Abstract
Background
Trypanosoma cruzi is the causative agent of Chagas disease. Chagas disease is an endemic infection that affects over 8 million people throughout Latin America and now has become a global challenge. The current pharmacological treatment of patients is unsuccessful in most cases, highly toxic, and no vaccines are available. The results of inadequate treatment could lead to heart failure resulting in death. Therefore, a vaccine that elicits neutralizing antibodies mediated by cell-mediated immune responses and protection against Chagas disease is necessary.
Methodology/Principal Findings
The “antigen capsid-incorporation” strategy is based upon the display of the T. cruzi epitope as an integral component of the adenovirus' capsid rather than an encoded transgene. This strategy is predicted to induce a robust humoral immune response to the presented antigen, similar to the response provoked by native Ad capsid proteins. The antigen chosen was T. cruzi gp83, a ligand that is used by T. cruzi to attach to host cells to initiate infection. The gp83 epitope, recognized by the neutralizing MAb 4A4, along with His6 were incorporated into the Ad serotype 5 (Ad5) vector to generate the vector Ad5-HVR1-gp83-18 (Ad5-gp83). This vector was evaluated by molecular and immunological analyses. Vectors were injected to elicit immune responses against gp83 in mouse models. Our findings indicate that mice immunized with the vector Ad5-gp83 and challenged with a lethal dose of T. cruzi trypomastigotes confer strong immunoprotection with significant reduction in parasitemia levels, increased survival rate and induction of neutralizing antibodies.
Conclusions/Significance
This data demonstrates that immunization with adenovirus containing capsid-incorporated T. cruzi antigen elicits a significant anti-gp83-specific response in two different mouse models, and protection against T. cruzi infection by eliciting neutralizing antibodies mediated by cell-mediated immune responses, as evidenced by the production of several Ig isotypes. Taken together, these novel results show that the recombinant Ad5 presenting T. cruzi gp83 antigen is a useful candidate for the development of a vaccine against Chagas disease.
Author Summary
Chagas disease is caused by the protozoan parasite Trypanosoma cruzi and has been detrimental to millions of people in Latin America since the early 1900s, and now this disease is having a global impact. Decades later, there is still no vaccine for this disease. Scientists have used the adenovirus vector in gene therapy as well as vaccine therapy for many diseases. In this study, adenovirus is used as the host vector for a potential vaccine against Chagas disease in a non-traditional way. Instead of the antigen being expressed as a transgene product, the antigen is incorporated on the capsid of the adenovirus to evoke an enhanced humoral immune response against T. cruzi. This study highlights the modified adenovirus' ability to elicit a strong anti-T. cruzi humoral response as well as providing protection against Trypanosoma cruzi infection. These findings illustrate the true potential of generating an effective T. cruzi vaccine by means of unique gene therapy-based techniques.
Introduction
Trypanosoma cruzi is the intracellular parasite that causes Chagas disease [1]. Chagas disease is an acute and chronic infection that affects over 8 million people throughout endemic regions in Mexico, Central and South America [2]. Recently, the disease has received increasing attention as an emerging health problem in North America, Europe, Japan and Australia due to international migrations from endemic areas to non-endemic areas [3], [4], posing a new significant worldwide health challenge. Over the decades, there has been an increase in the number of infected persons residing in the United States with over 300,000 reported cases [5]. T. cruzi is transmitted to the mammalian host at the site of a triatomine bug bite [6]. Nifurtimox and Benznidazol are current treatments for this infection [7]. These anti-parasitic drugs are 80% successful in curing the acute phase with severe side effects, but ineffective in curing the chronic phase. The results of inadequate treatment could lead to severe arrhythmias, congestive heart failure, and sudden death [8], [9], common pathological features of the disease. There is no vaccine against Chagas disease at present and progress has been slow despite several years of effort. Therefore, an improved treatment, such as a vaccine against Chagas disease is necessary.
Human adenovirus type 5 (Ad5) has been used as a vehicle for many preclinical and clinical vaccines such as Ebola, malaria, simian immunodeficiency virus (SIV), and human immunodeficiency virus (HIV) [10]–[13]. One advantage of using adenoviral vectors in gene therapy and vaccine approaches is the vectors' ability to efficiently infect a wide variety of cell types. Ad5 vectors also have the capability to contain DNA inserts up to 8 kilobases [14]–[17] and can transduce their genome in replicating and nonproliferating cells. Lastly, adenoviral vectors are easily manipulated using recombinant DNA techniques [18]. We sought out to utilize Ad5 and the “antigen capsid-incorporation” strategy [19] to design a novel vaccine approach against Chagas disease. This strategy consists of incorporating antigenic peptides within the capsid structure of the adenoviral vectors rather than an encoded transgene [16]. The capsid-incorporated antigen strategy can induce a robust humoral immune response to the presented antigen, similar to the response provoked by native Ad capsid proteins [20]–[22].
In one of our first publications, we utilized the antigen capsid-incorporation strategy and modified Ad5 to contain a seven amino acid region of the HIV glycoprotein 41. This incorporation was displayed within the hexon hypervariable region 2 of the virus capsid. We were able to generate a HIV epitope-specific humoral response through prime-boost immunization in BALB/c mice [16]. The goal of our current study was to use our incorporation strategy and design a recombinant Ad5 vector that presents a T. cruzi antigen to elicit epitope-specific humoral responses. Glycoprotein 83 (gp83), is a trans-sialidase like molecule used by T. cruzi trypomastigotes as a ligand to attach to host cells and initiate infection [23]. We have shown that the gp83 is expressed only in invasive trypomastigotes [24] and that blocking T. cruzi gp83 with MAb 4A4, that recognizes a conformational epitope on gp83, neutralizes trypomastigote cellular infection [24]. We also have shown that passive immunization with monovalent Fab fragments of the MAb 4A4 neutralizes T. cruzi infection in rodent models of Chagas disease challenged with a lethal dose of trypomastigotes [23]. Since passive immunization with neutralizing MAb 4A4 confers significant immune protection in mice infected with T. cruzi in terms of significant reduction of parasitemia and increased mice survival rate with respect to controls [23], we reasoned that a vaccine that induces neutralizing antibodies would provide immune protection. Therefore, to test this hypothesis we have chosen to incorporate the gp83 epitope recognized by the neutralizing MAb 4A4 [24] within the Ad5 major capsid protein hexon to evaluate whether this vaccine elicits an immune protective response. We provide evidence that the T. cruzi gp83 antigen was incorporated into the hypervariable region 1 (HVR1) of Ad5. We confirmed through mouse immunizations that the recombinant anti-T. cruzi vectors could elicit epitope-specific humoral immune responses in two different strains of mice. We also confirmed that upon challenge with trypomastigotes, the mice immunized with the antigen capsid-incorporated vector displayed a significant reduction in parasitemia, improvement in their survival rate and were able to elicit neutralizing antibodies.
Materials and Methods
Antibodies
Anti-His6 mouse monoclonal antibody (MAb) was purchased from GenScript (Piscataway, NJ). Anti-Penta-His MAb was purchased from Qiagen (Valencia, CA). Horseradish peroxidase (HRP)-conjugated goat anti-mouse secondary antibody was purchased from Millipore (Temecula, CA). Isotype-specific goat anti-mouse antibodies were purchased from Sigma-Aldrich (St. Louis, MO). Adenovirus fiber antibody (4D2) was purchased from Abcam (Cambridge, MA). Donkey anti-goat HRP-conjugated antibody was purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA).
Cell culture and parasites
Human embryonic kidney (HEK293) cells were obtained from and cultured in the medium recommended by the American Type Culture Collection (Manassas, VA). The cell line was incubated at 37°C and 5% CO2 under humidified conditions.
The Tulahuen strain of T. cruzi [25] was used. Blood trypomastigotes used for challenging immunized mice were obtained from infected mice as described [26]. T. cruzi trypomastigotes expressing green fluorescent protein (GFP) for cellular infection assays were generated as described [27].
Recombinant adenoviral construction
In order to generate recombinant adenovirus with the T. cruzi gp83 epitope (gp83 epitope generated from GenBank accession #: AY513728.1) as well as His6 incorporation genetically incorporated within Ad5 hexon HVR1, the following was performed: the DNA sequence corresponding to the mouse MAb 4A4 binding region of gp83 and His6 (24 amino acids) was generated by GenScript (Piscataway, NJ) and subcloned into the HVR1 region (the gp83 sequence replaced amino acids 139 to 144) of the H5/pH5S plasmid [28]. The resulting plasmid HVR1-gp83-18 was digested with EcoRI and PmeI. These resulting fragments containing the homologous recombination region and the hexon gene were purified, and then recombined with a SwaI-digested Ad5 backbone vector that lacks the hexon gene, pAd5/ΔH5 [29].
Rescue, purification and titration of recombinant Ad5 vector
To rescue the vector, the replicative-defective recombinant adenoviral genome was digested with PacI and transfected with PolyJet (SignaGen Laboratories, Rockville, MD) into the Ad5-E1-expressing HEK293 cells. Multi-step large-scale propagations of recombinant Ad5 vector were performed after the vector was rescued. To purify the rescued vector, two-step cesium chloride ultracentrifugation was employed, followed by dialysis against 1× PBS containing 10% glycerol. To titrate the purified vectors, physical titers expressed as viral particles (VPs) per ml were measured using absorbance at 260 nm. The infectious particles (IPs) per ml were determined by TCID50 assay [30]. Modifications of the hexon gene were confirmed by PCR analysis with the primers pVI 1581 fwd: 5′-TATGTGTGTCATGTATGCGT-3′ and 3′HVR5:5′-GGCATGTAAGAAATATGAGTGTCTGGG-3′. To confirm the gp83 epitope-His6 incorporation within the hexon gene, PCR analysis was performed with the following primers: gp83-18Fwd: 5′-TATGTGTGTCATGTATGCGT-3′ and gp83-18Rev: 5′-TAGGTTGTGATGGTGATGGTGATG-3′.
Western blot analysis
To analyze gp83 epitope and His6 expression, 5×109 VPs/vector were boiled and resolved on SDS-PAGE gels, followed by transfer onto polyvinylidene difluoride (PVDF) membranes, which were then blocked with 5% dry non-fat milk in Phosphate Buffered Saline with Tween 20 (PBST) buffer (1× PBS and, 0.05% Tween 20) for 1 hour. The membrane was incubated overnight at 4°C with His6 MAb (1∶5,000 dilution in blocking buffer, GenScript, Piscataway, NJ), then washed, and incubated with HRP-conjugated goat anti-mouse antibody (1∶5,000; Millipore, Temecula, CA). The proteins were detected by using 3′3′-diaminobenzidine tablets (Sigma-Aldrich, MO) [30].
Mice immunizations
BALB/C or C57BL/6 mice (6–8 weeks) were immunized with either Ad5 (control) or Ad5-gp83 to determine the gp83-specific immunogenicity. The groups of mice were intramuscularly immunized with the corresponding vector (1×1010 VP/mouse) at each time-point, with a two-week interval between prime, boost and reboost.
Whole virus ELISAs, indirect ELISAs, and sera-based isotype ELISAs
In order to investigate the exposure-display of gp83 epitope and His6 on the surface of the capsid, whole virus ELISAs were performed as described elsewhere [28]. Briefly, different amounts of the Ad5-gp83 or Ad5 (control) were immobilized and blocked. The immobilized vector was incubated with His6 MAb (1∶2,000, GenScript, Piscataway, NJ), followed by incubation with the HRP-conjugated goat anti-mouse antibody (1∶5,000, Millipore, Temecula, CA). ELISAs were developed with the SIGMAFAST OPD peroxidase substrate (Sigma-Aldrich, MO) and measured at OD 450 nm.
Indirect ELISA was performed to detect gp83-specfic total IgG levels. ELISA plates were coated with 10 µM gp83-18 peptide (KIYWKQPVEGTKSWTLSK, GenScript, Piscataway, NJ) in 100 µl of 50 mM carbonate buffer per well as previously described in [16]. Unbound peptide was removed by washing with PBST buffer (1× PBS and, 0.05% Tween 20). The plate was then blocked with 5% non-fat milk/PBST for 1 hour at room temperature and 1 µl of serum samples (diluted 1∶100) from control and immunized mice were applied to the plate and incubated for 2 hours at room temperature. The plate was then extensively washed and blocked again followed by incubation with the HRP-conjugated goat anti-mouse antibody (1∶5,000, Millipore, Temecula, CA). ELISA was performed as described above. The amount of anti-gp83 antibodies in the sera was calculated based on a standard curve of mouse IgG protein.
Sera-based IgG isotype ELISAs were performed to determine the magnitude of gp83- specific humoral response and relative isotype development. Briefly, 10 µM gp83-18 peptide (KIYWKQPVEGTKSWTLSK) was used to coat the plates as described above. Immunized sera were diluted 100 fold, applied to the plates, and incubated for 2 hours. Then goat anti-mouse isotypes, IgG1, IgG2a and IgG2b (1∶1,000; Sigma-Aldrich, MO) were added, followed by incubation with HRP-conjugated donkey anti-goat antibody (1∶5,000, Jackson ImmunoResearch, PA). The ELISAs were developed as above.
Mice challenge
Groups of five female C57BL/6 mice (Jackson Laboratory, 6 week-old) that were immunized with either Ad5 (control) or Ad5-gp83 as described above were challenged intraperitoneally with a lethal dose of 5×103 blood trypomastigotes obtained from near the peak of parasitemia of C57BL/6 mice with the clone 20A of the Tulahuen strain of T. cruzi. Parasitemia was monitored by placing 5 µl of tail blood under a coverslip and counting 100 high-powered fields, as described elsewhere [31]. Animals reaching the peak of parasitemia were euthanized because they were moribund. IACUC regulations do not allow maintaining suffering moribund animals. This is consistent with federal policy and regulations for Public Health Service (PHS) funded grants to ensure the humane care and use of animals in research. We used a standardized model of infecting C57BL/6 mice of the same age, weight and sex with a lethal dose of highly infective trypomastigote clone 20A of the Tulahuen strain [25], which present similar levels of parasitemia including when reaching its peak. The day on which moribund mice were euthanized was recorded.
Neutralization assay and fluorescent microscopy
To investigate whether vaccination of mice induce neutralizing antibodies, immunized or control mice were bled before challenging with T. cruzi to obtain serum in order to evaluate the ability of antibodies to neutralize T. cruzi infection of cardiomyocytes as described [32]. Green fluorescent protein(GFP)-expressing trypomastigotes, obtained as described previously [27], resuspended in phenol red-free DMEM were preincubated with the same aliquot of sera from immunized or control mice for 30 min at 37°C and parasites were then exposed in triplicate to cardiomyocyte monolayers in Lab Tech chambers at a ratio of 10 parasites/cell. At the end of the incubation period, unbound parasites were removed, complete fresh medium was added to the co-cultures, and parasite multiplication within cell monolayers at 72 h was determined fluorometrically as relative fluorescence units (RFU), as previously described [27], [32]. To microscopically visualize the effect of neutralizing antibodies on cellular infection, we also exposed GFP-expressing trypomastigotes to antibodies from immunized and control mice sera, as described above, and the parasites were then exposed to cardiomyocytes at a parasite-to-cell ratio of 10∶1 for 72 h, as described previously [27], [32]. Cells were fixed and stained with 4′,6-diamidino-2-phenylindole (DAPI) and Alexa Fluor 546 phalloidin for fluorescence confocal microscopy evaluation of infection [26], [33].
Ethics statement
Animal experiments were performed in strict accordance set forth in the Guide to the Care and Use of Laboratory Animals, DHEW Publication No. (NIH) 78-23 (Revised, 1985). The University of Alabama at Birmingham Institutional Animal Use and Care Committee approved the use of mice as described herein under the approved protocol number 13120997. Animal studies that were conducted at Meharry Medical College were conducted under the National Institutes of Health guidelines on the humane use and care of laboratory animals for biomedical research (Guide for the Care and Use of Laboratory Animals. NIH Publication No. 85-23. Revised 1985). Protocols were approved by the Meharry Medical College Institutional Animal Care and Use Committee.
Statistical analyses
All experiments were repeated three times. Descriptive statistics, such as means and standard deviations, or standard error of the mean, were computed to study variables of interest. Statistical analyses were performed with paired two-tailed Student t-test or nonpaired two-tailed Student t-test, assuming unequal variance. Statistical significance was defined as P<0.05.
Results
Generation and characterization of the modified Ad5 vector
Ad5 (control) and Ad5 vector containing the gp83 mouse MAb 4A4 antibody binding site (GenBank accession #: AY513728.1) (Ad HVR1-gp83-18, from this point forward, will be referred to as Ad5-gp83) as shown in the linear schematic model in Fig. 1A (2) were rescued and upscaled as previously described in [30]. Physical titers and infectious titers were determined to test the stability of the vectors. Based on those two assays, a viral particle/infectious particle (VP/IP) ratio was determined. A normal VP/IP ratio of unmodified Ad ranges from ∼10–30 [30]. We observed an increase in VP/IP ratios for Ad5-gp83 (ratio of 60) when compared to the Ad5 vector (ratio of 30) (Table 1). Based on these observations, the insertion of gp83-His6 epitope had minimal effect on the vector's stability.
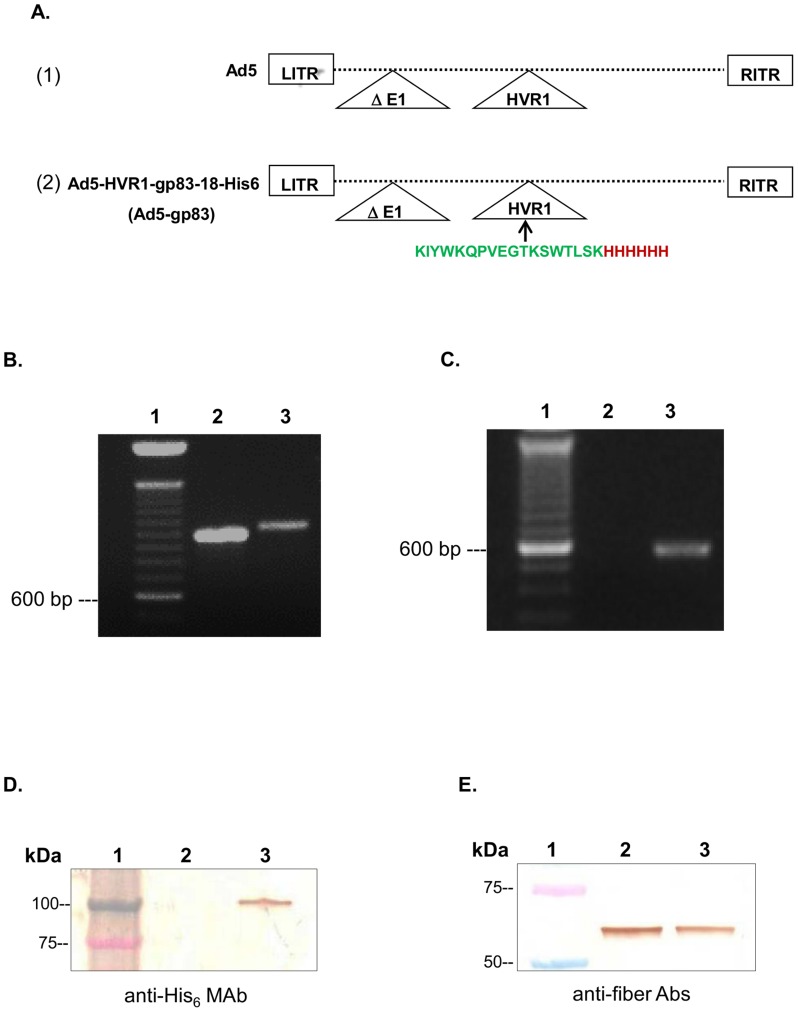
Figure 1. T. cruzi gp83 epitope and His6 epitope genetically incorporated into the HVR1 of Ad5.
Rescued vectors were upscaled and viral DNA was isolated and analyzed to confirm the modification of the relevant genes. A) Schematic of adenoviral genomes. (1) Ad5, a replication-defective adenovirus with unmodified hexon. (2) Ad5-gp83, Ad5 replication-defective genome containing the hexon modification. B) Hexon-specific PCR primers confirmed the presence of the hexon gene in all of the vectors. Lane 1, DNA ladder, lane 2, Ad5; and lane 3, Ad5-gp83. C) T. cruzi-His6-specific primers confirmed the incorporation of gp83 and His6 epitopes. Lane 1, DNA ladder, lane 2, Ad5; and lane 3, Ad5-gp83. D) Western blot analysis confirmed the presence of His6 incorporation within the modified vector. E) Western blot analysis confirmed the presence of fiber within the modified vector. In these assays, protein marker (lane 1), Ad5 (lane 2), and Ad5-gp83 (lane 3) were separated by 4–15% SDS-PAGE. The proteins were transferred to polyvinylidene fluoride (PVDF) membranes then incubated with either monoclonal antibodies to His6 or fiber. The binding was detected with a HRP-conjugated secondary antibody.
Table 1. Virological properties of vectors used in this study.
| Ad5 Vectors | Viral Particles (VP) | Infectious Particles (IP) | VP/IP |
| Ad5 | 2.38×1012 VP/ml | 7.9×1010 IP/ml | 30 |
| Ad5-HVR1-gp83-18 (Ad5-gp83) | 1.5×1012 VP/ml | 2.5×1010 IP/ml | 60 |
To confirm the antigen capsid-incorporation at the genomic level, PCR analysis was performed using genomic DNA from the purified virions. With respect to hexon-specific PCR, primers were designed to amplify a region upstream of HVR1 and downstream the HVR1 insertion site. Ad5 was found to have a wild type hexon producing a 982 base pairs (bp) fragment (Fig. 1B lane 2). The Ad5-gp83 construct revealed a 1036 bp fragment, suggesting the expected insertion (Fig. 1B lane 3). For the gp83-His6-specific PCR, primers were designed to amplify the specific region of the T. cruzi antigen-His6 insertion. As expected, the PCR for Ad5 did not produce a fragment (Fig. 1C lane 2), while Ad5-gp83 produced a 563 bp fragment indicating the incorporation of the gp83 epitope-His6 DNA within the hexon region (Fig. 1C lane 3).
After successful incorporation of the T. cruzi antigen gene, we next sought to verify expression of our incorporation at the protein level by Western blot analysis (Fig. 1D). In order to determine if the hexon modified vector displayed the T. cruzi gp83 epitope within the hexon region, Ad5 and Ad5-gp83 were subjected to Western blot analysis with His6 antibody. The His6 protein was detected as a 117 kDa protein band associated with Ad5-gp83 particles (Fig. 1D lane 3). The size of the 117 kDa band corresponds to the expected size of the Ad5 hexon protein with T. cruzi-His6 epitopes genetically incorporated into the HVR1. There was no His6 protein detected on Ad5 wild type particles (Fig. 1D lane 2). The data indicate that the T. cruzi antigen and His6 were expressed on the hexon gene.
As a control experiment, we sought to determine if the addition of the gp83 epitope onto the Ad5 capsid would dramatically affect the expression of any other capsid proteins. In brief, purified Ad5 and Ad5-gp83 were subjected to Western blot analysis with antibody to fiber protein. The fiber protein was detected as a monomer 64 kDa band associated with both vectors. Notably, the relative fiber protein expression levels for Ad5-gp83 vector were comparable to that of the control vector (Fig. 1E).
T. cruzi antigen capsid-incorporation was exposed on the surface of the virion
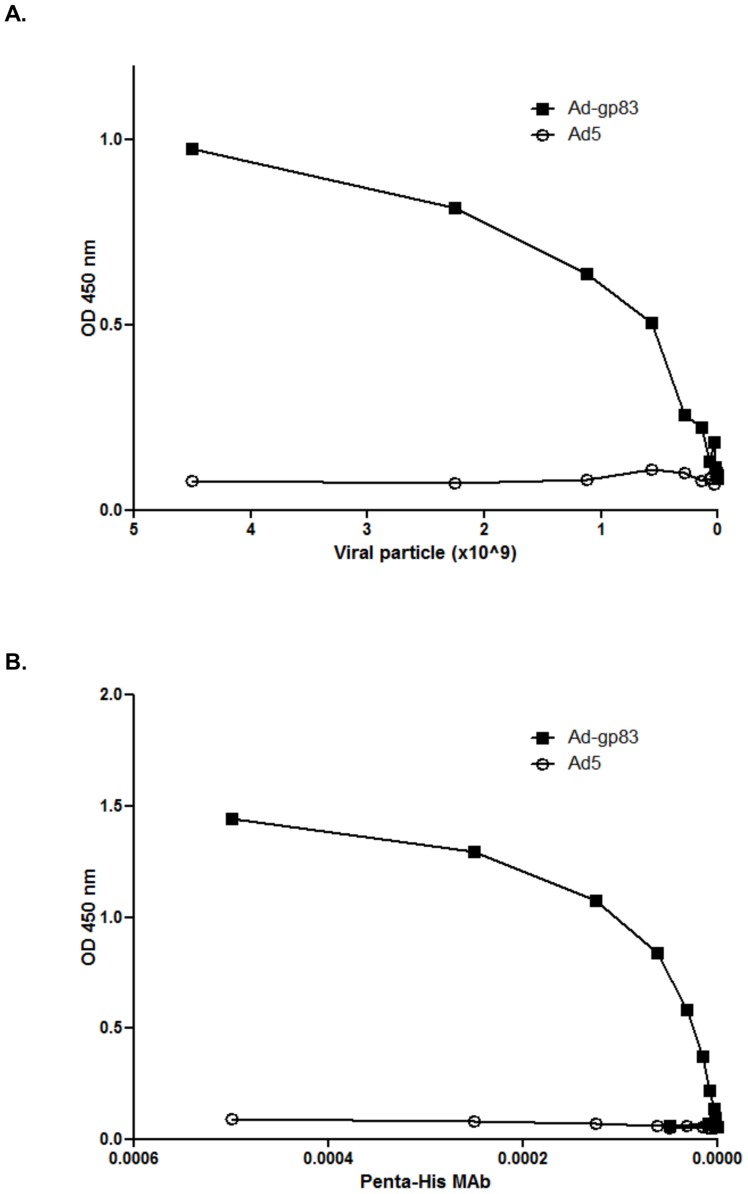
We performed an ELISA assay to verify that the T. cruzi antigen and His6 were accessible on the virion surface (Fig. 2). Serial dilutions of purified virus were immobilized into the wells of an ELISA plate and incubated with His6 MAb. The results showed substantial binding of the His6 antibody to the Ad5-gp83, whereas no binding was seen in response to Ad5 control (Fig. 2A).
Figure 2. Antigens are exposed on the virion surface.
A) Varying amounts (starting at 4.5×109 VP/mouse) of Ad5 or Ad5-gp83 were immobilized into the wells of ELISA plates and incubated with His6 MAb. B) Either Ad5 or Ad5-gp83 at 6×108 VP of were immobilized on an ELISA plate followed by varying dilutions of monoclonal antibody to His6 (starting at 1∶2,000 dilution). The binding was detected with a HRP-conjugated secondary antibody. OD was read at 450 nm with a microplate reader.
In order to determine the ability of the His6 antibody to bind to capsid-incorporated antigen in a dose-dependent manner a dose-response ELISA assay was performed with His6 MAb. A single concentration of Ad5 or Ad5-gp83 was applied to the ELISA plate, followed by the addition of serial dilutions of His6 MAb. As predicted, the His6 antibody bound to Ad5-gp83 in a dose dependent manner (Fig. 2B). These results indicate that the T. cruzi antigen and His6 epitope were properly exposed on the virion surface.
Anti-T. cruzi antibodies were produced in mice after immunization with modified vector
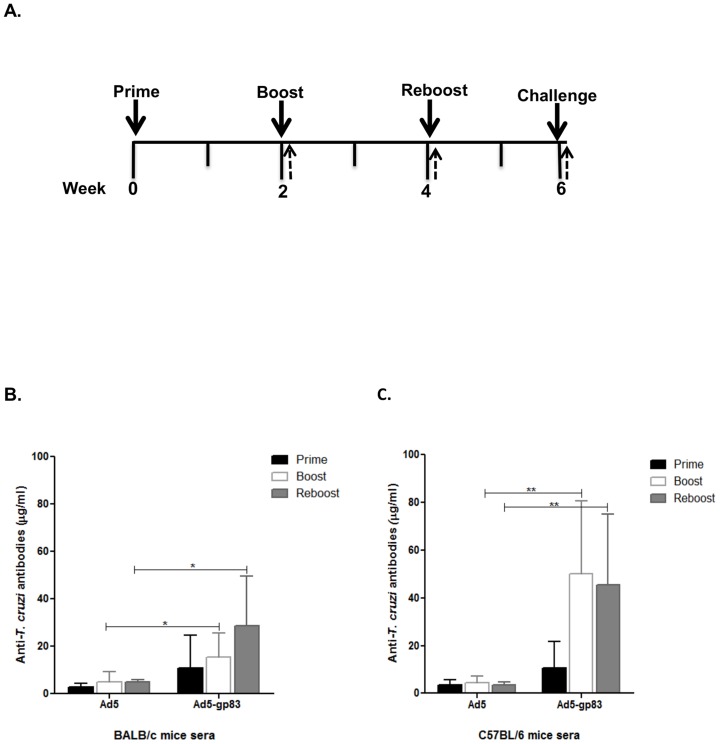
To measure T. cruzi-specific antibody responses elicited by the hexon-modified Ad5 vector, BALB/c and C57BL/6 mice were immunized with Ad5-gp83, according to the immunization schedule depicted in Fig. 3A. For comparison, mice were immunized with the same dose of Ad5 using the same immunization regimen. Sera were tested for antibodies against T. cruzi by ELISA (Fig. 3B and C). Anti-T. cruzi IgG levels increased significantly after boost (P≤0.05) and reboost (P≤0.05) in BALB/c mice immunized with the Ad5-gp83 compared to that of the control (Fig. 3B). There was no significant difference in immunoglobulin levels when comparing prime, boost and reboost in the Ad5-gp83 immunized BALB/c mice group. C57BL/6 mice immunized with the Ad5-gp83 vector as compared to Ad5 controls also showed a significant increase at boost (P≤0.01) and reboost (P≤0.01). There was a significant increase in immunoglobulin levels when comparing prime and boost (P≤0.05) as well as prime and reboost (P≤0.05) in the Ad5-gp83 immunized C57BL/6 mice group (statistical analyses not shown on graph). The antibody response at boost and reboost in the C57BL/6 mice were about 30% higher than that of the Ad5-gp83 BALB/C immunized mice (Fig. 3C).
Figure 3. Antigen capsid-incorporation vector elicits an in vivo T. cruzi humoral immune response.
BALB/c and C57BL/6 mice (n = 7) were primed, boosted, and reboosted with 1×1010 VP of Ad vectors. A) Immunization timeline showing when immunizations were performed (solid arrows) and sera was collected (dashed arrows). B) Post-prime, post-boost, and post-reboost BALB/c serum or C) Post-prime, post-boost, and post-reboost C57BL/6 serum was collected for ELISA binding assays. Ten µM of purified gp83 (KIYWKQPVEGTKSWTLSK) antigenic peptide was bound to ELISA plates. The plates were then incubated with serial diluted concentrations of immunized mice serum and the binding antibodies were detected with HRP conjugated secondary antibody. The amount of anti-gp83 antibodies in the sera was calculated based on a standard curve of mouse IgG protein. The values are expressed as the mean ± standard deviation. (*) = P≤0.05, and (**) = P≤0.01.
Levels of T. cruzi IgG subclasses, IgG1, IgG2a, and IgG2b, were assessed to determine the isotype of the humoral immune response being stimulated (Fig. 4). T. cruzi-specific peptide was bound to the ELISA plates which were then incubated with immunized mice sera, followed by isotype-specific antibodies, IgG1, IgG2a and IgG2b. Our vaccine strategy might also induce cell-mediated immune response based on the results of IgG subtypes obtained since the Th1 IFN-γ cytokine induces class switch to Ig2a, the Treg TGF-β cytokine induces class switch to IgG2b, and Th2 cytokine IL-4 induces class switch to IgG1 in mice. It appears that the predominant cell-mediated immune responses are mediated by T helper and Treg cells in our murine model.
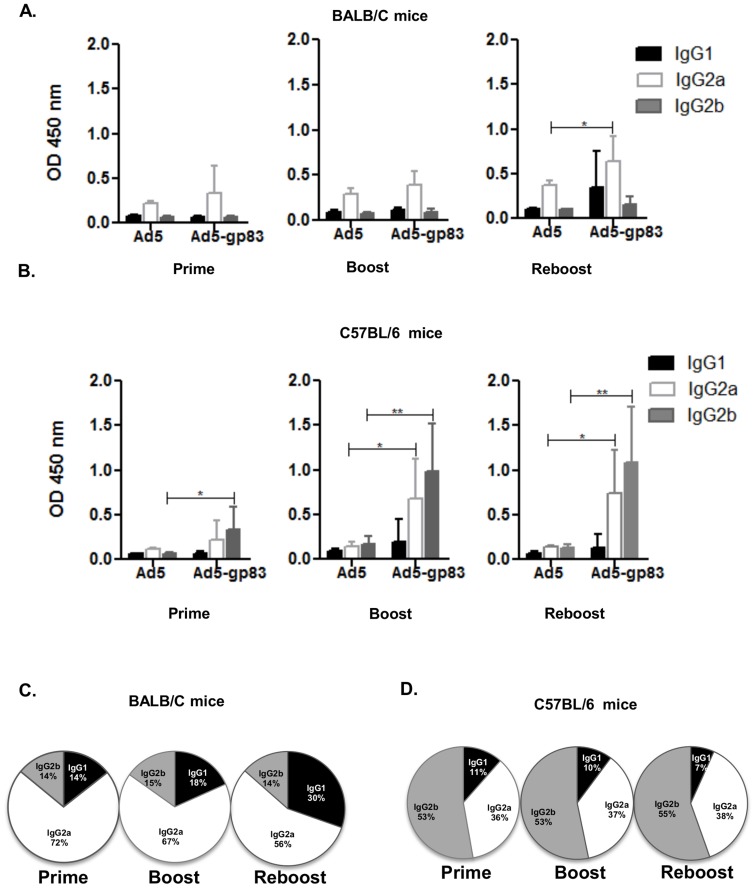
Figure 4. Antigen capsid-incorporation vector elicits an in vivo T. cruzi isotype-specific response.
Post-prime, post-boost, and post-reboost of BALB/c and C57BL/6 mice (n = 7) serum was used for the isotype-specific assays. A–B) Ten µM of purified gp83 (KIYWKQPVEGTKSWTLSK) antigenic peptide was bound to ELISA plates. Residual unbound peptide was washed from the plates. The plates were then incubated with immunized mice serum followed by isotype-specific antibodies. The binding was detected with HRP conjugated secondary antibodies. OD at 450 nm represents isotype-specific T. cruzi gp83 antibody levels in sera. The values are expressed as the mean ± standard deviation. (*) = P≤0.05, and (**) = P≤0.01. C–D) Pie charts illustrating the isotype distribution patterns post-prime, post-boost, and post-reboost for immunized BALB/c mice or C57BL/6 sera.
The sera from the BALB/c Ad5-gp83 immunized mice had detectable levels of IgG1, IgG2a, and IgG2b levels whereas IgG2a levels shows a significant increase at reboost compared to the control (P≤0.05) (Fig. 4A). The IgG1 and IgG2b levels stayed consistently low, although the data shows a slight increase in IgG1 at reboost but the increase was not significant. There was a significant increase in IgG1 levels when comparing prime and boost (P≤0.05), as well as a significant increase of IgG2b levels (P≤0.05) between prime and reboost in the Ad5-gp83 immunized BALB/c mice group (statistical analyses are not shown on graph). The sera from the Ad5-gp83 immunized BALB/c mice showed that IgG2a was the more dominant isotype (Fig. 4C). The sera from the Ad5-gp83 C57BL/6 immunized mice also had detectable levels of IgG1, IgG2a, and IgG2b (Fig. 4B). There was a significant increase of IgG2a levels at boost (P≤0.05) and reboost (P≤0.05) compared to that of the Ad5 control. Likewise, IgG2b levels showed a significant increase at prime (P≤0.05), boost (P≤0.01), as well as reboost (P≤0.01) compared to that of the Ad5 control. But surprisingly while IgG2a was the dominant isotype in the BALB/c Ad5-gp83 immunized mice, IgG2b was more dominant in the C57BL/6 Ad5-gp83 immunized mice (Fig. 4D). There was a significant increase in IgG2a levels between prime and boost (P≤0.05) and prime and reboost (P≤0.05) in the Ad5-gp83 C57BL/6 immunized mice group. There was also a significant increase in IgG2b levels between prime and boost (P≤0.05) as well as prime and reboost (P≤0.05) in the Ad5-gp83 C57BL/6 immunized mice group (statistical analyses are not shown on graph). The fact that we do not see an apparent difference between boost and reboost responses may suggest that in this model we may only need to give a boost immunization. The IgG subtype response obtained in immunized mice suggests Th1, Th2 and Treg-type responses.
Modified Ad5 vector contributed in protection from T. cruzi infection
Based on the higher immune response from the C57BL/6 mice, C57BL/6 mice were immunized with the either Ad5 or Ad5-gp83, according to the same immunization schedule depicted in Fig. 3. Two weeks after reboost the mice were injected with a lethal dose of blood trypomastigotes.
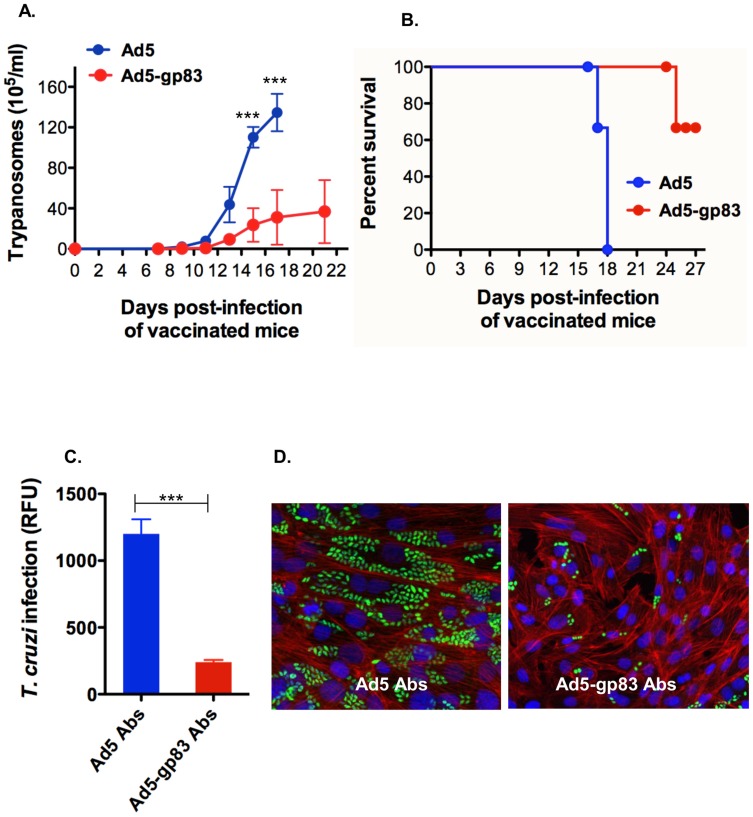
We observed that mice immunized with vector Ad5-gp83 and challenged with a lethal dose of T. cruzi blood trypomastigotes presented a significant reduction in the level of parasitemia with respect to the mice control group that received Ad5 vector alone (Fig. 5A). We also observed that there was an increase in the survival rate of mice immunized with Ad5-gp83 with respect to the control group of mice that received Ad5 (Fig. 5B). Anti-gp83 antibodies present in sera obtained from mice immunized with Ad5-gp83 before T. cruzi challenge were able to neutralize T. cruzi infection of cardiomyocytes in vitro with respect to sera obtained from mice immunized with Ad5 vector alone control (Fig. 5C). Fig. 5D shows a fluorescence microscopic observation of the neutralization of T. cruzi cellular infection resulting from the treatment of trypomastigotes with sera containing anti-gp83 antibodies from immunized mice with Ad5-gp83 with respect to sera from the control group of mice receiving Ad5. These results indicate that mice immunization with vector Ad5-gp83 confers strong immunoprotection as evident by the significant reduction in the level of parasitemia, increased survival rate and induction of neutralizing antibodies as compared to vaccine control mice.
Figure 5. Mice immunization with antigen capsid-incorporation vector protects against the challenge with T. cruzi and elicit neutralizing antibodies.
A) Parasitemia of vaccinated mice. C57BL/6 mice (5 per group, 6-week old) that were immunized with either Ad5 or Ad5-p83 were challenged intraperitoneally with a lethal dose of blood trypomastigotes (5×103) and the kinetics of parasitemia was determined in 5 µl of blood tail. Data represent the mean values ± SEM. B) Kaplan-Meier survival plot. C) Neutralization of T. cruzi infection of cardiomyocytes with Abs from vaccinated mice. Parasite multiplication within cell monolayers was estimated by determining the fluorescence level of parasites expressing green fluorescent protein, which is indicated as relative fluorescence units (RFU) at 72 hours of infection. Data represent the mean values ± SEM of the results from triplicate samples. (***) = P≤0.001. D) Fluorescence microscopic observation of the effect of neutralizing antibodies on cardiomyocyte infection by T. cruzi. Trypomastigotes expressing GFP were pre-treated with Abs and exposed to cardiomyocytes for 72 h as described in Material and Methods. Abs were obtained from vaccinated mice with either Ad or Ad5-gp83 before T. cruzi challenge. GFP-expressing amastigotes are seen inside host cells, host cell nuclei are stained blue, and cellular actin filaments are stained red.
Discussion
A safe and effective vaccine against T. cruzi has long been in demand, but is not available thus far. Efforts toward vaccine development against T. cruzi have been primarily focused on antigens that are expressed on the plasma membrane of the parasite, attached by a glycosylphosphatidylinositol (GPI) anchor [34]–[38]. The surface coat of the invasive trypomastigote form of T. cruzi contains trans-sialidases (TS), TS like molecules, mucins and other membrane proteins (reviewed in [34]). In the trypomastigote stage of T. cruzi, TS and TS like molecules are glycosylphosphatidylinositol-anchored non-integral membrane proteins that are crucial during the parasite's life cycle and for survival in the mammalian host [35]. A TS-like molecule which is commonly referred to as gp83 was selected for incorporation into our vaccine vector because we have demonstrated that blocking T. cruzi with anti-gp83 antibodies reduces trypanosome binding and invasion [36]. The gp83 is only expressed in invasive trypomastigotes but not on non-invasive epimastigotes [23]. Furthermore, our results indicate that the epitope recognized by the neutralizing antibody 4A4 on the gp83 of the trypomastigote is required for binding to phagocytic and non-phagocytic cells to trigger cellular infection [23]. The release of gp83 from trypomastigotes acts on cells to enhance cellular infection [37], [38]. Passive immunization of mice with monovalent Fab fragments of the MAb 4A4 confer strong protection and survival of mice challenged with a lethal dose of trypomastigotes [23]. In addition, we have extensively mapped the gp83 conformational epitope recognized by the neutralizing MAb 4A4 (Pratap et al., 2014 in preparation).
In this study, we examine the humoral responses to the T-cruzi gp83-specific antigen that is capsid-incorporated on Ad5 vectors. We generated a recombinant Ad5 vector with an epitope derived from T. cruzi gp83 incorporated into the HVR1 region of the major capsid protein hexon. We describe the construction and in vitro characterization of Ad5-gp83. The insertion of the T. cruzi epitope did not dramatically affect major capsid proteins, such as fiber, or the overall fitness of the vector. The modified vector had normal growth characteristics similar to those of the wild type Ad5. The insertion of the T. cruzi epitope was surface exposed via whole-virus ELISA assay demonstration. Importantly, our study provides evidence that immunization with the T. cruzi capsid-modified vector elicits a robust humoral antibody response in two different strains of mice and reduces experimental T. cruzi infection in C57BL/6 mice.
Generation of different IgG subclasses have been indicated in antibody responses [39]–[42] as well as CD8 responses [39], [43]–[46] after T. cruzi infection. In the present study, analysis of the IgG subclasses from the mice immunized with Ad5-gp83 indicated IgG2a as being the dominant response in immunized BALB/c mice (at post-boost and post-reboost time points) and IgG2b the dominant response in immunized C57BL/6 mice (at post-prime, post-boost, and post-reboost time points). In addition to eliciting neutralizing antibodies, the strong IgG2b response induced by the Ad5-gp83 vaccine may also facilitate antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), which are known antibody effector functions of IgG2b. Further study is required to determine if the antigen capsid-incorporation strategy is as effective at stimulating T. cruzi-specific cellular immunity as it has been shown here to stimulate humoral immunity.
Earlier works have applied trans-sialidase (TS) antigens for vaccine development against T. cruzi [47]–[51] and mucins [52]. Wizel et al. was the first to show inhibition of T. cruzi infection by using plasmid DNA of TS as a vaccine strategy. However, their findings only focused on the CD+8 T cell response [47]. Vasconcelos et al. used different combinations of a plasmid containing the gene encoding the catalytic domain of TS and the recombinant TS protein. While this study confirmed the CD+8 T cell-mediated immune response in BALB/c and C57BL/6 mice, none of the different combinations could protect highly susceptible mice (A/Sn) from T. cruzi infection [53]. Based on the promising findings of viral vectors used for vaccine therapy, other groups have chosen to use Ad as a vaccine vector and expressed TS and other T. cruzi proteins as a transgene [54]–[58]. Amastigote surface protein-2 (ASP-2) has also been a promising target for vaccine development against Chagas disease [59]–[61]. Our impending approach is to utilize the antigen capsid-incorporation strategy and develop Ad5 vectors that present ASP-2 epitopes to elicit a robust epitope-specific response. To date, the studies in our manuscript herein demonstrate the first time that the antigen capsid-incorporation strategy has been applied for a vaccine development against a T. cruzi parasite.
A major obstacle in using Ad5 for vaccine therapy is that the majority of the population has pre-existing immunity (PEI) resulting from natural exposure to the common cold [62]–[65]. The antigen capsid-incorporation strategy to some degree can circumvent PEI in BALB/c mice relative to boost and reboost (Fig. 3B). One of our future strategies to circumvent the PEI is to develop a chimeric Ad5 vector by replacing the entire Ad5 hexon with the hexon from Ad serotype 3. These chimeric vectors will either present and/or express T. cruzi antigens. This strategy will allow the evasion of Ad5 immunity, in addition; the dual antigen presentation could theoretically allow a more comprehensive vaccine effort which could potentially control T. cruzi infection via the humoral immune response and disease progression via the cellular immune response. Another future strategy to circumvent vector-mediated PEI is to develop T. cruzi vaccine vectors from rare adenovirus serotypes (e.g, Ad3, Ad35 or Ad36) [66]–[68].
A protein BLAST search using blastp on the UniProt database (http://www.uniprot.org/) and a tblastn for bioinformatics analysis of the T. cruzi genome databases of currently sequenced strains in the NCBI genomes and refseq databases (http://www.ncbi.nlm.nih.gov/refseq) revealed highly significant identity between the epitope recognized by MAb 4A4 and many surface proteins from multiple geographically diverse T. cruzi strains typically present in Brazil, Argentina, Chile, Venezuela, Bolivia, Colombia and Mexico (Pratap et. al., 2014 manuscript in preparation) [69], [70]. Accordingly, the identified strains with highly similar protein and nucleotide matches to the MAb 4A4 epitope are CL Brener, Y, Sylvio and Marinkellei (Brazil), CA1 (Argentina), Dm28C (Venezuela), Tulahuen (Chile), SO34 cL4 (Bolivia), Colombiana (Colombia) and MINOA (Mexico). These results suggest that vaccine development with the neutralizing MAb 4A4 epitope delivered by Ad5-gp83 could have a broad spectrum protective effect on T. cruzi strains differing in levels of drug resistance and a wide range of geographical coverage; thus being relevant as an important component in the development of a universal vaccine against T. cruzi.
In summary, we demonstrated that Ad5-gp83 would be useful in the development of vaccines against Chagas disease, due to the ability of the vector in triggering robust humoral immune responses to a T. cruzi antigen and in providing immune-protection against T. cruzi challenge by eliciting neutralizing antibodies. The antigen capsid-incorporation strategy is attractive for a complex parasite such as T. cruzi, because the adenovirus capsid is extremely amenable to the incorporations of multiple linear and discontinuous epitopes that represent parasite antigens at various life cycles. Therefore, we suggest that this strategy can be manipulated to introduce T. cruzi epitopes of immunological importance to induce a robust anti-T. cruzi humoral and cellular response. Furthermore, our current study can be viewed as a platform to introduce an effective vaccine methodology for other infectious diseases.
Acknowledgments
The authors would like to thank Shruti Sakhare for assisting with bioinformatics analysis and Alexandre Krendelchtchikov for his technical support. The authors would like to thank Dr. Paul A. Goepfert and the Goepfert laboratory for their insightful discussions of this article. The authors would also like to thank Dr. Casey D. Morrow and Ms. Adrienne Ellis for mentorship to ALF and administrative support.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. The findings described are freely available to other researchers in the manuscript or supporting information.
Funding Statement
This work was supported in part by National Institutes of Health grants 5T32AI7493-18 (ALF), 5R01AI089337-04 and UAB departmental funds (QLM), AI080580, AI007281, and MD007593 (FV), GM059994 (MFL), AI083925 (PNN). The Confocal Microscopy Facility used at Meharry was supported by National Institutes of Health grant MD007586. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Chagas C (1988) A short chronicle of the discovery of Chagas' disease. Pacing Clin Electrophysiol 11: 1108–1113. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization (2013) Chagas disease (American trypanosomiasis). [PMC free article] [PubMed] [Google Scholar]
- 3. Gascon J, Bern C, Pinazo MJ (2010) Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop 115: 22–27. [DOI] [PubMed] [Google Scholar]
- 4. Schmunis GA, Yadon ZE (2010) Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop 115: 14–21. [DOI] [PubMed] [Google Scholar]
- 5. Bern C, Kjos S, Yabsley MJ, Montgomery SP (2011) Trypanosoma cruzi and Chagas' Disease in the United States. Clin Microbiol Rev 24: 655–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rassi A Jr, Rassi A, Marin-Neto JA (2010) Chagas disease. Lancet 375: 1388–1402. [DOI] [PubMed] [Google Scholar]
- 7. Castro JA, Diaz de Toranzo EG (1988) Toxic effects of nifurtimox and benznidazole, two drugs used against American trypanosomiasis (Chagas' disease). Biomed Environ Sci 1: 19–33. [PubMed] [Google Scholar]
- 8. Urbina JA (2010) Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop 115: 55–68. [DOI] [PubMed] [Google Scholar]
- 9. Guedes PM, Silva GK, Gutierrez FR, Silva JS (2011) Current status of Chagas disease chemotherapy. Expert Rev Anti Infect Ther 9: 609–620. [DOI] [PubMed] [Google Scholar]
- 10. Ono T, Yamaguchi Y, Oguma T, Takayama E, Takashima Y, et al. (2012) Actively induced antigen-specific CD8+ T cells by epitope-bearing parasite pre-infection but not prime/boost virus vector vaccination could ameliorate the course of Plasmodium yoelii blood-stage infection. Vaccine 30: 6270–6278. [DOI] [PubMed] [Google Scholar]
- 11. Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, et al. (2003) Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol 77: 6305–6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liang X, Casimiro DR, Schleif WA, Wang F, Davies ME, et al. (2005) Vectored Gag and Env but not Tat show efficacy against simian-human immunodeficiency virus 89.6P challenge in Mamu-A*01-negative rhesus monkeys. J Virol 79: 12321–12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shiver JW, Emini EA (2004) Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu Rev Med 55: 355–372. [DOI] [PubMed] [Google Scholar]
- 14. Liu M, Acres B, Balloul JM, Bizouarne N, Paul S, et al. (2004) Gene-based vaccines and immunotherapeutics. Proc Natl Acad Sci U S A 101 Suppl 2: 14567–14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matthews QL, Sibley DA, Wu H, Li J, Stoff-Khalili MA, et al. (2006) Genetic incorporation of a herpes simplex virus type 1 thymidine kinase and firefly luciferase fusion into the adenovirus protein IX for functional display on the virion. Mol Imaging 5: 510–519. [PMC free article] [PubMed] [Google Scholar]
- 16. Matthews QL, Fatima A, Tang Y, Perry BA, Tsuruta Y, et al. (2010) HIV antigen incorporation within adenovirus hexon hypervariable 2 for a novel HIV vaccine approach. PLoS One 5: e11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tang Y, Wu H, Ugai H, Matthews QL, Curiel DT (2009) Derivation of a triple mosaic adenovirus for cancer gene therapy. PLoS One 4: e8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vorburger S, Hunt KK (2001) Adenoviral Gene Therapy. The Oncologist 7: 46–59. [DOI] [PubMed] [Google Scholar]
- 19. Matthews QL, Yang P, Wu Q, Belousova N, Rivera AA, et al. (2008) Optimization of capsid-incorporated antigens for a novel adenovirus vaccine approach. Virol J 5: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shiratsuchi T, Rai U, Krause A, Worgall S, Tsuji M (2010) Replacing adenoviral vector HVR1 with a malaria B cell epitope improves immunogenicity and circumvents preexisting immunity to adenovirus in mice. J Clin Invest 120: 3688–3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McConnell MJ, Danthinne X, Imperiale MJ (2006) Characterization of a permissive epitope insertion site in adenovirus hexon. J Virol 80: 5361–5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Worgall S, Krause A, Rivara M, Hee KK, Vintayen EV, et al. (2005) Protection against P. aeruginosa with an adenovirus vector containing an OprF epitope in the capsid. J Clin Invest 115: 1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Villalta F, Smith CM, Ruiz-Ruano A, Lima MF (2001) A ligand that Trypanosoma cruzi uses to bind to mammalian cells to initiate infection. FEBS Lett 505: 383–388. [DOI] [PubMed] [Google Scholar]
- 24. Villalta F, Lima MF, Ruiz-Ruano A, Zhou L (1992) Attachment of Trypanosoma cruzi to host cells: a monoclonal antibody recognizes a trypomastigote stage-specific epitope on the gp 83 required for parasite attachment. Biochem Biophys Res Commun 182: 6–13. [DOI] [PubMed] [Google Scholar]
- 25. Lima MF, Villalta F (1989) Trypanosoma cruzi trypomastigote clones differentially express a parasite cell adhesion molecule. Mol Biochem Parasitol 33: 159–170. [DOI] [PubMed] [Google Scholar]
- 26. Villalta F, Dobish MC, Nde PN, Kleshchenko YY, Hargrove TY, et al. (2013) VNI cures acute and chronic experimental Chagas disease. J Infect Dis 208: 504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lepesheva GI, Hargrove TY, Anderson S, Kleshchenko Y, Furtak V, et al. (2010) Structural insights into inhibition of sterol 14alpha-demethylase in the human pathogen Trypanosoma cruzi. J Biol Chem 285: 25582–25590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu H, Han T, Belousova N, Krasnykh V, Kashentseva E, et al. (2005) Identification of sites in adenovirus hexon for foreign peptide incorporation. J Virol 79: 3382–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu H, Dmitriev I, Kashentseva E, Seki T, Wang M, et al. (2002) Construction and characterization of adenovirus serotype 5 packaged by serotype 3 hexon. J Virol 76: 12775–12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gu L, Li ZC, Krendelchtchikov A, Krendelchtchikova V, Wu H, et al. (2013) Using multivalent adenoviral vectors for HIV vaccination. PLoS One 8: e60347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Villalta F, Kierszenbaum F (1983) Immunization against a challenge with insect vector, metacyclic forms of Trypanosoma cruzi simulating a natural infection. Am J Trop Med Hyg 32: 273–276. [DOI] [PubMed] [Google Scholar]
- 32. Johnson CA, Rachakonda G, Kleshchenko YY, Nde PN, Madison MN, et al. (2013) Cellular response to Trypanosoma cruzi infection induces secretion of defensin alpha-1, which damages the flagellum, neutralizes trypanosome motility, and inhibits infection. Infect Immun 81: 4139–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnson CA, Kleshchenko YY, Ikejiani AO, Udoko AN, Cardenas TC, et al. (2012) Thrombospondin-1 interacts with Trypanosoma cruzi surface calreticulin to enhance cellular infection. PLoS One 7: e40614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Villalta F, Madison MN, Kleshchenko YY, Nde PN, Lima MF (2008) Molecular analysis of early host cell infection by Trypanosoma cruzi. Front Biosci 13: 3714–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Agusti R, Couto AS, Campetella OE, Frasch AC, de Lederkremer RM (1997) The trans-sialidase of Trypanosoma cruzi is anchored by two different lipids. Glycobiology 7: 731–735. [DOI] [PubMed] [Google Scholar]
- 36. Villalta F, Lima MF, Howard SA, Zhou L, Ruiz-Ruano A (1992) Purification of a Trypanosoma cruzi trypomastigote 60-kilodalton surface glycoprotein that primes and activates murine lymphocytes. Infect Immun 60: 3025–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Villalta F, Zhang Y, Bibb KE, Burns JM Jr, Lima MF (1998) Signal transduction in human macrophages by gp83 ligand of Trypanosoma cruzi: trypomastigote gp83 ligand up-regulates trypanosome entry through the MAP kinase pathway. Biochem Biophys Res Commun 249: 247–252. [DOI] [PubMed] [Google Scholar]
- 38. Nde PN, Simmons KJ, Kleshchenko YY, Pratap S, Lima MF, et al. (2006) Silencing of the laminin gamma-1 gene blocks Trypanosoma cruzi infection. Infect Immun 74: 1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bryan MA, Guyach SE, Norris KA (2010) Specific humoral immunity versus polyclonal B cell activation in Trypanosoma cruzi infection of susceptible and resistant mice. PLoS Negl Trop Dis 4: e733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rowland EC, Mikhail KS, McCormick TS (1992) Isotype determination of anti-Trypanosoma cruzi antibody in murine Chagas' disease. J Parasitol 78: 557–561. [PubMed] [Google Scholar]
- 41. el Bouhdidi A, Truyens C, Rivera MT, Bazin H, Carlier Y (1994) Trypanosoma cruzi infection in mice induces a polyisotypic hypergammaglobulinaemia and parasite-specific response involving high IgG2a concentrations and highly avid IgG1 antibodies. Parasite Immunol 16: 69–76. [DOI] [PubMed] [Google Scholar]
- 42. d'Imperio Lima MR, Eisen H, Minoprio P, Joskowicz M, Coutinho A (1986) Persistence of polyclonal B cell activation with undetectable parasitemia in late stages of experimental Chagas' disease. J Immunol 137: 353–356. [PubMed] [Google Scholar]
- 43. Padilla AM, Bustamante JM, Tarleton RL (2009) CD8+ T cells in Trypanosoma cruzi infection. Curr Opin Immunol 21: 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoft DF, Eickhoff CS (2005) Type 1 immunity provides both optimal mucosal and systemic protection against a mucosally invasive, intracellular pathogen. Infect Immun 73: 4934–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tarleton RL (2007) Immune system recognition of Trypanosoma cruzi. Curr Opin Immunol 19: 430–434. [DOI] [PubMed] [Google Scholar]
- 46. Martin D, Tarleton R (2004) Generation, specificity, and function of CD8+ T cells in Trypanosoma cruzi infection. Immunol Rev 201: 304–317. [DOI] [PubMed] [Google Scholar]
- 47. Wizel B, Nunes M, Tarleton RL (1997) Identification of Trypanosoma cruzi trans-sialidase family members as targets of protective CD8+ TC1 responses. J Immunol 159: 6120–6130. [PubMed] [Google Scholar]
- 48. Costa F, Franchin G, Pereira-Chioccola VL, Ribeirao M, Schenkman S, et al. (1998) Immunization with a plasmid DNA containing the gene of trans-sialidase reduces Trypanosoma cruzi infection in mice. Vaccine 16: 768–774. [DOI] [PubMed] [Google Scholar]
- 49. Fujimura AE, Kinoshita SS, Pereira-Chioccola VL, Rodrigues MM (2001) DNA sequences encoding CD4+ and CD8+ T-cell epitopes are important for efficient protective immunity induced by DNA vaccination with a Trypanosoma cruzi gene. Infect Immun 69: 5477–5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Katae M, Miyahira Y, Takeda K, Matsuda H, Yagita H, et al. (2002) Coadministration of an interleukin-12 gene and a Trypanosoma cruzi gene improves vaccine efficacy. Infect Immun 70: 4833–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chou B, Hisaeda H, Shen J, Duan X, Imai T, et al. (2008) Critical contribution of immunoproteasomes in the induction of protective immunity against Trypanosoma cruzi in mice vaccinated with a plasmid encoding a CTL epitope fused to green fluorescence protein. Microbes Infect 10: 241–250. [DOI] [PubMed] [Google Scholar]
- 52. Serna C, Lara JA, Rodrigues SP, Marques AF, Almeida IC, et al. (2014) A synthetic peptide from Trypanosoma cruzi mucin-like associated surface protein as candidate for a vaccine against Chagas disease. Vaccine 32: 3525–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vasconcelos JR, Boscardin SB, Hiyane MI, Kinoshita SS, Fujimura AE, et al. (2003) A DNA-priming protein-boosting regimen significantly improves type 1 immune response but not protective immunity to Trypanosoma cruzi infection in a highly susceptible mouse strain. Immunol Cell Biol 81: 121–129. [DOI] [PubMed] [Google Scholar]
- 54. Vasconcelos JR, Bruna-Romero O, Araujo AF, Dominguez MR, Ersching J, et al. (2012) Pathogen-induced proapoptotic phenotype and high CD95 (Fas) expression accompany a suboptimal CD8+ T-cell response: reversal by adenoviral vaccine. PLoS Pathog 8: e1002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Buckner FS, Wipke BT, Van Voorhis WC (1997) Trypanosoma cruzi infection does not impair major histocompatibility complex class I presentation of antigen to cytotoxic T lymphocytes. Eur J Immunol 27: 2541–2548. [DOI] [PubMed] [Google Scholar]
- 56. Wrightsman RA, Manning JE (2000) Paraflagellar rod proteins administered with alum and IL-12 or recombinant adenovirus expressing IL-12 generates antigen-specific responses and protective immunity in mice against Trypanosoma cruzi. Vaccine 18: 1419–1427. [DOI] [PubMed] [Google Scholar]
- 57. Machado AV, Cardoso JE, Claser C, Rodrigues MM, Gazzinelli RT, et al. (2006) Long-term protective immunity induced against Trypanosoma cruzi infection after vaccination with recombinant adenoviruses encoding amastigote surface protein-2 and trans-sialidase. Hum Gene Ther 17: 898–908. [DOI] [PubMed] [Google Scholar]
- 58. Miyahira Y, Takashima Y, Kobayashi S, Matsumoto Y, Takeuchi T, et al. (2005) Immune responses against a single CD8+-T-cell epitope induced by virus vector vaccination can successfully control Trypanosoma cruzi infection. Infect Immun 73: 7356–7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Barbosa RP, Filho BG, Dos Santos LI, Junior PA, Marques PE, et al. (2013) Vaccination using recombinants influenza and adenoviruses encoding amastigote surface protein-2 are highly effective on protection against Trypanosoma cruzi infection. PLoS One 8: e61795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nogueira RT, Nogueira AR, Pereira MC, Rodrigues MM, Galler R, et al. (2011) Biological and immunological characterization of recombinant Yellow Fever 17D viruses expressing a Trypanosoma cruzi Amastigote Surface Protein-2 CD8+ T cell epitope at two distinct regions of the genome. Virol J 8: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Duan X, Yonemitsu Y, Chou B, Yoshida K, Tanaka S, et al. (2009) Efficient protective immunity against Trypanosoma cruzi infection after nasal vaccination with recombinant Sendai virus vector expressing amastigote surface protein-2. Vaccine 27: 6154–6159. [DOI] [PubMed] [Google Scholar]
- 62. Thacker EE, Timares L, Matthews QL (2009) Strategies to overcome host immunity to adenovirus vectors in vaccine development. Expert Rev Vaccines 8: 761–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chirmule N, Propert K, Magosin S, Qian Y, Qian R, et al. (1999) Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther 6: 1574–1583. [DOI] [PubMed] [Google Scholar]
- 64. Schagen FH, Ossevoort M, Toes RE, Hoeben RC (2004) Immune responses against adenoviral vectors and their transgene products: a review of strategies for evasion. Crit Rev Oncol Hematol 50: 51–70. [DOI] [PubMed] [Google Scholar]
- 65. Zaiss AK, Machado HB, Herschman HR (2009) The influence of innate and pre-existing immunity on adenovirus therapy. J Cell Biochem 108: 778–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tian X, Su X, Li H, Li X, Zhou Z, et al. (2011) Construction and characterization of human adenovirus serotype 3 packaged by serotype 7 hexon. Virus Res 160: 214–220. [DOI] [PubMed] [Google Scholar]
- 67. Soloff AC, Liu X, Gao W, Day RD, Gambotto A, et al. (2009) Adenovirus 5- and 35-based immunotherapy enhances the strength but not breadth or quality of immunity during chronic SIV infection. Eur J Immunol 39: 2437–2449. [DOI] [PubMed] [Google Scholar]
- 68. Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, et al. (2007) Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 81: 4654–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403–410. [DOI] [PubMed] [Google Scholar]
- 70. Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, et al. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. The findings described are freely available to other researchers in the manuscript or supporting information.