Abstract
Objectives
To compare clinical outcomes and mortality rates between Kimberley Indigenous, other Indigenous and non-Indigenous Australian patients on peritoneal dialysis (PD).
Design and participants
Patients commencing renal replacement therapy (RRT) with PD for the first time from 1 January 2003 to 31 December 2009 were retrospectively identified. Secondary data from medical records and the Australian and New Zealand Dialysis and Transplant Registry from 1 January 2003 to 31 December 2010 were used to compare outcomes between patients.
Main outcome measures
Time to first peritonitis; failure and death rates per 100 patient-years, hazard ratios, unadjusted and adjusted (for age, sex, comorbid conditions, PD not the first RRT modality used). Comparison of the two PD systems used in the Kimberley.
Results
Kimberley patients had significantly shorter median time to first peritonitis (11.2 versus 21.5 months), higher technique failure (46.0 versus 25.2 per 100 patient-years) and shorter median survival on PD (17.5 versus 22.4 months) but similar adjusted mortality (hazard ratio 1.32; 95% CI, 0.76-2.29) as non-Indigenous patients. They also had a significantly higher technique failure rate than other Indigenous patients (46.0 versus 31.4 per 100 patient-years) and nearly double the average peritonitis episodes previously reported for Indigenous Australians (2.0 versus 1.15 per patient-year).
Conclusions
PD can bring patients closer to home; however, it is relatively short term and potentially hazardous. PD remains an important therapy for suitable remote patients to get closer to home, providing they are fully informed of the options. The current expansion of safer Kimberley haemodialysis options needs to continue.
Keywords: Aboriginal, Indigenous, mortality rate, peritoneal dialysis, peritonitis, Torres Strait Islander
Introduction
In Australia, kidney failure occurs at extremely high rates among Aboriginal and Torres Strait Islander peoples (Indigenous Australians) in remote areas with rates up to 30 times the national average.1 Peritoneal dialysis (PD) has long been an important option for people from remote areas who are on renal replacement therapy (RRT) and wish to return to their home to live and dialyse.
In contrast to international studies,2–6 recent Australian reports have indicated that patient survival on PD appears to not be as good as patients receiving haemodialysis (HD) therapy,7,8 and outcomes are generally worse when compared with PD patients in other countries.9,10 Peritonitis continues to be a leading cause of technique failure and death for Australian PD patients.8,9,11 From 2002 to 2005, Australia's peritonitis rate of an episode every 19.4 patient months was only slightly better than the minimum recommendation of an episode every 18.0 patient months.9,12
A recent international review concluded that mortality, technique failure and peritonitis rates were generally higher among Indigenous PD patients compared with non-Indigenous patients.13 Locality is also important. Remote Indigenous PD patients have significantly shorter times to first peritonitis, higher technique failureand greater risk of all-cause mortality compared with metropolitan non-Indigenous patients in Australia.14
What is already known on this subject:
There is an epidemic of end-stage kidney disease among Indigenous Australians in remote areas of Australia, and peritoneal dialysis (PD) is an important option for people to return home to live and dialyse.
In Australia, peritonitis is the leading cause of technique failure and death, with Indigenous Australian PD patients having a shorter time to first peritonitis and on average nearly twice the number of episodes of peritonitis per year than non-Indigenous patients. Indigenous Australian PD patients from remote locations have a higher risk of peritonitis than metropolitan non-Indigenous patients.
Patient and technique survival is inherently biased by initial training, ongoing supervision, retraining and regular contact to detect problems early and avoid later more serious complications
At least 70% of patients originating from the remote Kimberley region received PD early in their treatment.15 Anecdotal evidence suggested that peritonitis and technique failure were common, and duration of PD was limited. Two PD systems have been used: UltraBag (Baxter, Old Toongabbie, NSW, Australia) until 2007 and Stay-Safe (Fresenius Medical Care, Milsons Point, NSW, Australia) starting in 2006. PD patient management and training also changed during 2007 from Royal Perth Hospital (RPH) with training in Perth, to Fresenius Medical Care with training either in Broome at the Kimberly Aboriginal Medical Services Council, or one of two training centres in suburban Perth. It had been suggested locally that increased peritonitis rates occurred following the change in systems. This paper therefore describes outcomes for Kimberley Indigenous Australians receiving PD treatment for the first time comparing them with other Australian PD patients, as well as comparing outcomes in Kimberley patients using the two systems.
Methods
Since 2003, it has been possible to identify and obtain accurate information on all Kimberley patients due to improved local renal services. Kimberley patients were identified based on extensive search and cross-referencing of records, including the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA).15 Patients were retrospectively identified for inclusion in the clinical outcomes and mortality analyses (Fig. 1).
FIGURE 1.
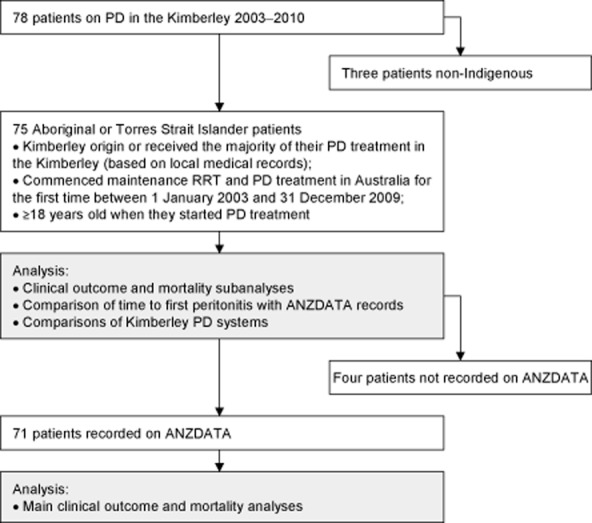
Selection process used to determine the Kimberley study populations and the analyses they were included in.
What this study adds:
Indigenous Australian PD patients from the Kimberley region had significantly higher recorded rates of technique failure than patients from elsewhere in Australia.
For Kimberley patients, there was a significant under-reporting of episodes of peritonitis on The Australia and New Zealand Dialysis and Transplant Registry, and they appear to have nearly double the episodes of peritonitis per patient year than previously reported for Indigenous Australian patients.
PD can bring Kimberley patients closer to home, but is relatively short term (median 17-22 months survival across Australia) and can have high levels of morbidity in remote areas. As a result of this study, there has been more caution in recommending PD and concurrently more support for geographically expanded and safer haemodialysis options in the Kimberley.
Using only ANZDATA records, we compared outcomes for Kimberley Indigenous PD patients to Indigenous Australian and non-Indigenous patients (≥18 years old) from elsewhere in Australia who had commenced maintenance RRT and PD treatment in Australia for the first time between 1 January 2003 and 31 December 2009. These patients were followed-up until 31 December 2010.
Collection of peritonitis data for Kimberley patients involved an extensive search of local patient, PathWest laboratory, RPH and ANZDATA records. Access to paper records for patients on UltraBag was limited because many patients with peritonitis were managed in Perth during their episodes. The records for some patients who had ceased PD were archived and not possible to access.
The extensive search of patient records identified a further four Kimberley patients not recorded as receiving PD on ANZDATA. To obtain a more accurate time to first peritonitis and to assess potential reporting discrepancies in ANZDATA, we compared peritonitis rates after adding these extra four patients and extra peritonitis information collected on all Kimberley patients. Using these data, we also compared Kimberley patients using UltraBag and those using Stay-Safe. Seven patients who started on UltraBag changed to Stay-Safe during 2006–2007, and for this comparison those patients were censored when they stopped using UltraBag.
Clinical outcomes
ANZDATA carries out regular surveys of patients on RRT. Comorbid conditions (diabetes, chronic lung disease, coronary artery disease, peripheral vascular disease, cerebrovascular disease) that were reported to ANZDATA were used to determine comorbid conditions at the start of treatment. Aboriginal or Torres Strait Islander identification was based on ANZDATA records.
We defined technique failure as cessation of PD due to all causes except death, transplantation and recovery of own renal function, consistent with ANZDATA reporting. PD failure and mortality rates are expressed as failures or deaths per 100 patient-years of PD, respectively.
Statistical analysis
Differences in baseline characteristics were compared with Kimberley Indigenous patients using χ2 test for categorical data, and Mann–Whitney tests for continuous data. Patients were censored for transplantation and recovery of own renal function. To determine median survival on PD patients were also censored for continuing on PD at the end of the study.
Cox proportional hazards ratios (HR) were determined for time to first peritonitis. There was no time group interaction for time to first peritonitis.
As the proportional hazards assumption was violated when assessing technique failure, log-rank test for equality of survivor functions was used to determine significance. Technique failure was also censored for death.
Poisson regression using marginal means was used to adjust death rates per 100 patient-years and Cox proportional hazards to adjust mortality for: age at start of PD, sex, presence of comorbid conditions and PD not the first RRT modality used.
All analyses were performed using Stata, version 13 (StataCorp, College Station, Texas, USA). Point estimates were presented with 95% CIs; P < 0.05 was considered statistically significant.
Ethics approval
Ethics approval was obtained from the Human Research Ethics Committee of The University of Western Australia and the Western Australian Aboriginal Health Information and Ethics Committee.
Results
A snapshot of the modality and location of Kimberley Indigenous patients receiving RRT is shown in Table 1. The demographic and baseline data for Australian patients recorded on ANZDATA are shown in Table 2.
TABLE 1.
Annual snapshot (at 31 December each year) of modality/location of Kimberley Indigenous RRT patients
| Modality/Location | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 |
|---|---|---|---|---|---|---|---|---|
| Home therapies | ||||||||
| Peritoneal dialysis | 23 | 19 | 19 | 16 | 17 | 24 | 18 | 10 |
| Home haemodialysis | 5 | 4 | 5 | 6 | 5 | 3 | 6 | 5 |
| Transplantation | 6 | 5 | 5 | 5 | 6 | 9 | 10 | 13 |
| Haemodialysis (HD) | ||||||||
| Kimberley† | 33 | 37 | 39 | 43 | 44 | 43 | 42 | 45 |
| non-Kimberley‡ | 11 | 20 | 17 | 23 | 29 | 33 | 33 | 34 |
| Total | 78 | 85 | 85 | 93 | 101 | 112 | 109 | 107 |
HD treatment in the Kimberley is provided by the Kimberley Satellite Dialysis Centre (10 chairs, up to 41 patients) in Broome and Derby Aboriginal Health Service (two chairs, up to eight patients) in Derby;
HD treatment outside the Kimberley is mainly provided in Perth, and 25 patients were on waiting lists to have therapy provided in the Kimberley at 31 December 2010. RRT, Renal replacement Therapy.
TABLE 2.
Demographic characteristics and baseline data of Australian patients ≥ 18 years old who commenced PD and RRT for the first time from 1 January 2003 to 31 December 2009
| Indigenous Australians | |||
|---|---|---|---|
| Characteristics | Kimberley | Rest of Australia | Non-Indigenous |
| No. of patients | 71 | 384 | 5285 |
| Median age at start of first PD (IQR) | 52.4 (44.8-61.7) | 53.1 (45.6-61.4) | 63.8 (51.1-73.1)* |
| Female (%) | 49 (69.0%) | 204 (53.1%)* | 2195 (41.5%)* |
| PD not first RRT modality used (%) | 17 (23.9%) | 186 (48.4%)* | 1834 (34.7%) |
| Comorbid conditions | |||
| Diabetes (%) | 56 (78.9%) | 300 (78.1%) | 2050 (38.8%)* |
| Chronic lung disease (%) | 11 (15.5%) | 72 (18.8%) | 795 (15.0%) |
| Coronary heart disease (%) | 30 (42.3%) | 189 (49.2%) | 1979 (37.5%) |
| Peripheral vascular disease (%) | 25 (35.2%) | 127 (33.1%) | 1244 (23.5%)* |
| Cerebrovascular disease (%) | 7 (9.9%) | 62 (16.2%) | 766 (14.5%) |
PD = peritoneal dialysis. RRT = Renal replacement Therapy. IQR = Interquartile range.
Significant at P < 0.05 compared with the group of Kimberley Indigenous patients.
Kaplan–Meier survival estimates for time to first peritonitis as recorded on ANZDATA are shown in Figure 2a. As shown in Table 3, both groups of Indigenous PD patients had significantly shorter times to first peritonitis and median survival, and higher technique failure than non-Indigenous patients. While Indigenous patients from the Kimberley and elsewhere had similar time to first peritonitis, patients from the Kimberley had significantly higher technique failure than those from elsewhere in Australia (Table 3).
FIGURE 2.
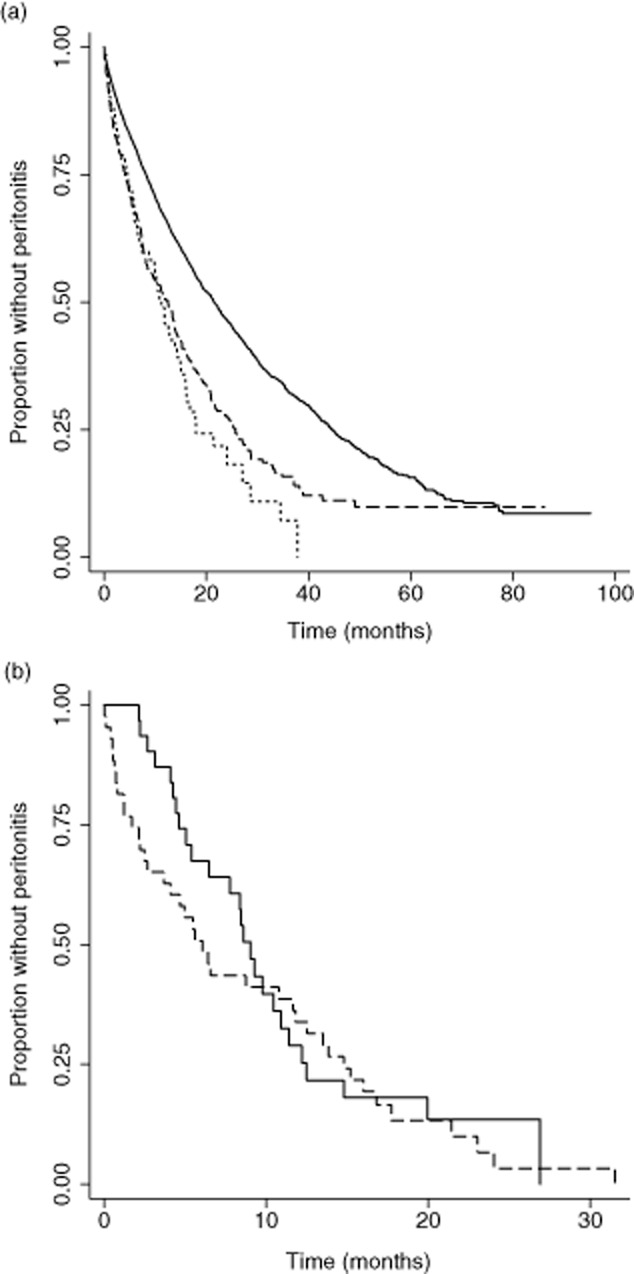
Kaplan–Meier survival estimates to first peritonitis during first peritoneal dialysis (PD) treatment. (a) Kimberley Indigenous patients and Indigenous and non-Indigenous patients from elsewhere in Australia (*P < 0.001 compared with the group of non-Indigenous Australian patients) ( ) non-Indigenous (n = 5285); (
) non-Indigenous (n = 5285); ( ) Indigenous – rest of Australia (n = 384)*; (
) Indigenous – rest of Australia (n = 384)*; ( ) Indigenous – Kimeberley (n = 71)*; (b) Kimberley Indigenous patients on the two PD systems used in Kimberley. Patients were ≥18 years old, commenced PD for the first time in Australia from 1 January 2003 to 31 December 2009 and followed up until 31 December 2010. (
) Indigenous – Kimeberley (n = 71)*; (b) Kimberley Indigenous patients on the two PD systems used in Kimberley. Patients were ≥18 years old, commenced PD for the first time in Australia from 1 January 2003 to 31 December 2009 and followed up until 31 December 2010. ( ) Baxter UltraBag (n = 32); (
) Baxter UltraBag (n = 32); ( ) Fresenius Medicare Stay–Safe (n = 43).
) Fresenius Medicare Stay–Safe (n = 43).
TABLE 3.
Clinical outcomes during first PD treatment of patients ≥18 years old who commenced PD and RRT for the first time in Australia from 1 January 2003 to 31 December 2009. Patients were followed up until 31 December 2010
| Indigenous Australians | |||
|---|---|---|---|
| Characteristics | Kimberley | Rest of Australia | Non-Indigenous |
| Patient-years | 113.1 | 645.7 | 9182.4 |
| Median time on PD during first PD treatment in months (IQR) | 17.3 (10.0-26.8) | 17.5 (7.4-29.1) | 16.9 (7.6-29.3) |
| Median survival on PD during first PD treatment in months (IQR)# | 17.5 (10.0-29.3)* | 19.8 (7.9-33.6)* | 22.5 (9.1-44.5) |
| Median time (months) to first peritonitis (IQR) | 11.2 (4.5-18.0)* | 12.5 (4.1-25.7)* | 21.5 (8.2-44.6) |
| Peritonitis hazard ratio (95% CI) | |||
| Crude | 2.0 (1.6-2.7)* | 1.7 (1.5-1.9)* | 1.00 |
| Adjusted† | 2.0 (1.5-2.6)* | 1.6 (1.4-1.8)* | 1.00 |
| Technique failure (95% CI) rate per 100 patient-years‡ | 46.0 (35.0-60.3)* | 31.3 (27.3-35.9)** | 25.2 (24.2-26.2) |
| Mortality (95% CI) | |||
| Crude | |||
| Death rate per 100 patient-years | 11.5 (6.7-19.8) | 16.3 (13.4-19.7)** | 12.8 (12.1-13.6) |
| Hazard ratio | 0.96 (0.56-1.66) | 1.28 (1.05-1.57)** | 1.00 |
| Adjusted† | |||
| Death rate per 100 patient-years§ | 16.0 (8.0-24.0) | 19.4 (15.8-23.1)* | 13.5 (12.7-14.2) |
| Hazard ratio¶ | 1.31 (0.76-2.29) | 1.49 (1.20-1.84)* | 1.00 |
Significant at P < 0.001 and
significant at P < 0.05 compared with the group of non-Indigenous Australian patients.
Censored for continuing on PD at the end of the study, transplantation and recovery of own renal function.
In the final Cox proportional hazards model the characteristics significantly (at P ≤ 0.05) associated with earlier time of first peritonitis are diabetes (HR, 1.13 (95% CI, 1.05-1.22)); and cardiovascular disease (HR, 1.12 (95% CI, 1.01-1.24)) and PD not the first RRT modality used (HR, 1.17 (95% CI, 1.09-1.27)).
Censored for death, transplantation and recovery of own renal function. Log-rank test for equality of survivor functions was used to determine significance.
Means for the covariates for the Poisson regression of adjusted death rate: age 60.4 years; 57.4% male; 41.9% with diabetes; 15.3% with chronic lung disease; 38.3% with coronary heart disease; 24.3% with peripheral vascular disease; 14.5% with cerebrovascular disease; and 35.5% PD not the first RRT modality used.
In the final Cox proportional hazards model the characteristics significantly (at P ≤ 0.05) associated with mortality are age (HR, 1.04 (95% CI, 1.04-1.05); female (HR, 0.86 (95% CI, 0.77-0.97)); diabetes (HR, 1.37 (95% CI, 1.22-1.54)); chronic lung disease (HR, 1.33 (95% CI, 1.17-1.52)); coronary heart disease (HR, 1.43 (95% CI, 1.26-1.62)); peripheral vascular disease (HR, 1.50 (95% CI, 1.32-1.70)); cerebrovascular disease (HR, 1.17 (95% CI, 1.02-1.34)); and PD not the first RRT modality used (HR, 1.53 (95% CI, 1.37-1.71)). CI, confidence interval; HR, Hazard ratio; IQR, interquartile range; PD, peritoneal dialysis; RRT, renal replacement therapy.
Based on ANZDATA records adjusted mortality rates and hazard ratios of Indigenous PD patients located elsewhere were significantly worse than non-Indigenous patients; however, there was no significant difference between Kimberley patients and non-Indigenous patients (Table 3). Adding the extra four Kimberley patients to the ANZDATA information did not alter this result.
We then assessed whether the high peritonitis and technique failure rate of Kimberley patients was associated with the change in PD systems. Thirty-two and 43 patients contributed 48.1 and 57.0 patient-years on UltraBag and Stay-Safe, respectively. Kaplan–Meier survival estimates to first peritonitis are shown in Figure 2b. The median time to first peritonitis was 9.0 (IQR 4.6–12.5) and 6.1 (IQR 1.7–14.8) months for patients on UltraBag and Stay-Safe, respectively. Overall, there was no significant difference in risk (HR 1.2 (95% CI, 0.72-1.95); P = 0.508), with an average of 2.0 episodes of peritonitis per patient year. One patient had nine episodes over 15 months.
Based on our audit of files in addition to ANZDATA information, we found that 13 (9 UltraBag, 4 Stay-Safe) of the 67 (19.4%) first episodes of peritonitis were not recorded in ANZDATA. Other discrepancies included 11 (three UltraBag, eight Stay-Safe) later episodes that were recorded in ANZDATA as first episodes, and six (one UltraBag, five Stay-Safe) first episodes recorded in ANZDATA did not match episodes recorded in patient notes. After adding the extra information on peritonitis, the median time to first peritonitis for Kimberley patients was reduced to 8.4 (IQR 3.1–13.8) from 11.2 (IQR 4.5–18.0) months.
Seven patients were switched from UltraBag to Stay-Safe. Five (71%) patients had peritonitis within 45 days of changing from UltraBag to Stay-Safe, five failed within nine months due to peritonitis, and three died within 11 months of the change while on PD.
Discussion
Kimberley Indigenous patients had significantly higher recorded rates of technique failure and shorter time to first peritonitis than non-Indigenous PD patients. There also appear to have been more episodes of peritonitis per patient year than previousl reported for Indigenous patients (2.0 versus 1.15).16 For Kimberley patients, there was also significant under-reporting of episodes of peritonitis on ANZDATA. Whether similar under-reporting occurs elsewhere in Australia is unknown.
Despite more than three times the number of episodes of peritonitis than previously reported for Australian PD patients (2.0 versus 0.6),17 median survival on PD for Kimberley patients was only five months shorter than non-Indigenous patients (17.5 versus 22.4 months). This was much lower than Canadian patients (Aboriginal: 40.1, non-Aboriginal: 45.5 months), although this study did not take into account changes in modality.18 Consistent with previous reports, adjusted mortality rates for Indigenous PD patients from elsewhere in Australia were also significantly higher than non-Indigenous patients.13,14,16 The mortality rates for Kimberley patients fell between these two groups.
There are a number of factors that impact on patient outcomes on PD, including patient selection, prophylaxis and timely treatment of infectious complications, social factors, patient education and support and clinical governance and professional standards.9 These are likely to be particularly relevant for those living in remote areas such as the Kimberley region, where reduced access to resources, health literacy discrepancies relevant to PD education and training, access to medical and nursing care, high turnover of staff, adherence to treatment protocols as well as socioeconomic disadvantage, are likely to play a role.13
In this population, there were few patients who, from a clinical perspective, had good outcomes on PD. However, patients may have a different view. For some a year at home on PD, even with episodes of peritonitis, may be preferred to having HD with less morbidity in Perth. A few patients appeared to do very well on PD. Unfortunately, we could not find reliable predictors to identify patients likely to have better outcomes on PD.
While patients using the Stay-Safe system did tend to have earlier first episodes of peritonitis, there were no statistical difference overall between the two systems. In addition despite our best efforts, there could have been greater undercounting of peritonitis in early (Ultrabag) as against later years of the study (Stay-Safe) due to more difficulty accessing medical records as described in the methods. More undercounting on the ‘Ultrabag’ system than on the Stay-Safe system may explain some of the non-statistically significant difference between the systems.
Despite no clear difference between systems overall, for the seven patients who changed system, three deaths and a trend to more and earlier post-conversion peritonitis episodes is concerning. We recommend extreme caution in the future before changing a patient from one PD system to another in this population.
Kimberley PD patients had higher adjusted death rates per 100 patient-years (16.0, 95% CI; 8.0–24.0) compared with an earlier study (2003–2007) of HD patients (10.2, 95% CI; 6.6–15.7).15 While these differences are not statistically significant, and these were different studies over different time periods, it raises concerns that mortality may be worse on PD than HD for Kimberley patients, as is seen in general in Australia (HR 1.10; 95% CI:1.06–1.16).7
Peritoneal dialysis continues to be a useful therapy; however, the demonstrated significantly increased morbidity, and a potential increase in mortality highlights some of the risks. At the time of the study, Kimberley patients accounted for 14.8% of the Indigenous PD person-years in Australia (see Table 3), while in the HD study, the Kimberley patients only accounted for 8.1% of Indigenous person-years on HD.15 This demonstrates very high levels of use of PD relative to HD for Kimberley patients at the time, due presumably to limited alternatives for patients on RRT to return closer to home. As a result of the experience reported in this study, there has been more caution in recommending PD in this population. Fortunately other options are expanding, with satellite HD now available in four Kimberley towns, and ‘home’ HD is provided effectively in secure clinic rooms in several locations.
PD remains an important therapy for suitable remote patients to get closer to home if they are fully informed of the risks. PD can bring Kimberley patients closer to home; however in Australia, it is relatively short term for most patients and potentially hazardous. Further work to improve the delivery of PD in the Kimberley and Australia more generally is required if use of this modality is to be expanded. The current and future expansion of safer haemodialysis options closer to home in the Kimberley is required to ensure long-term culturally safe care can be provided to all people who require RRT.
Acknowledgments
We thank the staff at the Kimberley Aboriginal Medical Services Council, Kimberley Renal Support Service, Western Australian Country Health Service and the Royal Perth Hospital for providing data for this study. We thank Professor Max Bulsara and Associate Professor Sharon Evans for the expert statistical advice.
References
- 1.Cass A, Cunningham J, Wang Z, Hoy W. Regional variation in the incidence of end-stage renal disease in Indigenous Australians. The Medical Journal of Australia. 2001;175:24–27. doi: 10.5694/j.1326-5377.2001.tb143507.x. [DOI] [PubMed] [Google Scholar]
- 2.Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Archives of Internal Medicine. 2011;171:110–118. doi: 10.1001/archinternmed.2010.352. [DOI] [PubMed] [Google Scholar]
- 3.Murphy SW, Foley RN, Barrett BJ, et al. Comparative mortality of hemodialysis and peritoneal dialysis in Canada. Kidney International. 2000;57:1720–1726. doi: 10.1046/j.1523-1755.2000.00017.x. [DOI] [PubMed] [Google Scholar]
- 4.Serkes KD, Blagg CR, Nolph KD, Vonesh EF, Shapiro F. Comparison of patient and technique survival in continuous ambulatory peritoneal dialysis (CAPD) and hemodialysis: a multicenter study. Peritoneal Dialysis International: Journal of the International Society for Peritoneal Dialysis. 1990;10:15–19. [PubMed] [Google Scholar]
- 5.Vonesh EF, Moran J. Mortality in end-stage renal disease: a reassessment of differences between patients treated with hemodialysis and peritoneal dialysis. Journal of the American Society of Nephrology. 1999;10:354–365. doi: 10.1681/ASN.V102354. [DOI] [PubMed] [Google Scholar]
- 6.Williams VR, Quinn R, Callery S, Kiss A, Oliver MJ. The impact of treatment modality on infection-related hospitalization rates in peritoneal dialysis and hemodialysis patients. Peritoneal Dialysis International: Journal of the International Society for Peritoneal Dialysis. 2011;31:440–449. doi: 10.3747/pdi.2009.00224. [DOI] [PubMed] [Google Scholar]
- 7.Marshall MR, Hawley CM, Kerr PG, et al. Home hemodialysis and mortality risk in Australian and New Zealand populations. American Journal of Kidney Diseases. 2011;58:782–793. doi: 10.1053/j.ajkd.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 8.Johnson DW, Dent H, Hawley CM, et al. Associations of dialysis modality and infectious mortality in incident dialysis patients in Australia and New Zealand. American Journal of Kidney Diseases. 2009;53:290–297. doi: 10.1053/j.ajkd.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 9.Jose MD, Johnson DW, Mudge DW, et al. Peritoneal dialysis practice in Australia and New Zealand: a call to action. Nephrology (Carlton, Vic.) 2011;16:19–29. doi: 10.1111/j.1440-1797.2010.01390.x. [DOI] [PubMed] [Google Scholar]
- 10.Ghali JR, Bannister KM, Brown FG, et al. Microbiology and outcomes of peritonitis in Australian peritoneal dialysis patients. Peritoneal Dialysis International: Journal of the International Society for Peritoneal Dialysis. 2011;31:651–662. doi: 10.3747/pdi.2010.00131. [DOI] [PubMed] [Google Scholar]
- 11.Brown F, Liu WJ, Kotsanas D, Korman TM, Atkins RC. A quarter of a century of adult peritoneal dialysis-related peritonitis at an Australian medical center. Peritoneal Dialysis International: Journal of the International Society for Peritoneal Dialysis. 2007;27:565–574. [PubMed] [Google Scholar]
- 12.Li PK, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Peritoneal Dialysis International: Journal of the International Society for Peritoneal Dialysis. 2010;30:393–423. doi: 10.3747/pdi.2010.00049. [DOI] [PubMed] [Google Scholar]
- 13.Prakash S. An international perspective on peritoneal dialysis among indigenous patients. Peritoneal Dialysis International: Journal of the International Society for Peritoneal Dialysis. 2011;31:390–398. doi: 10.3747/pdi.2010.00228. [DOI] [PubMed] [Google Scholar]
- 14.Lim WH, Boudville N, McDonald SP, Gorham G, Johnson DW, Jose M. Remote indigenous peritoneal dialysis patients have higher risk of peritonitis, technique failure, all-cause and peritonitis-related mortality. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association – European Renal Association. 2011;26:3366–3372. doi: 10.1093/ndt/gfr070. [DOI] [PubMed] [Google Scholar]
- 15.Marley JV, Dent HK, Wearne M, et al. Haemodialysis outcomes of Aboriginal and Torres Strait Islander patients of remote Kimberley region origin. The Medical Journal of Australia. 2010;193:516–520. doi: 10.5694/j.1326-5377.2010.tb04035.x. [DOI] [PubMed] [Google Scholar]
- 16.Lim WH, Johnson DW, McDonald SP. Higher rate and earlier peritonitis in Aboriginal patients compared to non-Aboriginal patients with end-stage renal failure maintained on peritoneal dialysis in Australia: analysis of ANZDATA. Nephrology (Carlton, Vic.) 2005;10:192–197. doi: 10.1111/j.1440-1797.2005.00374.x. [DOI] [PubMed] [Google Scholar]
- 17.Cho Y, Badve SV, Hawley CM, et al. The effects of living distantly from peritoneal dialysis units on peritonitis risk, microbiology, treatment and outcomes: a multi-centre registry study. BMC Nephrolology. 2012;13:41. doi: 10.1186/1471-2369-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sood MM, Komenda P, Sood AR, et al. Adverse outcomes among Aboriginal patients receiving peritoneal dialysis. Canadian Medical Association Journal. 2010;182:1433–1439. doi: 10.1503/cmaj.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]


