Abstract
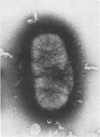
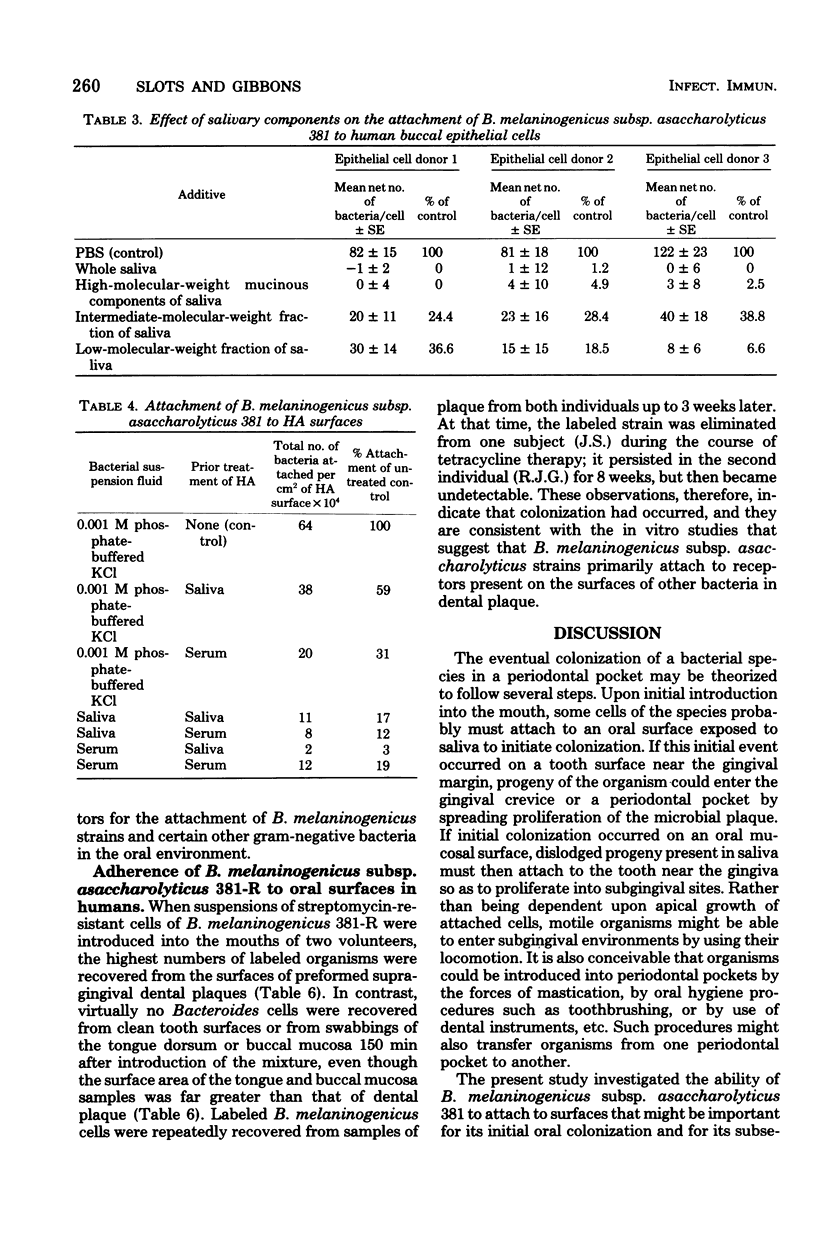
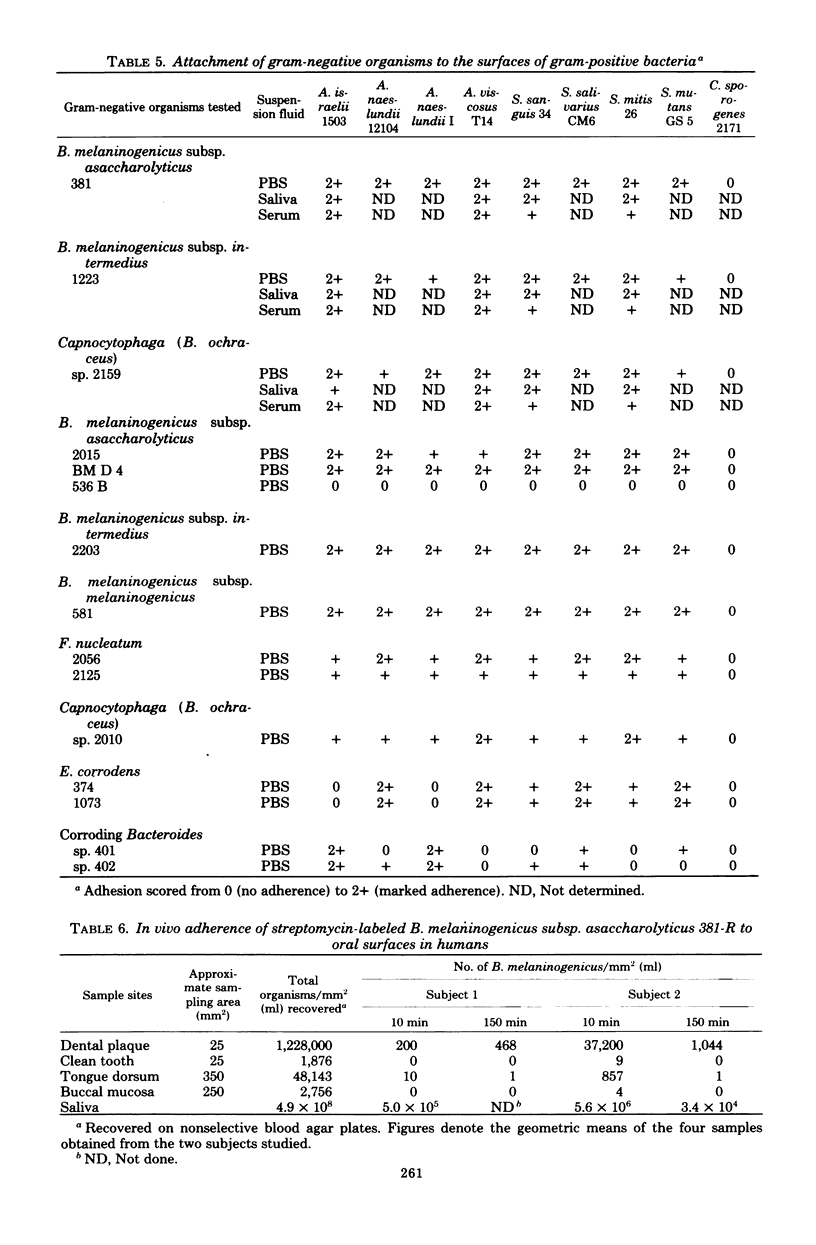
This investigation examined the ability of cells of Bacteroides melaninogenicus subsp. asaccharolyticus 381 to adhere to surfaces that might be important for its initial colonization of the mouth and its subsequent colonization in periodontal pockets. Of 48 asaccharolytic strains of B. melaninogenicus, 47 agglutinated human erythrocytes, whereas none of 20 fermentative strains, which included reference cultures of the subspecies intermedius and melaninogenicus, were active. Electron microscopy indicated that both asaccharolytic and fermentative strains possessed pili; hence, the presence of pili did not correlate with the hemagglutinating activities of B. melaninogenicus strains. Both asaccharolytic and fermentative B. melaninogenicus strains suspended in phosphate-buffered saline adhered in high numbers to buccal epithelial cells and to the surfaces of several gram-positive bacteria tested, including Actinomyces viscosus, A. naeslundii, A. israelii, Streptococcus sanguis, and S. mitis. B. melaninogenicus subsp. asaccharolyticus 381 also attached, but in comparatively low numbers, to untreated and to saliva-treated hydroxyapatite. Addition of clarified whole saliva to suspensions of strain 381 almost completely eliminated adherence to buccal epithelial cells and to hydroxyapatite surfaces, but saliva had no detectable effect on attachment to gram-positive plaque bacteria. Both fermentative and nonfermentative strains of B. melaninogenicus also attached in high numbers to crevicular epithelial cells derived from human periodontal pockets, but normal human serum strongly inhibited attachment. Serum also inhibited attachment of strain 381 to saliva- and serum-treated hydroxyapatite, but it had little effect upon attachment to gram-positive bacteria. These observations suggested that salivary and serum components would strongly inhibit the attachment of B. melaninogenicus cells to several oral surfaces, but not to the surfaces of certain gram-positive bacteria commonly present in human dental plaque. This was confirmed by an in vivo experiment in which streptomycin-labeled cells of B. melaninogenicus 381-R were introduced into the mouths of two volunteers. After 10 min, several hundred-fold higher numbers of the organism were recovered from preformed bacterial plaque present on teeth than from clean tooth surfaces or from the buccal mucosa and tongue dorsum. High numbers of B. melaninogenicus cells were also recovered from preformed plaque after 150 min, but virtually no cells of the organism were recovered from the other surfaces studied. These data suggest that the presence of dental plaque containing Actinomyces and other gram-positive bacteria may be essential for the attachment and colonization of B. melaninogenicus cells after their initial introduction into the mouth. Similarly, the presence of subgingival plaque containing gram-positive bacteria may be necessary for its secondary colonization in periodontal pockets.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILIT H. L., BALDWIN D. C., HUNT E. E., Jr THE INCREASING PREVALENCE OF GINGIVAL BACTEROIDES MELANINOGENICUS WITH AGE IN CHILDREN. Arch Oral Biol. 1964 Jul-Aug;9:435–438. doi: 10.1016/0003-9969(64)90028-7. [DOI] [PubMed] [Google Scholar]
- Bladen H., Hageage G., Pollock F., Harr R. Plaque formation in vitro on wires by gram-negative oral microorganisms (Veillonella). Arch Oral Biol. 1970 Feb;15(2):127–133. doi: 10.1016/0003-9969(70)90048-8. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M. Antigenic heterogeneity of gonococcal pili. J Exp Med. 1975 Jun 1;141(6):1470–1475. doi: 10.1084/jem.141.6.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D. I., Gibbons R. J., Spinell D. M. Characteristics of some high molecular weight constituents with bacterial aggregating activity from whole saliva and dental plaque. Caries Res. 1971;5(2):111–123. doi: 10.1159/000259739. [DOI] [PubMed] [Google Scholar]
- Kelstrup J. The incidence of bacteroides melaniogenicus in human gingival sulci, and its prevalence in the oral cavity at different ages. Periodontics. 1966 Jan-Feb;4(1):14–18. [PubMed] [Google Scholar]
- Listgarten M. A. Structure of the microbial flora associated with periodontal health and disease in man. A light and electron microscopic study. J Periodontol. 1976 Jan;47(1):1–18. doi: 10.1902/jop.1976.47.1.1. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. Importance of nutrition in gingival crevice microbial ecology. Periodontics. 1968 Dec;6(6):245–249. [PubMed] [Google Scholar]
- Manganiello A. D., Socransky S. S., Smith C., Propas D., Oram V., Dogon I. L. Attempts to increase viable count recovery of human supragingival dental plaque. J Periodontal Res. 1977 Mar;12(2):107–119. doi: 10.1111/j.1600-0765.1977.tb00113.x. [DOI] [PubMed] [Google Scholar]
- Newman M. G., Socransky S. S. Predominant cultivable microbiota in periodontosis. J Periodontal Res. 1977 Mar;12(2):120–128. doi: 10.1111/j.1600-0765.1977.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Newman M. G., Socransky S. S., Savitt E. D., Propas D. A., Crawford A. Studies of the microbiology of periodontosis. J Periodontol. 1976 Jul;47(7):373–379. doi: 10.1902/jop.1976.47.7.373. [DOI] [PubMed] [Google Scholar]
- Okuda K., Takazoe I. Haemagglutinating activity of Bacteroides melaninogenicus. Arch Oral Biol. 1974 May;19(5):415–416. doi: 10.1016/0003-9969(74)90184-8. [DOI] [PubMed] [Google Scholar]
- Ritz H. L. Microbial population shifts in developing human dental plaque. Arch Oral Biol. 1967 Dec;12(12):1561–1568. doi: 10.1016/0003-9969(67)90190-2. [DOI] [PubMed] [Google Scholar]
- SOCRANSKY S. S., GIBBONS R. J. REQUIRED ROLE OF BACTEROIDES MELANINOGENICUS IN MIXED ANAEROBIC INFECTIONS. J Infect Dis. 1965 Jun;115:247–253. doi: 10.1093/infdis/115.3.247. [DOI] [PubMed] [Google Scholar]
- Slots J. Microflora in the healthy gingival sulcus in man. Scand J Dent Res. 1977 May;85(4):247–254. doi: 10.1111/j.1600-0722.1977.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable microflora of advanced periodontitis. Scand J Dent Res. 1977 Jan-Feb;85(2):114–121. doi: 10.1111/j.1600-0722.1977.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable organisms in juvenile periodontitis. Scand J Dent Res. 1976 Jan;84(1):1–10. doi: 10.1111/j.1600-0722.1976.tb00454.x. [DOI] [PubMed] [Google Scholar]
- Socransky S. S., Manganiello A. D., Propas D., Oram V., van Houte J. Bacteriological studies of developing supragingival dental plaque. J Periodontal Res. 1977 Mar;12(2):90–106. doi: 10.1111/j.1600-0765.1977.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Theilade E., Wright W. H., Jensen S. B., Löe H. Experimental gingivitis in man. II. A longitudinal clinical and bacteriological investigation. J Periodontal Res. 1966;1:1–13. doi: 10.1111/j.1600-0765.1966.tb01842.x. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Pulkkinen A. J. Adherence as an ecological determinant for streptococci in the human mouth. Arch Oral Biol. 1971 Oct;16(10):1131–1141. doi: 10.1016/0003-9969(71)90042-2. [DOI] [PubMed] [Google Scholar]