Abstract
Cellular resistance to facultative intracellular parasites has been studied by determining the antimycobacterial activity and the amount of fatty acids in sera and in heptane extracts of freshly collected and 24-h-cultured normal and activated guinea pig alveolar macrophages and liver cells. The quantity and the antimycobacterial activity of extractable fatty acids were determined by gas chromatography and the agar plate diffusion test, respectively. These determinations showed that heptane extracts of activated cells inhibited the growth of BCG much more effectively than fractions prepared from normal cells; chromatographic analyses showed that extracts of activated cells contained six times more C16 and C18 long-chain fatty acids than did fractions of normal cells. Heptane extracts of 24-h-cultured cells and of their media showed that during incubation normal and activated cells release fatty acids into the culture media without apparent cell injury; in all experiments liver cells produced larger amounts of fatty acids than alveolar macrophages. Sera collected from activated guinea pigs inhibited the growth of BCG and contained two to five times more total fatty acids than did the growth-supporting normal serum. That bactericidal fatty acids are excreted into the tissue culture medium of incubated cells or into the blood of immunologically stimulated animals suggests that macrophages can exert antibacterial effects without phagocytosis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axline S. G. Functional biochemistry of the macrophage. Semin Hematol. 1970 Apr;7(2):142–160. [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Cellular hypersensitivity and cellular immunity in the pathogensis of tuberculosis: specificity, systemic and local nature, and associated macrophage enzymes. Bacteriol Rev. 1968 Jun;32(2):85–102. doi: 10.1128/br.32.2.85-102.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISLER D. M., VONMETZ E. ANTI-PASTEURELLA PESTIS FACTOR IN THE ORGANS OF NORMAL MICE AND GUINEA PIGS. I. BIOLOGIC CHARACTERISTICS. J Immunol. 1963 Aug;91:287–294. [PubMed] [Google Scholar]
- Eisler D. M., Heckly R. J. Possible mechanisms of action of an anti-Pasteurella pestis factor. J Bacteriol. 1968 Dec;96(6):1977–1981. doi: 10.1128/jb.96.6.1977-1981.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farshtchi D., Lewis V. J. Effects of three bacterial infections on serum lipids of rabbits. J Bacteriol. 1968 May;95(5):1615–1621. doi: 10.1128/jb.95.5.1615-1621.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERSHON Z., OLITZKI A. L. MONOCYTIN, A PROTECTING SUBSTANCE PRODUCED BY MURINE MONOCYTES. Proc Soc Exp Biol Med. 1965 May;119:32–36. doi: 10.3181/00379727-119-30090. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., O'Leary W. M., Kaye D. Serum concentrations of lipids in rabbits infected with Escherichia coli and Staphylococcus aureus. Proc Soc Exp Biol Med. 1970 Jan;133(1):309–313. doi: 10.3181/00379727-133-34463. [DOI] [PubMed] [Google Scholar]
- HIRSCH J., AHRENS E. H., Jr The separation of complex lipide mixtures by the use of silicic acid chromatography. J Biol Chem. 1958 Aug;233(2):311–20. [PubMed] [Google Scholar]
- Kanai K., Kondo E. Subcellular and intercellular aspects of tuberculous infection in reference to protection and sensitization. Jpn J Med Sci Biol. 1972 Jun;25(3):133–167. doi: 10.7883/yoken1952.25.133. [DOI] [PubMed] [Google Scholar]
- Karnovsky M. L., Shafer A. W., Cagan R. H., Graham R. C., Karnovsky M. J., Glass E. A., Saito K. Membrane function and metabolism in phagocytic cells. Trans N Y Acad Sci. 1966 Apr;28(6):778–787. doi: 10.1111/j.2164-0947.1966.tb03542.x. [DOI] [PubMed] [Google Scholar]
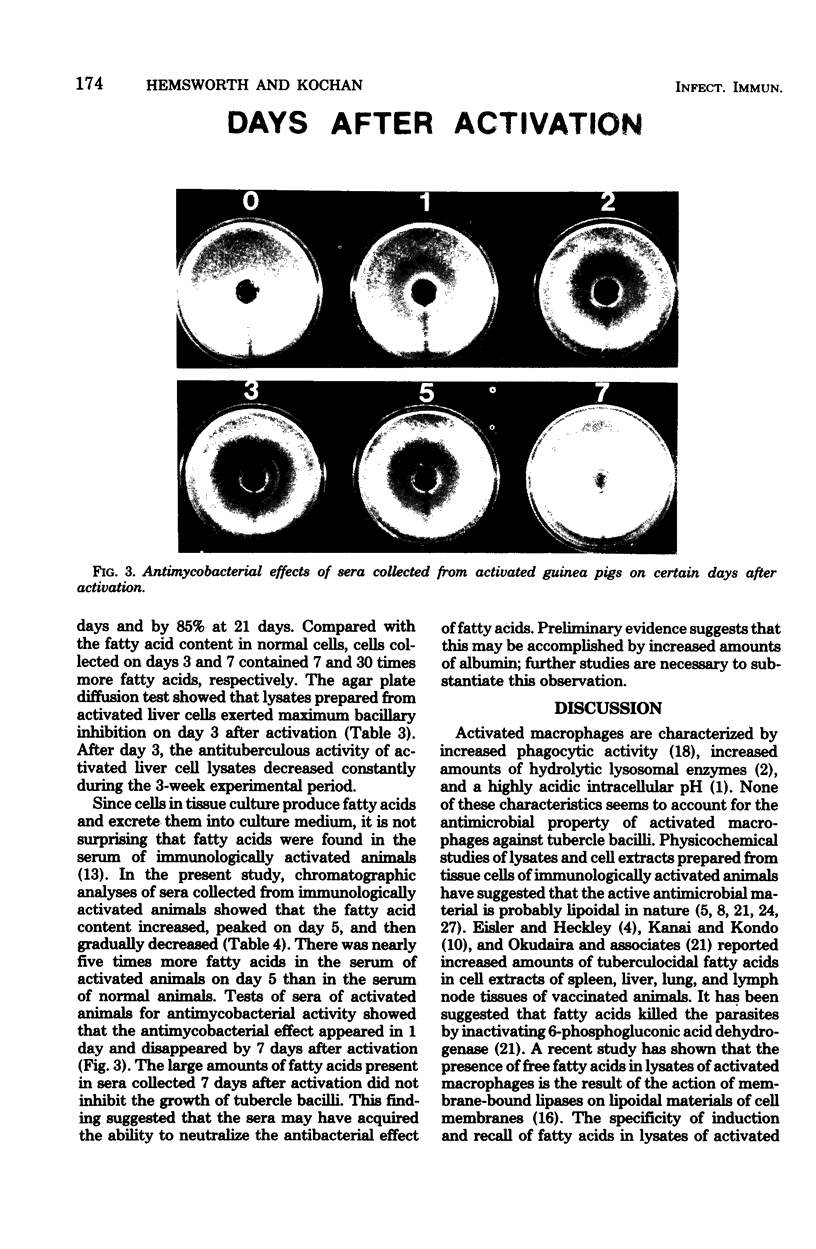
- Kochan I., Berendt M. Fatty acid-induced tuberculocidal activity in sera of guinea pigs treated with bacillus Calmette-Guérin and lipopolysaccharide. J Infect Dis. 1974 Jun;129(6):696–704. doi: 10.1093/infdis/129.6.696. [DOI] [PubMed] [Google Scholar]
- Kochan I., Golden C. A. Antimycobacterial effect of lysates prepared from immunologically activated macrophages. Infect Immun. 1973 Sep;8(3):388–394. doi: 10.1128/iai.8.3.388-394.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan I., Golden C. A. Immunological nature of antimycobacterial phenomenon in macrophages. Infect Immun. 1974 Feb;9(2):249–254. doi: 10.1128/iai.9.2.249-254.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan I., Pellis N. R., Pfohl D. G. Effects of normal and activated cell fractions on the growth of tubercle bacilli. Infect Immun. 1972 Aug;6(2):142–148. doi: 10.1128/iai.6.2.142-148.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEQUIRE V. S., HUTCHERSON J. D., HAMILTON R. L., GRAY M. E. The effects of bacterial endotoxin on lipide metabolism. I. The responses of the serum lipides of rabbits to single and repeated injections of Shear's polysaccharide. J Exp Med. 1959 Aug 1;110(2):293–309. doi: 10.1084/jem.110.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEMAN C. Influence of trace amounts of fatty acids on the growth of microorganisms. Bacteriol Rev. 1954 Jun;18(2):147–163. doi: 10.1128/br.18.2.147-163.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OREN R., FARNHAM A. E., SAITO K., MILOFSKY E., KARNOVSKY M. L. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963 Jun;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okudaira H., Kataoka T., Okada H., Furuse-Irie R., Kawachi S. Cytotoxic factor demonstrated in lymph node extract. J Biochem. 1970 Sep;68(3):379–394. doi: 10.1093/oxfordjournals.jbchem.a129368. [DOI] [PubMed] [Google Scholar]
- PATNODE R. A. Tissue fatty acids and their possible relationship to the natural resistance of rabbits to infection with human-type tubercle bacilli. Am Rev Tuberc. 1954 May;69(5):710–723. doi: 10.1164/art.1954.69.5.710. [DOI] [PubMed] [Google Scholar]
- Page R. C., Davies P., Allison A. C. Participation of mononuclear phagocytes in chronic inflammatory diseases. J Reticuloendothel Soc. 1974 May;15(5):413–438. [PubMed] [Google Scholar]
- Rastogi S., Agnihotri M. S., Agrawal R. C. Follow up study of serum free fatty acids (F.F.A.) in pulmonary tuberculosis. J Assoc Physicians India. 1973 Aug;21(8):681–685. [PubMed] [Google Scholar]
- Thoen C. O., Karlson A. G., Ellefson R. D. Effect of streptomycin therapy on serum lipid-lipoprotein profiles of rabbits infected with Mycobacterium bovis. J Infect Dis. 1973 Apr;127(4):408–414. doi: 10.1093/infdis/127.4.408. [DOI] [PubMed] [Google Scholar]
- YOUMANS G. P., YOUMANS A. S. Effect of mycosuppressin on the course of experimental tuberculosis in mice. J Bacteriol. 1962 Oct;84:701–707. doi: 10.1128/jb.84.4.701-707.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]