Abstract
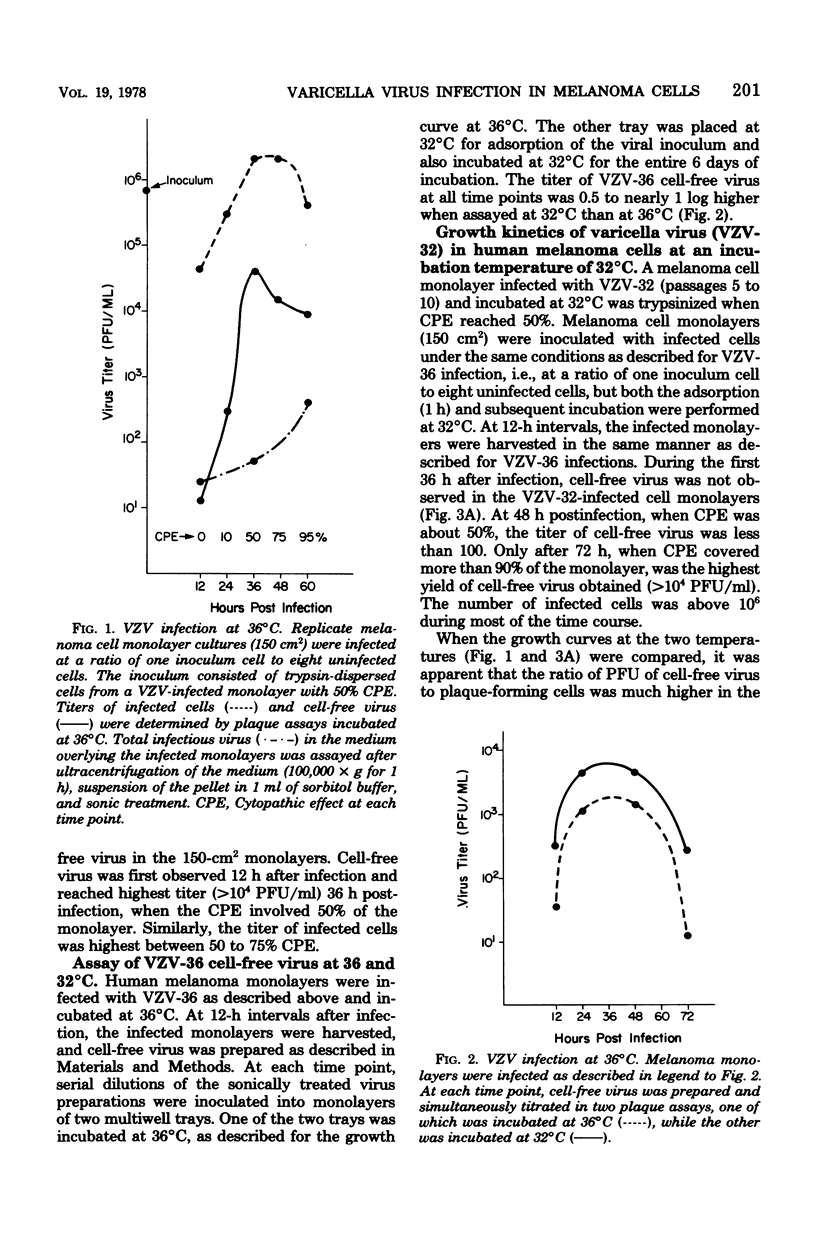
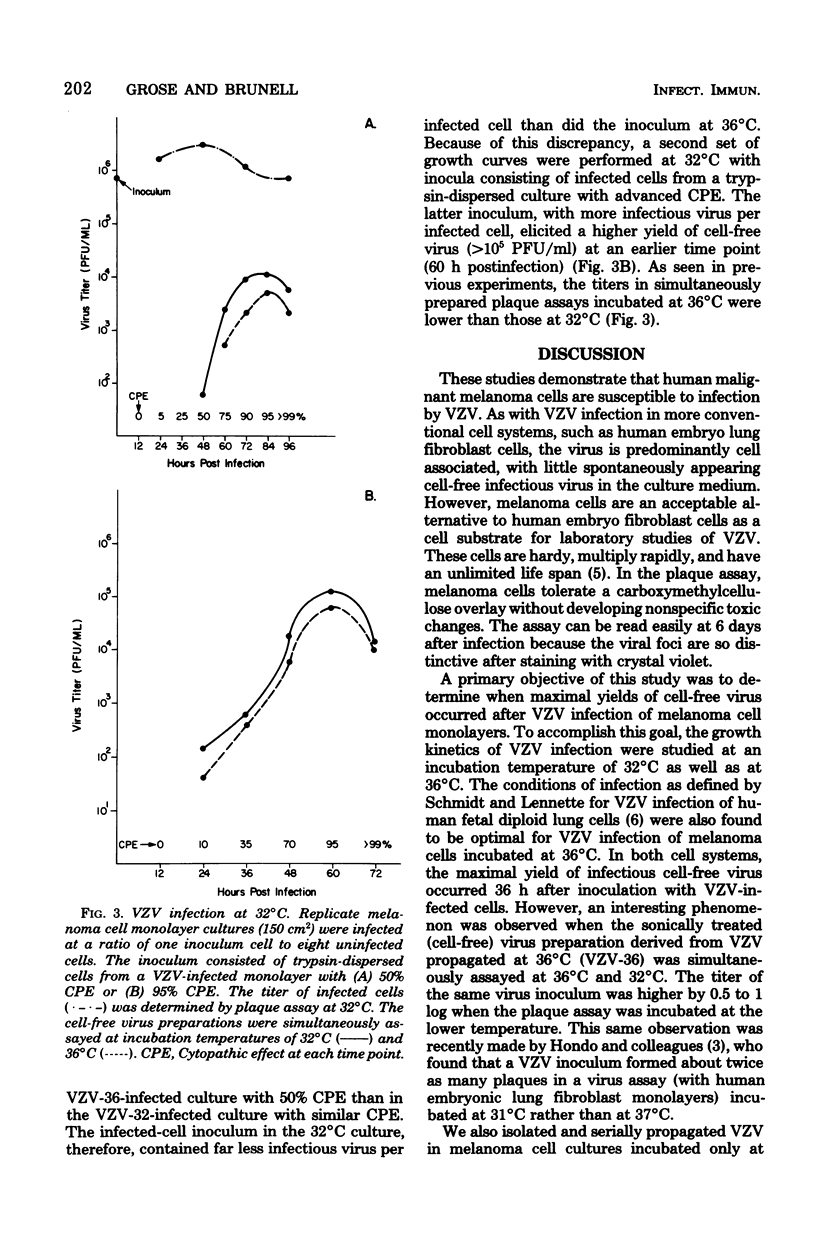
Cell lines derived from human malignant melanoma tumors are susceptible to infection with varicella-zoster virus (VZV). Within 5 days after inoculation of vesicular fluid, cytopathic changes appeared in melanoma cell monolayer cultures that were incubated at either 36 or 32 degrees C. The VZV isolates at the two temperatures were serially propagated by passage of trypsin-dispersed infected cells. A plaque assay was developed utilizing melanoma cell monolayers overlaid with nutrient medium containing carboxymethylcellulose. By this assay method, the growth cycle of a VZV isolate propagated at 36 degrees C was studied and compared with that of another VZV isolate grown at 32 degrees C. With equivalent infected-cell inocula at a ratio on one inoculum cell to eight uninfected cells, the yield of cell-free virus at an incubation temperature of 32 degrees C was slightly higher than at 36 degrees C, although the peak occurred 60 h, rather than 36 h, postinfection. It was also found that the titer of low-passage VZV propagated at 36 degrees C was 0.5 to 1 log higher when assayed at 32 degrees C rather than at 36 degrees C.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunell P. A. Separation of infectious Varicella-Zoster virus from human embryonic lung fibroblasts. Virology. 1967 Apr;31(4):732–734. doi: 10.1016/0042-6822(67)90207-3. [DOI] [PubMed] [Google Scholar]
- Carey T. E., Takahashi T., Resnick L. A., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma: mixed hemadsorption assays for humoral immunity to cultured autologous melanoma cells. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3278–3282. doi: 10.1073/pnas.73.9.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondo R., Shibuta H., Matumoto M. An improved plaque assay for varicella virus. Arch Virol. 1976;51(4):355–359. doi: 10.1007/BF01317939. [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B., Merigan T. C. A useful quantitative semimicromethod for viral plaque assay. Proc Soc Exp Biol Med. 1973 Apr;142(4):1174–1179. doi: 10.3181/00379727-142-37202. [DOI] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H. Improved yields of cell-free varicella-zoster virus. Infect Immun. 1976 Sep;14(3):709–715. doi: 10.1128/iai.14.3.709-715.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLER T. H. Serial propagation in vitro of agents producing inclusion bodies derived from varicella and herpes zoster. Proc Soc Exp Biol Med. 1953 Jun;83(2):340–346. doi: 10.3181/00379727-83-20354. [DOI] [PubMed] [Google Scholar]
- WELLER T. H., WITTON H. M., BELL E. J. The etiologic agents of varicella and herpes zoster; isolation, propagation, and cultural characteristics in vitro. J Exp Med. 1958 Dec 1;108(6):843–868. doi: 10.1084/jem.108.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams V., Gershon A., Brunell P. A. Serologic response to varicella-zoster membrane antigens measured by direct immunofluorescence. J Infect Dis. 1974 Dec;130(6):669–672. doi: 10.1093/infdis/130.6.669. [DOI] [PubMed] [Google Scholar]



