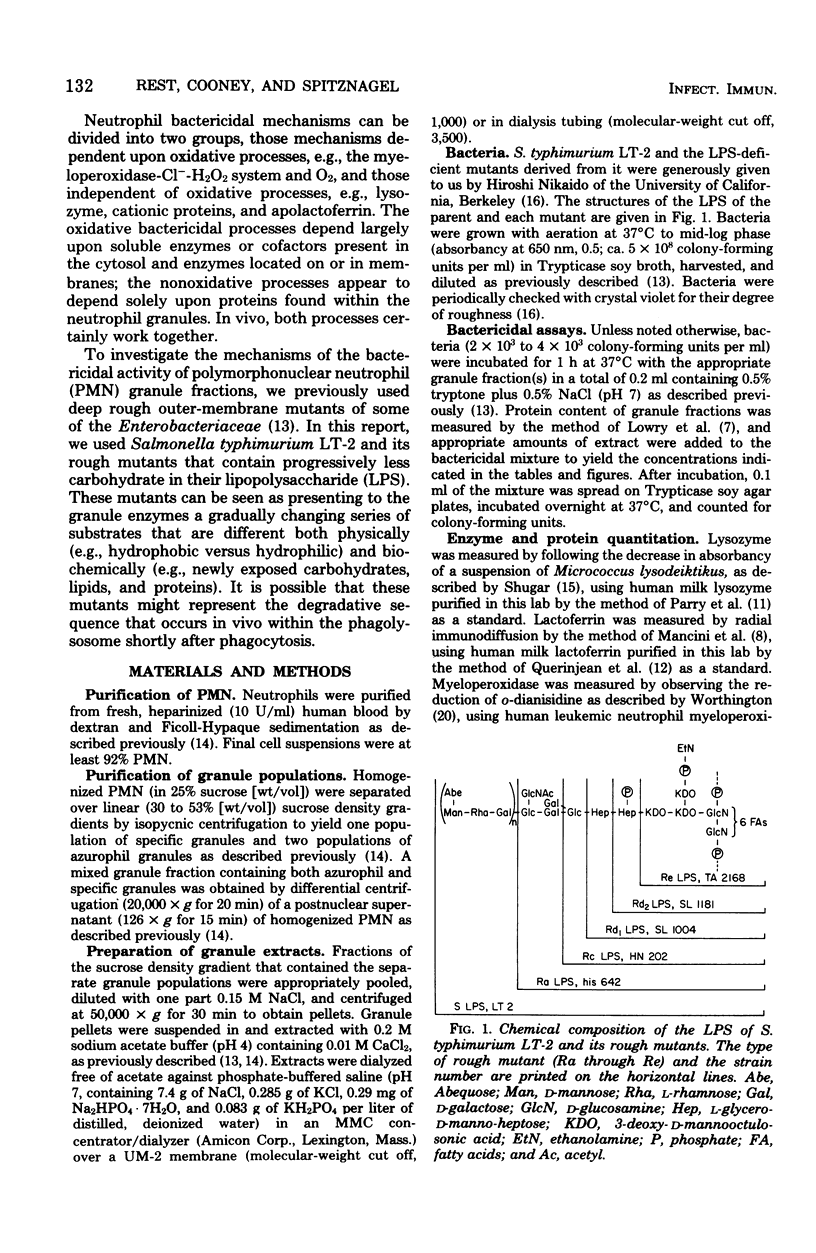
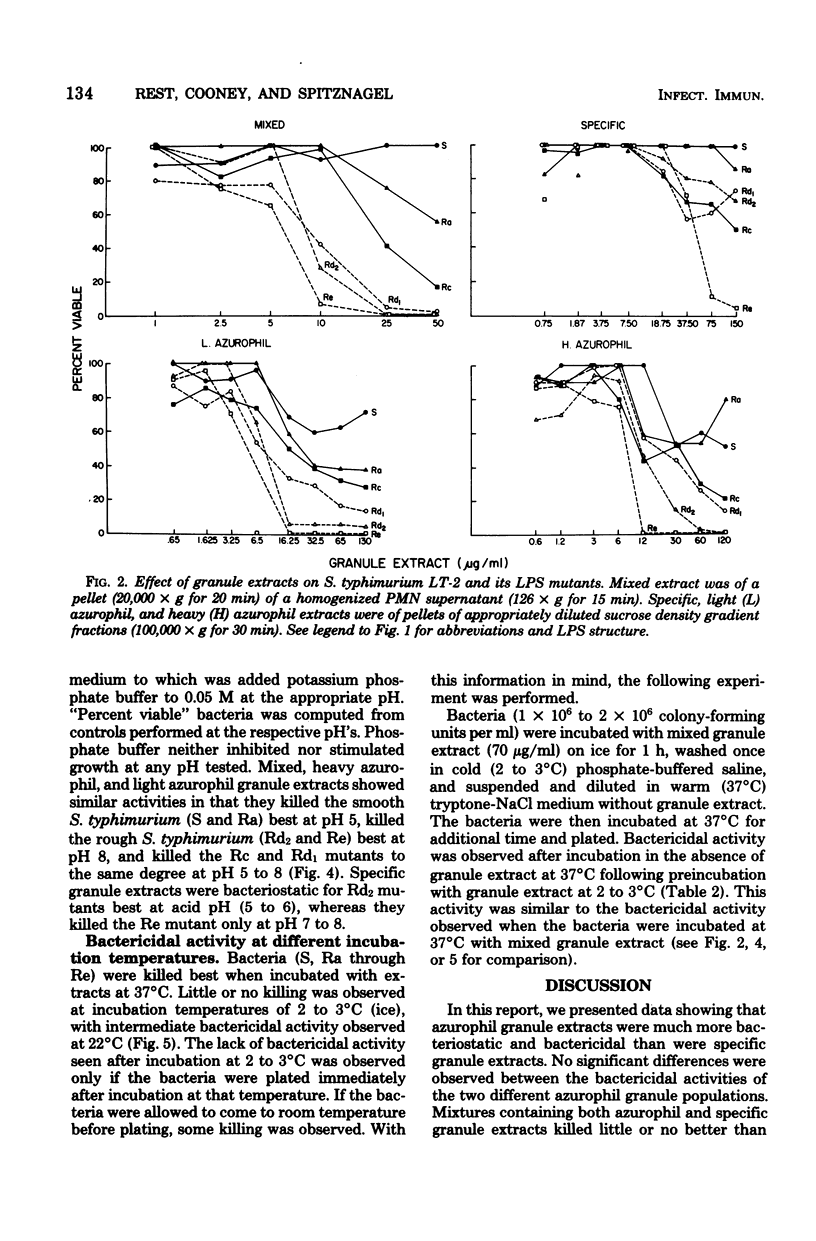
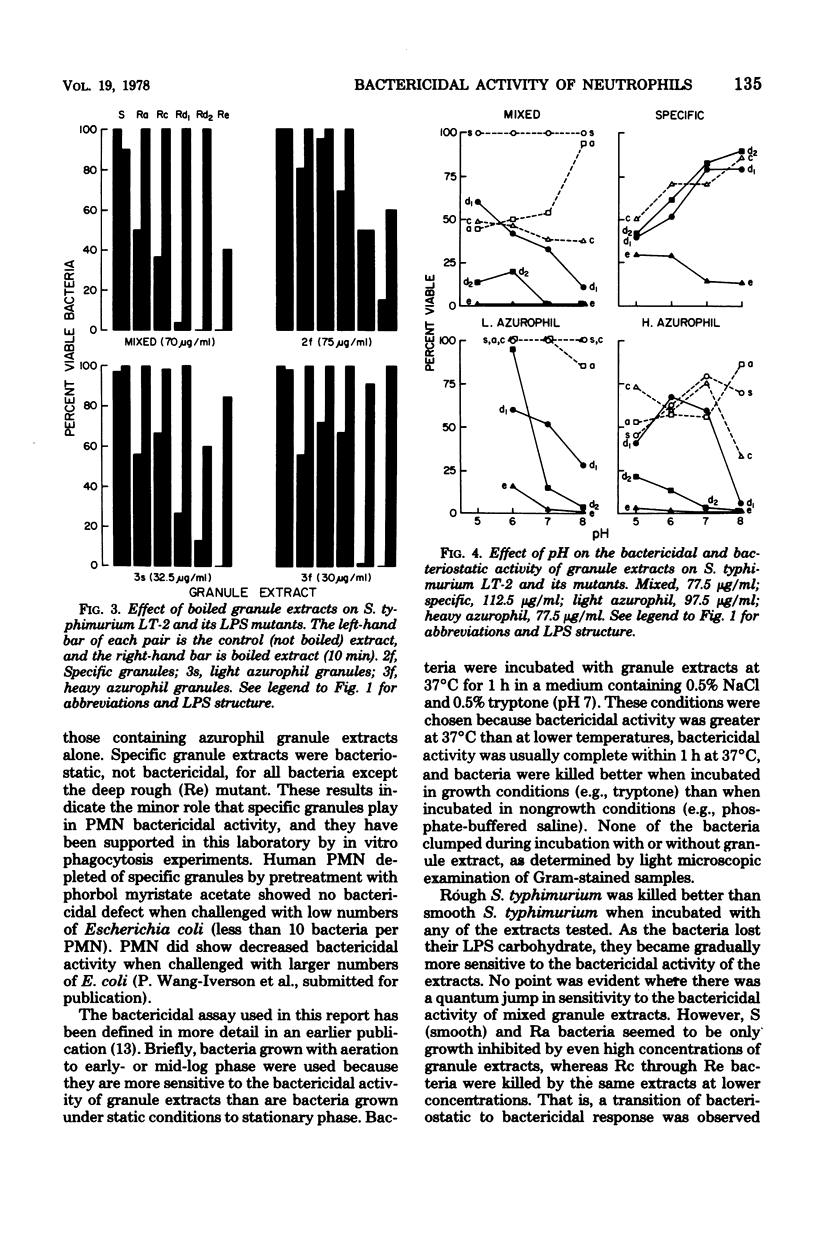
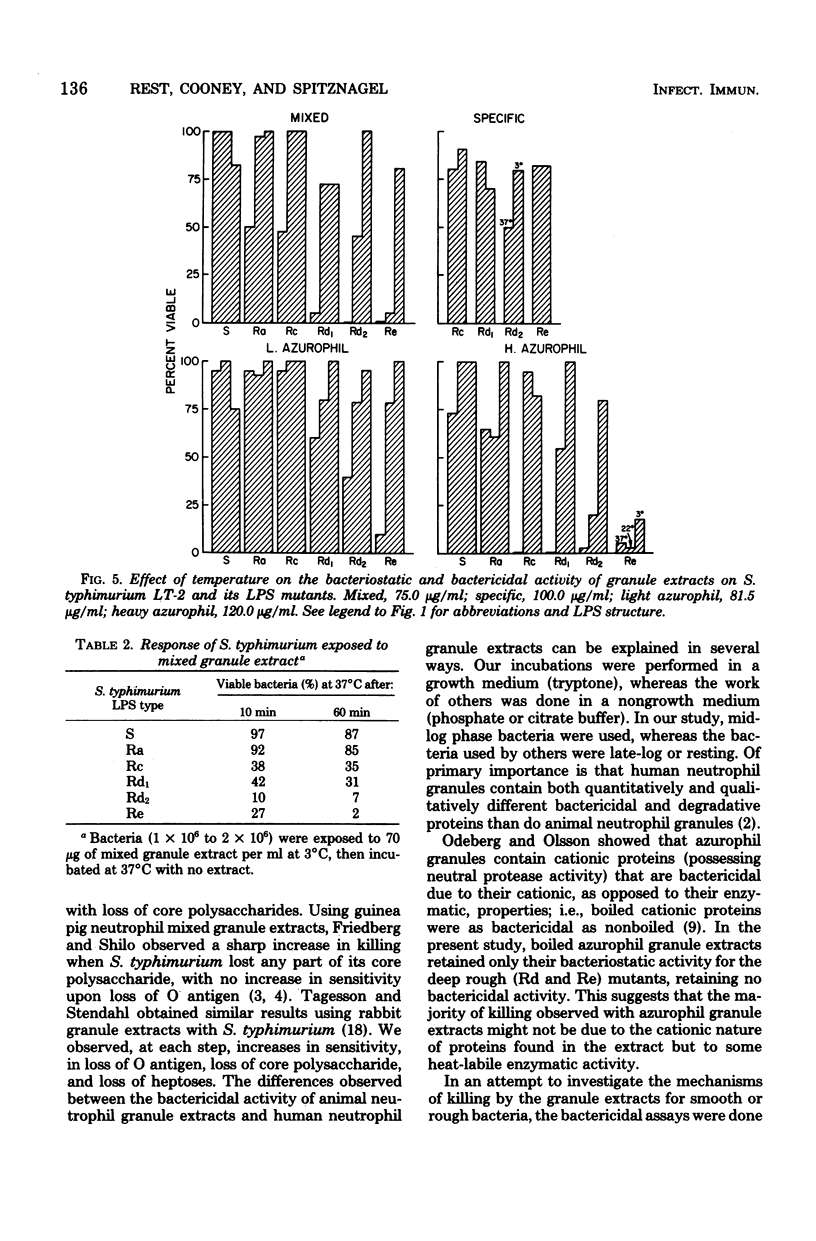
Abstract
Extracts of specific granules and azurophil granules from human neutrophils were tested for their bactericidal activity against various lipopolysaccharide mutants of Salmonella typhimurium LT-2. Three purified granule populations, one specific and two azurophil, were obtained by isopycnic centrifugation of homogenized neutrophils. Each was extracted with 0.2 M acetate buffer (pH 4), and the extracts were dialyzed against phosphate-buffered saline (pH 7) to remove acetate. These extracts contained ≥84% of the lysozyme, lactoferrin, or myeloperoxidase initially present in the whole granules. The S. typhimurium mutants possessed Ra, Rc, Rd1, Rd2, or Re lipopolysaccharide. As the carbohydrate content of the lipopolysaccharide decreased, the bacteria became increasingly more susceptible to the bactericidal activity of all granule extracts. Bactericidal activity of the extracts was in the order: mixed (azurophil + specific) ≥ azurophil » specific. Specific granules were bacteriostatic for S through Rd2 bacteria. They were bactericidal only for the Re mutant. Both azurophil granule populations were equally bactericidal. Extracts boiled for 30 min retained none of their bactericidal activity for any of the bacteria; however, they remained bacteriostatic for the deep rough (Rd2, Re) mutants. Bactericidal activity was dependent upon pH, in that mixed and azurophil granule contents killed the smooth parent and Ra mutant best at pH 5, the Rc and Rd1 mutants to the same degree at pH 5 to 8, and the deep rough mutants (Rd2 and Re) best at pH 8. Specific granule contents were most bacteriostatic for S through Rd2 bacteria at pH 5 and killed the Re mutant only at pH 8. Thus, as the S. typhimurium lipopolysaccharide content decreased, the bactericidal pH optimum increased. Killing by all extracts was dependent upon incubation temperature, with almost no bactericidal or bacteriostatic activity observed when bacteria and granule fractions were incubated on ice (2°C) and plated immediately. Intermediate killing was observed at 22°C. If bacteria were incubated with granule extracts at 2°C, washed free of extract, suspended in medium without extract, and reincubated at 37°C, killing was observed. This suggested that a component(s) of the extracts was sticking to the bacteria at 2°C but killing only at 37°C.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bretz U., Baggiolini M. Biochemical and morphological characterization of azurophil and specific granules of human neutrophilic polymorphonuclear leukocytes. J Cell Biol. 1974 Oct;63(1):251–269. doi: 10.1083/jcb.63.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune K., Spitznagel J. K. Peroxidaseless chicken leukocytes: isolation and characterization of antibacterial granules. J Infect Dis. 1973 Jan;127(1):84–94. doi: 10.1093/infdis/127.1.84. [DOI] [PubMed] [Google Scholar]
- Friedberg D., Friedberg I., Shilo M. Interaction of Gram-Negative Bacteria with the Lysosomal Fraction of Polymorphonuclear Leukocytes II. Changes in the Cell Envelope of Escherichia coli. Infect Immun. 1970 Mar;1(3):311–318. doi: 10.1128/iai.1.3.311-318.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg D., Shilo M. Interaction of Gram-Negative Bacteria with the Lysosomal Fraction of Polymorphonuclear Leukocytes I. Role of Cell Wall Composition of Salmonella typhimurium. Infect Immun. 1970 Mar;1(3):305–310. doi: 10.1128/iai.1.3.305-310.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane S. P., Peters T. J. Analytical subcellular fractionation of human granulocytes with reference to the localization of vitamin B12-binding proteins. Clin Sci Mol Med. 1975 Aug;49(2):171–182. doi: 10.1042/cs0490171. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leffell M. S., Spitznagel J. K. Association of lactoferrin with lysozyme in granules of human polymorphonuclear leukocytes. Infect Immun. 1972 Nov;6(5):761–765. doi: 10.1128/iai.6.5.761-765.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Antibacterial activity of cationic proteins from human granulocytes. J Clin Invest. 1975 Nov;56(5):1118–1124. doi: 10.1172/JCI108186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I., Olofsson T., Odeberg H. Myeloperoxidase-mediated iodination in granulocytes. Scand J Haematol. 1972;9(5):483–491. doi: 10.1111/j.1600-0609.1972.tb00974.x. [DOI] [PubMed] [Google Scholar]
- Parry R. M., Jr, Chandan R. C., Shahani K. M. Isolation and characterization of human milk lysozyme. Arch Biochem Biophys. 1969 Mar;130(1):59–65. doi: 10.1016/0003-9861(69)90009-5. [DOI] [PubMed] [Google Scholar]
- Querinjean P., Masson P. L., Heremans J. F. Molecular weight, single-chain structure and amino acid composition of human lactoferrin. Eur J Biochem. 1971 Jun 11;20(3):420–425. doi: 10.1111/j.1432-1033.1971.tb01408.x. [DOI] [PubMed] [Google Scholar]
- Rest R. F., Cooney M. H., Spitznagel J. K. Susceptibility of lipopolysaccharide mutants to the bactericidal action of human neutrophil lysosomal fractions. Infect Immun. 1977 Apr;16(1):145–151. doi: 10.1128/iai.16.1.145-151.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Spitznagel J. K. Subcellular distribution of superoxide dismutases in human neutrophils. Influence of myeloperoxidase on the measurement of superoxide dismutase activity. Biochem J. 1977 Aug 15;166(2):145–153. doi: 10.1042/bj1660145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHUGAR D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim Biophys Acta. 1952 Mar;8(3):302–309. doi: 10.1016/0006-3002(52)90045-0. [DOI] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Dalldorf F. G., Leffell M. S., Folds J. D., Welsh I. R., Cooney M. H., Martin L. E. Character of azurophil and specific granules purified from human polymorphonuclear leukocytes. Lab Invest. 1974 Jun;30(6):774–785. [PubMed] [Google Scholar]
- Tagesson C., Stendahl O. Influence of the cell surface lipopolysaccharide structure of Salmonella typhimurium on resistance to intracellular bactericidal systems. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Aug;81(4):473–480. doi: 10.1111/j.1699-0463.1973.tb02232.x. [DOI] [PubMed] [Google Scholar]
- West B. C., Rosenthal A. S., Gelb N. A., Kimball H. R. Separation and characterization of human neutrophil granules. Am J Pathol. 1974 Oct;77(1):41–66. [PMC free article] [PubMed] [Google Scholar]


