspect Biol. 2010;2:a001834. doi: 10.1101/cshperspect.a001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Yaron A, Bagri A, Tessier-Lavigne M, Rubenstein JL. Sorting of striatal and cortical interneurons regulated by semaphorin-neuropilin interactions. Science. 2001;293:872–875. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- Matsuoka RL, Nguyen-Ba-Charvet KT, Parray A, Badea TC, Chedotal A, Kolodkin AL. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature. 2011 doi: 10.1038/nature09675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. Development and plasticity of the inhibitory neocortical circuitry with an emphasis on the rodent barrel field cortex: a review. Can J Physiol Pharmacol. 1997;75:470–478. [PubMed] [Google Scholar]
- Mohler H. GABAA receptors in central nervous system disease: anxiety, epilepsy, and insomnia. J Recept Signal Transduct Res. 2006;26:731–740. doi: 10.1080/10799890600920035. [DOI] [PubMed] [Google Scholar]
- Mok H, Shin H, Kim S, Lee JR, Yoon J, Kim E. Association of the kinesin superfamily motor protein KIF1Balpha with postsynaptic density-95 (PSD-95), synapse-associated protein-97, and synaptic scaffolding molecule PSD-95/discs large/zona occludens-1 proteins. J Neurosci. 2002;22:5253–5258. doi: 10.1523/JNEUROSCI.22-13-05253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani T, Minaki Y, Kumai M, Ono Y. Helt determines GABAergic over glutamatergic neuronal fate by repressing Ngn genes in the developing mesencephalon. Development. 2007;134:2783–2793. doi: 10.1242/dev.02870. [DOI] [PubMed] [Google Scholar]
- Nam CI, Chen L. Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc Natl Acad Sci U S A. 2005;102:6137–6142. doi: 10.1073/pnas.0502038102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecho-Vrieseling E, Sigrist M, Yoshida Y, Jessell TM, Arber S. Specificity of sensory-motor connections encoded by Sema3e-Plxnd1 recognition. Nature. 2009;459:842–846. doi: 10.1038/nature08000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Whitford KL, Dijkhuizen PA, Vitalis T, Ghosh A. Control of cortical interneuron migration by neurotrophins and PI3-kinase signaling. Development. 2002;129:3147–3160. doi: 10.1242/dev.129.13.3147. [DOI] [PubMed] [Google Scholar]
- Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, et al. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron. 2009;63:628–642. doi: 10.1016/j.neuron.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Powell EM, Mars WM, Levitt P. Hepatocyte growth factor/scatter factor is a motogen for interneurons migrating from the ventral to dorsal telencephalon. Neuron. 2001;30:79–89. doi: 10.1016/s0896-6273(01)00264-1. [DOI] [PubMed] [Google Scholar]
- Pozas E, Ibanez CF. GDNF and GFRalpha1 promote differentiation and tangential migration of cortical GABAergic neurons. Neuron. 2005;45:701–713. doi: 10.1016/j.neuron.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange O, Wong TP, Gerrow K, Wang YT, El-Husseini A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci U S A. 2004;101:13915–13920. doi: 10.1073/pnas.0405939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles E, Acampora D, Gogoi R, Tuorto F, Papalia A, Guillemot F, et al. Otx2 controls identity and fate of glutamatergic progenitors of the thalamus by repressing GABAergic differentiation. J Neurosci. 2006;26:5955–5964. doi: 10.1523/JNEUROSCI.1097-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rallu M, Corbin JG, Fishell G. Parsing the prosencephalon. Nat Rev Neurosci. 2002;3:943–951. doi: 10.1038/nrn989. [DOI] [PubMed] [Google Scholar]
- Rao A, Craig AM. Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron. 1997;19:801–812. doi: 10.1016/s0896-6273(00)80962-9. [DOI] [PubMed] [Google Scholar]
- Rosales CR, Osborne KD, Zuccarino GV, Scheiffele P, Silverman MA. A cytoplasmic motif targets neuroligin-1 exclusively to dendrites of cultured hippocampal neurons. Eur J Neurosci. 2005;22:2381–2386. doi: 10.1111/j.1460-9568.2005.04400.x. [DOI] [PubMed] [Google Scholar]
- Rouaux C, Arlotta P. Fezf2 directs the differentiation of corticofugal neurons from striatal progenitors in vivo. Nat Neurosci. 2010;13:1345–1347. doi: 10.1038/nn.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph J, Zimmer G, Steinecke A, Barchmann S, Bolz J. Ephrins guide migrating cortical interneurons in the basal telencephalon. Cell Adh Migr. 2010;4:400–408. doi: 10.4161/cam.4.3.11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Yamagata M. Many paths to synaptic specificity. Annu Rev Cell Dev Biol. 2009;25:161–195. doi: 10.1146/annurev.cellbio.24.110707.175402. [DOI] [PubMed] [Google Scholar]
- Schapitz IU, Behrend B, Pechmann Y, Lappe-Siefke C, Kneussel SJ, Wallace KE, et al. Neuroligin 1 is dynamically exchanged at postsynaptic sites. J Neurosci. 2010;30:12733–12744. doi: 10.1523/JNEUROSCI.0896-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Schuurmans C, Guillemot F. Molecular mechanisms underlying cell fate specification in the developing telencephalon. Curr Opin Neurobiol. 2002;12:26–34. doi: 10.1016/s0959-4388(02)00286-6. [DOI] [PubMed] [Google Scholar]
- Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 2000;288:1796–1802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- Siddiqui TJ, Craig AM. Synaptic organizing complexes. Curr Opin Neurobiol. 2010 doi: 10.1016/j.conb.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer HS, Minzer K. Neurobiology of Tourette's syndrome: concepts of neuroanatomic localization and neurochemical abnormalities. Brain Dev. 2003;25(Suppl 1):S70–S84. doi: 10.1016/s0387-7604(03)90012-x. [DOI] [PubMed] [Google Scholar]
- Skutella T, Nitsch R. New molecules for hippocampal development. Trends Neurosci. 2001;24:107–113. doi: 10.1016/s0166-2236(00)01717-3. [DOI] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Sudhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein E, Savaskan NE, Ninnemann O, Nitsch R, Zhou R, Skutella T. A role for the Eph ligand ephrin-A3 in entorhino-hippocampal axon targeting. J Neurosci. 1999;19:8885–8893. doi: 10.1523/JNEUROSCI.19-20-08885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto F, Tsuboi M, Kamiya H, Mizuno H, Kiyama Y, Komai S, et al. Interactions between plexin-A2, plexin-A4, and semaphorin 6A control lamina-restricted projection of hippocampal mossy fibers. Neuron. 2007;53:535–547. doi: 10.1016/j.neuron.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Tamas G, Buhl EH, Lorincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3:366–371. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- Terauchi A, Johnson-Venkatesh EM, Toth AB, Javed D, Sutton MA, Umemori H. Distinct FGFs promote differentiation of excitatory and inhibitory synapses. Nature. 2010;465:783–787. doi: 10.1038/nature09041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse B, Thisse C. Functions and regulations of fibroblast growth factor signaling during embryonic development. Dev Biol. 2005;287:390–402. doi: 10.1016/j.ydbio.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic signaling: the positive side of negative feedback. Curr Opin Neurobiol. 2007;17:318–324. doi: 10.1016/j.conb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twelvetrees AE, Yuen EY, Arancibia-Carcamo IL, MacAskill AF, Rostaing P, Lumb MJ, et al. Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin. Neuron. 2010;65:53–65. doi: 10.1016/j.neuron.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemori H. Weaving the neuronal net with target-derived fibroblast growth factors. Dev Growth Differ. 2009;51:263–270. doi: 10.1111/j.1440-169X.2008.01079.x. [DOI] [PubMed] [Google Scholar]
- Umemori H, Linhoff MW, Ornitz DM, Sanes JR. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell. 2004;118:257–270. doi: 10.1016/j.cell.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, et al. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- Waites CL, Craig AM, Garner CC. Mechanisms of vertebrate synaptogenesis. Annu Rev Neurosci. 2005;28:251–274. doi: 10.1146/annurev.neuro.27.070203.144336. [DOI] [PubMed] [Google Scholar]
- Wassef A, Baker J, Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol. 2003;23:601–640. doi: 10.1097/01.jcp.0000095349.32154.a5. [DOI] [PubMed] [Google Scholar]
- Xu Q, Guo L, Moore H, Waclaw RR, Campbell K, Anderson SA. Sonic hedgehog signaling confers ventral telencephalic progenitors with distinct cortical interneuron fates. Neuron. 2010;65:328–340. doi: 10.1016/j.neuron.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer G, Garcez P, Rudolph J, Niehage R, Weth F, Lent R, et al. Ephrin-A5 acts as a repulsive cue for migrating cortical interneurons. Eur J Neurosci. 2008;28:62–73. doi: 10.1111/j.1460-9568.2008.06320.x. [DOI] [PubMed] [Google Scholar]