Abstract
Chondroitin sulfate (CS) was administered orally to BALB/c mice immunized intraperitoneally with ovalbumin (OVA) and/or dinitrophenylated OVA. The titers of antigen-specific IgE and IgG1 in mouse sera were determined. The antigen-specific IgE production by mice fed ad libitum with CS was significantly inhibited. We also examined the effect of feeding CS on immediate-type hypersensitivity. One hour after antigen stimulation, the ears of mice fed with CS swelled less than those of the control mice. Furthermore, the rise in serum histamine in the mice fed with CS under active systemic anaphylaxis was significantly lower than that in the controls. We next examined the pattern of cytokine production by splenocytes from mice followed by re-stimulation with OVA in vitro. The splenocytes from the mice fed with CS produced less interleukin (IL)-5, IL-10, and IL-13 than those from the control group. In contrast, the production of interferon-γ and IL-2 by the splenocytes of mice fed with CS was not significantly different from those in the control mice. In addition, the production of transforming growth factor-β from the splenocytes of mice fed with CS was significantly higher than that of the control mice. Furthermore, we showed that the percentages of CD4+ cells, CD8+ cells, and CD4+CD25+ cells in the splenocytes of mice fed with CS are significantly higher than those of the control. These findings suggest that oral intake of CS inhibits the specific IgE production and antigen-induced anaphylactic response by up-regulating regulatory T-cell differentiation, followed by down-regulating the Th2 response.
The incidence of type I allergic disorders has been increasing worldwide, particularly, hypersensitivity to food and airborne allergens (1–4). The mechanism of type I allergy includes a series of events (5, 6), namely, production of antigen-specific IgE, binding of IgE to the FcεRI receptor on mast cells or basophils, cross-linking of IgE with newly absorbed allergens, and release of chemical mediators such as histamine and leukotrienes from cells. Inhibition of any of the steps in this sequence leads to the attenuation of allergic symptoms. Several drugs, such as corticosteroids, epinephrine, histamine antagonists, and leukotriene synthesis inhibitors, interfere with the above reactions (7). However, the effect of these drugs is short-lived; thus, a more fundamental means of preventing allergies is desirable.
Chondroitin sulfate (CS)3 is one of a family of structurally complex, sulfated, linear polysaccharides called glycosaminoglycans. CS is composed of a repeating disaccharide unit of the structure, [→4)GlcA(β1→3)GalNAc(β1→]n, where GlcA is glucuronic acid and GalNAc is N-acetylgalactosamine (8). CS contains, on average, one sulfate group per disaccharide unit at either the C-4 or C-6 positions of GalNAc (9–11). CS is an integral component of the proteoglycans found localized on cell surfaces and in the extracellular matrix and is important for cell-cell communications (9, 12, 13). Recently, there have been a number of reports on the biochemical activities of orally administrated exogenous CS, including its anti-inflammatory and chondroprotective properties (12–19). It has been proposed that these activities result from an increase in the biosynthesis of connective tissue components, such as hyaluronan, at disease sites (20, 21). Both in vitro and in vivo studies have shown that CS regulates the formation of new cartilage by stimulating the chondrocyte synthesis of collagen, proteoglycans and hyaluronan (22, 23).
Polysaccharides such as CS are poorly absorbed through the digestive system (24, 25). Therefore, we examined the half-life of CS in the circulatory system and demonstrated it to be 3–15 min, based on the pharmacokinetic study of intravenously administrated CS (26). Accordingly, it appears unlikely that orally administered CS is systemically distributed to connective tissues such as cartilage and skin and that exogenously administered CS actually directly stimulates chondrocyte synthesis of extracellular matrix components. This suggests that the mechanism of action of orally administrated CS might be mediated by other systems, such as the immunological system (27). Our laboratory has already shown that CS up-regulates the in vitro antigen-specific Th1 immune response on murine splenocytes sensitized with ovalbumin (OVA) and that CS suppresses the antigen-specific IgE responses. In addition, we have characterized the structure of CS chains required for these immunological effects (28, 29). These studies suggest that the CS intake could control the IgE-mediated allergic response and Th2 response-mediated inflammatory diseases. However, no in vivo studies, on the effect of CS intake on the immune system, have yet been performed.
In the present study, we examined the effect of CS intake on the production of specific IgE antibody and specific IgG antibody in OVA-sensitized mice. We also examined the effect of CS intake on antigen-induced anaphylactic response, such as ear swelling, and active systemic anaphylaxis in OVA-sensitized mice. Furthermore, to clarify the mechanism of inhibition of specific IgE production, we examined the pattern of cytokine production by splenocytes from mice fed with CS. In addition, to further assess the involvement of the immunological process of CS intake, we analyzed the differentiation in splenocytes, Peyer’s patch (PP) cells, mesenteric lymph node (MLN) cells, and intestinal intraepithelial lymphocytes (IELs) using flow cytometry (FCM).
EXPERIMENTAL PROCEDURES
Animals and Administration Protocols
Inbred specific pathogen-free BALB/c mice (female, 6 weeks of age) were purchased from Charles River Japan (Yokohama, Japan). The mice were maintained in a temperature (23–25 °C)-, humidity (40–60%)-, and light-controlled environment with free access to an MF diet (Japan SLC Co. Ltd., Shizuoka, Japan) andwater. They were acclimatized for at least 1 week before the start of the study. The CS-fed group had 400 mg/kg/day of CS by daily gavage for 4 weeks or free access to 2% CS ad libitum for 4 weeks, respectively. The control group for CS by gavage had saline by daily gavage for 4 weeks, and the control group for 2%CS ad libitum had free access to water ad libitum for 4 weeks. The care and use of the experimental animals in this study followed “The Ethical Guidelines of Animal Care, Handling and Termination” prepared by the National Institute of Health Sciences of Japan.
Reagents
CS samples (chondroitin 6-sulfate, average molecular weight Mr 31,000, from shark cartilage) were a kind gift from San Ei-Gen FFI Co. (Osaka, Japan). OVA (grade V, chicken egg) was purchased from Sigma Co. Dinitrophenylated (DNP)-OVA was purchased from Cosmobio Co., Ltd. (Tokyo, Japan). RPMI 1640 was purchased from ICN Biomedicals (Aurora, OH). Fetal bovine serum (FBS) was purchased from Sanko Junyaku (Tokyo, Japan), and it was heat-inactivated at 56 °C for 30 min prior to use.
NMR and Compositional Analysis of CS
The determination of unsaturated disaccharides, prepared from CS, was performed on the lyase-digested samples using high-performance liquid chromatography (ΔDi-0S, 3.9%; ΔDi-4S, 24.5%; ΔDi-6S, 53.9%; ΔDi-diSE, 1.7%; ΔDi-diSB, 0.6%; ΔDi-diSD, 15.3%; and ΔDi-triS, 0.2%) (30, 31).
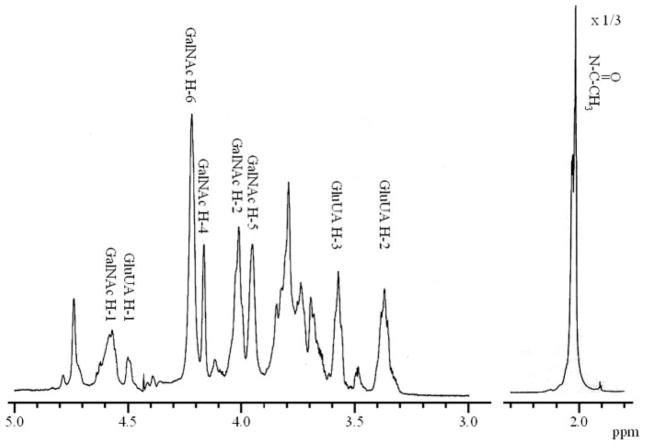
1H NMR spectroscopy was performed using the conditions previously described by Toida et al. (10). The CS sample (~1–3 mg) was kept in a desiccator over phosphorus pentoxide in vacuo overnight at room temperature. The thoroughly dried sample was then dissolved in 500 μl of 2H2O (100%, Aldrich Japan, Tokyo), and passed through a 0.45-μm syringe filter and transferred to an NMR tube. 1D and 2D NMR experiments were performed using a JNM-ECP600 spectrometer of the Japan Electron Optical Laboratory (JEOL) equipped with a 5-mm field gradient, tunable probe with standard JEOL software at 333 K for all the experiments with 500-μl samples. The HO2H signal was suppressed by presaturation during 3 or 1.5 s for the 1D or 2D spectra, respectively. To obtain the 2D spectra, 512 experiments resulting in 1024 data points for a spectral width of 2,000 Hz were measured, and the time domain data were multiplied after zero filling (data matrix size, 1K × 1K) with a shifted sine-bell window functions for 2D double quantum-filtered and triple quantum filtered-COSY, NOESY, or TOCSY experiments. An MLEV-17 mixing sequence of 100 ms was used for the 2D TOCSY experiments, and for the NOESY experiments mixing times of 150, 250, and 500 ms were used. The purity of CS sample was thought to be >95% based on the compositional and NMR analyses (Fig. 1).
FIGURE 1. 1H NMR spectrum of CS recorded in 2H2O at 333 K.
The peaks are labeled according to the main corresponding residues in the structure and the resonating proton. The height of the NHCOCH3 peak has been reduced to one-third to be in scale.
Sensitization Protocols
Seven days after starting the administration of CS, all the mice were immunized intraperitoneally with OVA (20 μg/mice) and/or DNP-OVA (10 μg/mice) with an alum (Al(OH)3, Nagahama LSL Co. Ltd., Shiga, Japan) adjuvant. One week later, blood was collected and serum obtained. Ten days after the first immunization, the mice were given boosters using the same doses of the antigens. A week after the second immunization, all the mice were killed by cervical dislocation, their spleens aseptically removed, and cell suspensions made by passing them through a sterile cell strainer (BD Biosciences). Blood was withdrawn and serum obtained. The serum samples were stored at −20 °C prior to analysis (33, 34). For the study for the induction of active systemic anaphylactic shock, the experimental procedure after the second immunization is described below.
Induction of Active Systemic Anaphylactic Shock and Measurement of Histamine in Plasma
The mice immunized with OVA were challenged intraperitoneally with 0.2 ml of phosphate-buffered saline (PBS) containing OVA (1 mg/mouse) to induce anaphylactic shock. Ten minutes after antigen challenge, the mice were killed, and blood was collected to obtain serum for the histamine determination. Serum histamine concentrations were measured using the post-column high-performance liquid chromatography method described by Kawasaki et al. (35).
Ear Swelling Assay
Ten days after the second immunization, the ear thickness (time 0) of the mice was measured with an upright dial gauge (Ozaki Mfg. Co. Ltd., Tokyo, Japan). Ear swelling responses were elicited by applying 10 μl of antigen (1% picryl chloride in acetone) to the ventral sides of the left ears of the mice (33). Ear thickness was measured at 1 h after picryl chloride challenge, and the increase in ear thickness from time 0 was determined.
Antibody (Antigen-specific IgE and IgG1 Isotypes) Titer Determination
The mouse serum titers (reciprocal of serum dilution with fluorescence intensity at 50% of the maximum level) of anti-OVA IgE, IgG1, and IgG2a were determined in triplicate in a 96-well microtiter plate by the method of Teshima et al. with some modifications (34, 36). Fifty microliters of OVA (Cosmobio Co., 20 μg/ml) in 50 mM sodium carbonate buffer, pH 9.5, was added to each well of a 96-well microtiter plate and incubated overnight at 4 °C. Each well was washed four times with 200 μl of PBS containing 0.05% Tween 20 (PBS/Tween). Two hundred microliters of 0.1% casein in PBS was added, the plates were incubated for 1 h at room temperature, and then each well was washed as described above. Fifty microliters of antiserum was added to each well, the plates were incubated for 1 day at 4 °C, and then each well was washed as before. Fifty microliters of rabbit anti-mouse IgE/IgG1/IgG2a (10−3 dilution in PBS containing 0.1% casein, Yamasa Shoyu Co., Ltd., Chiba, Japan) was added to each well, the plates were incubated for 1 h at room temperature, and then each well was washed as before. Fifty microliters of sheep anti-rabbit-IgG-β-galactosidase conjugate (10−3 dilution in PBS containing 0.1% casein, Amersham Biosciences) was added to each well, the plates were incubated for 1 h at room temperature, and then each well was washed as before. The wells were incubated for 1 h at 37 °C with 100 μl of PBS containing 0.1 mM 4-methylumbelliferone-β-galactoside (Sigma). Finally, 25 μl of 1 M anhydrous sodium carbonate was added to each well. The fluorescence intensity of the liberated 4-methylumbelliferone was monitored by a Titertek Fluoroskan reader (Flow Laboratories, Inc., Costa Mesa, CA).
Cytokine Assays of Splenocytes
Splenocytes were collected from the removed spleens of all the OVA-immunized mice, and the cells (5×106 cells/ml) from each spleen were cultured in triplicate on a 24-well culture plate and re-stimulated with OVA at a final concentration of 100 μg/ml at 37 °C for 3 days (28). The levels of interleukin (IL)-4, IL-5, IL-10, and IL-12 (p70), transforming growth factor-β (TGF-β), and interferon (IFN)-γ in the culture medium (RPMI 1640) after 3 days of co-culture with OVA were measured with an OptEIA mouse cytokine enzyme-linked immunosorbent assay set (BD Pharmingen). Absorbance was measured at 450 nm with a microplate reader (E-max, Molecular Devices, Sunnyvale, CA).
FCM Analysis of Splenocytes
The collected splenocytes from mice were washed twice in PBS containing 2% FBS, and then cell staining analysis was carried out in the following way (28, 34). Briefly, 100 μl of splenocytes at 2.0 × 106 cells/ml in PBS containing 2% FBS and 0.1% NaN3 was mixed with 1 μl of anti-CD16/CD32 monoclonal antibody (BD Pharmingen, San Diego, CA) in a tube and reacted for 5 min at 4 °C. Fluorescently labeled antibodies at 0.0125 μg/10 μl were added to the above tubes; mixed and then incubated for 30 min at 4 °C in the dark. After staining, the cells were washed three times with PBS. After washing, the cells were poured into FCM tubes in PBS containing 2% FBS and 0.1% NaN3 in a total volume of 500 μl. We defined CD4+CD8− cells as helper T cells and CD4−CD8+ cells as cytotoxic T cells in the present study. The target for detection was CD3 as the T cell, and CD45R/B220+ as the B cell, the surface antigens of the respective cell populations.
FCM analysis was performed with a FACSCalibur™ flow cytometer (BD Biosciences) (23). Splenocytes were stained with peridinin chlorophyll protein-labeled anti-mouse CD3ε (145–2C11, BD Pharmingen), fluorescein isothiocyanate (FITC)-labeled anti-mouse CD4 (H129.19, BD Pharmingen), phycoerythrin (PE)-labeled anti-mouse CD8α (53–6.7, BD Pharmingen), FITC-labeled anti-mouse CD25 (PC61, BD Pharmingen), and PE-labeled anti-mouse CD45R/B220 (RA3–6B2) antibodies. Fluorescence overlap was compensated electronically by using splenocytes stained with single colors, and then 10,000 cells were acquired and stored for each analysis. The data were analyzed using CellQuest® software.
Isolation of IELs
The IELs were isolated as previously described by Nagafuchi (37, 38). Briefly, the small intestine was removed from the mice. Once free of the luminal contents, it was turned inside-out with the aid of polyethylene tubing. Each reversed intestine was cut into four segments, and the segments were placed in a 50-ml polypropylene conical tube containing 45 ml of Hanks’ balanced salt solution (Invitrogen) containing 5% FBS (Sigma). The tube was shaken at 37 °C for 45 min (horizontal position on an orbital shaker at 135 rpm), and the cell suspension was passed through a glass-wool column to remove any adherent cells. The cells were then suspended in 30% (w/v) Percoll (Amersham Biosciences) and centrifuged for 20 min at 1,800 rpm. The cell pellet was collected, and then the IELs were purified by density-gradient centrifugation using Percoll as the separation medium (1,800 rpm, 20 min). The IELs were recovered at the 44 and 70% Percoll interphases. More than 90% of the IELs were recovered.
Isolation of the PP Cells and MLN Cells
The PP and MLN cells were recovered from OVA-immunized mice by dissection from the small intestines, and single-cell suspensions were prepared by pressing the PPs and the MLNs through a nylon mesh Cell Strainer (BD Falcon, Franklin Lakes, NJ). The cell suspension was centrifuged and washed three times in RPMI 1640 containing 10% FBS (39).
FCM Analysis of the PP Cells, MLN Cells, and IELs
A three-color analysis of the PP cells, MLN cells, and IEL subsets was performed (39). The antibodies used for the FCM were FITC-labeled anti-mouse Integrin β7 chain (M293, BD Pharmingen), FITC-labeled goat F(ab′)2 anti-mouse IgM plus IgG plus IgA (H plus L) (Southern Biotechnology Associates, Inc., Birmingham, AL), peridinin chlorophyll protein-labeled anti-mouse CD3ε, FITC- and peridinin chlorophyll protein-labeled antimouse CD4, PE-labeled anti-mouse CD8α, FITC-labeled anti-mouse CD8β (53-5.8, BD Pharmingen), FITC-labeled anti-mouse TCRαβ (H57-597, Immunotech, Marseille, France), PE-labeled anti-mouse TCRγδ (GL3, Immunotech), FITC-labeled anti-mouse CD25, and PE-labeled anti-mouse CD45R/B220 antibodies. All incubations were performed in the dark. A single cell suspension of lymphocytes in BD Pharmingen Stain Buffer containing 2% FBS was incubated with 50 μl of properly diluted monoclonal antibody at 4 °C for 30 min. The cells were washed in Hanks’ balanced salt solution and recovered by centrifugation. After staining, a total of at least 10,000 cells was analyzed using a FACSCalibur™. The data were analyzed using CellQuest® software.
Statistical Analysis
All values are expressed as means±S.E. Statistical comparisons were performed by using the Student’s t test or Scheffe’s method after an analysis of variances. In all cases, the probability (p) values below 0.05 were considered significant.
RESULTS
Body Weight and the Amount of Feed Intake
We first examined whether CS intake by gavage or orally ad libitum affect the body weight and feed intake of the mice. There was no significant difference in the body weight and the amount of feed intake between the control group and the CS-fed group for both administration methods during the experimental period. No specific symptoms were observed in the CS group during the study.
Specific IgE and IgG Production in Vivo
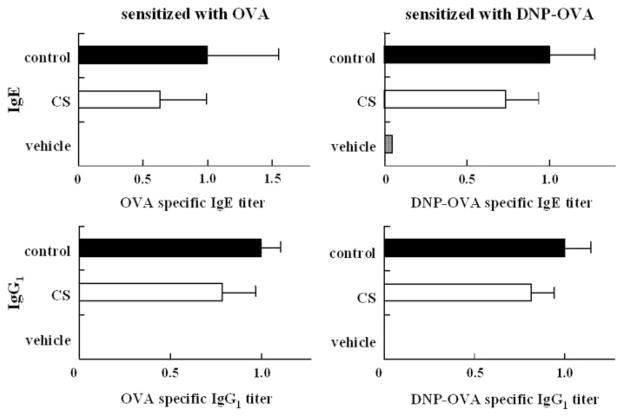
The group of mice administered CS by gavage and immunized with DNP-OVA or OVA showed a serum titer of DNP-specific IgE antibodies and OVA-specific IgE antibodies lower than those in the control group, although the specific IgE antibodies of both the CS group and the control were not significantly different (Fig. 2).
FIGURE 2. Effects of CS on the serum antigen-specific antibody titer in secondary immune response.
Bars represent mean values ± S.D. of five mice. CS (400 mg/kg/day) was administered by gavage from the first immunization to 7 days after the second immunization.
In contrast, in the group of mice administered CS orally ad libitum and immunized with OVA, the serum titers of OVA-specific IgE antibodies and OVA-specific IgG1 antibodies were significantly lower than those in the control group (Fig. 3). This result suggests that the oral ad libitum administration of CS inhibits the production of OVA-specific IgE and OVA-specific IgG1 antibodies. We postulate that the successive oral intake of CS is more effective for the inhibition of OVA-specific IgG1 antibody production or that the inhibitory effects are dependent on the oral intake amount of CS.
FIGURE 3. Effects of CS on the serum antigen-specific antibody titer in secondary immune response.
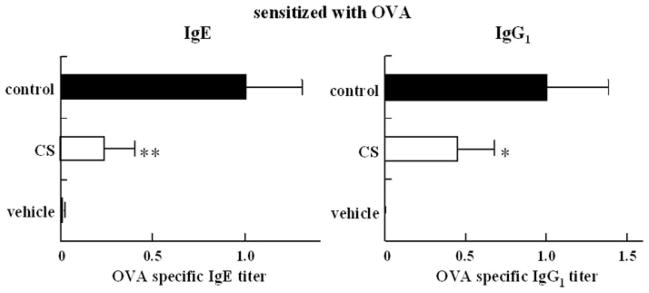
Bars represent mean values ± S.D. of 10 mice. CS was administered orally ad libitum from 7 days before the first immunization to 7 days after the second immunization. Asterisks indicate the significance of difference from the control value (**, p < 0.01; *, p < 0.05).
Antigen-induced Immediate-type Ear Swelling Assay
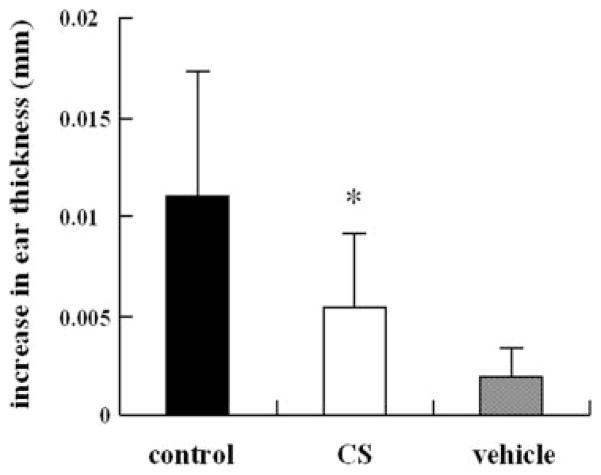
The reduction of specific IgE antibody production should inhibit the antigen-induced anaphylactic response in the immediate-type hypersensitivity. Therefore, we examined the effect of CS on the ear swelling response at 1 h after antigen stimulation as a test of immediate-type hypersensitivity (33). In the control group sensitized with DNP-OVA, the ear thickness significantly increased 1 h after antigen stimulation. The increase in ear thickness observed in the group orally administered CS was significantly lower than that of the control group (Fig. 4).
FIGURE 4. Effects of CS on the immunized mice ear swelling responses 1 h after epicutaneous challenge with picryl chloride.
Bars represent mean values ± S.D. of five mice (ten ears). CS was administered orally ad libitum from the first immunization. *, p < 0.05 compared with control (saline).
Histamine Levels in Mice Plasma under Active Systemic Anaphylaxis
All the mice challenged intraperitoneally with 1 mg of OVA exhibited anaphylactic shock, but the response in the CS-fed group appeared to be weaker than that in the control group (data not shown). A marked increase in serum histamine levels occurred in all the mice undergoing OVA challenge (Fig. 5). The rise in serum histamine levels in the CS-fed group under active systemic anaphylaxis was significantly lower than that in control group.
FIGURE 5. Effect of CS on the serum histamine concentrations in the OVA-challenged mice.
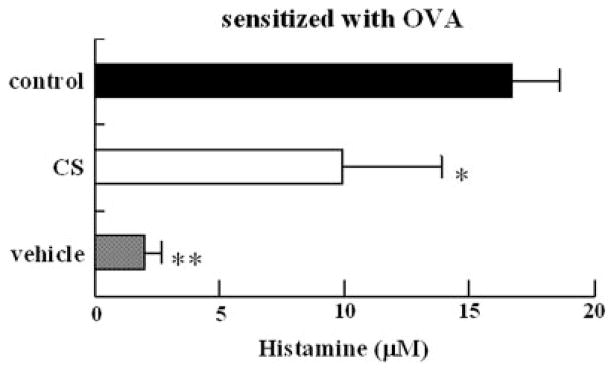
Serum samples were collected after 10-min intraperitoneal administration of OVA. Bars represent mean values ±S.D. of four mice. CS was orally ad libitum administered from the first immunization to the time of the active systemic anaphylaxis test. Mice were challenged with 1 mg of antigen. Asterisks indicate the significance of difference from the control value (**, p< 0.01; *, p < 0.05).
Production of Cytokine from Splenocytes in Vitro
To clarify the mechanisms involved in the inhibition of DNP-specific IgE production in mice, we investigated the cytokine production of splenocytes from mice stimulated with DNP-OVA in vitro. As shown in Fig. 6, the levels of IFN-γ and IL-2 produced by the splenocytes of the CS-fed group by gavage were not significantly different from those of the control group (Fig. 6). In contrast, the levels of IL-5, IL-10, IL-13, and Th2 type cytokines produced by the splenocytes of the CS-fed group by gavage were lower than those of the control group. The transforming growth factor-β level of the splenocytes from the CS-fed groups by gavage was significantly higher than that of the control group.
FIGURE 6. Effects of CS administration on the cytokineproduction of splenocytes in vitro.
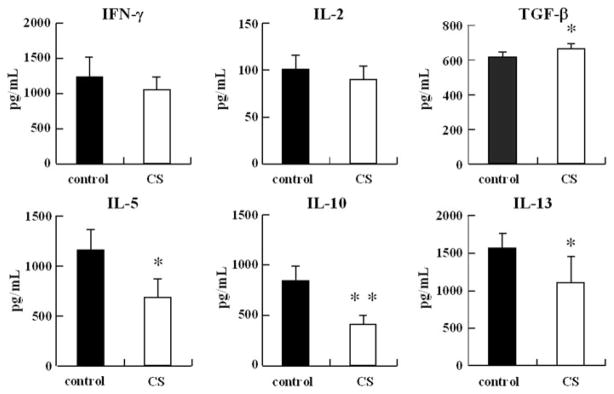
BALB/cmice (n=5) were intraperitoneally injected on days 0 and 10 with 20μg of OVA and 2mg of Al(OH)3 at a total volume of 400μl, and CS was orally ad libitum administered from 7 days before first immunization to 7 days after the second immunization. Splenocytes (5.0×106 cells/ml)were collected on day 18 and were co-cultured with OVA (final, 100μg/ml). The amounts of cytokines in the supernatant were measured by enzyme-linked immunosorbent assay. Asterisks indicate the significance of difference from the control value (**, p<0.01; *, p<0.05). Bars represent mean values (±S.D.) for six wells.
FCM Analysis of Splenocytes
To investigate the effect of CS on the differentiation of splenocytes in the OVA-sensitized mice fed with CS, we undertook phenotypic analysis of splenocytes using FCM. The target for detection was CD3+ for the T-cell and CD45R/B220+ for the B-cell surface antigens of the respective cell populations. Table 1 shows the percentages of CD3+, CD45R/B220+, CD4+, CD8+, and CD4+CD25+ cells in the population of splenocytes.
TABLE 1. Percentage of splenocytes expressing surface antigenic markers in each group.
Data are means ± S.D. (% (gated)), n = 8. CS was administered orally ad libitum from 7 days before the first immunization to 7 days after the second immunization. The control group had ad libitum free access to water.
| CD3+CD45R/B220−, T cell | CD3−CD45R/B220+, B cell | CD4+CD8−, CD4 | CD4−CD8+, CD8 | CD4+CD25+ | |
|---|---|---|---|---|---|
| CS | 38.0 ± 2.8 | 52.9 ± 2.0 | 27.7 ± 2.0a | 11.8 ± 0.7a | 4.4 ± 0.5b |
| Control | 36.2 ± 2.8 | 53.2 ± 2.6 | 25.2 ± 1.3 | 10.8 ± 0.9 | 3.9 ± 0.3 |
Significantly different from control at p < 0.05.
Significantly different from control at p < 0.01.
The percentage of T cells in the splenocytes from the control mice was 36.2%, whereas those of mice fed with CS was 38.0%. The percentage of B cells in the splenocytes from the control was not significantly different from those of the mice fed with CS. Furthermore, to assess whether CS affects the differentiation of the T-cell subsets, we analyzed the expression of CD4+ T cells and CD8+ T cells. The percentage of CD4+ T cells in the splenocytes from the mice fed with CS (27.7%) was significantly higher than that of the control mice (25.2%). The percentage of CD8+ T cells in the splenocytes from the mice fed with CS (11.8%) also was significantly higher than that of the control mice (10.8%). In addition, as shown in Fig. 7, the percentage of CD4+ CD25+ T cells, regulatory T cells (Tregs), in the splenocytes from the mice fed with CS (4.4%) also was significantly higher than that of the control mice (3.9%).
FIGURE 7. Expression of CD4 and CD25 on splenocytes from the BALB/c mice.
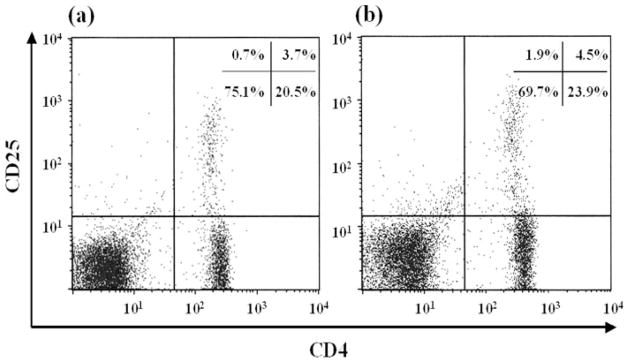
Splenocytes were isolated from the control group (a) and the CS group (b). The histograms are representatives of five independent experiments.
FCM Analysis of PP Cells, MLN Cells, and IELs
To examine the intestinal immune system involved in the inhibition of specific IgE production by the CS intake, the PP cells, MLN cells, and IELs were isolated from the small intestine of the OVA-sensitized mice and the OVA-sensitized mice fed CS, and their lymphocytes were analyzed by FCM. For the PP cells, as shown in Table 2, the proportion of T cells from the mice fed CS was significantly higher than those of the control. In contrast, the proportion of B cells from the mice fed with CS was significantly lower than those of control. Furthermore, the proportion of CD4+ T cells and CD8+ T cells in the PP cells from the mice fed with CS was significantly higher than that of the control mice. These results suggest that CS induces the differentiation of CD4+ T cells and CD8+ T cells in the PP cells.
TABLE 2. Percentage of PP cells expressing surface antigenic markers in each group.
Data are means ± S.D. (% (gated)), n = 4. CS was administered orally ad libitum from 7 days before the first immunization to 7 days after the second immunization. The control group had ad libitum free access to water.
| CD3+CD45R/B220−, T cell | CD3−CD45R/B220+, B cell | CD4+CD8−, CD4 | CD4−CD8+, CD8 | CD4+CD25+ | |
|---|---|---|---|---|---|
| CS | 32.5 ± 2.0a | 60.8 ± 2.9a | 23.9 ± 0.6b | 5.0 ± 0.4a | 2.2 ± 0.4 |
| Control | 24.3 ± 2.1 | 70.4 ± 2.0 | 18.2 ± 3.6 | 2.7 ± 0.9 | 2.3 ± 1.0 |
Significantly different from the control group at p < 0.06.
Significantly different from the control group at p < 0.05.
For the MLN cells, as shown in Table 3, the proportion of T cells, B cells, CD4+ T cells, and CD8+ T cells from the control was not significantly different from those of the mice fed with CS. However, the proportion of CD4+ CD25+ T cells from the mice fed with CS was significantly higher than that of the control mice as with splenocytes.
TABLE 3. Percentage of MLN cells expressing surface antigenic markers in each group.
Data are means ± S.D. (% (gated)), n = 4. CS was administered orally ad libitum from 7 days before the first immunization to 7 days after the second immunization. The control group had ad libitum free access to water.
| CD3+CD45R/B220−, T cell | CD3−CD45R/B220+, B cell | CD4+CD8−, CD4 | CD4−CD8+, CD8 | CD4+CD25+ | |
|---|---|---|---|---|---|
| CS | 72.0 ± 2.3 | 26.4 ± 2.2 | 55.6 ± 4.1 | 14.9 ± 0.2 | 6.6 ± 0.1a |
| Control | 70.2 ± 1.0 | 28.4 ± 1.1 | 53.3 ± 1.7 | 14.0 ± 0.6 | 6.1 ± 0.4 |
Significantly different from control at p < 0.05.
For the IELs, as shown in Table 4, the proportion of the CD4+ T cells in the IELs of the OVA-sensitized mice fed with CS (ad libitum and 400 mg/kg/day by gavage) were significantly higher than that of the control. The proportion of the TCRαβ-T cells in the IELs of the OVA-sensitized mice fed with CS (ad libitum) also was significantly higher than that of the control mice, and, although the proportion of TCRαβ-T cells of the OVA-sensitized mice fed with CS (400 mg/kg/day) appears to be higher than that of the control mice, the values are not significantly different. These results suggest that CS induces the differentiation of TCRαβ-T cells and CD4+ cells in the IELs and directly or indirectly affects part of the gut-associated lymphoid tissue without the absorption of CS through the digestive tract.
TABLE 4. Percentage of IELs expressing surface antigenic markers in each group.
Data are means ± S.D. (% (gated)), n = 5. CS (ad libitum) was administered orally ad libitum from 7 days before the first immunization to 7 days after the second immunization. CS (400 mg/kg/day) was administered by gavage from the first immunization to 7 days after the second immunization. The control group had ad libitum free access to water.
| TCRαβ | TCRγδ | CD8αα | CD8αβ | CD4 | |
|---|---|---|---|---|---|
| CS (ad libitum) | 53.4 ± 4.4a | 32.8 ± 4.1 | 45.9 ± 6.7 | 22.6 ± 4.5 | 17.5 ± 5.2a |
| CS (400 mg/kg/day) | 49.1 ± 3.8 | 28.9 ± 5.3 | 47.2 ± 2.8 | 20.3 ± 2.6 | 13.8 ± 3.0a |
| Control | 46.8 ± 3.8 | 31.7 ± 1.7 | 50.7 ± 1.7 | 17.8 ± 1.9 | 9.7 ± 1.1 |
Significantly different from control at p < 0.05.
DISCUSSION
We first showed that feeding CS to mice inhibited specific IgE and IgG1 production in response to DNP-OVA in vivo, but DNP-specific IgG1 production was not significantly affected. Also, feeding CS to mice immunized with DNP-OVA significantly inhibited the immediate ear swelling response induced by antigen stimulation.
CD4+ helper T cells are subpopulations of two cell types, Th1 and Th2, defined based on their different patterns of cytokine secretion (40, 41). The balance of these two types of cells is considered to be important in maintaining homeostasis in the host. Once this balance becomes disturbed, various immunological diseases, such as allergies and intestinal inflammation, can occur due to circumvention of the host defense mechanisms. Thus, the regulation of these two types of cells seems to be important for preserving the host immune response, including IgE and cytokine production.
Th1 cells can secrete IFN-γ and IL-2, whereas Th2 cells can produce IL-4, IL-5, and IL-13. IFN-γ and IL-12 induce differentiation to Th1 from Th0 cells, whereas IL-4 induces differentiation to Th2. Therefore, an increase in IFN-γ and IL-12 could shift the Th1/Th2 cell balance to predominantly Th1, and an increase in IL-4, IL-5, and IL-13 could shift the balance to predominantly Th2 (42–45). In the present study, we showed that the levels of IFN-γ and IL-12 produced by splenocytes from the CS-fed group were not significantly different from that from the control group, whereas the levels of IL-5, IL-10, and IL-13 produced by splenocytes from the group fed with CS were lower than those from the control group. Thus, these results suggest that feeding CS to mice reduces the differentiation of Th2. Consequently, it is thought that feeding CS inhibits specific IgE production in mice. This would suggest that feeding CS mainly inhibits antigen-specific Th2 differentiation and only slightly affects antigen nonspecific differentiation.
It is difficult to accept that orally administered CS is systemically distributed to connective tissues such as cartilage and skin and that exogenously administered CS actually directly stimulates chondrocyte synthesis of extracellular matrix components, because CS is poorly absorbed through the digestive system (24, 25). Moreover, the half-life of CS in the circulatory system is only 3–15 min, based on the pharmacokinetic study of intravenously administrated CS (26). This suggests that the systemic effects of orally administrated CS might instead be mediated by the intestinal immunological system, including gut-associated lymphoid tissue (46). Our studies have already shown that CS up-regulates the in vitro antigen-specific Th1 immune response on murine splenocytes sensitized with OVA and that CS suppresses the antigen-specific IgE responses; in addition, we have characterized the structure of CS chains responsible for the immunological effects (28, 29). These studies suggest that CS can directly affect the differentiation of the lymphocytes and that a therapeutic use of CS to control the IgE-mediated allergic response could be expected.
However, to date, the underlying mechanism of the systemic Th2 down-regulation on CS intake has remained unclear. It has been thought that intact foreign food antigens infrequently induce clinical symptoms, because tolerance develops in most individuals, even though they routinely penetrate the gastrointestinal tract. Husby et al. (47, 48) demonstrated that oral feeding of food substances leads to immunological tolerance induction in human subjects. We suggest that the underlying immunological mechanism, by which CS intake effects systemic immunity, is involved in the oral tolerance system. Several mechanisms have been proposed for the development of oral tolerance, ranging from the deletion of antigen-specific T cells (49, 50) to immune deviation (51, 52), induction of anergy (53), CD8+ suppressor T cells (54), and suppression by Tregs. Recent studies suggest that various antigen-presenting cells, especially intestinal epithelial cells and various dendritic cells, and Tregs play a central role in oral tolerance (55). Five different Tregs have been identified in conjunction with intestinal immunity: Th3 cells, a population of CD4+ cells that secrete transforming growth factor-β; Tr1 cells, CD4+ cells that secrete IL-10; CD4+CD25+ T cells; CD8+ suppressor T cells; and γδ T cells of IELs. Early studies suggested that CD8+ suppressor T cells are important to oral tolerance (54). Many recent studies have focused on self-tolerance as maintained by CD4+CD25+ Tregs, which constitute 3–10% of peripheral CD4+ T cells in normal naïve mice (2, 55, 56). Oral antigen administration impairs the development of effector/memory Th2 cells and germinal centers, class switching to IgE (51, 57). In this study, we show that the CS intake appears to promote the induction of Tregs differentiation, such as CD4+CD25+ T cells, CD8+ T cells, and Th3 cells, which can secrete transforming growth factor-β in the spleen and MLN. Therefore, our findings suggest that the promotion of systemic differentiation of Tregs by CS intake could result in the inhibition of Th2 cell differentiation and specific-IgE production followed by the IgE-mediated allergic responses.
Improved hygienic conditions reducing early-life exposure to microbes have been associated with a heightened risk of allergic diseases. The composition of the microbial gut colonization early in life is especially crucial for healthy maturation of the naïve immune system (58). Some researchers have shown that probiotics provide a microbial stimulus by means of cultures of beneficial live microorganisms characteristic of the healthy infant gut (58). In addition, there are some studies showing that prebiotics, the preventive usage of oligosaccharides, including oligofructose and oligogalactose in allergy or inflammation disease, have demonstrated efficacy in animals and in adults. Commensal microflora contains a number of components such as peptidoglycans on the cell membrane that can activate innate and adaptive immunity. Unlimited immune activation in response to signals from commensal bacteria could pose the risk of inflammation and immune response to mucosal microflora, thus requiring precise regulatory control. The mucosal immune system has developed specialized regulatory, anti-inflammatory mechanisms for eliminating or tolerating non-dangerous food and airborne antigens and commensal micro-organisms (59). Furthermore, a series of pattern recognition receptors, toll-like receptors on gut lymphoid and epithelial cells that interact with bacterial molecular patterns (e.g. endotoxin (lipopolysaccharide), flagellin, etc.), help to modulate intestinal innate immunity and an appropriate adaptive immune response (60). The PP cells and IELs have been studied as a major inductive site for mucosal immunity within the small intestine.
In this study, we also show that CS intake affects the change in the population of PP cells and IELs in mice. CS intake up-regulated the differentiation of CD4+ T cells and CD8+ T cells in the PP cells and up-regulated the differentiation of CD4+ T cells and TCRαβ-expressed T cells, even though the population of TCRγδ-expressed T cells are unchanged in the IELs. Considering the previous findings and the present findings, CS might directly affect the surface of the PP cells and IELs, indirectly affect the PP cells and IELs by regulating the antigen-presenting functions of the intestinal epithelial cells and dendritic cells, or regulate the mucosal immune system to the systemic immune system. Many results support the concept that the dendritic cells are the inducers of Tregs differentiation under certain circumstances (54). Therefore, CS might regulate the maturation of dendritic cells through the mucosal immune system.
Matsuzaki et al. (61) reported that feeding the Lactobacillus casei strain Shirota to mice effectively inhibited IgE production in response to OVA in vitro. They also reported that the levels of cytokines produced by Th1 cells increased and that those of cytokines produced by Th2 cells decreased due to feeding the L. casei strain Shirota to mice (62). Shibata et al. (32) reported that the feeding of chitin, a polymer of N-acetyl-D-glucosamine, down-regulates serum IgE levels in mice through the induction of a Th1 response.
It should be noted that the effect of CS intake on mice immunized with OVA appears to be similar to those of feeding the L. casei strain Shirota or feeding chitin to mice immunized with OVA. We suggest that these studies are associated with the induction of Tregs in systemic immunity through the intestinal mucosal immunological system. The effect of CS intake after immunization is an important subject for potential therapeutic use in patients with allergy. Further studies are required to more fully elucidate the effects of CS intake on the immune system.
In conclusion, we show that CS intake has an anti-allergic effect, preventing or reducing immediate-type hypersensitivity by up-regulating Tregs differentiations followed by down-regulating the Th2 response. However, additional studies are necessary to show that CS can directly or indirectly stimulate the intestinal immunological system and host defenses. If this can be demonstrated, then the use of CS as a dietary supplement to stimulate a balanced and appropriately effective mucosal immune system will be justified.
Acknowledgments
We thank Dr. Y. Kikuchi and Dr. R. Teshima for teaching us the technique of splenocyte culture and Dr. Mamoru Totsuka and Dr. Kiyoshi Yamada for teaching us the technique of isolating IELs.
Footnotes
The work was supported in part by grants from the Japan Health Sciences Foundation, the Ministry of Health, Labour and Welfare of Japan, and a Grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The abbreviations used are: CS, chondroitin sulfate; OVA, ovalbumin; DNP, dinitrophenylated; IL, interleukin; IFN, interferon; IELs, intraepithelial lymphocytes; PP, Peyer’s patch; MLN, mesenteric lymph node; FBS, fetal bovine serum; PBS, phosphate-buffered saline; FCM, flow cytometry; FITC, fluorescein isothiocyanate; PE, phycoerythrin; Tregs, regulatory T cells.
References
- 1.Marks DR, Marks LM. Postgrad Med. 1993;93:191–196. doi: 10.1080/00325481.1993.11701608. [DOI] [PubMed] [Google Scholar]
- 2.Sampson HA. J Allergy Clin Immunol. 2004;113:805–819. doi: 10.1016/j.jaci.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal MK, Swanson MC, Reed CE, Yunginger JW. J Allergy Clin Immunol. 1984;74:687–693. doi: 10.1016/0091-6749(84)90231-8. [DOI] [PubMed] [Google Scholar]
- 4.Yunginger JW. Ann Allergy. 1992;69:87–96. [PubMed] [Google Scholar]
- 5.Matsuo N, Yamada K, Shoji K, Mori M, Sugano M. Allergy. 1997;52:58–64. doi: 10.1111/j.1398-9995.1997.tb02546.x. [DOI] [PubMed] [Google Scholar]
- 6.Kawabe H, Hayashi H, Hayashi O. Biochem Biophys Res Commun. 1987;143:467–474. doi: 10.1016/0006-291x(87)91377-5. [DOI] [PubMed] [Google Scholar]
- 7.Bochner BS, Lichtenstein LM. New Engl J Med. 1991;324:1785–1790. doi: 10.1056/NEJM199106203242506. [DOI] [PubMed] [Google Scholar]
- 8.Roden L. In: The Biochemistry of Glycoproteins and Proteoglycans. Lennarz WJ, editor. 1980. pp. 267–371. [Google Scholar]
- 9.Kimata K, Okayama M, Oohira A, Suzuki S. Mol Cell Biochem. 1973;1:211–228. doi: 10.1007/BF01659331. [DOI] [PubMed] [Google Scholar]
- 10.Toida T, Toyoda H, Imanari T. Anal Sci. 1993;9:53–58. [Google Scholar]
- 11.Scott JE. Pathol Biol (Paris) 2001;49:284–289. doi: 10.1016/s0369-8114(01)00152-3. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher JT, Lyon M, Steward WP. Biochem J. 1986;236:313–325. doi: 10.1042/bj2360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourin MC, Ohlin AK, Lane D, Stenflo J, Lindahl U. J Biol Chem. 1988;263:8044–8052. [PubMed] [Google Scholar]
- 14.Rovetta G. Drugs Exp Clin Res. 1991;17:53–57. [PubMed] [Google Scholar]
- 15.Kelly GS. Altern Med Rev. 1998;3:27–39. [PubMed] [Google Scholar]
- 16.Omata T, Segawa Y, Itokazu Y, Inoue N, Tanaka Y. Arzneim Forsch. 1999;49:577–581. doi: 10.1055/s-0031-1300465. [DOI] [PubMed] [Google Scholar]
- 17.Conrozier T. Presse Med. 1998;27:1862–1865. [PubMed] [Google Scholar]
- 18.Bourgeois P, Chales G, Dehais J, Delcambre B, Kuntz JL, Rozenberg S. Osteoarthr Cartil. 1998;6:25–30. doi: 10.1016/s1063-4584(98)80008-3. [DOI] [PubMed] [Google Scholar]
- 19.Beren J, Hill SL, Diener-West M, Rose NR. Exp Biol Med. 2001;226:144–151. doi: 10.1177/153537020122600213. [DOI] [PubMed] [Google Scholar]
- 20.Theoharides TC, Patra P, Boucher W, Letourneau R, Kempuraj D, Chiang G, Jeudy S, Hasse L, Athanasiou A. Br J Pharmacol. 2000;131:1039–1049. doi: 10.1038/sj.bjp.0703672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bali JP, Cousse H, Neuzil E. Semin Arthritis Rheum. 2001;31:58–68. doi: 10.1053/sarh.2000.24874. [DOI] [PubMed] [Google Scholar]
- 22.McCarty MF, Russell AL, Seed MP. Med Hypotheses. 2000;54:798–802. doi: 10.1054/mehy.1999.0954. [DOI] [PubMed] [Google Scholar]
- 23.Ishizeki K, Hiraki Y, Kubo M, Nawa T. Int J Dev Biol. 1997;41:83–89. [PubMed] [Google Scholar]
- 24.Conte A, Volpi N, Palmieri L, Bahous I, Ronca G. Arzneim Forsch. 1995;45:918–925. [PubMed] [Google Scholar]
- 25.Baici A, Horler D, Moser B, Hofer H, Fehr K, Wagenhauser F. Rheumatol Int. 1992;12:81–88. doi: 10.1007/BF00290259. [DOI] [PubMed] [Google Scholar]
- 26.Sakai S, Onose J, Nakamura N, Toyoda H, Toida T, Imanari I, Linhardt RJ. Anal Biochem. 2002;302:169–174. doi: 10.1006/abio.2001.5545. [DOI] [PubMed] [Google Scholar]
- 27.Wrenshall LE, Stevens RB, Cerra FB, Platt JL. J Leukocyte Biol. 1999;66:391–400. doi: 10.1002/jlb.66.3.391. [DOI] [PubMed] [Google Scholar]
- 28.Sakai S, Akiyama H, Harikai N, Toyoda H, Toida T, Maitani T, Imanari T. Immunol Lett. 2002;84:211–216. doi: 10.1016/s0165-2478(02)00181-5. [DOI] [PubMed] [Google Scholar]
- 29.Akiyama H, Sakai S, Linhardt RJ, Goda Y, Toida T, Maitani T. Biochem J. 2004;382:269–278. doi: 10.1042/BJ20031851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toyoda H, Shinomiya K, Yamanashi S, Koshiishi I, Imanari T. Anal Sci. 1988;4:381–384. [Google Scholar]
- 31.Toyoda H, Yamamoto H, Ogino N, Toida T, Imanari T. J Chromatgr A. 1999;830:197–201. [Google Scholar]
- 32.Shibata Y, Foster LA, Bradfield JF, Myrvik QN. J Immunol. 2000;164:1314–1321. doi: 10.4049/jimmunol.164.3.1314. [DOI] [PubMed] [Google Scholar]
- 33.Akiyama H, Hoshino K, Tokuzumi M, Teshima R, Mori H, Inakuma T, Ishiguro Y, Goda Y, Sawada J, Toyoda M. Biol Pharm Bull. 1999;22:551–555. doi: 10.1248/bpb.22.551. [DOI] [PubMed] [Google Scholar]
- 34.Sato Y, Akiyama H, Suganuma H, Watanabe T, Nagaoka MH, Inakuma T, Goda Y, Maitani T. Biol Pharm Bull. 2004;27:978–984. doi: 10.1248/bpb.27.978. [DOI] [PubMed] [Google Scholar]
- 35.Kawasaki M, Toyoda M, Teshima R, Sawada J, Saito Y. Biol Pharm Bull. 1994;17:1321–1325. doi: 10.1248/bpb.17.1321. [DOI] [PubMed] [Google Scholar]
- 36.Teshima R, Kawase M, Tanaka T, Hirai K, Sato M, Sawada J, Ikebuchi H, Ichinoe M, Terao T. J Agric Food Chem. 1990;38:1618–1622. [Google Scholar]
- 37.Nagafuchi S, Totsuka M, Hachimura S, Goto M, Takahashi T, Yajima T, Kuwata T, Kaminogawa S. Biosci Biotechnol Biochem. 2000;64:1459–1465. doi: 10.1271/bbb.64.1459. [DOI] [PubMed] [Google Scholar]
- 38.Akiyama H, Sato Y, Watanabe T, Nagaoka MH, Yoshioka Y, Shoji T, Kanda T, Yamada K, Totsuka M, Teshima R, Sawada J, Goda Y, Maitani T. FEBS Letters. 2005;579:4485–4491. doi: 10.1016/j.febslet.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 39.Okunuki H, Teshima R, Harikai N, Sakai S, Akiyama H, Maitani T, Sawada J. Biol Pharm Bull. 2003;26:1260–1265. doi: 10.1248/bpb.26.1260. [DOI] [PubMed] [Google Scholar]
- 40.Mosmann TR, Coffman RL. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 41.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. J Immunol. 1986;175:5–14. [PubMed] [Google Scholar]
- 42.Coffman RL, Carty J. J Immunol. 1986;136:949–954. [PubMed] [Google Scholar]
- 43.Swain SA, Weiberg AD, English M, Huston G. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 44.Mcknight AJ, Zimmer GJ, Fogelman I, Wolf SF, Abbas AK. J Immunol. 1994;152:2172–2179. [PubMed] [Google Scholar]
- 45.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Nat Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 46.Mowat AM. Immunol Today. 1987;8:93–98. doi: 10.1016/0167-5699(87)90853-X. [DOI] [PubMed] [Google Scholar]
- 47.Husby S, Mestecky J, Moldoveanu Z, Holland S, Elson CO. J Immunol. 1994;152:4663–4670. [PubMed] [Google Scholar]
- 48.Husby S. J Pediatr Gastroenterol Nutr. 2000;30:13–19. doi: 10.1097/00005176-200001001-00003. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Nature. 1995;376:177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 50.Gutgemann I, Fahrer AM, Altman JD, Davis MM, Chien YH. Immunity. 1998;8:667–673. doi: 10.1016/s1074-7613(00)80571-3. [DOI] [PubMed] [Google Scholar]
- 51.McMenamin C, McKersey M, Kuhnlein P, Hunig T, Holt PG. J Immunol. 1995;154:4390–4394. [PubMed] [Google Scholar]
- 52.Alpan O, Bachelder E, Isil E, Arnheiter H, Matzinger P. Nat Immunol. 2004;5:615–622. doi: 10.1038/ni1077. [DOI] [PubMed] [Google Scholar]
- 53.Van Houten N, Blake SF. J Immunol. 1996;157:1337–1341. [PubMed] [Google Scholar]
- 54.Mowat AM, Parker LA, Beacock-Sharp H, Millington OR, Chirdo F. Ann N Y Acad Sci. 2004;1029:1–8. doi: 10.1196/annals.1309.001. [DOI] [PubMed] [Google Scholar]
- 55.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. J Clin Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 57.Sugai M, Gonda H, Kusunoki T, Katakai T, Yokota Y, Shimizu A. Nat Immunol. 2003;4:25–30. doi: 10.1038/ni874. [DOI] [PubMed] [Google Scholar]
- 58.Kalliomaki M, Isolauri E. Curr Opin Allergy Clin Immunol. 2003;3:15–20. doi: 10.1097/00130832-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Tlaskalova-Hogenova H, Stepankova R, Hudcovic T, Tuckova L, Cukrowska B, Lodinova-Zadnikova R, Kozakova H, Rossmann P, Bartova J, Sokol D, Funda DP, Borovska D, Rehakova Z, Sinkora J, Hofman J, Drastich P, Kokesova A. Immunol Lett. 2004;93:97–108. doi: 10.1016/j.imlet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 60.Forchielli ML, Walker WA. Br J Nutr. 2005;93:41–48. doi: 10.1079/bjn20041356. [DOI] [PubMed] [Google Scholar]
- 61.Matsuzaki T, Yamazaki R, Hashimoto S, Yokokura T. J Dairy Sci. 1998;81:48–53. doi: 10.3168/jds.S0022-0302(98)75549-3. [DOI] [PubMed] [Google Scholar]
- 62.Shida K, Makino K, Morishita A, Takamizawa K, Hachimura S, Ametani A, Sato T, Kumagai Y, Habu S, Kaminogawa S. Int Arch Allergy Immunol. 1998;115:278–287. doi: 10.1159/000069458. [DOI] [PubMed] [Google Scholar]