Abstract
To determine the proangiogenesis effect of series of saccharides and a synthetic oligosaccharide and potential mechanisms, an in vitro 3-dimensional endothelial cell sprouting (3D-ECS) assay and the chick chorioallantoic membrane (CAM) model were used. We demonstrated that a sulfated oligosaccharide significantly promotes the endothelial capillary network initiated by vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (b-FGF). Furthermore, although the capillary network initiated by VEGF and b-FGF lasts no more than 7 days, addition of a sulfated oligosaccharide significantly amplifies angiogenesis and stabilizes the capillary network of new blood vessels. In the CAM model, sulfated oligosaccharide also stimulated angiogenesis. In both the CAM and the 3D-ECS assay, structure–function studies reveal that increased saccharide chain length up to the hexa- to decasaccharide show optimal proangiogenesis efficacy. In addition, the sulfation and molecular shape (branched vs linear) of oligosaccharide are important for sustained proangiogenesis efficacy. Data indicate that chemically defined synthetic oligosaccharides can play an important role in regulation of capillary structure and stability, which may contribute to future advances in therapeutic angiogenesis. The proangiogenesis efficacy of an oligosaccharide is mediated via integrin αvβ3 and involves mitogen-activated protein kinase signaling mechanisms.
Keywords: angiogenesis, oligosaccharides, αvβ3 integrin, growth factors, mitogen-activated protein kinase
Control of angiogenesis is a complex process involving local release of vascular growth factors, the extracellular matrix, adhesion molecules, and metabolic factors.1–3 Mechanical forces within blood vessels may also contribute to angiogenesis.2 The principal classes of endogenous growth factors implicated in new blood vessel growth are the basic fibroblast growth factor (b-FGF) family and vascular endothelial growth factor (VEGF).4 The mitogen-activated protein kinase signal transduction cascade (MAPK; extracellular signal-regulated kinases 1 and 2, ERK1/2) is involved both in VEGF-induced gene expression and in control of proliferation of vascular endothelial cells.4
The effects of heparin on angiogenesis are controversial, with some studies claiming stimulatory and other studies claiming inhibitory effects. In the setting of heparin-derived compounds, data have suggested a biphasic effect of unfractionated heparin (UFH) but inhibition of angiogenesis by low-molecular-weight heparin (LMWH).5,6
The use of either VEGF or b-FGF strategies in experimental and clinical settings to promote neovascularization has demonstrated limited success.7–10 The present investigation examined the proangiogenesis efficacy of saccharide-based heparin analogs using a 3-dimensional microvascular endothelial sprouting assay in vitro11–13 and the chick chorioallantoic membrane (CAM) model on ovo.14–16
METHODS
Reagents
All common reagents were obtained from Sigma Chemical Company (St. Louis, MO); PD 98059 was obtained from Calbiochem (La Jolla, CA). Human b-FGF and VEGF were obtained from Invitrogen (Carlsbad, CA). Purified human fibrinogen was obtained from the New York Blood Bank (New York, NY). The monoclonal antibody against the αvβ3 integrin, LM609, was obtained from Chemicon (San Diego, CA), and the small molecule αvβ3 antagonist, XT199, was obtained from PRI (Albany, NY).
Oligosaccharides Tested in Angiogenesis Assays
Heparin and LMWH are shown to modulate angiogenesis.17–23 Heparin-derived oligosaccharides were prepared by the controlled, partial depolymerization of bovine lung heparin with heparin lyase 1.24 The resulting oligosaccharides were fractionated on the basis of size and charge to afford homogenous, structurally defined oligosaccharides ranging from disaccharide (dp 2) to decasaccharide (dp 10).24 The persulfated β (1→3) L-fucose tetrasaccharides (Ch 1) was synthesized in 10 steps from 1-thio-β-L-fucopyranoside.25 The unsulfated and persulfated β (1→6) D-glucose tetrasaccharides (Ch 4 and Ch 5 and Ch 6 and Ch 7) were synthesized in 11 and 12 steps, respectively, from isopropyl 1-thio-β-D-glucopyranoside.26 The persulfated β (1→4) 3-O-methyl-D-xylose hexasaccharide (Ch 3) was synthesized in 17 steps from D-xylose (H. He and Y. Du, unpublished data). The branched unsulfated and persulfated β (1→3, 6) glucose trisaccharides (Ch 8 and Ch 9, respectively) were synthesized from methyl 4,6-O-benzylidine-D-glucopyranoside in 5 and 6 steps, respectively.27 The doubly branched persulfated and unsulfated β (1→3, 6) D-glucose hexasaccharides (Ch 2 and Ch 10) were synthesized from phenyl 2, 4-di-O-acetyl-1-thio-β-D-glucopyranside in 6 and 7 steps, respectively.27 All oligosaccharides were pure (>90%) by capillary electrophoresis28 and polyacrylamide gel electrophoresis29 and showed electrospray ionization-mass spectrometry and nuclear magnetic resonance spectra consistent with structure.
3-Dimensional Angiogenesis Assay
In vitro 3-dimensional sprout angiogenesis of human dermal microvascular endothelial cells (HDMECs) cultured on microcarrier beads coated with fibrin was carried out. A microcarrier in vitro angiogenesis assay previously designed to investigate bovine pulmonary artery endothelial cell angiogenesis behavior in bovine fibrin gels11–13 was modified for the study of human microvascular endothelial cell angiogenesis in a 3-dimensional extracellular matrix environment.
Confluent HDMEC (passages 5–10) were mixed with gelatin-coated Cytodex-3 beads with a ratio of 40 cells per bead. Suspended cells and beads (150–200 beads per well for 24-well plate) with 5 mL endothelial basal medium (EBM) + 15% normal human serum were mixed gently every hour for the first 4 h; the mixture culture was then incubated overnight in a CO2 incubator. The next day, 10 mL fresh EBM + 15% human serum were added for another 3 h. Before experiments, 500 μL phosphate-buffered saline (PBS) in a well of 24-well plate and 100 μL endothelial cell-bead culture solution were added to the PBS. The number of beads per well was counted, and the concentration of beads per endothelial cell was calculated.
Human fibrinogen, isolated as previously described,13 was dissolved in cell culture media at a concentration of 1 mg/mL (pH 7.4) and sterilized by filtering through a 0.22-μm filter. A solution of pure fibrinogen solution (1 mg/mL) was prepared in EBM medium with or without angiogenesis factors or the agent to be tested. As a positive control, a mixture of standard growth factors (30 ng/mL VEGF + 25 ng/mL b-FGF) was used. Endothelial cells-beads were washed with EBM medium twice, and endothelial cells-beads were added to fibrinogen solution. For each condition, the experiment was carried out in triplicate. Endothelial cells-beads in fibrinogen solution were mixed gently, 2.5 μL human thrombin (0.05 U/μL) was added, and 300 μL was immediately transferred to each well of the 24-well plate. The fibrinogen solution polymerizes in 5 to 10 min; after 20 min, EBM + 20% normal human serum + 10 μg/mL aprotinin were added. The plate was incubated in a CO2 incubator overnight to allow for the HDMEC to invade the fibrin gel and form tubes. All cells were pretreated with subthreshold concentrations of b-FGF (1.25 ng/mL) and VEGF (2.5 ng/mL).
The angiogenesis response was monitored visually and recorded by video image capture. Specifically, capillary sprout formation was observed and recorded with a Nikon Diaphot-TMD inverted microscope (Nikon, Melville, NY) equipped with an incubator housing with a Nikon NP-2 thermostat and Sheldon #2004 carbon dioxide flow mixer. The microscope was directly interfaced to a video system consisting of a Dage-MTI CCD-72S video camera and Sony 12-in PVM-122 video monitor linked to a Macintosh G3 computer. The images were captured at various magnifications using Adobe Photoshop. The effect of the proangiogenesis factors on sprout angiogenesis was quantified visually by determining the number and percent of endothelial cell-beads with capillary sprouts. One hundred beads (5–6 random low-power fields) in each triplicate well were counted for each experimental condition. All experiments were repeated at least 3 times. To locate the nucleus of HDMEC, the fibrin or collagen gel was fixed with methanol/acetone (1:1) and stained with 0.001% phalloidin iodine, a fluorescence-labeled actin that binds to the nucleus.
Chick Chorioallantoic Membrane Model of Angiogenesis
Neovascularization was examined in the CAM model, as previously described.14–16 Briefly, 10-d-old chick embryos were purchased from Spafas (Preston, CT) and incubated at 37°C with 55% relative humidity. A hypodermic needle was used to make a small hole in the shell at the air sac, and a second hole was made on the broadside of the egg, directly over an avascular portion of the embryonic membrane that was identified by candling. A false air sac was created beneath the second hole by the application of negative pressure at the first hole, causing the CAM to separate from the shell. A window, approximately 1.0 cm2, was cut in the shell over the dropped CAM with a small crafts grinding wheel (Dremel, Racine, WI), allowing direct access to the underlying CAM. Either b-FGF or VEGF at 1 to 2 μg was used as a standard proangiogenesis factor to induce new blood vessel branches on the CAM of the 10-d-old embryos. Sterile disks of #1 filter paper (Whatman International, Maidstone, UK) were pre-treated with 3 mg/mL cortisone acetate and air dried under sterile conditions. Oligosaccharides, b-FGF, VEGF or control vehicle, and inhibitors were then applied to the disks, and the disks were allowed to dry. The disks were then suspended in PBS and placed on growing CAMs. Filters treated with saccharide analogs and/or b-FGF were placed on the first day of the 3-d incubation; 1 d later, the MAPK cascade inhibitor, PD 98059, or the αvβ3 integrin antagonist, XT199, was added to CAMs topically by means of the filter disks.
Microscopic Analysis of CAM Sections
After incubation at 37°C with 55% relative humidity for 3 d, the CAM tissue directly beneath each filter disk was resected from control and treated CAM samples. Tissues were washed 3 times with PBS, placed in 35-mm Petri dishes (Nalge Nunc, Rochester, NY), and examined under an SV6 stereo-microscope (Karl Zeiss, Thornwood, NY) at 50× magnification. Digital images of CAM sections exposed to filters were collected, using a 3-CCD color video camera system (Toshiba America, New York), and analyzed with Image-Pro software (Media Cybernetics, Silver Spring, MD). The numbers of vessel branch points contained in a circular region equal to the area of each filter disk were counted. One image was counted in each CAM preparation, and findings from 8 CAM preparations were analyzed for each treatment condition. Each experiment was performed 3 times. The resulting angiogenesis index is the mean ± SD of new branch points in each treatment set.
Statistical Analysis
Statistical analysis was performed by 1-way analysis of variance comparing experimental with respective control groups. Statistical significance was based on P < 0.05.
RESULTS
3-Dimensional Angiogenesis Assay for Heparin Oligosaccharides
A 3-dimensional in vitro assay method was used to screen compounds for angiogenic activity. Gelatin-coated beads were used to support endothelial cells in a fibrin gel matrix (Fig. 1). The cells on these beads were treated with subthreshold levels of VEGF (1.25 ng/mL) and b-FGF (2.5 ng/mL) in the presence and absence of the agent to be tested; angiogenesis response was recorded by video image capture, and capillary sprouts were counted. No significant sprouting was demonstrated at the subthreshold levels of VEGF (1.25 ng/mL) plus b-FGF (2.5 ng/mL).
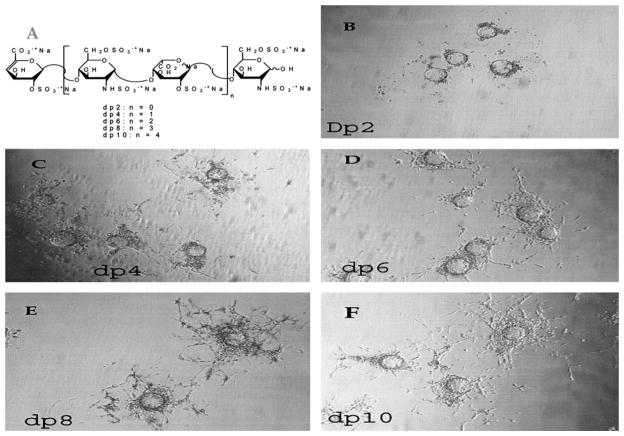
FIGURE 1.
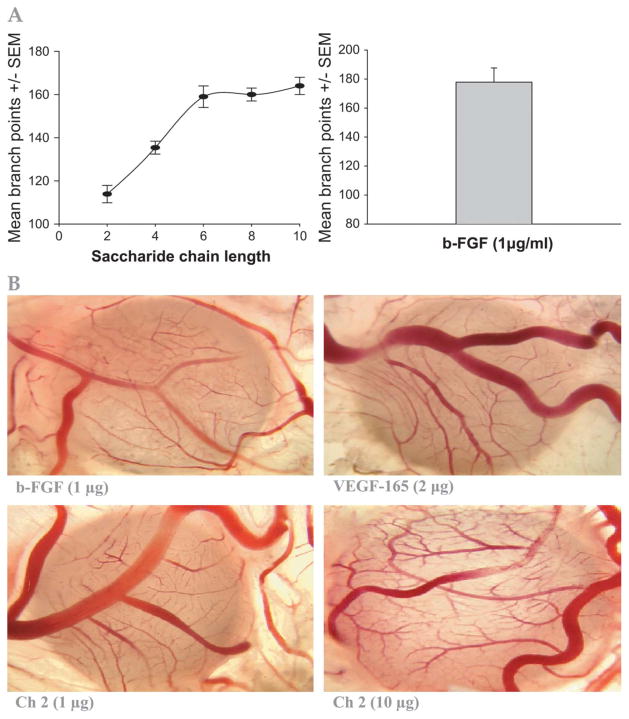
In vitro angiogenesis assay of structurally defined, homogenous heparin-derived oligosaccharides in the presence of VEGF and b-FGF. A, Oligosaccharide structure. B, Beads with disaccharide (dp 2). C, Beads with tetrasaccharide (dp 4). D, Beads with hexasaccharide (dp 6). E, Beads with octasaccharide (dp 8). F, Beads with decasaccharide (dp 10). CAM data for the same oligosaccharide afforded 115 ± 6, 135 ± 9, 160 ± 11, 161 ± 8, 165 ± 12 (new branch points ± SEM) for oligosaccharides dp 2, dp 4, dp 6, and dp 8, respectively, as shown in Fig. 6A.
Heparin oligosaccharides of defined structure and size were prepared (Fig. 1A). These linear oligosaccharides have a charge density of –2/saccharide unit (3 sulfo groups and 1 carboxyl group per disaccharide repeating unit). Oligosaccharides ranging from disaccharide (dp 2) to decasaccharide (dp 10) were examined using the 3-dimensional in vitro assay at a concentration of 10 μg/mL (Fig. 1B–F). These defined heparin oligosaccharides exhibited proangiogenic activity that clearly increased with chain size.
Angiogenic Activity of Linear Synthetic Oligosaccharides
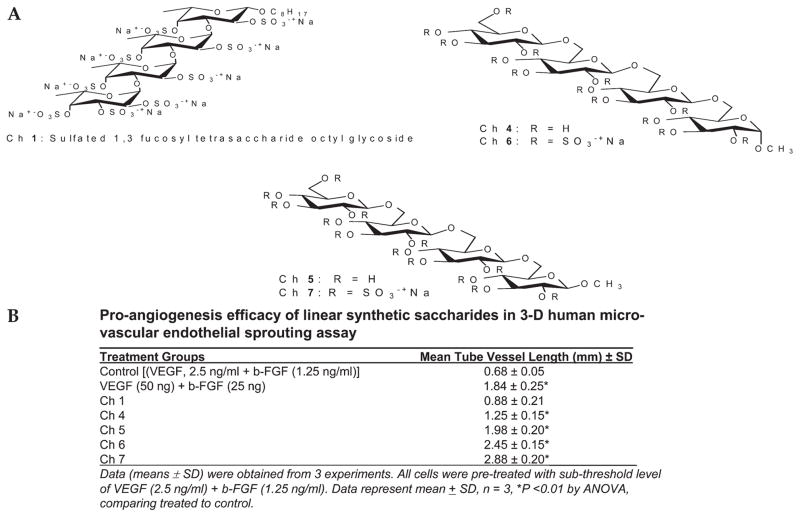
We examined the in vitro angiogenic activity of synthetically prepared analogs of heparin oligosaccharides. We began with linear tetrasaccharides, composed of various saccharide units and having a charge density of 0 to –3/saccharide unit (Fig. 2A, B). A linear fucose tetrasaccharide (Ch 1) with a charge density of –2/saccharide unit showed no angiogenic activity. Two linear glucose tetrasaccharides (Ch 6 and Ch 7) with a charge density of –3/saccharide unit showed potent proangiogenic activity, whereas their unsulfated counterparts (Ch 4 and Ch 5 with a charge density of 0/saccharide unit) showed only slight proangiogenic activity. A linear xylose hexasaccharide (Ch 3) having a charge density of – 1/saccharide unit showed no angiogenic activity (Fig. 3A, B).
FIGURE 2.
Evaluation of linear synthetic oligosaccharides using 3D on ovo angiogenesis assay in the presence of subthresh-old concentration of VEGF (1.25 ng/mL) and b-FGF (2.5 ng/mL). A, Structure of Ch 1, Ch 4, Ch 5, Ch 6, and Ch 7. B, Proangiogenesis activity of Ch 1, Ch 4, Ch 5, Ch 6, and Ch 7 in the 3D HMDEC sprouting assay. Data represent mean ± SD, n = 3.
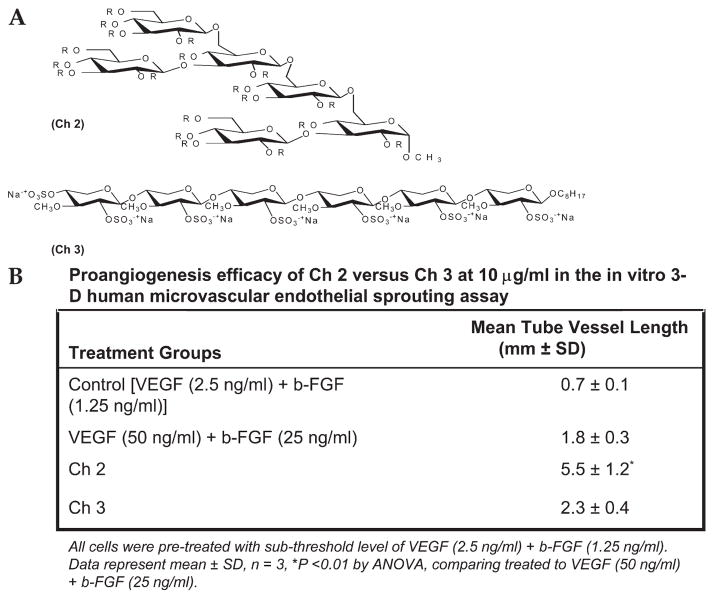
FIGURE 3.
Comparison of linear and branched sulfated synthetic oligosaccharides in 3D on ovo angiogenesis assay in the presence of VEGF and b-FGF. A, Structures of Ch 2 and 3. B, Control with VEGF and b-FGF but no added oligosaccharide, Ch 2, or Ch 3 with subthreshold b-FGF (2.5 ng/mL) and VEGF (1.25 ng/mL). Data showed significant increase in tube length (P < 0.01) with the sulfated hexasaccharides Ch 2 but not Ch 3 as compared to control.
Angiogenic Activity of Branched Synthetic Oligosaccharides
A doubly branched glucose hexasaccharide (Ch 2, having a charge density of –3/saccharide unit) showed highly potent proangiogenic activity in the 3-dimensional in vitro assay (Fig. 3B). A linear hexasaccharide (Ch 3, having a charge density of –1/saccharide unit) showed no angiogenic activity.
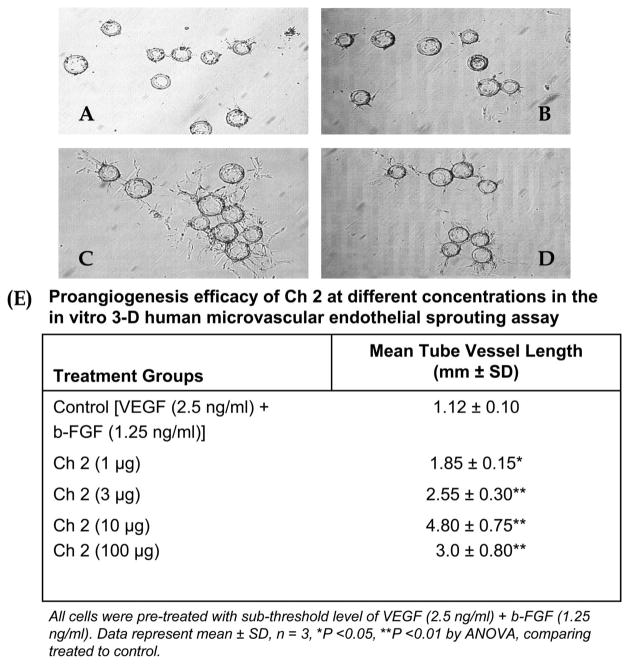
The most active proangiogenic oligosaccharide (Ch 2) was evaluated at a variety of concentrations (Fig. 4A–D). A concentration response curve was obtained, with peak activity observed at a concentration of 10 μg/mL (Fig. 4E). A 10-fold increase in concentration to 100 μg/mL showed no significant change in activity in the 3-dimensional in vitro assay. Using the optimum concentration of 10 μg/mL, the time dependence of the proangiogenic activity of Ch 2 was examined. A maximum effect in the 3-dimensional in vitro assay was observed between days 5 and 7. Moreover, in the absence of Ch 2, a maximum proangiogenic effect for VEGF (25 ng/mL) and b-FGF (30 ng/mL) alone, observed at day 2, was similar to that observed for VEGF and b-FGF in the presence of 10 μg/mL of Ch 2 (Fig. 5). Although the activity of maximal concentrations of VEGF (25 ng/mL) and b-FGF (30 ng/mL) alone declined after day 2, the activity of VEGF and b-FGF in the presence of 10 μg/mL of Ch 2 continued to increase, reaching a maximum between days 5 and 7.
FIGURE 4.
Concentration response of the proangiogenic activity of Ch 2 measured using the 3D in vitro assay in the presence of VEGF and b-FGF at day 7. A, Activity at 0 μg/mL. B, Activity at 1 μg/mL. C, Activity at 10 μg/mL. D, Activity at 100 μg/mL. E, Table demonstrating the concentration-dependent effect on the proangiogenesis efficacy of the saccharides vs subthreshold VEGF (1.25 ng/mL) plus b-FGF (2.5 ng/mL; control) in the 3D angiogenesis sprouting assay.
FIGURE 5.
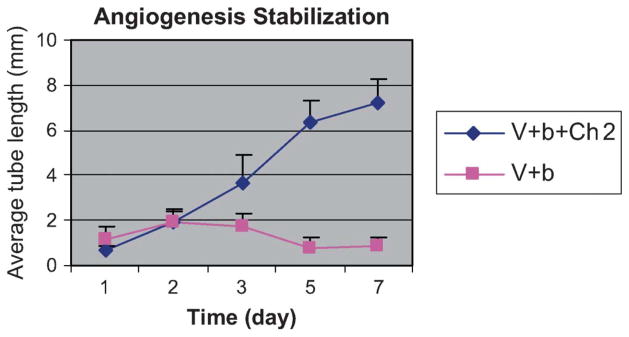
The average tube length measured using the 3D in vitro assay as a function of time. VEGF (50 ng/mL) and b-FGF (25 ng/mL) control (■) and VEGF (1.25 ng/mL), b-FGF (2.5 ng/mL) plus Ch 2 (◆). b = b-FFG; V = VEGF.
CAM Angiogenesis Assay for Heparin Oligosaccharides
The on ovo CAM assay gave results identical to those observed in the 3-dimensional endothelial cell sprouting (3D-ECS) assay (Fig. 1), showing an increase in angiogenic effect from disaccharide (dp 2) to hexasaccharide (dp 6), with no significant differences observed from hexasaccharide (dp 6) to decasaccharide (dp 10). Data in the CAM are given in the legend to Figure 1.
Angiogenic Activity of Synthetic Oligosaccharides
The results obtained in the on ovo CAM assay further confirmed the potent proangiogenic effect of Ch 2 (Fig. 6A). In addition, the effect of chain length on the proangiogenesis efficacy was demonstrated in the CAM assay, with a maximal increase in new vessel branch points with the hexasaccharide (Fig. 6B).
FIGURE 6.
A, Effect of heparin oligosaccharide chain length on angiogenesis (mean branch points) as compared to b-FGF (1 μg/mL) in the on ovo CAM model. Data represent mean ± SD, n = 8/group. B, Representative illustration for the proangiogenesis effect of sulfated saccharide (Ch 2) vs b-FGF or VEGF in the CAM model. CAMs (n = 8/group/experiment) were exposed to sulfated Ch 2 analogs at 1 or 10 μg vs 1.0 μg b-FGF or 20 μg VEGF for 3 d. Sulfated saccharide Ch 2, VEGF, or b-FGF resulted in maximal increase in blood vessel branching, as shown in these representative images.
Angiogenic Activity of Branched Synthetic Oligosaccharides
The doubly branched, unsulfated hexasaccharide analog of Ch 2 and Ch 8 showed substantially reduced proangiogenic activity as compared to their respective sulfated saccharides, Ch 10 and Ch 9 (Table 1). Singly branched glucose trisaccharides, Ch 8 and Ch 9, containing the 3 saccharides closest to the reducing end of Ch 2 and Ch 10, represent a simplification of these studies. The singly branched unsulfated trisaccharide Ch 8 showed diminished proangiogenic activity compared with Ch 10 (data not shown).
TABLE 1.
Effect of Sulfation on the Proangiogenesis Efficacy of Saccharides in the Chorioallantoic Membrane Model
| Treatment | Mean Branch Points ± SEM |
|---|---|
| PBS (control) | 78 ± 9 |
| Ch 10 (10 μg) | 123 ± 10 |
| Ch 2 (10 μg) | 177 ± 11* |
| Ch 8 (10 μg) | 115 ± 6 |
| Ch 9 (10 μg) | 162 ± 7* |
Data represent mean ± SEM, n = 8 per group;
P < 0.01.
Ch 10 and Ch 8 are the corresponding non-sulfated saccharides to the sulfated saccharides Ch 2 and Ch 9.
Preliminary Mechanistic Evaluation of Proangiogenic Effect of Potent Oligosaccharide
Initial studies on the mechanism of action of Ch 2 were undertaken next. The role of the ERK1/2 in the signal transduction pathway in the stimulation of angiogenesis by Ch 2 was assessed first. Studies on ERK1/2 inhibition were carried out in the 3D-ECS assay. Oligosaccharide Ch 2 at 10 μg caused a significant increase in tube length and number of migrating cells, an effect that was blocked by PD 98059 (P < 0.01; Table 2). Next, the role of the integrin αvβ3 in the stimulation of angiogenesis by Ch 2 was studied. Angiogenesis promoted by Ch 2 (10 μg) in the presence of subthreshold levels of VEGF and b-FGF was significantly (P < 0.01) blocked by the αvβ3 integrin antagonist XT199 (Table 2).30,31 Similar results were shown when using the αvβ3 antibody LM609 (data not shown). These data suggest that the proangiogenic effects of sulfated saccharides begin at the plasma membrane αvβ3 integrin and involve activation of the ERK1/2.
TABLE 2.
Effect of MAP Kinase (ERK1/2) Inhibitor (PD9805) or αvβ3 Integrin Antagonist (XT199) on the Proangiogenesis Efficacy of Branched Sulfated Hexasaccharide Ch 2 in the Chorioallantoic Membrane Model
Effects on APTT
Lack of anticoagulant effect of Ch 2 and the other linear or branched oligosaccharides at 1 to 10 μg on APTT was demonstrated as compared to standard heparin or LMWH (data not shown).
DISCUSSION
Glycosaminoglycans have been implicated in the binding and activation of a variety of growth factors, cytokines, and chemokines. Reported data have suggested that certain oligosaccharides promote specific members of the FGF family that are important for wound repair and angiogenesis.32 In the present investigation, initiation and maintenance of blood vessel generation by sulfated and unsulfated oligosaccharides was assessed in the 3D-ECS assay. Heparin shows variable concentration-dependent proangiogenic and antiangiogenic effects; LMWHs are antiangiogenic; and heparin oligosaccharides ranging from tetrasaccharide (dp 4) to decasaccharide (dp10) are potent proangiogenic agents. Structure–activity relationship studies on synthetic oligosaccharides show that sulfated trisaccharides to hexasaccharides, having a charge density of –1.5 to –3/saccharide unit, show the most potent proangiogenic activity as demonstrated by the 3D-ECS. Moreover, branched sulfated hexasaccharide (Ch 2) shows potent and sustained proangiogenic activity, suggesting that it both promotes and stabilizes angiogenesis (Figs. 3–5). It is interesting to note that certain sulfated oligosaccharides showed antitumor activity in a sarcoma-180 mouse model.27 This is in contrast to the potent proangiogenesis activity in the CAM and in the 3D-ECS for sulfated oligosaccharides, as shown in this report. The effect on tumor weight based on the study design27 cannot be explained and is difficult to ascertain because of the lack of details about the model. The study performed by Du et al27 focused on the chemistry aspects and did not clearly provide a detailed description of the model; the effect on sarcoma tumor weight was marginal at doses ranging from 2.5 to 10 mg/kg. In addition, the study did not provide any data on the effect on tumor angiogenesis. The present study showed that in the absence of sulfated oligosaccharide both b-FGF and VEGF were effective only in initiating but not in sustaining blood vessel formation (Fig. 5). The proangiogenic activity of Ch 2 was confirmed using the on ovo CAM assay (Fig. 6B). Preliminary studies on the mechanism of action of the sulfated, branched synthetic hexasaccharide Ch 2 suggested the involvement of MAPK, ERK1/2 (Table 2). Upstream of MAPK, it appears that proangiogenic integrin acceptors are also involved in the proangiogenic activity of Ch 2. Additional studies will be required to fully understand both the angiogenesis-promoting and angiogenesis-stabilization activity of this compound.
Heparin and the related heparan sulfate have important functions as regulators of blood vessel growth and regression.5,17 This regulatory activity may be explained based on multiple mechanisms in which heparin and heparan sulfate interact with peptide growth factors. For example, heparin and heparan sulfate have a high affinity for angiogenic growth factors, such as b-FGF and VEGF, as well as for angiogenic inhibitors, such as thrombospondin and platelet factor 4. In addition, heparin and sulfated oligosaccharides stabilize b-FGF and other growth factors. Also, FGFs and thrombospondin are stored in the extracellular matrix bound to heparan sulfate; fragments of heparin or heparan sulfate may act as natural chaperones to shuttle b-FGF or other growth factors to different cellular compartments. Moreover, heparin-like low-affinity receptors on the surface of ECs (and other cells) prepare FGFs for binding to their specific high-affinity receptors. In addition, heparin may downregulate angiogenesis by releasing endogenous antiangiogenesis molecules, such as tissue factor pathway inhibitor or reducing tissue factor generation through the blockade of P-, L-, and E-selectins, which in turn would downregulate angiogenesis.6,18,19
Khorana et al21 provide evidence that the antiangiogenic effect of heparin in vitro is size dependent. If their findings also apply on ovo, then further refinements of heparin size may be necessary to optimize its antitumor effects. Because inhibition of angiogenesis has the potential to suppress processes such as wound healing or vascular remodeling, the benefits and risks of these novel heparin fractions require careful assessment. In contrast and in agreement with our results, Montesano et al,33 using a similar in vitro assay, reported that combinations of VEGF and hyaluronan oligosaccharides resulted in synergistic effect in inducing bovine microvascular endothelial cells to invade a 3D collagen gel and form capillary-like tubes, with an optimal effect at ≈0.5 to 2 μg/mL.
CONCLUSION
Data demonstrated that the sulfation and molecular shape (branched vs linear) of oligosaccharide are important for sustained proangiogenesis efficacy. Data indicate that chemically defined synthetic oligosaccharides can play an important role in regulation of capillary structure and stability, which may contribute to future advances in therapeutic angiogenesis. Furthermore, the proangiogenesis efficacy of oligosaccharide is mediated via integrin αvβ3 and involves MAPK signaling mechanisms.
References
- 1.Carmeliet P, Collen D. Molecular basis of angiogenesis: role of VEGF and VE-cadherin. Ann N Y Acad Sci. 2000;902:249–262. doi: 10.1111/j.1749-6632.2000.tb06320.x. [DOI] [PubMed] [Google Scholar]
- 2.Tomanek RJ, Schatteman GC. Angiogenesis: new insights and therapeutic potential. Anat Rec. 2000;261:126–135. doi: 10.1002/1097-0185(20000615)261:3<126::AID-AR7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Mousa SA. Angiogenesis promoters and inhibitors: potential therapeutic implications. Mol Med Today. 1996;2:140–142. doi: 10.1016/1357-4310(96)88781-8. [DOI] [PubMed] [Google Scholar]
- 4.Hudlicka O. Factors involved in capillary growth in the heart. Mol Cell Biochem. 1995;147:57–68. doi: 10.1007/BF00944784. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J, Weisz PB, Joullie MM, et al. Control of angiogenesis with synthetic heparin substitutes. Science. 1989;243:1490–1493. doi: 10.1126/science.2467380. [DOI] [PubMed] [Google Scholar]
- 6.Mousa SA, Mohamed S. Antiangiogenic mechanisms and efficacy of the low molecular weight heparin, tinzaparin: anti-cancer efficacy. Oncol Rep. 2004;12:683–688. [PubMed] [Google Scholar]
- 7.Khurana R, Simons M, Martin JF, et al. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation. 2005;112:1813–1824. doi: 10.1161/CIRCULATIONAHA.105.535294. [DOI] [PubMed] [Google Scholar]
- 8.Shah PB, Losordo DW. Non-viral vectors for gene therapy: clinical trials in cardiovascular disease. Adv Genet. 2005;54:339–361. doi: 10.1016/S0065-2660(05)54014-8. [DOI] [PubMed] [Google Scholar]
- 9.Presta M, Dell’Era P, Mitola S, et al. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Ruel M, Sellke FW. Angiogenic protein therapy. Semin Thorac Cardiovasc Surg. 2003;15:222–235. doi: 10.1016/s1043-0679(03)70002-1. [DOI] [PubMed] [Google Scholar]
- 11.Feng X, Clark RA, Galanakis D, et al. Fibrin and collagen differentially regulate human dermal microvascular endothelial cell integrins: stabilization of alphav/beta3 mRNA by fibrin1. J Invest Dermatol. 1999;113:913–919. doi: 10.1046/j.1523-1747.1999.00786.x. [DOI] [PubMed] [Google Scholar]
- 12.Nehls V, Drenckhahn D. A microcarrier-based cocultivation system for the investigation of factors and cells involved in angiogenesis in three-dimensional fibrin matrices in vitro. Histochem Cell Biol. 1995;104:459–466. doi: 10.1007/BF01464336. [DOI] [PubMed] [Google Scholar]
- 13.Nehls V, Drenckhahn D. A novel, microcarrier-based in vitro assay for rapid and reliable quantification of three-dimensional cell migration and angiogenesis. Microvasc Res. 1995;50:311–322. doi: 10.1006/mvre.1995.1061. [DOI] [PubMed] [Google Scholar]
- 14.Dupont E, Falardeau P, Mousa SA, et al. Antiangiogenic and antimetastatic properties of Neovastat (AE-941), an orally active extract derived from cartilage tissue. Clin Exp Metastasis. 2002;19:145–153. doi: 10.1023/a:1014546909573. [DOI] [PubMed] [Google Scholar]
- 15.Kim S, Bell K, Mousa SA, et al. Regulation of angiogenesis in vivo by ligation of integrin α5β1 with the central cell-binding domain of fibronectin. Am J Pathol. 2000;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colman RW, Pixley RA, Sainz IM, et al. Inhibition of angiogenesis by antibody blocking the action of proangiogenic high-molecular-weight kininogen. J Thromb Haemost. 2003;1:164–170. doi: 10.1046/j.1538-7836.2003.00025.x. [DOI] [PubMed] [Google Scholar]
- 17.Folkman J. Regulation of angiogenesis: a new function of heparin. Biochem Pharmacol. 1985;34:905–909. doi: 10.1016/0006-2952(85)90588-x. [DOI] [PubMed] [Google Scholar]
- 18.Mousa SA, Mohamed S. Inhibition of endothelial cell tube formation by the low molecular weight heparin, tinzaparin, is mediated by tissue factor pathway inhibitor. Thromb Haemost. 2004;92:627–633. doi: 10.1160/TH04-02-0069. [DOI] [PubMed] [Google Scholar]
- 19.Borsig L, Wong R, Feramisco J, et al. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci U S A. 2001;98:3352–3357. doi: 10.1073/pnas.061615598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Presta M, Leali D, Stabile H, et al. Heparin derivatives as angiogenesis inhibitors. Curr Pharmac Design. 2003;9:553–566. doi: 10.2174/1381612033391379. [DOI] [PubMed] [Google Scholar]
- 21.Khorana AA, Sahni A, Altland OD, et al. Heparin inhibition of endothelial cell proliferation and organization is dependent on molecular weight. Arterioscler Thromb Vasc Biol. 2003;23:2110–2115. doi: 10.1161/01.ATV.0000090671.56682.D7. [DOI] [PubMed] [Google Scholar]
- 22.Folkman J, Langer R, Linhardt RJ, et al. Angiogenesis inhibition and tumor regression caused by heparin or a heparin fragment in the presence of cortisone. Science. 1983;221:719–725. doi: 10.1126/science.6192498. [DOI] [PubMed] [Google Scholar]
- 23.Raman R, Venkataraman G, Ernst S, et al. Structural specificity of heparin binding in the fibroblast growth factor family of proteins. Proc Natl Acad Sci U S A. 2003;100:2357–2362. doi: 10.1073/pnas.0437842100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pervin A, Gallo C, Jandik KA, et al. Preparation and structural characterization of large heparin-derived oligosaccharides. Glycobiology. 1995;5:83–95. doi: 10.1093/glycob/5.1.83. [DOI] [PubMed] [Google Scholar]
- 25.Hua Y, Du Y, Yu G, et al. Synthesis and biological activities of octyl 2,3-di-O-sulfo-α-L-fucopyranosyl-(1→3)-2-O-sulfo-α-L-fucopyranosyl-(1→4)-2,3-di-O-sulfo-α-L-fucopyranosyl-(1→3)-2-O-sulfo-α-L-fuco-pyranosyl-(1→4)-2,3-di-O-sulfo-β-L-fucopyranoside. Carbohydr Res. 2004;339:2083–2090. doi: 10.1016/j.carres.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 26.He H, Yang F, Du Y. Synthesis of natural β-D-(1→3)-glucopyranosyl oligosaccharides. Carbohydr Res. 2002;337:1673–1678. doi: 10.1016/s0008-6215(02)00276-8. [DOI] [PubMed] [Google Scholar]
- 27.Du Y, Gu G, Hua Y, et al. Synthesis and antitumor activities of glucan derivatives. Tetrahedron. 2004;60:6345–6351. [Google Scholar]
- 28.Koketsu M, Linhardt RJ. Electrophoresis for the analysis of acidic oligosaccharides. Anal Biochem. 2000;283:136–145. doi: 10.1006/abio.2000.4649. [DOI] [PubMed] [Google Scholar]
- 29.Edens RE, Al-Hakim A, Weiler JM, et al. Gradient polyacrylamide gel electrophoresis for determination of the molecular weights of heparin preparations and low-molecular-weight heparin derivatives. J Pharm Sci. 1992;81:823–827. doi: 10.1002/jps.2600810821. [DOI] [PubMed] [Google Scholar]
- 30.Bishop GG, McPherson JA, Sanders JM, et al. Selective alpha(v)beta(3)-receptor blockade reduces macrophage infiltration and restenosis after balloon angioplasty in the atherosclerotic rabbit. Circulation. 2001;103:1906–1911. doi: 10.1161/01.cir.103.14.1906. [DOI] [PubMed] [Google Scholar]
- 31.Mousa SA, O’Connor LJ, Bergh JJ, et al. The proangiogenic action of thyroid hormone analogue GC-1 is initiated at an integrin. J Cardiovasc Pharmacol. 2005;46:356–360. doi: 10.1097/01.fjc.0000175438.94906.a0. [DOI] [PubMed] [Google Scholar]
- 32.Taylor KR, Rudisill JA, Gallo RL. Structural and sequence motifs in dermatan sulfate for promoting fibroblast growth factor-2 (FGF-2) and FGF-7 activity. J Biol Chem. 2005;280:5300–5306. doi: 10.1074/jbc.M410412200. [DOI] [PubMed] [Google Scholar]
- 33.Montesano R, Kumar S, Orci L, et al. Synergistic effect of hyaluronan oligosaccharides and vascular endothelial growth factor on angiogenesis in vitro. Lab Invest. 1996;75:249–262. [PubMed] [Google Scholar]