Abstract
The PI3K/Akt pathway plays a crucial role in the pathogenesis of multiple myeloma (MM) in the bone marrow (BM) milieu. However, efficacy of selective and potent Akt inhibition has not yet been fully elucidated. In this study, we therefore examined the biologic impact of selective and potent Akt inhibition by a novel allosteric inhibitor TAS-117. TAS-117 induced significant growth inhibition, associated with downregulation of phosphorylated Akt (p-Akt), selectively in MM cell lines with high baseline p-Akt. Cytotoxicity of TAS-117 was also observed in patients MM cells, but not in normal peripheral blood mononuclear cells. Importantly, TAS-117 induced significant cytotoxicity in MM cells even in the presence of BM stromal cells, associated with inhibition of IL-6 secretion. Oral administration of TAS-117 significantly inhibited human MM cell growth in murine xenograft models. TAS-117 triggered apoptosis and autophagy, as well as induction of endoplasmic reticulum (ER) stress response with minimal expression of CHOP, a fatal ER-stress marker. Importantly, TAS-117 enhanced bortezomib-induced cytotoxicity, associated with increased CHOP and PARP cleavage and blockade of bortezomib-induced p-Akt, suggesting that TAS-117 augments bortezomib-induced ER stress and apoptotic signaling. Carfilzomib-induced cytotoxicity was similarly enhanced by TAS-117. Importantly, TAS-117 enhanced bortezomib-induced cytotoxicity in vivo, associated with prolonged host survival. Our results show that selective and potent Akt inhibition by TAS-117 triggers anti-MM activities in vitro and in vivo, as well as enhances cytotoxicity of proteasome inhibition, providing the preclinical framework for clinical evaluation of selective Akt inhibitors, alone and in combination with proteasome inhibitors in MM.
Introduction
Multiple myeloma (MM) is characterized by excess plasma cells in the bone marrow (BM), lytic bone lesions, and immunodeficiency, associated with monoclonal protein in the blood and/or urine. In spite of recent advances in treatment, including high-dose therapy and novel agents such as bortezomib, thalidomide, and lenalidomide, MM remains incurable due to development of drug resistance in the BM microenvironment (1–4). The phosphatidylinositol 3-kinase (PI3K)/Akt pathway plays a crucial role in survival, growth, and drug resistance of different types of cancers including MM (5–8). PI3K is activated via upstream tyrosine kinase-associated receptors by various growth factors and/or cytokines, leading to phosphorylation and activation of the serine-threonine protein kinase Akt. Many other proteins, including GSK3α/β and FKHR proteins, are phosphorylated by activated Akt, and regulate downstream signaling cascades to maintain cell proliferation, survival, and protection against apoptosis (5, 9, 10). Moreover, Akt also targets mammalian target of rapamycin (mTOR) pathway, which regulates cell metabolism and autophagy (11, 12).
In MM, the BM microenvironment triggers PI3K/Akt signaling cascade via both physical interaction of MM cells with BM stromal cells (BMSCs) and soluble factors secreted from BMSCs, including IL-6 and IGF-1 (13–16). Since activated PI3K/Akt signaling mediates MM cell drug resistance, targeting Akt is a strategy to overcome drug resistance (7, 17, 18). For example, we have shown that bortezomib activates Akt (17), and that inhibition by a non-selective Akt inhibitor perifosine (19, 20) can synergistically enhance bortezomib-induced cytotoxicity. Perifosine also induces antitumor activity in vivo in a murine xenograft model of human MM (17). Moreover, a recent study has shown the in-vitro efficacy of a selective Akt inhibitor MK-2206 (21). However, since perifosine is not selective to Akt signaling (22) and MK-2206 was evaluated only in vitro, the biologic impact and preclinical efficacy of selective and potent Akt inhibition in MM has not yet been fully elucidated.
In this study, we therefore examine the biologic impact of selective and potent Akt inhibition in MM cells both in vitro and in vivo by a novel small molecule inhibitor TAS-117. These studies provide the framework of clinical trials of TAS-117, alone and in combination with proteasome inhibitors, to improve patient outcome in MM.
Materials and Methods
Reagents
TAS-117 is trans-3-amino-1methyl-3-[4-(3-phenyl-5H-imidazo[1,2-c]pyrido[3,4-e][1,3]oxazin-2-yl)phenyl]-cyclobutanol. Its chemical structure is shown in Supplementary Fig. S1A. TAS-117-HCl is TAS-117 monohydrochrolide. Bortezomib, carfilzomib, and perifosine were obtained from Selleck Chemicals. Recombinant human IL-6, IGF-1, and TNF-α were obtained from R&D system.
Human MM cell lines and primary cells
For details on human MM cell lines and primary cells, see Supplementary Materials and Methods.
Growth inhibition assay
The inhibitory effect of TAS-117 on MM cell growth was assessed by measuring 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide (MTT, Sigma-Aldrich) dye absorbance, as previously described (17), or using CellTiter-Glo Luminescent Cell Viability Assay (Promega) according to manufacturer’s instructions. To measure proliferation of MM cells with or without BMSCs, the rate of DNA synthesis was measured by [3H]-thymidine (Perkin-Elmer) uptake, as previously described (23).
Western blotting
MM cells were treated with or without novel or conventional agents; cells were then harvested, washed, and lysed, as in prior studies (17, 24). Cell lysates were subjected to SDS-PAGE, transferred to membranes, and immunoblotted with antibodies. For details on antibodies, see Supplementary Materials and Methods. Protein expression was quantified using ImageJ (National Institutes of Health).
Cell cycle analysis and detection of apoptosis by flow cytometry
For cell cycle analysis, MM cells treated with or without drug were harvested, washed with phosphate-buffered saline (PBS), fixed with 70% ethanol, and treated with 10 μg/mL RNase (Roche Diagnosis). Cells were then stained with propidium iodide (PI; Sigma-Aldrich). Detection of apoptotic cells was done with the annexin V/detection kit (Immunotech/Beckman Coulter), as previously described (25). Cell-cycle or apoptotic profiles were analyzed on a BD FACS Canto™ II (BD Biosciences) using FACSDiva (BD Biosciences), with ModFit LT software for cell cycle analysis. Annexin V-FITC1 positive and PI negative cells were considered as early apoptotic, whereas positivity for both annexin V-FITC1 and PI was associated with late apoptosis or necrosis.
ELISA
IL-6 secretion by human BMSCs was assessed by enzyme-linked immunosorbent assay (ELISA). BMSCs were cultured in 96-well plates, with or without TAS-117. After 24 hours, supernatants were harvested and subjected to ELISA using DuoSet ELISA Development Kit (R&D Systems), according to manufacturer’s instructions.
Murine xenograft models of human MM
For details on murine xenograft models of human MM, see Supplementary Materials and Methods.
Statistical analysis
Statistical significance was determined by Student’s t-test, or Dunnett’s multiple comparison test especially for tumor volume. The minimal level of significance was p<0.05. Survival was assessed using Kaplan-Meier curves and log-rank analysis. The combined effect of TAS-117 with bortezomib or carfilzomib was analyzed by isobologram analysis using the CompuSyn software program (ComboSyn, Inc.).
Results
TAS-117 selectively inhibits Akt and induces cytotoxicity in MM cells with high baseline phosphorylation of Akt
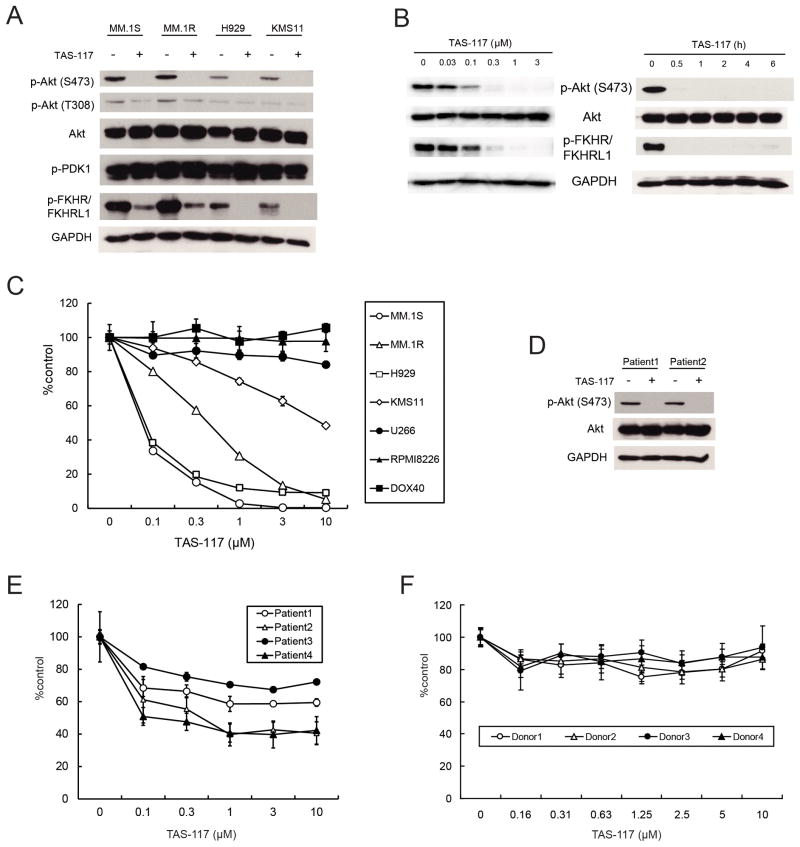
We first examined the impact of inhibition of p-Akt by a small molecule allosteric Akt inhibitor TAS-117, which inhibits Akt1, 2, and 3 with IC50 values of 4.8, 1.6, and 44 nM, respectively, with no or only minimal inhibitory activities against other protein and lipid kinases including PI3K, PDK1, and mTOR in enzyme assays at 1 μM (Supplementary Fig. S1B and S1C). Moreover, in the IP-kinase assay using lysates prepared from MM.1S cells, TAS-117 inhibited Akt kinase activity but not p-Akt (Supplementary Fig. S1D), indicating that Akt activity is directly inhibited. TAS-117 blocked basal phosphorylation of Akt and downstream p-FKHR/FKHRL1 (Fig. 1A) in MM cells with high baseline p-Akt (Supplementary Fig. S2), but did not inhibit autophosphorylation of PDK1 which phosphorylates Akt at Thr308. This inhibitory effect was dose-dependent, with rapid downregulation of p-Akt within 0.5 h (Fig. 1B). Furthermore, TAS-117 did not inhibit other crucial pathways in MM; indeed phosphorylation of STAT3 and ERK was increased (Supplementary Fig. S3), as previously reported for another allosteric Akt inhibitor (21). These data indicate that TAS-117 selectively inhibits Akt activity in MM cells. Next we examined cytotoxicity of TAS-117 in MM cells. TAS-117 induced significant growth inhibition in MM cell lines with high baseline p-Akt, but not in cell lines with low baseline p-Akt, suggesting that high baseline p-Akt reflects sensitivity to selective Akt inhibition with TAS-117 (Fig. 1C and Supplementary Fig. S2). Importantly, TAS-117 inhibited baseline p-Akt in patient MM cells (Fig. 1D); and induced cytotoxicity in patient MM cells, but not in normal mononuclear cells from healthy donors (Fig. 1E and F). Moreover, TAS-117 reduced the proportion of side population (SP) cells (26) associated with significant cytotoxicity and inhibition of p-Akt (Supplementary Fig. S4A, S4B and S4C), suggesting that TAS-117 may also target tumor-initiating cells in MM.
Figure 1. TAS-117 blocks Akt phosphorylation associated with inhibition of MM cell growth.
(A) MM.1S, MM.1R, H929, and KMS11 cells were treated with or without TAS-117 (1 μM) for 6 h. Whole cell lysates were subjected to western blotting using phospho (p)-Akt (Ser473 and Thr308), Akt, p-PDK1, p-FKHR/FKHRL1, and GAPDH Abs.
(B) MM.1S cells were treated with TAS-117 (0–3 μM) for 6 h. MM.1S cells were also treated with TAS-117 (1 μM) for the indicated times. Whole cell lysates were subjected to western blotting using p-Akt (Ser473), Akt, p-FKHR/FKHRL1, and GAPDH Abs.
(C) MM cell lines were cultured with TAS-117 (0–10 μM) for 72 h. Cell viability was assessed by MTT assay of triplicate cultures, expressed as percentage of untreated control. Data represent mean ± SD.
(D) Primary MM cells isolated from two patients were treated with or without TAS-117 (1 μM) for 6 h. Whole cell lysates were subjected to western blotting using p-Akt (Ser473), Akt, and GAPDH Abs.
(E–F) Primary MM cells isolated from four patients (E) and mononuclear cells isolated from four healthy donors (F) were cultured with TAS-117 (0–10 μM) for 48 h. Cell viability was assessed by MTT assay of triplicate cultures, expressed as percentage of untreated control. Data represents mean ± SD.
TAS-117 abrogates the cytoprotective effect of the bone marrow microenvironment associated with Akt inhibition in both MM cells and BMSCs
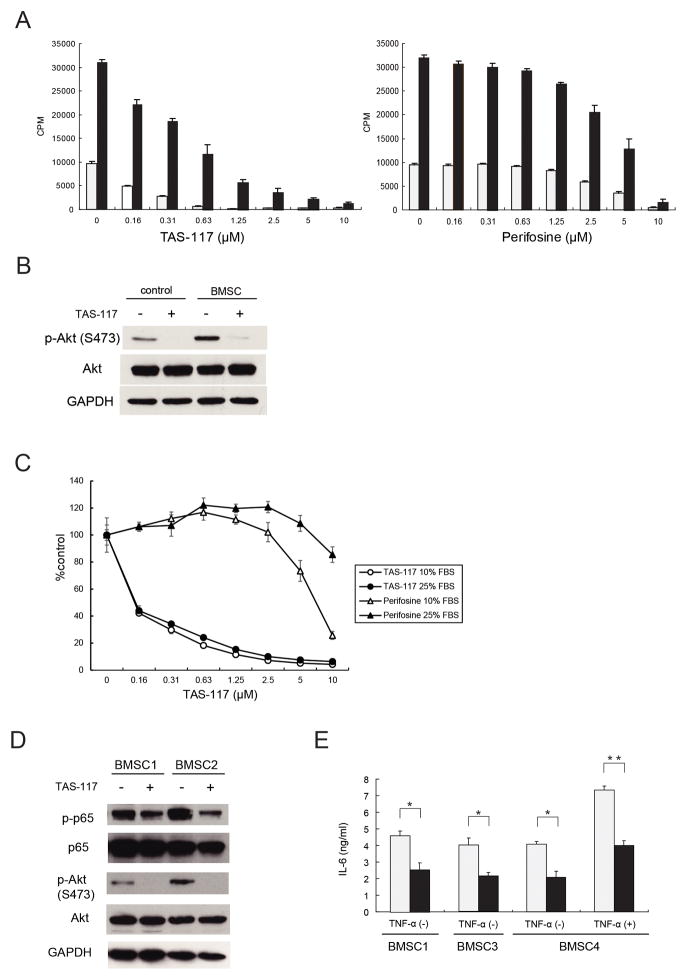
The BM microenvironment induces growth, survival, and drug resistance in MM cells via cytokines such as IL-6 and IGF-1 or adhesion of MM cells to BMSCs (15, 16). We next therefore examined whether TAS-117 abrogates the cytoprotective effects conferred by BMSCs or cytokines. TAS-117 (0.16–10 μM) induced MM growth inhibition evaluated by [3H]-thymidine uptake in a dose-dependent manner, even in the presence of BMSCs; in contrast, perifosine did so only at high doses (>1 μM) (Fig. 2A). Importantly, induction of Akt phosphorylation when MM.1S cells were cultured with conditioned media derived from BMSCs was blocked by TAS-117 (Fig. 2B). Similar results were obtained when MM cells were stimulated with IL-6 and IGF-1 (Supplementary Fig. S5A), and MTT assay showed that neither cytokines blocked cytotoxicity induced by TAS-117 (Supplementary Fig. S5B). These results suggest that TAS-117 induces significant antitumor activity against MM cells even in the BM microenvironment, associated with significant inhibition of p-Akt, and that TAS-117 has more potent inhibitory effect than perifosine in the BM milieu. Moreover, increasing serum concentrations in culture from 10% to 25% attenuated cytotoxicity induced by perifosine but not by TAS-117 (Fig. 2C), further confirming potency of TAS-117 in the BM microenvironment.
Figure 2. TAS-117 abrogates the cytoprotective effect of the bone marrow microenvironment associated with Akt inhibition in both MM cells and BMSCs.
(A) MM.1S cells were cultured for 48 h with increasing concentrations of TAS-117 (left) or perifosine (right) in the presence (■) or absence (□) of BMSCs. Cell proliferation was assessed by [3H]-thymidine uptake of quadruplicate cultures. Data represent the mean ± SD [3H]-thymidine incorporation (CPM).
(B) MM.1S cells were treated with or without TAS-117 (0.5 μM) for 6 h with normal medium (control) or conditioned medium derived from culture supernatant of BMSCs (BMSC). Whole cell lysates were subjected to western blotting using p-Akt (Ser473), Akt, and GAPDH Abs.
(C) MM.1S cells were cultured for 48 h with increasing concentrations of TAS-117 or perifosine in RPMI-1640 containing 10% or 25% FBS. Cell growth was assessed by CellTiter-Glo Luminescent Cell Viability Assay of quadruplicate cultures, expressed as percentage of untreated control. Data represents mean ± SD. (D) BMSCs from two MM patients were treated with or without TAS-117 (2 μM) for 6 h. Whole cell lysates were subjected to western blotting using p-p65, p65, p-Akt (Ser473), Akt, and GAPDH Abs.
(E) BMSCs derived from three MM patients were cultured for 24 h with control medium (□) or TAS-117 (2 μM) (■) in the presence or absence of TNF-α (0.25 ng/ml). IL-6 in culture supernatant was measured by ELISA of triplicate cultures. Data represents mean ± SD. * p< 0.01, ** p< 0.001.
Since Akt mediates NF-κB activity (14), we hypothesized that Akt inhibition by TAS-117 led to NF-κB inhibition, which resulted in decreased secretion of IL-6 from BMSCs. TAS-117 inhibited phosphorylation of baseline Akt in BMSCs, associated with reduced phosphorylation of NF-κB p65 (Fig. 2D). Importantly, TAS-117 significantly inhibited baseline secretion of IL-6 as well as TNF-α-induced secretion of IL-6 from BMSCs (Fig. 2E), without affecting cell viability (Supplementary Fig. S6). These results indicate that Akt inhibition attenuates IL-6 secretion from BMSCs by modulating NF-κB pathway, leading to growth inhibition of MM cells.
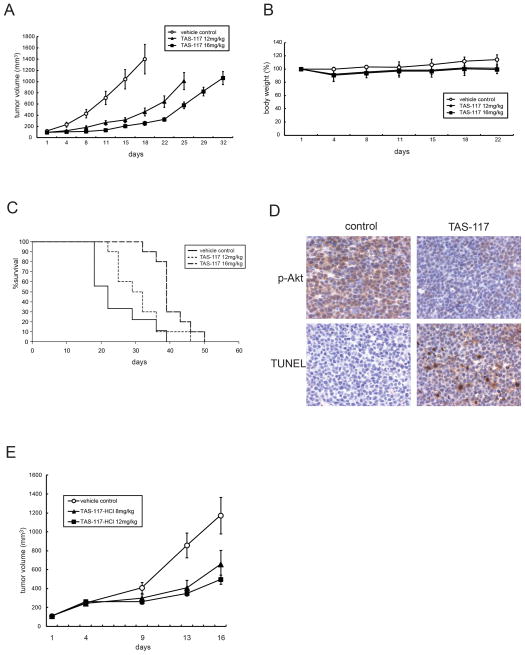
TAS-117 inhibits human MM cell growth in vivo
To evaluate the in-vivo impact of TAS-117 treatment, we used a subcutaneous xenograft model of human MM in immunodeficient mice. Oral administration of TAS-117 at daily doses of both 12 mg/kg and 16 mg/kg 5 days a week significantly reduced MM.1S tumor growth versus vehicle control (at both doses, on day 18, p<0.001; Fig. 3A). TAS-117 treatment for 3 weeks was well tolerated, without significant weight loss (Fig. 3B). Importantly, overall survival of TAS-117-treated mice was significantly prolonged in both 12 mg/kg and 16 mg/kg cohorts (p<0.05, and p<0.001 versus control, respectively; Fig. 3C). To examine target inhibition by TAS-117 in vivo, we performed immunohistochemical analysis of tumors. The expression of p-Akt was inhibited in tumors harvested from TAS-117- (16 mg/kg) treated mice after 5 days of treatment. TUNEL-positive apoptotic cells were increased by the treatment (Fig. 3D), indicating that TAS-117-induced apoptosis in MM cells is associated with inhibition of p-Akt.
Figure 3. TAS-117 inhibits tumor growth in murine xenograft models of human MM.
(A–D) SCID mice were injected subcutaneously with 5×106 MM.1S cells and treated for 21 days with 12 mg/kg oral TAS-117 daily for 5 days a week (n=10); 16 mg/kg oral TAS-117 daily for 5 days a week (n=10); or vehicle as a control (n=9).
(A) Tumor volume was calculated from caliper measurements every 3–4 days, and data represent mean ± SE.
(B) Body weight of mice was expressed as percentage of baseline. Data represent mean ± SD.
(C) Survival was evaluated from the first day of treatment using Kaplan-Meier curves.
(D) Tumors harvested from TAS-117- (16 mg/kg) and vehicle control- treated mice after 5 days of treatment were subjected to immunohistochemical analysis using p-Akt (Ser473) and TUNEL assay.
(E) SCID mice were injected subcutaneously with 1×107 H929 cells and treated daily for 14 consecutive days with 8 mg/kg oral TAS-117-HCl (n=10); 12 mg/kg oral TAS-117-HCl (n=10); or vehicle alone as a control (n=10). Tumor volume was calculated from caliper measurements every 3–5 days, and data represent mean ± SE.
Using the H929 xenograft model, we further confirmed significant growth inhibition by TAS-117-HCl on day 16 in both 8 mg/kg and 12 mg/kg cohorts (p<0.05, and p<0.01 versus control, respectively; Fig. 3E). These data suggest that the growth inhibitory effect by TAS-117 in vivo is not cell-line specific. In this study, we measured plasma exposure levels of TAS-117 in mice. At the dose of 8 mg/kg, it was 0.860, 1.311, and 0.019 μM at 1, 4, and 24 h post oral administration, respectively. At the dose of 12 mg/kg, it was 3.695, 2.069, and 0.016 μM at 1, 4, and 24 h post oral administration, respectively.
TAS-117 triggers cytotoxicity by inducing apoptosis and autophagy
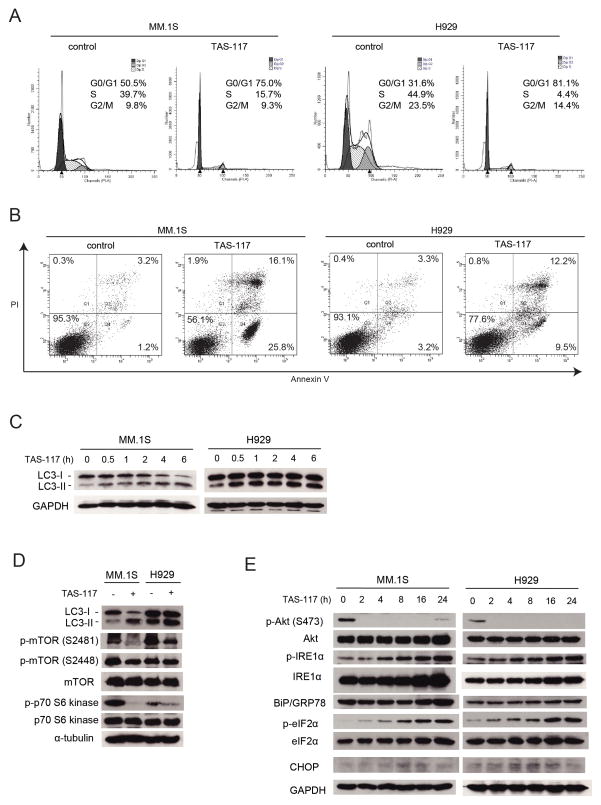
To elucidate the mechanism of cytotoxicity triggered by TAS-117, we performed FACS analysis. PI staining showed increased G0/G1 phase in both MM.1S and H929 cells after culture with TAS-117 for 24 h (Fig. 4A). Moreover, annexin V/PI staining showed increased annexin-positive apoptotic MM.1S and H929 cells after culture with TAS-117 for 48 h (Fig. 4B). Apoptosis induction was also confirmed by cleavage of caspase-8, 3, and PARP (Supplementary Fig. S7). These results suggest that cytotoxicity triggered by TAS-117 is associated with G0/G1 arrest, followed by apoptosis.
Figure 4. TAS-117 triggers G0/G1 arrest followed by apoptosis, associated with induction of autophagy and endoplasmic reticulum stress response.
(A) MM.1S and H929 cells were treated with or without TAS-117 (0.5 μM) for 24 h and then subjected to propidium iodide (PI) staining for cell cycle analysis by flow cytometry.
(B) MM.1S and H929 cells were treated with or without TAS-117 (1 μM) for 48 h. Apoptotic cells were analyzed by flow cytometry using annexin V/PI staining.
(C) MM.1S and H929 cells were treated with TAS-117 (1 μM) for the indicated times. Whole cell lysates were then subjected to western blotting using LC3A/B and GAPDH Abs. Note: the GAPDH blot in MM.1S cells is the same as that in Figure 1B (right panel) because same cell lysates were used in these experiments.
(D) MM.1S and H929 cells were treated with or without TAS-117 (1 μM) for 6 h. Whole cell lysates were subjected to western blotting using LC3A/B, p-mTOR (Ser2481 and Ser2448), mTOR, p-p70 S6 kinase, p70 S6 kinase, and α-tubulin Abs.
(E) MM.1S cells were treated with or without TAS-117 (1 μM), and H929 cells were treated with or without TAS-117 (0.5 μM), for the indicated times. Whole cell lysates were subjected to western blotting using p-Akt (Ser473), Akt, p-IRE1α, IRE1α, BiP/GRP78, p-eIF2α, eIF2α, CHOP, and GAPDH Abs.
Since increase in annexin-positive cells or cleavage of caspase and PARP induced by TAS-117 seems modest compared with significant cytotoxicity in MM.1S and H929 observed in Fig. 1C, we hypothesized that TAS-117 also induces other pathways leading to cell death. Since Akt inhibition leads to induction of autophagy (27), we next examined whether TAS-117 induced autophagy in MM cells. LC3-II was induced by TAS-117 within 6 h in both MM.1S and H929 cells (Fig. 4C), and autophagosome formation was also observed (Supplementary Fig. S8), suggesting early induction of autophagy. We further investigated expression of molecules in mTOR pathway mediating autophagy, downstream of Akt. While phosphorylation of mTOR at Ser2448 was only modestly reduced, phosphorylation of p70 S6 kinase was significantly reduced by TAS-117 treatment, suggesting that mTORC1 activity was inhibited (Fig. 4D). These results indicate that TAS-117 may induce autophagy via inhibition of Akt-mTOR pathway.
The accumulation of misfolded proteins in the endoplasmic reticulum (ER) causes ER stress and triggers the cellular adaptive unfolded protein response (UPR) (28–30). Akt inhibition induces phosphorylation of eukaryotic translation initiation factor 2 (eIF2α), which is mediated by PKR-like ER kinase (PERK) in the UPR (31). Interestingly, TAS-117 increased phosphorylation of eIF2α and inositol-requiring enzyme 1α (IRE1α) as well as induced IRE1α and molecular chaperone BiP/GRP78, indicating that both PERK and IRE1α pathways are activated (Fig. 4E). Importantly, induction of C/EBP homologous protein (CHOP), a transcription factor leading to apoptosis due to uncompensated ER stress (32), was only modest. These results suggest that the ER stress-induced apoptosis may only minimally contribute to significant cytotoxicity of TAS-117.
TAS-117 enhances bortezomib-induced cytotoxicity and fatal ER stress in MM cells
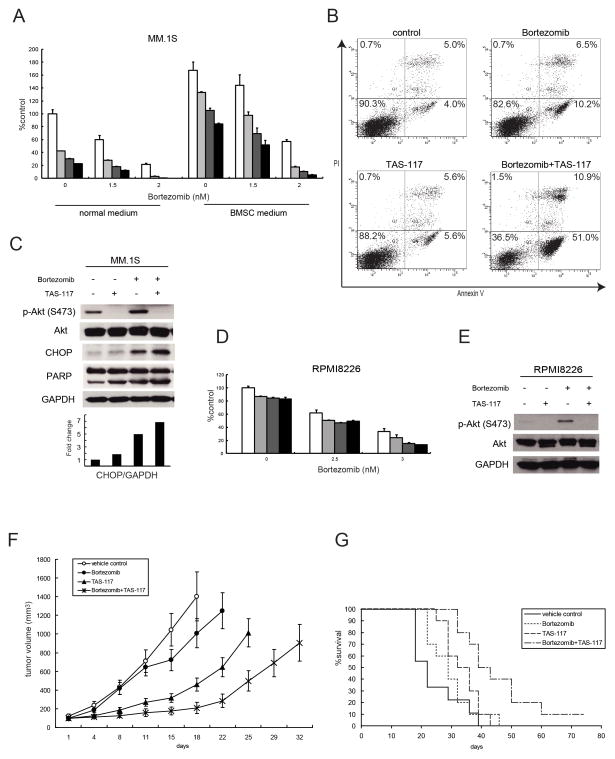
Bortezomib is an effective proteasome inhibitor for MM therapy (33) which induces the terminal UPR, leading to apoptosis (34). Moreover, we have previously shown that bortezomib activates Akt; and conversely, that Akt inhibition by perifosine with bortezomib triggers synergistic MM cell cytotoxicity (17). Therefore we next examined the efficacy of TAS-117 in combination with bortezomib. In MM.1S cells expressing high baseline p-Akt, bortezomib caused increased p-Akt which was inhibited by TAS-117. TAS-117 enhanced growth inhibition triggered by bortezomib, even in the presence of conditioned media derived from BMSCs (Fig. 5A). Combination indices (CI) indicated synergistic cytotoxicity (CI<1.0) at higher doses of combination therapy (Supplementary Table S1). Furthermore, annexin V/PI staining showed that TAS-117 strongly enhanced apoptosis induced by bortezomib (Fig. 5B). Importantly, western blotting showed that CHOP expression induced by bortezomib was enhanced by TAS-117 and associated with increased PARP cleavage (Fig. 5C), suggesting that MM cells are dying, at least in part, due to ER stress-induced apoptosis. Moreover, TAS-117 with bortezomib induced synergistic cytotoxicity (CI<1.0) even in RPMI8226 cells with low baseline p-Akt (Fig. 5D and Supplementary Table S2). As in MM.1S cells, bortezomib induced p-Akt in RPMI8226 cells, which was blocked by TAS-117 (Fig. 5E). These results suggest that TAS-117 can enhance bortezomib-induced cytotoxicity in MM cells by inhibiting bortezomib-induced activation of Akt, regardless of baseline levels of p-Akt, and enhancing ER stress-induced apoptosis.
Figure 5. TAS-117 enhances bortezomib-induced cytotoxicity in vitro and in vivo.
(A) MM.1S cells were cultured for 48 h with bortezomib (0–2 nM) in combination with TAS-117 0 μM (□), 0.25 μM (
 ), 0.5 μM (
), 0.5 μM (
 ), 1 μM (■), in normal medium or conditioned medium derived from culture supernatant of BMSCs (BMSC medium). Cell growth was assessed by MTT assay of triplicate cultures, expressed as percentage of untreated control. Data represents mean ± SD.
), 1 μM (■), in normal medium or conditioned medium derived from culture supernatant of BMSCs (BMSC medium). Cell growth was assessed by MTT assay of triplicate cultures, expressed as percentage of untreated control. Data represents mean ± SD.
(B) MM.1S cells were treated with or without TAS-117 (1 μM), bortezomib (2 nM), or the combination for 24 h. Apoptotic cells were analyzed by flow cytometry using annexin V/PI staining.
(C) MM.1S cells were treated with or without bortezomib (10 nM) in the presence or absence of TAS-117 (0.5 μM) for 8 h. Whole cell lysates were subjected to western blotting using p-Akt (Ser473), Akt, CHOP, PARP, and GAPDH Abs. The graph represents fold changes of CHOP density relative to GAPDH.
(D) RPMI8226 cells were cultured for 48 h with bortezomib (0–3 nM) in combination with TAS-117 0 μM (□), 0.25 μM (
 ), 0.5 μM (
), 0.5 μM (
 ), 1 μM (■). Cell viability was assessed by MTT assay of triplicate cultures, expressed as percentage of untreated control. Data represents mean ± SD.
), 1 μM (■). Cell viability was assessed by MTT assay of triplicate cultures, expressed as percentage of untreated control. Data represents mean ± SD.
(E) RPMI8226 cells were treated with or without bortezomib (10 nM) in the presence or absence of TAS-117 (1 μM) for 8 h. Whole cell lysates were subjected to western blotting using p-Akt (Ser473), Akt, and GAPDH Abs.
(F–G) SCID mice were injected subcutaneously with 5×106 MM.1S cells and treated for 21 days with 0.5 mg/kg subcutaneous bortezomib twice a week (n=10); 12 mg/kg oral TAS-117 5 days a week (n=10); 0.5 mg/kg subcutaneous bortezomib twice a week and 12 mg/kg oral TAS-117 5 days a week (n=10); or vehicle alone as a control (n=9). Note: we performed the in-vivo studies using MM.1S cells at the same time for Figure 3A–D, so the vehicle control and TAS-117 (12 mg/kg) groups are commonly used.
(F) Tumor volume was calculated from caliper measurements every 3–4 days, anddata represent mean ± SE.
(G) Survival was evaluated from the first day of treatment using Kaplan-Meier curves.
TAS-117 enhances bortezomib-induced MM cytotoxicity in vivo
To determine whether the combination of TAS-117 with bortezomib is effective in vivo, we tested this combination using the subcutaneous xenograft model of human MM in mice. Treatment of TAS-117 in combination with low dose bortezomib for 3 weeks significantly inhibited MM cell growth versus control (day 18, p<0.001), versus low dose bortezomib alone (day 22, p<0.001), and versus TAS-117 alone (day 22, p<0.01; Fig. 5F). Importantly, overall survival in TAS-117 with low dose bortezomib-treated mice was significantly prolonged versus control (p<0.001), versus low dose bortezomib alone (p<0.01), and versus TAS-117 alone (p<0.05) treated cohorts (Fig. 5G). Importantly, combination treatment of TAS-117 with bortezomib was tolerable without weight loss (Supplementary Fig. S9). These results demonstrate that TAS-117 in combination with bortezomib induces promising anti-MM activity and tolerability in vivo.
TAS-117 enhances carfilzomib-induced cytotoxicity and fatal ER stress in MM cells
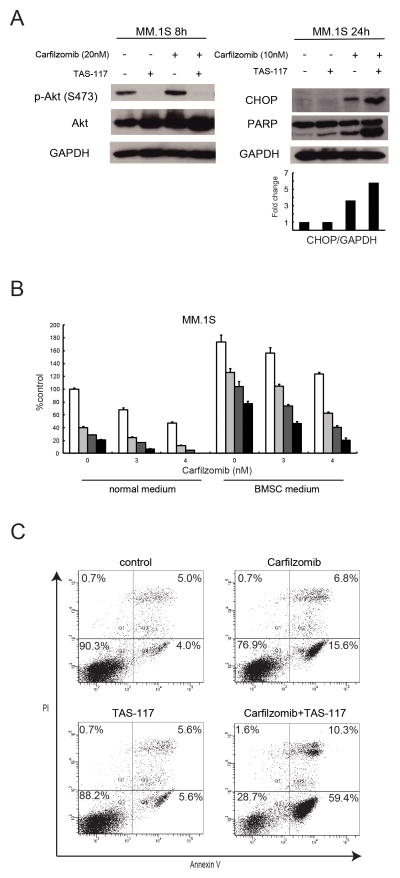
Carfilzomib, a second generation proteasome inhibitor which irreversibly inhibits chymotryptic activity (35), has been approved by the US Food and Drug Administration to treat MM resistant to bortezomib (36). As with bortezomib, carfilzomib also enhanced p-Akt in MM.1S cells, which was blocked by TAS-117 (Fig. 6A, left). Moreover, CHOP expression was induced by carfilzomib indicating ER stress, which was enhanced by TAS-117 and associated with enhanced PARP cleavage (Fig. 6A, right). MTT assay showed that TAS-117 enhanced cytotoxicity induced by carfilzomib, even in the presence of conditioned medium derived from BMSCs (Fig. 6B). The combination of TAS-117 with carfilzomib is more synergistic than with bortezomib (Supplementary Table S3). Apoptotic cells induced by carfilzomib were significantly increased in combination with TAS-117, detected by annexin V/PI staining (Fig. 6C). These results suggest that TAS-117 augments carfilzomib-induced apoptosis, at least in part, via increased ER stress. As shown in combination with bortezomib, TAS-117 in combination with carfilzomib induced synergistic cytotoxicity in RPMI8226 cells with low baseline of p-Akt (Supplementary Fig. S10A and Supplementary Table S4), associated with inhibition of p-Akt triggered by carfilzomib (Supplementary Fig. S10B), suggesting the potential clinical utility of this combination regardless of baseline levels of p-Akt.
Figure 6. TAS-117 enhances cytotoxicity induced by carfilzomib.
(A) MM.1S cells were treated with or without carfilzomib (10 or 20 nM) in the presence or absence of TAS-117 (0.5 μM) for 8 or 24 h. Whole cell lysates were subjected to western blotting using p-Akt (Ser473), Akt, CHOP, PARP, and GAPDH Abs. The graph represents fold changes of CHOP density relative to GAPDH.
(B) MM.1S cells were cultured for 48 h with carfilzomib (0–4 nM) in combination with TAS-117 0 μM (□), 0.25 μM (
 ), 0.5 μM (
), 0.5 μM (
 ), 1 μM (■), in normal medium or conditioned medium derived from culture supernatant of BMSCs (BMSC medium). Cell growth was assessed by MTT assay of triplicate cultures, expressed as percentage of untreated control. Data represents mean ± SD.
), 1 μM (■), in normal medium or conditioned medium derived from culture supernatant of BMSCs (BMSC medium). Cell growth was assessed by MTT assay of triplicate cultures, expressed as percentage of untreated control. Data represents mean ± SD.
(C) MM.1S cells were treated with or without TAS-117 (1 μM), carfilzomib (5 nM), or the combination for 24 h. Apoptotic cells were analyzed by flow cytometry using annexin V/PI staining. Note: we performed the FACS analysis for the combination of TAS-117 with bortezomib or carfilzomib at the same time for Figure 5B, so the control and TAS-117 panels are commonly used.
Discussion
PI3K/Akt pathway plays a crucial role in MM cell growth, survival, and drug resistance (7, 11). Many MM cell lines express high baseline levels of phosphorylated Akt; moreover, Akt is also activated in patient MM cells (37). Perifosine interacts with the cell membrane and modulates intracellular growth signaling pathways, and has been developed as an Akt inhibitor for clinical application (19, 20, 38). Indeed we have previously shown its efficacy using both in-vitro and in-vivo preclinical MM models (17) and in phase I/II clinical trial (39); however, it is not highly potent or selective, and phase III clinical trials performed in MM (38) were terminated due to slow accrual and limited resources; therefore, there remains a need for a potent selective Akt inhibitor. More recently, a selective Akt inhibitor MK-2206 has been reported to inhibit MM growth in vitro, either alone or in combination with mTORC1 inhibitor, PI3K inhibitor, or MEK1/2 inhibitor, suggesting the clinical utility of a selective Akt inhibitor in MM (21). In the current report, we demonstrate the efficacy of a novel potent and selective Akt inhibitor TAS-117 in vitro and in vivo. TAS-117 does not inhibit PDK1 and mTOR, the kinases responsible for phosphorylation of Akt at Thr308 and Ser473, respectively, in enzyme assays. TAS-117 does inhibit phosphorylation of p-FKHR/FKHRL1 downstream of Akt, but not PDK1 autophosphorylation upstream of Akt in MM cells. In addition, in vitro Akt IP-kinase assay shows direct inhibition of Akt by TAS-117 in MM cells. These data indicate that the target of TAS-117 in the PI3K/Akt pathway is Akt. TAS-117 induces significant growth inhibition in MM cell lines with high baseline p-Akt, SP cells, and patient MM cells, without affecting normal mononuclear cells. Importantly, TAS-117 is more potent than perifosine in high-serum conditions and in coculture with BMSCs. Finally, TAS-117 inhibits human MM cell growth in vivo in xenograft models. Our data therefore demonstrate the efficacy of TAS-117 against MM cells with high baseline p-Akt both in vitro and in vivo, suggesting its potential clinical utility as a single agent especially for a subset of patient population with high baseline p-Akt. Akt phosphorylation of MM cells may be used as a biomarker for patient selection, as similarly suggested in the study of MK-2206 (21).
The BM microenvironment triggers activation of multiple signaling cascades in MM cells including MEK/ERK, JAK2/STAT, and PI3K/Akt (15, 16). Specifically, Akt is activated by cytokines such as IL-6 and IGF-1 in the BM milieu. Importantly, TAS-117 induces significant cytotoxicity in MM cells even in the presence of BMSCs or cytokines, associated with blockade of Akt activity. IL-6 secretion from BMSCs is also inhibited by TAS-117, associated with NF-κB inhibition. These data further support potential clinical efficacy of TAS-117, since it can overcome the survival and growth advantage conferred in the context of the BM. In addition, the Akt pathway in MM cells with low baseline p-Akt in vitro can be activated in the BM milieu, indicating possible broad application in clinical practice.
We have shown that TAS-117 induces multiple apoptosis, autophagy, and ER stress cell signaling pathways. Autophagy is observed within 6 h of TAS-117 treatment. In contrast, apoptosis is observed at 24–48 h. These results suggest that early cytotoxic effects of TAS-117 are associated with autophagy, whereas late cytotoxic effects are related to apoptosis. Interestingly, TAS-117 induces p-IRE1α, IRE1α, BiP/GRP78, and p-eIF2α, which are upregulated by the UPR as an adaptive cellular response to ER stress. In contrast, the induction of CHOP, a fatal ER-stress marker, is only modest, indicating that TAS-117 alone induces ER stress and the UPR as an adaptive response. A recent study also shows that phosphorylation of eIF2α in the PERK signaling pathway is a cellular adaptive response to Akt inhibition (31). Our data further demonstrate that both PERK and IRE1α pathways are activated by Akt inhibition in MM cells, indicating global ER stress triggered by TAS-117. Crosstalk between Akt pathway, ER stress, and the subsequent UPR is being delineated in ongoing studies.
Proteasome inhibitors play a central role in MM treatment. Bortezomib, the first FDA-approved proteasome inhibitor, is a major advance in MM; however, some patients do not respond and most patients acquire resistance with time (4). Though perifosine was reported to act synergistically with bortezomib (17), it has not been elucidated whether selective Akt inhibition triggers synergy with bortezomib. Importantly, we demonstrate that selective Akt inhibition by TAS-117 enhances bortezomib-induced cytotoxicity. Moreover, TAS-117 blocks p-Akt induced by bortezomib, suggesting a potential strategy to overcome bortezomib-resistance. We also show that TAS-117 enhances cytotoxicity induced by carfilzomib, and blocks carfilzomib-induced activation of Akt. Furthermore, TAS-117 enhances proteasome inhibitor-induced cytotoxicity even in RPMI8226 cells with low baseline p-Akt, since proteasome inhibition by bortezomib or carfilzomib can increase p-Akt, which is blocked by TAS-117. Taken together, these results suggest that combinations of Akt inhibitors with proteasome inhibitors represent a promising strategy for MM therapy regardless of baseline p-Akt level in tumors.
Interestingly, CHOP expression induced by bortezomib is enhanced by TAS-117: it enhances uncompensated ER stress induced by bortezomib, leading to apoptosis. Carfilzomib also induces CHOP expression, which is similarly enhanced by TAS-117. Our data therefore demonstrate that the selective Akt inhibitor TAS-117 enhances fatal ER stress induced by proteasome inhibitors in MM. We have recently reported that the IRE1α endoribonuclease inhibitor MKC-3946 blocks XBP1 splicing and thereby enhances fatal ER stress induced by bortezomib (40). These combinations of ER-stress enhancers with proteasome inhibitors hold great clinical promise.
In summary, we demonstrate that selective and potent Akt inhibition by TAS-117 triggers anti-MM activities in vitro and in vivo, as well as enhances cytotoxicity induced by proteasome inhibition. Our results provide the preclinical framework for clinical trials of selective Akt inhibitors such as TAS-117, alone and in combination with proteasome inhibitors, to improve patient outcome in MM.
Supplementary Material
Acknowledgments
Grant Support
This work was supported by the National Institutes of Health grants (SPORE-P50100707, PO1-CA078378, and RO1CA050947). N.M. was a recipient of the Kano-grant from the Japanese Society of Myeloma. K.C.A. is an American Cancer Society Clinical Research Professor.
Footnotes
Disclosure of Potential Conflicts of Interest
T.S. is an employee at TAIHO PHARMACEUTICAL CO., LTD. P.G.R. serves on advisory boards to Celgene, Millennium, Johnson & Johnson, Novartis, and Bristol-Myers Squibb. T.U. is the Executive Director of the Tsukuba Research Center and is a member of the Board of TAIHO PHARMACEUTICAL CO., LTD. K.C.A. serves on advisory boards to Celgene, Onyx, Sanofi-Aventis, and Gilead. The remaining authors declare no competing financial interests.
References
- 1.Laubach J, Richardson P, Anderson K. Multiple myeloma. Annu Rev Med. 2011;62:249–64. doi: 10.1146/annurev-med-070209-175325. [DOI] [PubMed] [Google Scholar]
- 2.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–60. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 3.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–20. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahindra A, Laubach J, Raje N, Munshi N, Richardson PG, Anderson K. Latest advances and current challenges in the treatment of multiple myeloma. Nat Rev Clin Oncol. 2012;9:135–43. doi: 10.1038/nrclinonc.2012.15. [DOI] [PubMed] [Google Scholar]
- 5.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 6.Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–9. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- 7.Younes H, Leleu X, Hatjiharissi E, Moreau AS, Hideshima T, Richardson P, et al. Targeting the phosphatidylinositol 3-kinase pathway in multiple myeloma. Clin Cacer Res. 2007;13:3771–5. doi: 10.1158/1078-0432.CCR-06-2921. [DOI] [PubMed] [Google Scholar]
- 8.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–62. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 9.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–44. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 11.Pene F, Claessens YE, Muller O, Viguie F, Mayeux P, Dreyfus F, et al. Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase pathways in the proliferation and apoptosis in multiple myeloma. Oncogene. 2002;21:6587–97. doi: 10.1038/sj.onc.1205923. [DOI] [PubMed] [Google Scholar]
- 12.Janku F, McConkey DJ, Hong DS, Kurzrock R. Autophagy as a target for anticancer therapy. Nat Rev Clin Oncol. 2011;8:528–39. doi: 10.1038/nrclinonc.2011.71. [DOI] [PubMed] [Google Scholar]
- 13.Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20:5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- 14.Mitsiades CS, Mitsiades N, Poulaki V, Schlossman R, Akiyama M, Chauhan D, et al. Activation of NF-kappaB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene. 2002;21:5673–83. doi: 10.1038/sj.onc.1205664. [DOI] [PubMed] [Google Scholar]
- 15.Hideshima T, Anderson KC. Molecular mechanisms of novel therapeutic approaches for multiple myeloma. Nat Rev Cancer. 2002;2:927–37. doi: 10.1038/nrc952. [DOI] [PubMed] [Google Scholar]
- 16.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–98. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 17.Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053–62. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda H, Hideshima T, Fulciniti M, Perrone G, Miura N, Yasui H, et al. PI3K/p110{delta} is a novel therapeutic target in multiple myeloma. Blood. 2010;116:1460–8. doi: 10.1182/blood-2009-06-222943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2:1093–103. [PubMed] [Google Scholar]
- 20.Ruiter GA, Zerp SF, Bartelink H, van Blitterswijk WJ, Verheij M. Anti-cancer alkyl-lysophospholipids inhibit the phosphatidylinositol 3-kinase-Akt/PKB survival pathway. Anticancer Drugs. 2003;14:167–73. doi: 10.1097/00001813-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Ramakrishnan V, Kimlinger T, Haug J, Painuly U, Wellik L, Halling T, et al. Anti-myeloma activity of Akt inhibition is linked to the activation status of PI3K/Akt and MEK/ERK pathway. PLoS One. 2012;7:e50005. doi: 10.1371/journal.pone.0050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gills JJ, Dennis PA. Perifosine: update on a novel Akt inhibitor. Curr Oncol Rep. 2009;11:102–10. doi: 10.1007/s11912-009-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda H, Hideshima T, Fulciniti M, Lutz RJ, Yasui H, Okawa Y, et al. The monoclonal antibody nBT062 conjugated to cytotoxic Maytansinoids has selective cytotoxicity against CD138-positive multiple myeloma cells in vitro and in vivo. Clin Cancer Res. 2009;15:4028–37. doi: 10.1158/1078-0432.CCR-08-2867. [DOI] [PubMed] [Google Scholar]
- 24.Hideshima T, Neri P, Tassone P, Yasui H, Ishitsuka K, Raje N, et al. MLN120B, a novel IkappaB kinase beta inhibitor, blocks multiple myeloma cell growth in vitro and in vivo. Clin Cancer Res. 2006;12:5887–94. doi: 10.1158/1078-0432.CCR-05-2501. [DOI] [PubMed] [Google Scholar]
- 25.Cirstea D, Hideshima T, Rodig S, Santo L, Pozzi S, Vallet S, et al. Dual inhibition of akt/mammalian target of rapamycin pathway by nanoparticle albumin-bound-rapamycin and perifosine induces antitumor activity in multiple myeloma. Mol Cancer Ther. 2010;9:963–75. doi: 10.1158/1535-7163.MCT-09-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jakubikova J, Adamia S, Kost-Alimova M, Klippel S, Cervi D, Daley JF, et al. Lenalidomide targets clonogenic side population in multiple myeloma: pathophysiologic and clinical implications. Blood. 2011;117:4409–19. doi: 10.1182/blood-2010-02-267344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Degtyarev M, De Maziere A, Orr C, Lin J, Lee BB, Tien JY, et al. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J Cell Biol. 2008;183:101–16. doi: 10.1083/jcb.200801099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 29.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–30. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 30.Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008;8:663–74. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- 31.Mounir Z, Krishnamoorthy JL, Wang S, Papadopoulou B, Campbell S, Muller WJ, et al. Akt determines cell fate through inhibition of the PERK-eIF2alpha phosphorylation pathway. Sci Signal. 2011;4:ra62. doi: 10.1126/scisignal.2001630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–59. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–98. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 34.Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–16. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demo SD, Kirk CJ, Aujay MA, Buchholz TJ, Dajee M, Ho MN, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67:6383–91. doi: 10.1158/0008-5472.CAN-06-4086. [DOI] [PubMed] [Google Scholar]
- 36.Lawasut P, Chauhan D, Laubach J, Hayes C, Fabre C, Maglio M, et al. New proteasome inhibitors in myeloma. Curr Hematol Malig Rep. 2012;7:258–66. doi: 10.1007/s11899-012-0141-2. [DOI] [PubMed] [Google Scholar]
- 37.Hsu J, Shi Y, Krajewski S, Renner S, Fisher M, Reed JC, et al. The AKT kinase is activated in multiple myeloma tumor cells. Blood. 2001;98:2853–5. doi: 10.1182/blood.v98.9.2853. [DOI] [PubMed] [Google Scholar]
- 38.Richardson PG, Eng C, Kolesar J, Hideshima T, Anderson KC. Perifosine, an oral, anti-cancer agent and inhibitor of the Akt pathway: mechanistic actions, pharmacodynamics, pharmacokinetics, and clinical activity. Expert Opin Drug Metab Toxicol. 2012;8:623–33. doi: 10.1517/17425255.2012.681376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richardson PG, Wolf J, Jakubowiak A, Zonder J, Lonial S, Irwin D, et al. Perifosine plus bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma previously treated with bortezomib: results of a multicenter phase I/II trial. J Clin Oncol. 2011;29:4243–9. doi: 10.1200/JCO.2010.33.9788. [DOI] [PubMed] [Google Scholar]
- 40.Mimura N, Fulciniti M, Gorgun G, Tai YT, Cirstea D, Santo L, et al. Blockade of XBP1 splicing by inhibition of IRE1alpha is a promising therapeutic option in multiple myeloma. Blood. 2012;119:5772–81. doi: 10.1182/blood-2011-07-366633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.