Abstract
OBJECTIVES
To assess the association between self-reported noncancer pain and 5-year mortality.
DESIGN
Cohort.
SETTING
Community-dwelling older adults.
PARTICIPANTS
Canadian Study of Health and Aging 1996 wave.
MEASUREMENTS
Registrar of Vital Statistics–established 5-year mortality. Noncancer pain was assessed using the 5-point verbal descriptor scale, dichotomized into no or very mild versus moderate, severe, or very severe pain. Frailty was the accumulation of health deficits. Cognitive status (Modified Mini-Mental State Examination) and depressed mood (five-item mental health screening questionnaire) were also assessed. Multivariable logistic regression and Cox proportional hazards were used to analyze the relationship between pain and 5-year mortality.
RESULTS
Of 5,703 participants, 4,694 (82.3%) had complete data for analysis; 1,663 of these (35.4%) reported moderate, severe, or very severe pain, and 1,343 (28.6%) had died at 5-year follow-up. Four hundred ninety-six of those who died (29.8%) reported moderate, severe, or very severe pain and 847 (27.9%) no or very mild pain. Multivariate logistic analysis found that individuals with moderate, severe, or very severe pain had lower odds of 5-year mortality than those with no or very mild pain (odds ratio = 0.78, 95% confidence interval (CI) = 0.66–0.92; P < .001). The risk of death was lower in persons reporting moderate or greater pain than in those with no or very mild pain (HR = 0.85, 95% CI = 0.75–0.96; P = .01). An interaction between pain and sex explained this effect. Men with pain were not significantly more likely than men without pain to die (HR = 1.00, 95% CI = 0.84–1.19; P = .99), whereas women without pain (HR = 0.54, 95% CI = 0.47–0.63; P < 0.01) and women with pain (HR = 0.40; CI = 0.33–0.47; P < .01) had less risk of death than men without and with pain, respectively.
CONCLUSION
Older women with pain were less likely to die within 5 years than older women without pain, men in pain, or men without pain.
Keywords: pain, mortality, older adult
Noncancer pain is common in community-dwelling older adults, with prevalence rates of 30% to 50% reported.1–5 The high frequency of pain that older persons experience has been attributed to an increase in the number of comorbid conditions that accompany aging and is associated with nociceptive and neuropathic pain, such as osteoarthritis, osteoporosis, and postherpetic neuralgia.6–8 Clinically significant pain—usually designated as moderate intensity or higher—results in substantial short-term morbidity,9 including functional disability, depressed mood, sleep disturbances, and social vulnerability.10–12
Longer-term pain-related outcomes have been less well established, particularly in older populations. Persistent pain may be associated with important adverse long-term outcomes including cognitive decline, the development of frailty, and mortality.13–15 Mortality risk may stem from a combination of factors, such as pain-related morbidity, the interaction between pain and concomitant comorbidity, low physical activity, and poor adherence to complex medical regimens.15–20 Most prior work on pain and mortality has focused on younger populations and reported inconsistent findings.16–19 Such inconsistencies are attributed to differences in pain ascertainment, study designs and populations, linkages of different data sources, and adjustments for available confounders (e.g., not controlling for functional status, mood, and cognitive status).16–19 At the same time, older populations in pain may have a survival advantage through not-yet-appreciated mechanisms such as a predisposition for adaptation and resilience.
The purpose of the current study was to delineate the independent association between noncancer pain and 5-year mortality in an older population. The Canadian Study of Health and Aging (CSHA) was used to analyze this relationship and overcome many of the shortcomings of previously published studies. The CSHA is a nationally representative survey of community-dwelling older adults that captures well-validated measures of pain and 5-year mortality, along with important potential confounders.21,22 Based upon available research and clinical experience, it was hypothesized that clinically significant noncancer pain would be associated with a greater likelihood of 5-year mortality. A better understanding of long-term pain–related outcomes can better inform management practices for a growing number of older adults in pain.
METHODS
Population
This is a secondary data analysis of the CSHA, a prospective, nationally representative, observational study of men and women aged 65 and older. A detailed description of the survey’s objectives, design, and methodologies were published elsewhere.21,22 In brief, the original cohort was established in 1992, with subsequent waves of data collected in 1996 and 2001. Individuals aged 65 and older were recruited on the basis of an age-stratified random sample in 36 urban and surrounding rural areas in all 10 Canadian provinces. Trained research personnel conducted interviews in participants’ homes that included an assessment of health, comorbidity, mood, physical abilities, and cognitive function.
This study used data from the 1996 (second wave) of CSHA because this was the only wave that included an assessment of pain, the primary independent variable of interest. To avoid cancer-related pain, participants who had been diagnosed with cancer in the past year were excluded from the analysis. Additional participants were excluded if they had missing data from a pain assessment, from any component of the frailty index (FI), or on 5-year mortality. The older adult population in Canada is much more homogeneous than in the United States, so the sample was 98.8% Caucasian. All participants provided written informed consent, and the CSHA protocol was approved at each participating institution in Canada. The University of Chicago’s Biological Sciences Division institutional review board reviewed and approved the research protocol for this secondary analysis.
Measures
Five-Year Mortality
Deaths in the subsequent 5 years were identified through the Registrar of Vital Statistics in each province.
Pain
Pain was assessed using the 5-point verbal descriptor scale (VDS). The reliability and validity of the VDS has been established in older adults.23,24 Participants were asked, “How much bodily pain have you had during the past 4 weeks?” Response categories were presented on a card in ascending order (1 = none, 2 = very mild, 3 = moderate, 4 = severe, and 5 = very severe). Participants selected the category that best characterized their pain experience over that time. An analytical variable for pain was constructed by dichotomizing the original response categories (1 = no pain and very mild pain and 2 = moderate pain, severe pain, and very severe pain). The latter category of pain intensity has been documented in the literature to adversely affect health in older persons.9,13,25
Frailty
The FI, a well-validated deficit accumulation approach, was calculated to characterize each participant’s overall health status.26 It includes 33 self-reported items in the domains of self-reported health (one item), medical comorbidity (17 items), functional limitation (14 items), and social support (one item). The variables poor self-assessed health, eyesight, hearing, hypertension, heart or circulatory problems, stroke or effects of stroke, arthritis or rheumatism, Parkinson’s disease, eye trouble, ear trouble, dental problems, chest problems, stomach problems, bladder control problems, bowel control problems, trouble with feet or ankles, trouble with skin, fractures, needing help to eat, needing help to dress and undress, needing help to take care of appearances, needing help to walk, needing help to get in and out of bed, needing help to take a bath or shower, needing help to go to the toilet, needing help in shopping, needing help to prepare own meals, needing help to do housework, needing help to take medications, needing help to handle own finances, needing help to use telephone, needing help to get to places out of walking distance, and lives alone are included in the FI. Each item is given a scored based on whether it was reported as absent (0) or present (1). For each individual, the FI was calculated by summing all of the item scores, yielding a range from 0 to 33. This approach provides an overall measure of vulnerability, with higher scores indicating greater health burden.27
Other Measures
Additional covariates included participant demographic characteristics (age, sex, ethnicity, education), a depression screen (five-item mental health screening questionnaire), and an assessment of cognitive status (modified Mini Mental State Examination (3MS)).28–30 The five-item mental health screening questionnaire asks five questions on a 6-point scale. For example, respondents are queried, “Have you felt downhearted and blue?” and presented with responses ranging from none of the time to all of the time. Higher scores indicate more symptoms of depression, and total scores range from 5 to 30, with scores of 12 or higher indicating depressed mood.28 Cognitive status was assessed using the 3MS, with a score less than 78 indicating cognitive impairment.29,30
Statistical Analysis
Summary statistics of individual characteristics were analyzed according to pain status (no or very mild vs moderate, severe, or very severe). T-tests were used to test group differences for continuous variables and chi-square analysis for categorical variables. Chi-square analysis was used to assess the relationship between pain and 5-year mortality. Multivariable logistic regression modeling was used to examine the association between pain (explanatory variable) and 5-year mortality (outcome variable). Cox proportional hazards regressions were fitted to evaluate the relationship between pain status and time to death in months from the 1996 evaluation to 5 years later. Three regression models were entered stepwise according to group (block style) for each approach. Variable selection for inclusion in each model was made a priori based upon the expertise of the authors and a literature review. Three models were tested. Model 1 adjusted for demographic variables (age, sex, race, education); Model 2 adjusted for demographic variables and the FI; and Model 3 adjusted for demographic variables, the FI, and depression and cognitive status.
An interaction term between sex and pain and 5-year mortality and time to death was evaluated, given that women generally report more pain and live longer than men.1–5,16–19 An interaction between pain and frailty was also examined because variables in the FI are associated with pain and shorten survival.14,31 A sensitivity analysis was also conducted to assess whether categorizing of pain intensity affected the study findings. The pain intensity responses were regrouped into three categories (no pain; very mild pain; and moderate, severe, or very severe pain; this division of pain intensity was prevalent enough in each category to make meaningful comparisons), and the relationship between pain and 5-year mortality was reexamined using logistic regression modeling. Odds ratios (ORs) and hazard ratios (HRs) are reported with 95% confidence intervals (CIs). Statistical analysis was conducted using SPSS version 20.0.0 (IBM, Armonk, NY).
RESULTS
Five thousand seven hundred three community-dwelling participants completed the second wave of the CSHA; 154 of these (2.7%) had a missing pain response, 149 (2.6%) reported cancer in the previous year, 699 (12.3%) had missing data in at least one component of the FI, and 229 (4.0%) did not have 5-year mortality available. The final sample size was 4,694 participants or 82.3% of the original sample.
Table 1 displays participant characteristics according to pain status; 1,663 (35.4%) reported moderate or greater pain. Participants with moderate or greater pain tended to be older (79.9 vs 79.3, P = .003) and female (68.2% vs 56.0%, P < .001) than those with no or very mild pain. Persons with moderate or greater pain on average had a higher degree of frailty (deficit accumulation) than persons with no or mild pain (6.6 vs 4.3 deficits, P < .001). Persons with moderate or grater pain were also more likely to report depressed mood than those with no or mild pain (36.5% vs 16.4%, P < .001). The proportions of persons with cognitive impairment were statistically similar between those reporting moderate or greater pain and those reporting no or very mild pain (12.1% vs 13.1%, P = .33).
Table 1.
Participant Characteristics According to Non-cancer Pain Self-Report (N = 4,694)
| Characteristic | No or Very Mild Pain, n = 3,031 (64.6%) | Moderate or Greater Pain, n = 1,663 (35.4%) | P-Value |
|---|---|---|---|
| Age, mean±SD, range | 79.3 ± 6.1 (70–103) | 79.9 ± 6.1 (70–105) | .003 |
| Female, % | 56.0 | 68.2 | <.001 |
| Caucasian, % | 98.9 | 98.8 | .77 |
| Education, % | |||
| ≤8th grade | 28.5 | 32.0 | .02 |
| High school | 44.2 | 43.6 | |
| <High school | 27.3 | 24.4 | |
| Frailty, mean ± SDa | 4.3 ± 2.7 | 6.6 ± 3.3 | <.001 |
| Depressed mood, %b | 16.4 | 36.5 | <.001 |
| Cognitive impairment, %c | 12.1 | 13.1 | .33 |
A composite measure of self-reported health (1 item), medical comorbidity (17 items), functional abilities (14 items), and social support (1 item); range 0 to 33, higher scores indicating poorer health.
Designated using the five-item mental health screening questionnaire; total score range 0–30, scores ≥12 indicate depressed mood.
According to the modified Mini Mental State Examination; scores ≤77 indicate cognitive impairment.
SD = Standard Deviation.
Table 2 displays the unadjusted relationship between self-reported pain and 5-year mortality. Five years after the 1996 interview, 3,351 (71.4%) were alive, and 1,343 (28.6%) had died. Of participants who reported no or mild pain, 2,184 (72.1%) were living 5 years later, and 847 (27.9%) were dead. Of participants who reported moderate or greater pain, 1,167 (70.2%) were living 5 years later, and 496 (29.8%) were dead. The report of moderate or greater pain was not significantly associated with 5-year mortality (Pearson chi-square = 1.86, P = .18).
Table 2.
Relationship Between Self-Reported Noncancer Pain and 5-Year Mortality (N = 4,694)
| Mortality | No or Very Mild Pain, n = 3,031 (64.6%) | Moderate or Greater Pain, n = 1,663 (35.4%) | P-Value |
|---|---|---|---|
| n (%) | |||
| Alive, n = 3,351 (71.4%) | 2,184 (72.1) | 1,167 (70.2) | .18 |
| Dead, n = 1,343 (28.6%) | 847 (27.9) | 496 (29.8) | |
Table 3 displays the multivariate logistic regression analysis of self-reported pain and 5-year mortality. In Model 1, adjusted only for demographic characteristics, moderate or greater pain was associated with greater odds of dying than no or very mild pain in the next 5 years (OR = 1.17, 95% CI = 1.03–1.33; P < .001); in Model 2, which included demographic characteristics plus the FI, moderate or greater pain was associated with lower odds of dying in the next 5 years (OR = 0.81, 95% CI = 0.69–0.94; P < .001); and in Model 3, in which demographic characteristics and the FI plus mood and cognitive status were included, moderate or greater pain was associated with lower odds of dying in the next 5-years (OR = 0.78, 95% CI = 0.66–0.92; P < .001).
Table 3.
Logistic Regression of 5-Year Mortality According to Pain, Participant Demographic Characteristics, Frailty, Depressed Mood, and Cognitive Impairment (N = 4,694)
| Explanatory Variable | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Odds Ratio (95% Confidence Interval) | |||
| Age | 1.15 (1.14–1.17)a | 1.12 (1.11–1.14)a | 1.12 (1.10–1.13)a |
| Female | 0.46 (0.40–0.53)a | 0.41 (0.36–0.48)a | 0.41 (0.35–0.48)a |
| Caucasian | 0.64 (0.33–1.21) | 0.56 (0.29–1.17) | 0.61 (0.29–1.26) |
| Education (reference <high school) | |||
| High school | 0.95 (0.82–1.10) | 1.03 (0.89–1.21) | 1.20 (0.99–1.42) |
| College | 0.82 (0.69–0.97)a | 0.89 (0.74–1.07) | 1.08 (0.89–1.32) |
| Moderate pain | 1.17 (1.03–1.33)a | 0.81 (0.69–0.94)a | 0.78 (0.66–0.92)a |
| Frailtyb | — | 1.18 (1.15–1.20)a | 1.18 (1.15–1.21)a |
| Depressed moodc | — | — | 1.23 (1.03–1.47)a |
| Cognitive impairmentd | — | — | 2.35 (1.90–2.90)a |
P < .01.
A composite measure of self-reported health (1 item), medical comorbidity (17 items), functional abilities (14 items), and social support (1 item); scores range from 0 to 33, with higher scores indicating poorer health.
A summed response score of ≥2 on the five-item mental health screening questionnaire (range 0–30).
According to the Modified Mini-Mental State Examination; scores of ≤77 indicate cognitive impairment.
Each 1-point increase on the FI was associated with greater odds of death in the next 5 years (OR = 1.18, 95% CI = 1.15–1.21; P < .001). Persons with depressed mood (OR = 1.23, 95% CI = 1.03–1.47; P < .001) and cognitive impairment (OR = 2.35, 95% CI = 1.90, 2.90; P < .001) had greater odds of 5-year mortality than those without. Model 3 was well calibrated, with a nonsignificant Hosmer-Lemeshow test (chi-square = 5.05, P = .75).
Neither the interaction between pain, sex, and 5-year mortality (OR = 0.76, 95% CI = 0.55–1.10; P = .10) nor that between pain, frailty, and 5-year mortality (OR 1.03, 95% CI = 0.98–1.08; P = .32) was statistically significant. In the sensitivity analysis, the regrouping of pain intensity showed that very mild (OR = 0.86, 95% CI = 0.70–1.05; P = .13) and moderate, severe, or very severe pain (OR = 0.74, 95% CI = 0.62–0.88; P < .001) were associated with lower odds of 5-year mortality than no pain.
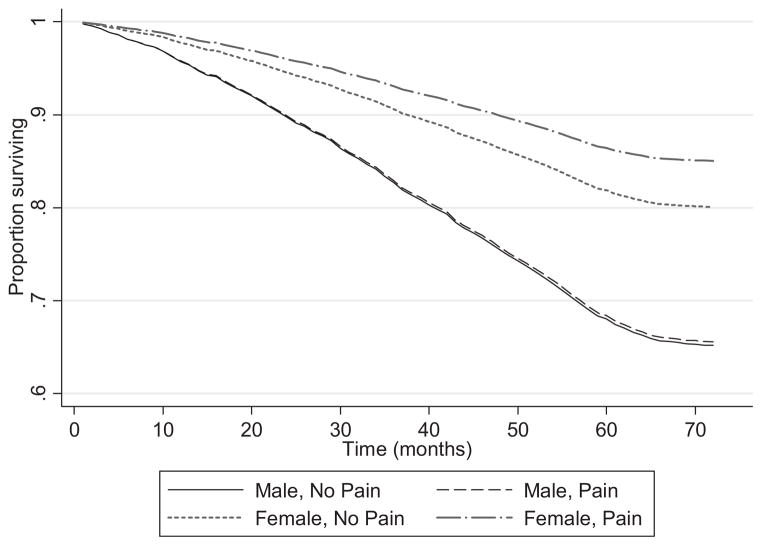
Table 4 displays the Cox proportional hazards regression of mortality measured in months over a 5-year period according to pain status for each of the three models. Of the 4,476 persons for whom time-to-event data were available, 1,234 (27.6%) died; the rest were censored after 5 years of follow-up. As in the logistic regression analysis, a significant relationship existed between pain and mortality. In Model 3, in which demographic characteristics, the FI, mood, and cognitive status were included, the relative risk of death over a 5-year period was significantly lower in persons reporting moderate or greater pain than in those with no or very mild pain (HR = 0.85, 95% CI = 0.75–0.96; P = .01). A significant interaction between pain and sex explained this effect. Men with pain were not significantly more likely than men without pain to die over the 5-year period (HR = 1.00, 95% CI = 0.84–1.19; P = .99), whereas women without (HR = 0.54, 95% CI = 0.47–0.63; P = .01) and with (HR = 0.40; CI = 0.33–0.47; P < .001) pain were significantly less likely to die (Figure 1). The interaction term between pain, frailty, and mortality over the 5-year period was not significant (HR = 0.99, 95% CI = 0.96–1.03; P = 71).
Table 4.
Cox Proportional Hazards Regression of Mortality over 5 Years According to Pain, Participant Demographic Characteristics, Frailty, Depressed Mood, and Cognitive Impairment (N = 4,476)
| Explanatory Variable | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | |||
| Age | 1.12 (1.11–1.12)a | 1.09 (1.08–1.10)a | 1.08 (1.07–1.09)a |
| Female | 0.55 (0.50–0.61)a | 0.50 (0.44–0.56)a | 0.48 (0.43–0.54)a |
| Caucasian | 0.68 (0.41–1.13) | 0.60 (0.35–1.04) | 0.66 (0.36–1.19) |
| Education (reference <high school) | |||
| High school | 0.92 (0.82–1.03) | 1.01 (0.89–1.15) | 1.13 (0.99–1.29) |
| College | 0.82 (0.71–0.93)a | 0.86 (0.75–1.01) | 0.99 (0.85–1.16) |
| Moderate pain | 1.13 (1.02–1.25)a | 0.85 (0.76–0.96)a | 0.85 (0.75–0.96)a |
| Frailtyb | — | 1.12 (1.10–1.14)a | 1.11 (1.10–1.14)a |
| Depressed moodc | — | — | 1.20 (1.05–1.38)a |
| Cognitive impairmentd | — | — | 1.75 (1.52–2.03)a |
P < .01.
A composite measure of self-reported health (1 item), medical comorbidity (17 items), functional abilities (14 items), and social support (1 item); scores range from 0 to 33, with higher scores indicating poorer health.
A summed response score of ≥2 on the 5-item mental health screening questionnaire (range 0–30).
According to the modified Mini-Mental State Examination; scores of ≤77 indicate cognitive impairment.
Figure 1.
Survival according to sex and pain status.
DISCUSSION
This large, representative, prospective cohort study found that self-report of clinically meaningful pain (moderate intensity or higher) was associated with lower likelihood of death 5 years later than self-report of no or mild pain. A significant interaction between pain and sex explained this effect, with older women with clinically significant pain being less likely to die within 5 years than older women without pain, men in pain, or men without pain. These findings do not support the initial hypothesis.
To the knowledge of the authors, this study is the first to specifically examine the relationship between pain and 5-year mortality solely in an older adult population. The findings differ from those of previous studies in younger populations in which significant pain was generally not found to be associated with subsequent death once the analysis was adjusted for concomitant morbidity.18–20 The apparent “protective” association between moderate or greater pain and future mortality remained robust after adjustment for known confounders (frailty, mood, and cognition), with a significant interaction between pain and sex, with women in pain being less likely to die over the 5 years than women without pain, men in pain, and men without pain.
Persistent noncancer pain should not automatically be equated with suffering, especially in older adults. Two prior studies support this idea.32,33 A cross-sectional analysis of older adults with chronic low back pain demonstrated that pain duration is inversely associated with disability measured according to self-report and performance-based instruments. That is, the longer the duration of pain (in this study, on average more than a decade), the less-disabled participants were.32 It was also found that older adults with persistent pain were more likely to have larger social networks than those without pain. Others have demonstrated that older adults with persistent pain report better mental health, less fear avoidance, less passive coping, and more life control than younger adults with pain.33 Assuming the participants in the CSHA who reported pain at baseline had persistent pain, it is likely that they, too, had called upon a number of unmeasured resources to confer the survival advantage demonstrated. Thus, rather than pain itself being protective, it may have been the internal and external resources that participants had garnered over the years that bolstered their resilience. It is important to recognize that the apparently counterintuitive findings of the current study and of others highlight that older adults in pain may adapt in yet-unappreciated ways, which adds to the small body of literature demonstrating that older adults with persistent pain should be viewed and managed differently than younger individuals with persistent pain.
Another potential explanation is that persons with clinically significant persistent pain access the healthcare system more frequently than persons without pain, which leads to better identification and management of medical problems. Research showing that persons with pain use the healthcare system more often than those without supports this supposition, although the evidence base specific to older adults according to sex is less well established.34–36 In addition to pain assessment and management, these visits may lead to greater adherence to preventive recommendations, particularly among women, along with better management of common chronic medical conditions such as hypertension, hypercholesterolemia, and diabetes mellitus that are known to increase mortality risk.
Another possibility is that, in contrast to younger persons, clinically significant persistent pain may serve a protective role particularly in women. Many sensory functions deteriorate with aging, including hearing, vision, and vibratory or position sense, diminishing an older person’s ability to respond and adapt to the environment. These decrements have been associated with functional decline, falls, and mortality.37,38 Older adults who experience clinically significant persistent pain may possess a more-dynamic and -responsive nociceptive system, so that they more appropriately and rapidly respond to health threats. Moreover, these individuals could be more vigilant and their risk-taking behavior more congruent with their health status. Although this theory challenges the long-held tenet that persistent pain has no intrinsic value, a recent randomized controlled trial of a nerve growth factor antibody given to adults with moderate to severe knee osteoarthritis pain further supports it.39 Nerve growth factor blockade significantly decreased pain intensity and simultaneously appeared to predispose to joint damage, often in the unaffected knee, that was serious enough to warrant joint replacement. Thus, clinically significant pain in these participants appeared to have a protective effect.
Although the current study has several strengths, including a large sample size drawn from a nationally representative sample that incorporated a validated pain measure, the following limitations are also acknowledged. A portion of the sample had missing data (17.7%), mostly in the frailty measure, and therefore was excluded from the analyses. It might be that their exclusion biased the findings because individuals with missing data for these variables tended to be older and had more comorbidities than those with complete data. In addition, the data collected within the CSHA limited the study because not all potential confounders could be controlled, such as the use of pain medication. Some pharmacological therapies to treat pain, particularly nonsteroidal antiinflammatory drugs and opioids, have been associated with greater mortality.40 The lower mortality associated with more-significant levels of pain in the current study’s cohort could be a result of less exposure to potentially toxic medications, although at the time of this study, the use of opioids for noncancer pain in community-dwelling older adults was infrequent. Also, access to pain location or the underlying etiology of pain was not available, so it was not possible to quantify how this might have affected the findings. Another potential limitation is that the pain data represent only one point in time, diminishing the ability to capture persistent pain, although pain status in older adults remains relatively constant over time.41 It is likely, therefore, that the pain data represent an accurate proxy of participants’ overall pain status.
Contrary to most prior evidence and the hypothesis, older women reporting moderate or greater pain had a significantly lower likelihood of dying within 5 years than women without pain and men with and without pain after controlling for other confounding factors. In particular, the adjustment for frailty, which itself is strongly associated with mortality, strengthens this apparent negative association between pain and mortality. Although the mechanism by which pain appears to provide this “protective” effect remains uncertain, this study does not support the common assertion that noncancer pain is associated with greater mortality. The findings inform conversations with older adults in pain, whereby negative consequences can be balanced with potential advantages facilitating coping strategies, although the findings do not mitigate the importance or need for healthcare providers to assess and manage pain, but instead to appreciate that clinically significant pain may afford an opportunity to address important pain-associated comorbidities (e.g., anxiety, depression, sleep and appetite disturbance) that threaten quality of life and other comorbidities that threaten lifespan. Prospective studies of persistent pain in older adults are sorely needed to better understand its relationship with outcomes over time.
Acknowledgments
All the data reported here were gathered using public funding from the National Health Research Development Program, which administrated a grant from the Seniors’ Independence Research program (6606–3954-MC(S)). Funding for these analyses came from a career development award from the National Institute on Aging (K23AG029815) and from the Canadian Institutes of Health Research through an operating grant (MOP-62823). Dr. Chin was supported by a National Institute of Diabetes and Digestive and Kidney Diseases Midcareer Investigator Award in Patient-Oriented Research (K24 DK071933) and the Chicago Center for Diabetes Translation Research (P30 DK092949). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the National Institute on Aging, the Centers for Disease Control and Prevention, or the National Center for Health Statistics.
Footnotes
Conflict of Interest: None of the authors maintain a personal or financial conflict of interest.
Author Contributions: All authors participated in study concept and design, acquisition of subjects and data, analysis and interpretation of data, and preparation of manuscript.
Sponsor’s Role: The National Institute on Aging had no role in the design, methods, participant recruitment, data collection, analysis, and preparation of the paper.
References
- 1.Maxwell CJ, Dalby DM, Slater M, et al. The prevalence and management of current daily pain among older home care clients. Pain. 2008;138:208–216. doi: 10.1016/j.pain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Jakobsson U, Klevsgard R, Westergren A, et al. Old people in pain: A comparative study. J Pain Symptom Manage. 2003;26:625–636. doi: 10.1016/s0885-3924(03)00145-3. [DOI] [PubMed] [Google Scholar]
- 3.Smith AK, Cenzer IS, Knight SJ, et al. The epidemiology of pain during the last 2 years of life. Ann Intern Med. 2010;153:563–569. doi: 10.1059/0003-4819-153-9-201011020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wajnberg A, Ornstein K, Zhang M, et al. Symptom burden in chronically ill homebound individuals. J Am Geriatr Soc. 2013;61:126–131. doi: 10.1111/jgs.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thielke SM, Whitson H, Diehr P, et al. Persistence and remission of musculoskeletal pain in community-dwelling older adults: Results from the Cardiovascular Health Study. J Am Geriatr Soc. 2012;60:1393–1400. doi: 10.1111/j.1532-5415.2012.04082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laiho K, Tuohimehto J, Tilvis R. Prevalence of rheumatoid arthritis and musculoskeletal disease in the elderly population. Rheumatol Int. 2001;20:85–87. doi: 10.1007/s002960000087. [DOI] [PubMed] [Google Scholar]
- 7.Bianch ML, Orsini MR, Saraifoger S, et al. Quality of life in post-menopausal osteoporosis. Health Qual Life Outcomes. 2005;3:78. doi: 10.1186/1477-7525-3-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimberlin DK, Whitley RJ. Varicella-Zoster vaccine for the prevention of herpes zoster. N Engl J Med. 2007;356:1338–1343. doi: 10.1056/NEJMct066061. [DOI] [PubMed] [Google Scholar]
- 9.Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: What is moderate pain in millimeters? Pain. 1997;72:95–97. doi: 10.1016/s0304-3959(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 10.Weiner DK, Haggerty CL, Kritchvesky SB, et al. How does low back pain impact physical function in independent, well-functioning older adults? Evidence from the Health ABC cohort and implications for the future. Pain Med. 2003;4:311–320. doi: 10.1111/j.1526-4637.2003.03042.x. [DOI] [PubMed] [Google Scholar]
- 11.AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc. 2002;50:1–20. doi: 10.1046/j.1532-5415.50.6s.1.x. [DOI] [PubMed] [Google Scholar]
- 12.Shega JW, Andrew M, Hemmerich J, et al. The relationship of pain and cognitive impairment with social vulnerability. An analysis of the Canadian Study of Health and Aging. Pain Med. 2012;13:190–197. doi: 10.1111/j.1526-4637.2011.01309.x. [DOI] [PubMed] [Google Scholar]
- 13.Weiner DK, Rudy TE, Morrow L, et al. The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Med. 2006;7:60–70. doi: 10.1111/j.1526-4637.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- 14.Blyth BM, Rochat S, Cumming RG, et al. Pain, frailty, and comorbidity on older men? The CHAMP study. Pain. 2008;140:224–230. doi: 10.1016/j.pain.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Karp JF, Shega JW, Morone N, et al. Advances in understanding the mechanisms and management of persistent pain in older adults: The critical role of descending inhibition. Br J Anesth. 2008;101:111–120. doi: 10.1093/bja/aen090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macfarlane GJ, McBeth J, Silman AJ. Widespread body pain and mortality: Prospective population based study. BMJ. 2001;323:1–5. doi: 10.1136/bmj.323.7314.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macfarlane GJ, Jones GT, Knekt P, et al. Is the report of widespread body pain associated with long-term increased mortality? Data from the Mini-Finland Health Survey. Rheumatology. 2007;46:805–807. doi: 10.1093/rheumatology/kel403. [DOI] [PubMed] [Google Scholar]
- 18.Anderson HI. Increased mortality among individuals with chronic widespread pain relates to lifestyle factors: A prospective population-based study. Disabil Rehabil. 2009;31:1980–1987. doi: 10.3109/09638280902874154. [DOI] [PubMed] [Google Scholar]
- 19.Torrance N, Elliot AM, Lee AJ, et al. Severe chronic pain is associated with increased 10-year mortality. A cohort linkage study. Eur J Pain. 2010;14:380–386. doi: 10.1016/j.ejpain.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Krein SL, Heisler M, Piettie JD, et al. The effects of chronic pain on diabetes patients’ self-management. Diabetes Care. 2005;28:65–70. doi: 10.2337/diacare.28.1.65. [DOI] [PubMed] [Google Scholar]
- 21.Canadian Study of Health and Aging Working Group. Canadian Study of Health and Aging: Study methods and prevalence of dementia. Can Med Assoc J. 1994;150:899–913. [PMC free article] [PubMed] [Google Scholar]
- 22.McDowell I, Hill G, Lindsay J. An overview of the Canadian Study of Health and Aging. Int Psychogeriatr. 2001;13(Suppl 1):7–18. doi: 10.1017/s1041610202007949. [DOI] [PubMed] [Google Scholar]
- 23.Taylor LJ, Harris J, Epps CD, et al. Psychometric evaluation of selected pain intensity scales for use with cognitively impaired and intact older adults. Rehabil Nurs. 2005;30:55–61. doi: 10.1002/j.2048-7940.2005.tb00360.x. [DOI] [PubMed] [Google Scholar]
- 24.Chibnall JT, Tait RC. Pain assessment in cognitively impaired and unimpaired older adults: A comparison of four scales. Pain. 2001;92:173–186. doi: 10.1016/s0304-3959(00)00485-1. [DOI] [PubMed] [Google Scholar]
- 25.Shega JW, Ersek M, Herr K, et al. The multidimensional experience of non-cancer pain in older persons: Does cognitive status matter? Pain Med. 2010;11:1680–725. doi: 10.1111/j.1526-4637.2010.00987.x. [DOI] [PubMed] [Google Scholar]
- 26.Mitnitski AB, Song X, Rockwood K. The estimation of relative fitness and frailty in community-dwelling older adults using self-report data. J Gerontol A Biol Sci Med Sci. 2004;59A:627–532. doi: 10.1093/gerona/59.6.m627. [DOI] [PubMed] [Google Scholar]
- 27.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62A:738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 28.Berwick DM, Murphy JM, Goldman PA, et al. Performance of a five-item mental health screening. Med Care. 1991;29:169–176. doi: 10.1097/00005650-199102000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) Exam. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 30.Bland RC, Newman SC. Mild dementia or cognitive impairment: The Modified Mini-Mental State Examination (3MS) as a screen for dementia. Can J Psychiatry. 2001;46:506–510. doi: 10.1177/070674370104600604. [DOI] [PubMed] [Google Scholar]
- 31.Shega JW, Dale W, Andrews M, et al. Persistent pain and frailty: A case for pain homeostenosis? J Am Geriatr Soc. 2012;60:113–117. doi: 10.1111/j.1532-5415.2011.03769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiner DK, Rudy TE, Kim Y, et al. Do medical factors predict disability in older adults with persistent low back pain? Pain. 2004;112:214–220. doi: 10.1016/j.pain.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 33.Wittink HM, Rogers WH, Lipman AG, et al. Older and younger adults in pain management programs in the United States: Differences and similarities. Pain Med. 2006;7:151–163. doi: 10.1111/j.1526-4637.2006.00113.x. [DOI] [PubMed] [Google Scholar]
- 34.Dworkin RH, Panarites CJ, Armstrong EP, et al. Healthcare utilization in people with postherpetic neuralgia and painful diabetic neuropathy. J Am Geriatr Soc. 2011;59:827–836. doi: 10.1111/j.1532-5415.2011.03403.x. [DOI] [PubMed] [Google Scholar]
- 35.Harrold LR, Yood RA, Straus W, et al. Challenges of estimating health service utilization for osteoarthritis patients on a population level. J Rheumatol. 2002;29:1931–1936. [PubMed] [Google Scholar]
- 36.Berger A, Sadosky A, Dukes E, et al. Clinical characteristics and patterns of healthcare utilization in patients with painful neuropathic disorders in UK general practice: A retrospective cohort study. BMC Neurol. 2012;12:8. doi: 10.1186/1471-2377-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kulmala J, Viljanen A, Sipila S, et al. Poor vision accompanied with other sensory impairments as a predictor of falls in older women. Age Ageing. 2009;38:162–167. doi: 10.1093/ageing/afn228. [DOI] [PubMed] [Google Scholar]
- 38.Lee DJ, Gomez-Marin O, Lam BL, et al. Severity of concurrent vision and hearing impairment and mortality. J Aging Health. 2007;19:382–396. doi: 10.1177/0898264307300174. [DOI] [PubMed] [Google Scholar]
- 39.Lane NE, Schnitzer TJ, Birbara CA, et al. Tenezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363:1521–1531. doi: 10.1056/NEJMoa0901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solomon DH, Rassen JA, Glynn RJ, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2010;170:1979–1986. doi: 10.1001/archinternmed.2010.450. [DOI] [PubMed] [Google Scholar]
- 41.Mossey JM, Gallagher RM. The longitudinal impact of comorbid chronic pain and chronic depression over 2 years in continuing care retirement community residents. Pain Med. 2004;5:335–348. doi: 10.1111/j.1526-4637.2004.04041.x. [DOI] [PubMed] [Google Scholar]