Abstract
High-level microsatellite instability (MSI-high) is found in approximately 15% of all colorectal adenocarcinomas (CRCs) and in at least 20% of right-sided cancers. It is most commonly due to somatic hypermethylation of the MLH1 gene promoter region, with familial cases (Lynch syndrome) representing only 2–3% of CRCs overall. In contrast to CRC, MSI-high in appendiceal adenocarcinomas is rare. Only four MSI-high appendiceal carcinomas and one MSI-high appendiceal serrated adenoma have been previously reported, and the prevalence of MSI in the appendix is unknown. We identified 108 appendiceal carcinomas from M. D. Anderson Cancer Center in which MSI status had been assessed by immunohistochemistry for the DNA mismatch repair proteins MLH1, MSH2, MSH6, and PMS2 (n=83), polymerase chain reaction (n=7), or both (n=18). Three cases (2.8%) were MSI-high and one was MSI-low. The three MSI-high cases included: 1) a poorly differentiated nonmucinous adenocarcinoma with loss of MLH1/PMS2 expression, lack of MLH1 promoter methylation, and lack of BRAF gene mutation, but no detected germline mutation in MLH1 from a 39-year-old man; 2) an undifferentiated carcinoma with loss of MSH2/MSH6, but no detected germline mutation in MSH2 or TACSTD1, from a 59-year-old woman; and 3) a moderately differentiated mucinous adenocarcinoma arising in a villous adenoma with loss of MSH2/MSH6 expression, in a 38-year-old man with a strong family history of CRC who declined germline testing. When the overall group of appendiceal carcinomas was classified according to histologic features and precursor lesions, the frequencies of MSI-high were: 3 of 108 (2.8%) invasive carcinomas, 3 of 96 (3.1%) invasive carcinomas that did not arise from a background of goblet cell carcinoid, and 0 of 12 (0%) signet ring and mucinous carcinomas arising in goblet cell carcinoid tumors. These findings, in conjunction with the previously reported MSI-high appendiceal carcinomas, highlight the low prevalence of MSI in the appendix as compared to the right colon and suggest that MLH1 promoter methylation is not a mechanism for microsatellite instability in this location.
INTRODUCTION
Approximately 15% of colorectal carcinomas (CRCs) display high-level microsatellite instability (MSI).1 MSI results from defective DNA mismatch repair and is characterized by widespread accumulation of mutations in nucleotide repeats, some of which are located in the coding regions of cancer-associated genes such as TGFβRII, PTEN, BAX, and others.2 CRCs with MSI arise in both familial and sporadic settings. Familial cases, representing 2–3% of all CRC,3, 4, 5 carry germline mutations in one of the DNA mismatch repair genes – MLH1, MSH2, MSH6, or PMS2 – or rarely a deletion in the last exon of EPCAM that causes heritable methylation of MSH2.6 In contrast, the more common CRCs with sporadic MSI are overwhelmingly due to epigenetic silencing of the MHL1 gene by hypermethylation of its promoter; this hypermethylation usually occurs in a background of more generalized methylation of CpG islands, the so-called CpG island methylator phenotype or CIMP.7 Sporadic MSI CRCs tend to be right-sided tumors, occur in older females and have characteristic histologic features including mucinous phenotype, tumor-infiltrating lymphocytes, Crohn’s-like peritumoral reaction, and poor differentiation.8, 9 Identification of MSI CRCs can potentially serve three goals: 1) the detection of patients with Lynch syndrome, 2) a prognostic marker for improved cancer-related survival, and 3) a predictive marker for resistance to chemotherapeutic agents including 5-FU, cisplatin and carboplatin, and sensitivity to irinotecan.10 However, both the prognostic and predictive values of MSI are fraught with controversy, possibly due to the lumping of familial and sporadic MSI CRCs in various studies.7
The association between mucinous tumors and MSI in the right colon does not appear to extend to tumors of the appendix. Mucinous neoplasms – whether benign, malignant, or of uncertain malignant potential – are the most common non-endocrine appendiceal tumors, followed by intestinal-type adenocarcinomas.11, 12, 13 However, most series have found no evidence of MSI in mucinous and nonmucinous appendiceal tumors. 11, 14, 15, 16, 17, 18 Furthermore, familial cases of appendiceal carcinoma (outside of adenomatous polyposis19) are extremely rare and could be coincidental.20, 21 Several case reports of MSI analysis performed on appendiceal tumors from patients with suspected Lynch syndrome were negative. 20, 22, 23, 24 To our knowledge, only four individual cases of MSI-high appendiceal carcinomas and one case of an appendiceal serrated adenoma with MSI have been previously reported.25, 26, 27, 28, 29 Only one of these patients had documented Lynch syndrome.25
In this study we report the unusual phenotypic and genetic findings in three new patients with MSI-high appendiceal carcinomas and contrast these with the total group of 108 appendiceal carcinomas which have undergone MSI workup in our institution. Our data provide prevalence estimates for appendiceal MSI and suggest – in contrast to the colorectum – that hypermethylation is not a mechanism for genetic instability in the appendix.
MATERIALS AND METHODS
Study Population
We searched the computerized Surgical Pathology and Molecular Diagnostics Laboratory files at M. D. Anderson Cancer Center (MDACC), Houston, TX using the terms “microsatellite” or “MSI” and “appendix” or “appendiceal” to identify appendiceal tumors that had been subjected to immunohistochemistry and/or PCR for MSI analysis. Cases were excluded if the primary site of origin was debatable (e.g., tumors involving both cecum and appendix without a clear epicenter in the appendix).
Gender and age at time of diagnosis were recorded for all patients. In cases that were positive for MSI, we also reviewed the patients’ personal and family histories of carcinoma, clinical follow-up, and results of germline mutational testing (if performed).
This study was approved by the institutional review board at MDACC.
Histologic Evaluation
Appendiceal carcinomas were classified in three manners: 1) according to precursor lesion (i.e., adenoma/cystadenoma, goblet cell carcinoid, or no precursor identified), 2) by histologic type (i.e., mucinous, signet ring cell, or nonmucinous/NOS), and 3) by degree of differentiation (well-, moderately-, or poorly differentiated). Differentiation was assessed by percentage of gland formation according to established criteria: well differentiated (>95% gland-forming), moderately differentiated (50–95% gland-forming), poorly differentiated (5–50% gland-forming or signet ring cell type), and undifferentiated (<5% glands).30 Tumors with signet ring cells floating in pools of mucin were classified as mucinous type, poorly differentiated.
Immunohistochemistry
Immunohistochemistry was performed on 4 μm thick formalin-fixed and paraffin-embedded tissue sections using antibodies directed against MLH1 (mouse monoclonal antibody clone G168-728 at a dilution of 1:300, Cell Marque, Rocklin, CA), MSH2 (mouse monoclonal antibody clone FE11 at a dilution of 1:100, Calbiochem, La Jolla, CA), MSH6 (mouse monoclonal antibody clone 44 at a dilution of 1:300, BD Transduction Laboratories, San Jose, CA), and PMS2 (mouse monoclonal antibody clone A16-4 at a dilution of 1:125, BD Transduction Laboratories). (In three cases evaluated prior to early 2005, only immunostaining for MLH1, MSH2, and MSH6 was performed.) Sections of normal human colon were used as controls. Immunohistochemical expression of each mismatch repair protein was considered intact if there was at least patchy nuclear staining of the neoplastic cells, and lost when there was complete absence of nuclear staining of the neoplastic cells despite internal control positivity (stromal cells, lymphocytes, and non-neoplastic crypt epithelium, if present).
Molecular Studies
Lesional and nonlesional tissue was manually microdissected from 10 μm thick unstained tissue sections, and genomic DNA was isolated using the DNA Mini Extraction Kit (Qiagen, Valencia, CA) or the PicoPure DNA Extraction Kit (MDS Analytical Technologies, Toronto, Ontario, Canada). MSI status was evaluated by fluorescence-labeled microsatellite marker polymerase chain reaction (PCR), followed by capillary electrophoresis fragment size analysis using an ABI 3130 sequencer and Genescan software (Applied Biosystems, Foster City, CA). Seven microsatellite markers were employed, including the National Cancer Institute panel (BAT25, BAT26, D2S123, D5S346, D17S250) with the addition of BAT40 and TGFβRII. (Six cases evaluated prior to 2008 did not include TGFβRII.) Tumors were classified as MSI-high when allelic shift was observed in 3 or more markers, MSI-low when allelic shift involved 1 or 2 markers, and microsatellite-stable (MSS) when none of the markers showed allelic shift. Molecular studies were performed in the Clinical Laboratory Improvement Amendment (CLIA)-certified Molecular Diagnostics Laboratory in the Division of Pathology and Laboratory medicine at MDACC.
RESULTS
Prevalence of MSI
We identified 108 invasive appendiceal adenocarcinomas with MSI studies performed between 2003 and 2012 (both PCR and immunohistochemistry in 18 cases, PCR only in 7, and immunohistochemistry only in 83). The histologic features of the tumors are summarized in Table 1. All but two cases were tested at the request of the patients’ oncologists or surgeons. The other two had MSI testing ordered by the pathologist due to unusual histologic features; one of these cases was MSI-high (see below), and the other was MSS.
TABLE 1.
MSI analysis in 108 appendiceal ADCAs
| ADCA type | Precursor lesion
|
|||||
|---|---|---|---|---|---|---|
| None identified | Adenoma/cystadenoma | Goblet cell carcinoid | ||||
| No. cases | MSI-high | No. cases | MSI-high | No. cases | MSI-high | |
| Signet ring (non-mucinous) | 14 | 0% | 0 | -- | 9 | 0% |
| Mucinous | ||||||
| Well differentiated | 12 | 0% | 8 | 0% | 0 | -- |
| Moderately differentiated | 17 | 0% | 12 | 1 (8%) | 2 | 0% |
| Poorly differentiated | 8a | 0% | 2 | 0% | 1 | 0% |
| Intestinal/non-mucinous | ||||||
| Well differentiated | 0 | -- | 1 | 0% | 0 | -- |
| Moderately differentiated | 7 | 0% | 11 | 0%b | 0 | -- |
| Poorly/undifferentiated | 4 | 2 (50%) | 0 | -- | 0 | -- |
One with neuroendocrine features and one with admixed squamous and neuroendocrine carcinoma
One of eleven cases was MSI-low (shift in 1 of 7 microsatellite markers) with intact MLH1, MSH2, MSH6, PMS2 immunostains ADCA, adenocarcinoma; MSI, microsatellite instability
The total patient population was comprised of 53 (49%) men and 55 (51%) women with a mean age of 48.1 years (range 17 – 75 years). Three patients had MSI-high appendiceal tumors (prevalence of 2.8%). These patients are presented in more detail below. One additional patient had MSI-low – with allelic shift in one of 7 markers – but immunohistochemistry for MLH1, MSH2, MSH6, and PMS2 was intact.
MSI-High Appendiceal Carcinomas
Patient 1
This 39-year-old man presented with right lower quadrant abdominal pain and underwent open appendectomy for presumed acute appendicitis. The distal aspect of the resected appendix harbored a poorly differentiated, nonmucinous adenocarcinoma of approximately 2 cm (Fig. 1); severe acute appendicitis with perforation was also present. Adenocarcinoma itself penetrated the visceral peritoneum and was associated with multifocal lymphovascular invasion; no precursor lesion was identified. One month later a right hemicolectomy revealed metastatic carcinoma in 3 pericolonic lymph nodes; there was no evidence of metastatic adenocarcinoma upon surgical examination of the abdominal cavity, and no residual carcinoma near the appendectomy site (final stage: pT4a pN1 cM0). The patient received adjuvant chemotherapy, but less than 2 months after completion a computed tomography (CT) scan revealed recurrent disease involving the right ureter. Despite aggressive surgical intervention and conventional chemotherapy, he eventually developed multiple sites of metastatic disease – including bony and intrathecal metastases – and died 3 years after his initial diagnosis.
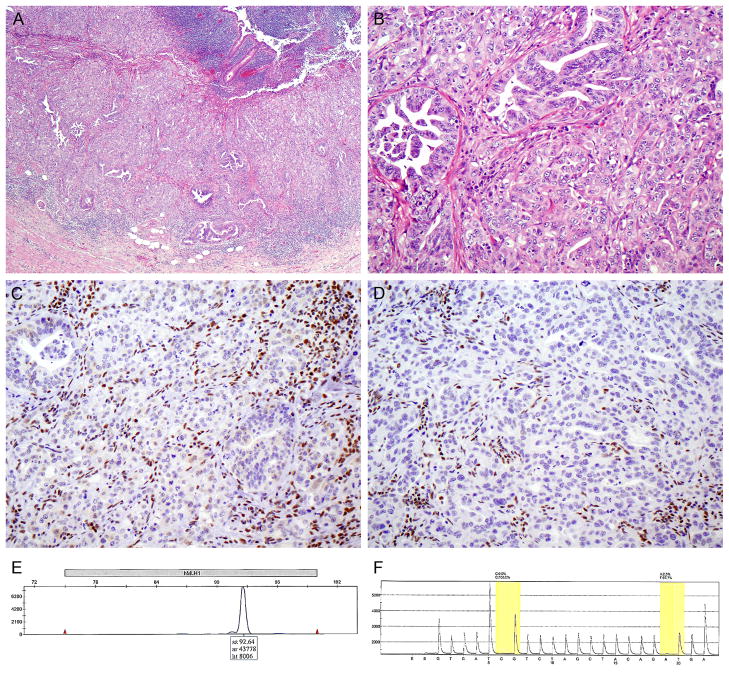
FIGURE 1.
Patient 1. Low- (A) and high-power (B) views of a poorly differentiated nonmucinous appendiceal adenocarcinoma. There is loss of MLH1 (C) and PMS2 (D) expression in the carcinoma cells, whereas nuclear expression is retained in the intervening stromal and inflammatory cells. (E) Lack of MLH1 promoter methylation, evidenced by amplification only of the unmethylated promoter sequence by PCR. (F) Lack of BRAF mutation in codons 595–600 of exon 15; codons 468–474 of exon 11 were also amplified and were negative for mutation (not shown).
MSI testing by immunohistochemistry and PCR was requested because of the patient’s age and his family history of cancer, which included colorectal carcinoma in his maternal grandmother and paternal grandfather. Immunostaining revealed complete loss of MLH1 and PMS2 expression in the tumor, with retained expression of MSH2 and MSH6. By PCR, the tumor was MSI-high with allelic shifts in 4 of 7 markers (BAT25, BAT40, D2S123, and D17S250). These findings prompted testing for hypermethylation of the MLH1 gene promoter and BRAF gene mutations, both of which were negative, thus arguing against sporadic loss of MLH1 expression and suggesting a germline mutation in the MLH1 gene. However, comprehensive germline mutational testing of MLH1 (including sequencing of all 19 exons and gene dosage analysis to detect large deletions, duplications, and other genomic rearrangements) was negative.
Patient 2
This 59-year-old woman underwent abdominal CT because of worsening right lower quadrant abdominal pain and was found to have an appendiceal mass. Gross examination of the subsequent right hemicolectomy revealed an 8.0 × 2.8 × 2.5 cm papillary tumor which filled the lumen of a 10 cm long appendix. Microscopically, the tumor was comprised of an undifferentiated carcinoma with numerous tumor-infiltrating lymphocytes and neutrophils, and sheet-like growth that bore a resemblance to neuroendocrine carcinoma (Fig. 2); however, immunostaining for synaptophysin and chromogranin was negative. No precursor lesion was identified. Carcinoma infiltrated the subserosal adipose tissue and was metastatic to 3 regional lymph nodes. Surgical examination of the abdomen and radiologic studies were negative for distant metastases (final tumor stage pT3 pN1 cM0). The patient went on to receive adjuvant chemotherapy and has remained disease-free for over 3 years.
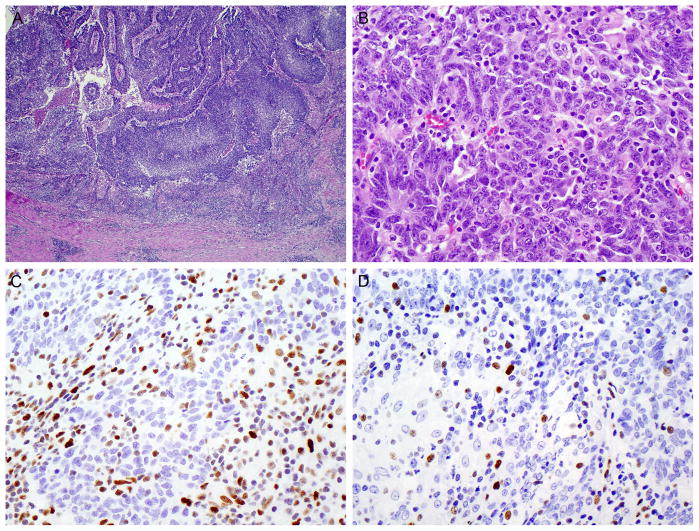
FIGURE 2.
Patient 2. (A) Low-power appearance of an undifferentiated, medullary-type carcinoma with vague papillary fronds projecting into the appendiceal lumen. (B) Sheet-like growth at high-power. (C) Loss of MSH2 expression in the tumor, but intact expression in the tumor-infiltrating lymphocytes. (D) Loss of MSH6.
The unusual histologic appearance of the tumor prompted further immunohistochemical staining, which revealed loss of MSH2 and MSH6 (but retained expression of MLH1 and PMS2) in the neoplastic cells. This finding implied high-level MSI due to germline or somatic MSH2 dysfunction with secondary loss of MSH6 immunoexpression. The patient’s family history was negative for cancer, but – because of the rarity of sporadic MSH2 mutations – she underwent germline mutational testing of MSH2. Comprehensive mutational testing, which included sequencing of all 16 exons of MSH2 and gene dosage analysis to detect large deletions or duplications in MSH2 and TACSTD1, was negative.
Patient 3
This 38-year-old man presented to an emergency center with abdominal pain due to ruptured appendicitis. The initial appendectomy specimen and subsequent right hemicolectomy performed 3 weeks later revealed a moderately differentiated mucinous adenocarcinoma (exact size unspecified), arising from an appendiceal villous adenoma (Fig. 3). Adenocarcinoma focally involved the serosal surface in an area of rupture but there was no lymphovascular invasion and 23 regional lymph nodes were negative. No metastases were initially detected at surgery or by radiologic staging (tumor stage pT4a, pN0, cM0). Six months after completion of adjuvant chemotherapy, surveillance CT scans detected new abdominal masses. The patient received additional chemotherapy and eventually underwent incomplete cytoreductive surgery for large volume peritoneal and infiltrative metastases. He was alive with progressive disease at last contact, 15 months after initial appendectomy.
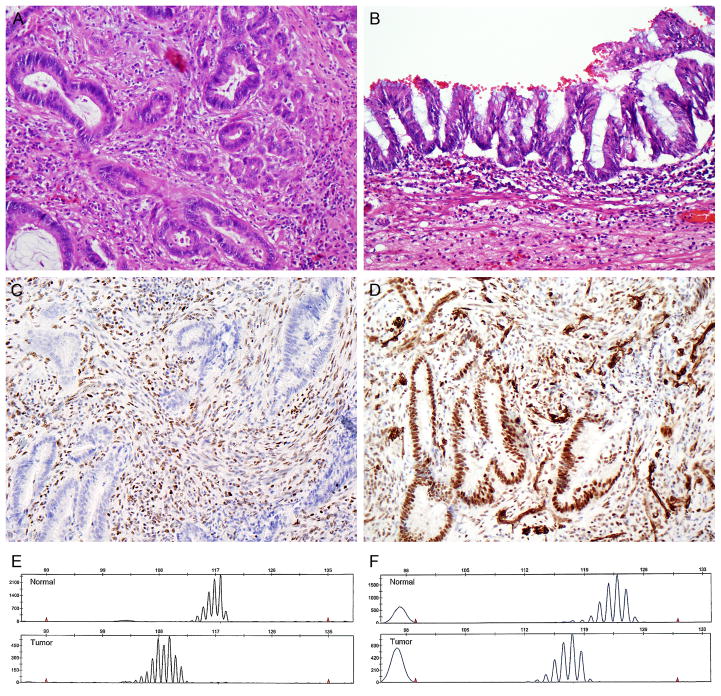
FIGURE 3.
Patient 3. Moderately differentiated adenocarcinoma (A), arising from an appendiceal villous adenoma (B). Loss of MSH2 (C) but retained expression of MLH1 (D) in the tumor. High-level microsatellite instability, with allelic shifts in 7 of 7 tested microsatellite markers. BAT 25 (E) and BAT26 (F) are shown.
Immunohistochemistry and PCR for MSI assessment were requested because of the patient’s age and strong family history of colorectal carcinoma (both parents and twelve maternal relatives with colon cancer). The tumor demonstrated complete loss of MSH2/MSH6 expression and was MSI-high, with allelic shift in 7 of 7 markers. The patient, however, declined further genetic counseling and germline mutational testing.
DISCUSSION
Our data highlight marked differences in both the frequency and mechanism of MSI in appendiceal versus colonic adenocarcinomas – particularly right-sided colonic carcinomas – despite the common embryologic origin of the appendix, cecum, and right colon (all of midgut derivation, with the appendix developing initially as a “bud” from the cecum31). In a study of 257 unselected CRCs from the Mayo Clinic, Rochester, MN, Cunningham et al. found high-level MSI in 51 (20%) and highlighted the strong association between right-sided tumors and MSI; 37% of proximal tumors, but only 5.1% of distal tumors, were MSI-high.32 At the lower end of the published spectrum of MSI prevalence in CRC, Aaltonen et al. reported defective DNA mismatch repair (MMR) in 63 (12%) of 509 Finnish patients with CRC.3 This discrepancy might be explained at least in part by differences between the two studies in the percentages of proximal and distal tumors, since 29% of carcinomas proximal to the splenic flexure in Aaltonen’s series had defective DNA MMR, as compared to only 4.1% of their distal CRCs. Multiple other series have confirmed that approximately 15% of CRCs overall – and at least 20% of proximal tumors – are MSI-high.1, 2
These figures are in marked contrast to appendiceal carcinomas, where MSI is rare. We found high-level MSI in three appendiceal adenocarcinomas, representing just 2.8% of 108 invasive appendiceal adenocarcinomas and 3.1% of 96 invasive adenocarcinomas that did not arise from a background of goblet cell carcinoid. It could be argued that the true frequency of MSI in appendiceal tumors is higher than we detected, due to the fact that many of our cases were evaluated by immunohistochemistry only, rather than immunohistochemistry plus PCR. Immunohistochemistry is nearly 100% sensitive for the detection of MSI that is due to hypermethylation of the MLH1 gene promoter, because this leads to a complete lack of MLH1 protein expression. However, some germline mutations in MLH1, MSH2, or other MMR genes may not be detectable by immunostaining and the MSI-high status of these tumors could be “missed” if PCR was not performed concomitantly. This is particularly true of some missense mutations that result in defective DNA mismatch repair despite varying degrees of retained protein expression. In CRC, the sensitivity of immunohistochemistry in screening for Lynch syndrome is thought to be in the range of 85%–95%,4, 33 but has been reported to be as high as 100% when the staining pattern is interpreted by specialists and when PCR is performed in cases where staining is indeterminate (e.g., fainter staining of tumor nuclei as compared to internal control nuclei).34 Therefore, it is unlikely that any significant numbers of MSI-high appendiceal tumors were missed in this study.
To our knowledge, only four other appendiceal adenocarcinomas with MSI – and one MSI-high serrated adenoma of the appendix – have been previously reported in the English literature. These included: 1) pT1 N0 moderately differentiated adenocarcinoma with loss of MSH2/MSH6 expression and MSI-high by PCR, in a 29-year-old man with Lynch syndrome due to germline A636P MSH2 mutation,25 2) moderately differentiated colonic-type adenocarcinoma with loss of MSH2/MSH6 expression, confined to the appendix, in a 26-year-old woman with a history of synovial sarcoma,27 3) invasive adenocarcinoma arising from a sessile serrated polyp, with loss of MLH1 expression in both the adenocarcinoma and polyp but MSI-high by PCR only in the carcinoma component,29 and 4) pT4a pN2a invasive mucinous carcinoma with MSI-high by PCR and loss of both MLH1 and MSH2 expression, in a 42-year-old man without a family history of cancer.26 (The appendiceal serrated adenoma was MSI-high with allelic shifts in 3 of 5 microsatellite markers, but no immunohistochemistry or mutational testing of individual MMR genes was performed.28) These cases and other reported investigations of MSI in appendiceal tumors are summarized in Table 2.
TABLE 2.
Previously reported investigations of appendiceal MSI
| Ref. | Histology | No. cases | Reason for evaluation | Method of MSI analysis | Result |
|---|---|---|---|---|---|
| Karamurzin25 | Moderately diff ADCA | 1 | Known HNPCC | PCR; IHC (MLH1, MSH2, MSH6, PMS2) | MSI-high with MSH2/MSH6 loss |
| Komm26 | Mucinous ADCA | 1 | Young age (42 y) | PCR; IHC (MLH1, MSH2); germline testing (MLH1, MSH2) | MSI-high with MLH1/MSH2 loss; germline testing negative |
| Racek20 | Mucinous ADCA | 2 | Two siblings with appendiceal ADCA | PCR; IHC (MLH1, MSH2, MSH6, PMS2); germline testing (MLH1, MSH2, MSH6) | Neg. |
| Freeman22 | Well diff ADCA | 1 | Young age (37 y) + family history | Germline mutational testing (MLH1, MSH2) | Neg. |
| Zauber18 | Low grade mucinous tumor | 31 | Series | PCR | Neg. |
| Yoon17 | Mucinous ADCA | 15 | Series | IHC (MLH1) | Neg. |
| “ ” | Mucinous tumor, UMP | 23 | “ ” | “ ” | Neg. |
| “ ” | Mucinous adenoma | 32 | “ ” | “ ” | Neg. |
| Hampel15 | ADCA | 1 | Series | PCR; IHC (MLH1, MSH2, MSH6, PMS2) | Neg. |
| Yantiss29 | Mucinous or serrated ADCA near serrated polyp | 4 | Series | PCR; IHC (MLH1, MSH2) | MSI-high with MLH1 loss (1 of 4) |
| “ ” | Serrated adenoma | 16 | “ ” | “ ” | Neg. |
| “ ” | SSP with dysplasia | 5 | “ ” | “ ” | Neg. |
| “ ” | SSP, no dysplasia | 15 | “ ” | “ ” | Neg. |
| “ ” | HPP | 18 | “ ” | “ ” | Neg. |
| “ ” | Cystadenoma | 17 | “ ” | “ ” | Neg. |
| Gologan14 | Mucinous ADCA | 4 | Series | PCR; IHC (MLH1, MSH2) | Neg. |
| Soilleux24 | Mixed HPP/serrated adenoma | 1 | Synchronous ovarian + uterine ADCA | IHC (MLH1, MSH2, MSH6, PMS2) | Neg. |
| Misdraji27 | Well-to-moderately diff colonic-type ADCA | 9 | Series | IHC (MLH1, MSH2, MSH6, PMS2) | MSH2/MSH6 loss (1 of 9) |
| “ ” | Low grade mucinous tumor, confined to appendix | 18 | “ ” | “ ” | Neg. |
| “ ” | Low grade mucinous tumor, extra-appendiceal spread | 8 | “ ” | “ ” | Neg. |
| Rossi23 | Mixed small cell and intestinal-type ADCA | 1 | Unusual tumor histology | PCR | Microsatellite alterations in 2/19 markers (?MSI-low) |
| Rudzki28 | Serrated adenoma | 1 | Synchronous ovarian ADCA | PCR | MSI-high |
| Kabbani16 | Mucinous and nonmucinous ADCA | 30 | Series | PCR | Neg. |
| Lyda38 | Mucinous cystadenoma | 1 | UC with multiple synchronous tumors | PCR | Neg. |
ADCA, adenocarcinoma; HPP, hyperplastic polyp; IHC, immunohistochemistry; MSI, microsatellite instability; SSP, sessile serrated polyp; UC, ulcerative colitis; UMP, uncertain malignant potential.
Most MSI-high CRCs are associated with loss of MLH1/PMS2 expression by immunohistochemistry, and most of these cases with MLH1 loss are due to epigenetic hypermethylation of the MLH1 gene promoter. In a large study of 1,061 population-based cases of CRC, 60% of the MSI-high tumors had MLH1 methylation.35 Of 313 cases in that study that were MSI-high and had available immunohistochemistry for MLH1, MSH2, MSH6, and PMS2, 216 (69%) demonstrated loss of MLH1 expression; 165 (76%) of these tumors were methylated and 51 (24%) were unmethylated. Less commonly, there was loss of another MMR protein (21%) or no evidence of MMR protein loss despite an MSI-high phenotype (9.6%). Overall, only 12–14% of population-based patients (but 70% of high-risk clinic-based patients) with MSI-high CRC had identifiable germline MMR mutations.35 Multiple other studies have confirmed that germline MMR mutation/Lynch syndrome accounts for a minority (1.9%–5%) of CRC in the general population. 1, 3, 32, 36
Although the numbers are small, the mechanism of MSI in appendiceal cancers studied to date appears different than that of CRC, especially right-sided colonic carcinomas. Among our 3 cases, only one had MLH1/PMS2 loss while the other two had absence of MSH2/MSH6. (Because the stability of PMS2 and MSH6 proteins depends upon intact MLH1 and MSH2, respectively, these cases are pathogenically due to dysfunction of MLH1 and MSH2 with secondary loss of PMS2 and MSH6 immunoexpression.) Further, our tumor with MLH1 loss lacked MLH1 methylation, and lacked BRAF mutation (which is present in at least half of sporadic CRCs with methylation-associated MSI-high). Similarly, in previously reported MSI-high appendiceal carcinomas, MSH2 loss accounted for 2 of 4 cases,25, 27 loss of both MLH1 and MSH2 accounted for 1 of 4,26 and MLH1 loss accounted for only 1 of 4.29 The tumor with both MLH1 and MSH2 loss also lacked MLH1 methylation and BRAF mutation, while the other case with MLH1 loss was not tested for hypermethylation or BRAF mutation. Therefore, in contrast to the frequent MLH1 loss and infrequent MSH2 loss found in MSI-high CRCs, 5 of 7 (71%) reported MSI-high appendiceal carcinomas have shown MSH2 loss, only 3 of 7 (43%) have shown MLH1 loss, and neither of the 2 tested cases had MLH1 methylation or BRAF mutation.
While MLH1 loss in CRC can be either sporadic or germline, loss of MSH2, MSH6, or isolated loss of PMS2 is usually reflective of a germline MMR gene mutation.27, 36 Additionally, CRCs with MLH1 loss that is not associated with MLH1 hypermethylation or BRAF mutation –or both – are considered to be highly suggestive of Lynch syndrome; in a recent literature review, for example, Parsons and colleagues reported the presence of BRAF V600E mutations in CRCs from only 1.4% of patients with Lynch syndrome and methylation of the “C region” of MLH1 in only 6% of MLH1 mutation carriers.37 However, both of our patients who underwent mutational testing for Lynch syndrome were negative, while the third (whose family history was most suggestive of Lynch syndrome) declined genetic counseling. Of the 3 previously reported patients with MSI-high appendiceal carcinomas who underwent genetic testing, only one was confirmed to have Lynch syndrome while the other two were negative. Thus, Lynch syndrome has been identified in only one of 5 (20%) MSI-high appendiceal carcinomas with immunohistochemical or molecular findings suggestive of germline mutations in MLH1 or MSH2. Even though mutational analysis for Lynch syndrome was negative in the remaining patients, it is still possible that these MSI-high appendiceal carcinomas are germline in nature.
In summary, the results of this study highlight several features of MSI in appendiceal neoplasms. First, MSI-high is 5-fold less common in appendiceal carcinomas than in CRCs overall (~3% vs. 15%) and at least 6-fold less common than in right sided colon carcinomas (~3% vs. ≥20%). Second, unlike CRC, MLH1 promoter methylation does not appear to play a role in the genesis of microsatellite instability in this location (at least based on the small number of reported cases to date). Finally, the same immunohistochemical/molecular alterations that would strongly suggest Lynch syndrome in CRC (e.g., MSH2/MSH6 loss, or MLH1/PMS2 loss without MLH1 promoter methylation or BRAF mutation) are less specific in appendiceal carcinomas. Taken together, these findings suggest that routine screening for MSI/Lynch syndrome detection in appendiceal carcinomas would be of very low yield.
Footnotes
Disclosures: The authors have no conflicts of interest or funding to disclose.
References
- 1.Vilar E, Gruber SB. Microsatellite instability in colorectal cancer - the stable evidence. Nat Rev Clin Oncol. 2010;7:153–162. doi: 10.1038/nrclinonc.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iacopetta B, Grieu F, Amanuel B. Microsatellite instability in colorectal cancer. Asia Pac J Clin Oncol. 2010;6:260–269. doi: 10.1111/j.1743-7563.2010.01335.x. [DOI] [PubMed] [Google Scholar]
- 3.Aaltonen LA, Salovaara R, Kristo P, et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med. 1998;338:1481–1487. doi: 10.1056/NEJM199805213382101. [DOI] [PubMed] [Google Scholar]
- 4.Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 5.Salovaara R, Loukola A, Kristo P, et al. Population-based molecular detection of hereditary nonpolypsis colorectal cancer. J Clin Oncol. 2000;18:2193–2200. doi: 10.1200/JCO.2000.18.11.2193. [DOI] [PubMed] [Google Scholar]
- 6.Ligtenberg MJ, Kuiper RP, Chan TL, et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3′ exons of TACSTD1. Nat Genet. 2009;41:112–117. doi: 10.1038/ng.283. [DOI] [PubMed] [Google Scholar]
- 7.Hughes LA, Khalid-de Bakker CA, Smits KM, et al. The CpG island methylator phenotype in colorectal cancer: progress and problems. Biochim Biophys Acta. 2012;1825:77–85. doi: 10.1016/j.bbcan.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Alexander J, Watanabe T, Wu TT, et al. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001;158:527–535. doi: 10.1016/S0002-9440(10)63994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yearsley M, Hampel H, Lehman A, et al. Histologic features distinguish microsatellite-high from microsatellite-low and microsatellite-stable colorectal carcinomas, but do not differentiate germline mutations from methylation of the MLH1 promoter. Hum Pathol. 2006;37:831–838. doi: 10.1016/j.humpath.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Laghi L, Malesci A. Microsatellite instability and therapeutic consequences in colorectal cancer. Dig Dis. 2012;30:304–309. doi: 10.1159/000337003. [DOI] [PubMed] [Google Scholar]
- 11.McGory ML, Maggard MA, Kang H, et al. Malignancies of the appendix: beyond case series reports. Dis Colon Rectum. 2005;48:2264–2271. doi: 10.1007/s10350-005-0196-4. [DOI] [PubMed] [Google Scholar]
- 12.Misdraji J, Young RH. Primary epithelial neoplasms and other epithelial lesions of the appendix (excluding carcinoid tumors) Semin Diagn Pathol. 2004;21:120–133. doi: 10.1053/j.semdp.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Sugarbaker PH. Epithelial appendiceal neoplasms. Cancer J. 2009;15:225–235. doi: 10.1097/PPO.0b013e3181a9c781. [DOI] [PubMed] [Google Scholar]
- 14.Gologan A, Krasinskas A, Hunt J, et al. Performance of the revised Bethesda guidelines for identification of colorectal carcinomas with a high level of microsatellite instability. Arch Pathol Lab Med. 2005;129:1390–1397. doi: 10.5858/2005-129-1390-POTRBG. [DOI] [PubMed] [Google Scholar]
- 15.Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783–5788. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabbani W, Houlihan PS, Luthra R, et al. Mucinous and nonmucinous appendiceal adenocarcinomas: different clinicopathological features but similar genetic alterations. Mod Pathol. 2002;15:599–605. doi: 10.1038/modpathol.3880572. [DOI] [PubMed] [Google Scholar]
- 17.Yoon SO, Kim BH, Lee HS, et al. Differential protein immunoexpression profiles in appendiceal mucinous neoplasms: a special reference to classification and predictive factors. Mod Pathol. 2009;22:1102–1112. doi: 10.1038/modpathol.2009.74. [DOI] [PubMed] [Google Scholar]
- 18.Zauber P, Berman E, Marotta S, et al. Ki-ras gene mutations are invariably present in low-grade mucinous tumors of the vermiform appendix. Scand J Gastroenterol. 2011;46:869–874. doi: 10.3109/00365521.2011.565070. [DOI] [PubMed] [Google Scholar]
- 19.Koorey D, Basha NJ, Tomaras C, et al. Appendiceal adenocinoma complicating adenomatous polyposis in a young woman with a de novo constitutional reciprocal translocation t(5;8)(q22;p23.1) J Med Genet. 2000;37:71–75. doi: 10.1136/jmg.37.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Racek AR, Rabe KG, Wick MJ, et al. Primary appendiceal mucinous adenocarcinoma in two first-degree relatives: case report and review. Hered Cancer Clin Pract. 2011;9:1–4. doi: 10.1186/1897-4287-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shih IM, Yan H, Speyrer D, et al. Molecular genetic analysis of appendiceal mucinous adenomas in identical twins, including one with pseudomyxoma peritonei. Am J Surg Pathol. 2001;25:1095–1099. doi: 10.1097/00000478-200108000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Freeman HJ. Duplicated appendix complicated by appendiceal cancer. World J Gastroenterol. 2011;17:135–136. doi: 10.3748/wjg.v17.i1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi G, Bertolini F, Sartori G, et al. Primary mixed adenocarcinoma and small cell carcinoma of the appendix. A clinicopathologic, immunohistochemical, and molecular study of a hitehrto unreported tumor. Am J Surg Pathol. 2004;28:12331239. doi: 10.1097/01.pas.0000128666.89191.48. [DOI] [PubMed] [Google Scholar]
- 24.Soilleux E, Arends MJ, Cluroe AD. Multiple mucinous tumours. Pathology. 2005;37:91–92. doi: 10.1080/00313020400023479. [DOI] [PubMed] [Google Scholar]
- 25.Karamurzin Y, Zeng Z, Stadler ZK, et al. Unusual DNA mismatch repair-deficient tumors in Lynch syndrome: a report of new cases and review of the literature. Hum Pathol. 2012;43:1677–1687. doi: 10.1016/j.humpath.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Komm M, Kronawitter-Fesl M, Kremer M, et al. Primary mucinous adenocarcinoma of the vermiform appendix with high grade microsatellite instability. J Cancer. 2011;2:302–306. doi: 10.7150/jca.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Misdraji J, Burgart LJ, Lauwers GY. Defective mismatch repair in the pathogenesis of low-grade appendiceal mucinous neoplasms and adenocarcinomas. Mod Pathol. 2004;17:1447–1454. doi: 10.1038/modpathol.3800212. [DOI] [PubMed] [Google Scholar]
- 28.Rudzki Z, Zazula M, Bialas M, et al. Synchronous serrated adenoma of the appendix and high-grade ovarian carcinoma: a case demonstrating different origin of the two neoplasms. Pol J Pathol. 2002;53:29–34. [PubMed] [Google Scholar]
- 29.Yantiss RK, Panczykowski A, Misdraji J, et al. A comprehensive study of nondysplastic and dysplastic serrated polyps of the vermiform appendix. Am J Surg Pathol. 2007;31:1742–1753. doi: 10.1097/PAS.0b013e31806bee6d. [DOI] [PubMed] [Google Scholar]
- 30.Edge SB, Byrd DR, Carducci MA, et al. AJCC Cancer Staging Manual. 7. New York, NY: Springer; 2009. [Google Scholar]
- 31.Schumpelick V, Dreuw B, Ophoff K, et al. Appendix and cecum. Embryology, anatomy, and surgical applications. Surg Clin North Am. 2000;80:295–318. doi: 10.1016/s0039-6109(05)70407-2. [DOI] [PubMed] [Google Scholar]
- 32.Cunningham JM, Kim CY, Christensen ER, et al. The frequency of hereditary defective mismatch repair in a prospective series of unselected colorectal carcinomas. Am J Hum Genet. 2001;69:780–790. doi: 10.1086/323658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limburg PJ, Harmsen WS, Chen HH, et al. Prevalence of alterations in DNA mismatch repair genes in patients with young-onset colorectal cancer. Clin Gastroenterol Hepatol. 2011;9:497–502. doi: 10.1016/j.cgh.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Overbeek LI, Ligtenberg MJ, Willems RW, et al. Interpretation of immunohistochemistry for mismatch repair proteins is only reliable in a specialized setting. Am J Surg Pathol. 2008;32:1246–1251. doi: 10.1097/pas.0b013e31816401bb. [DOI] [PubMed] [Google Scholar]
- 35.Poynter JN, Siegmund KD, Weisenberger DJ, et al. Molecular characterization of MSI-H colorectal cancer by MLH1 promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol Biomarkers Prev. 2008;17:3208–3215. doi: 10.1158/1055-9965.EPI-08-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Lier MG, Leenen CH, Wagner A, et al. Yield of routine molecular analyses in colorectal cancer patients ≤70 years to detect underlying Lynch syndrome. J Pathol. 2012;226:764–774. doi: 10.1002/path.3963. [DOI] [PubMed] [Google Scholar]
- 37.Parsons MT, Buchanan DD, Thompson B, et al. Correlation of tumour BRAF mutations and MLH1 methylation with germline mismatch repair (MMR) gene mutation status: a literature review assessing utility of tumour features for MMR variant classification. J Med Genet. 2012;49:151–157. doi: 10.1136/jmedgenet-2011-100714. [DOI] [PubMed] [Google Scholar]
- 38.Lyda MH, Noffsinger A, Belli J, et al. Multifocal neoplasia involving the colon and appendix in ulcerative colitis: pathological and molecular features. Gastroenterology. 1998;115:1566–1573. doi: 10.1016/s0016-5085(98)70037-x. [DOI] [PubMed] [Google Scholar]