Abstract
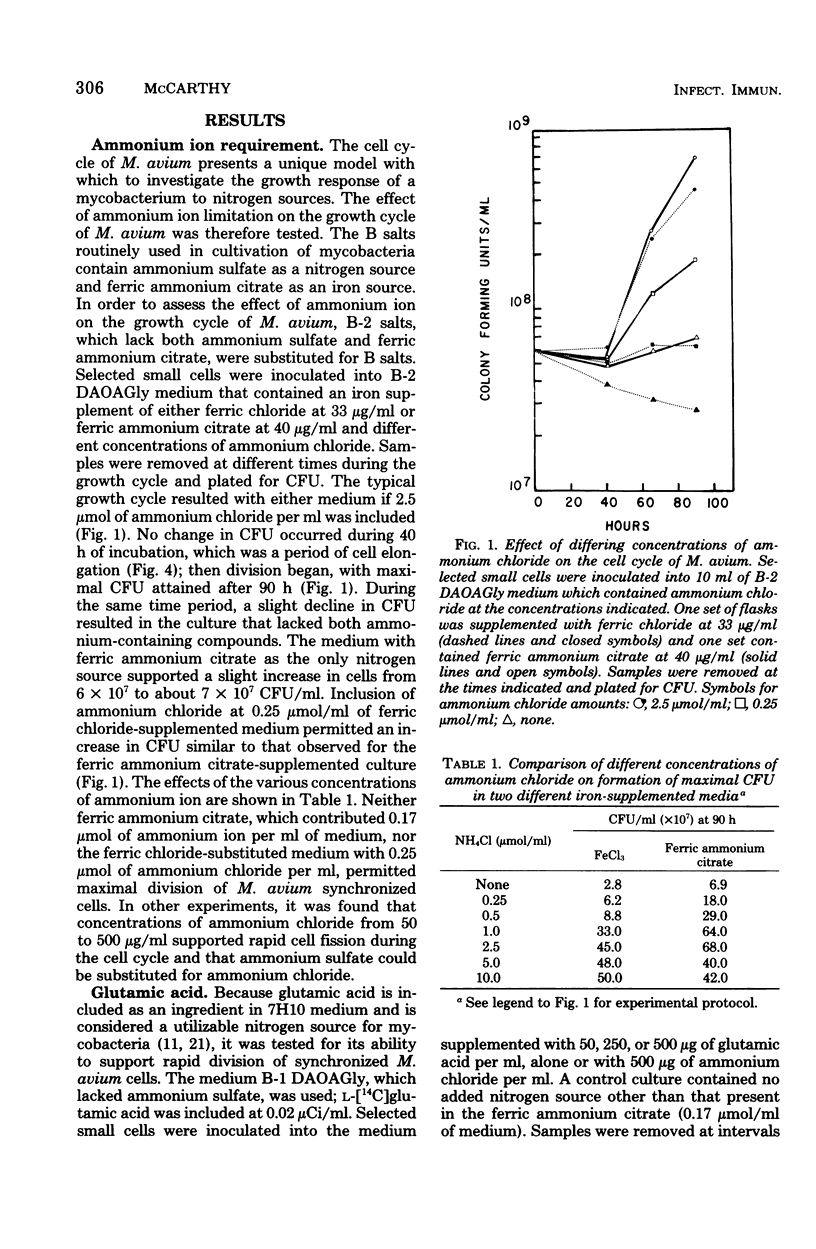
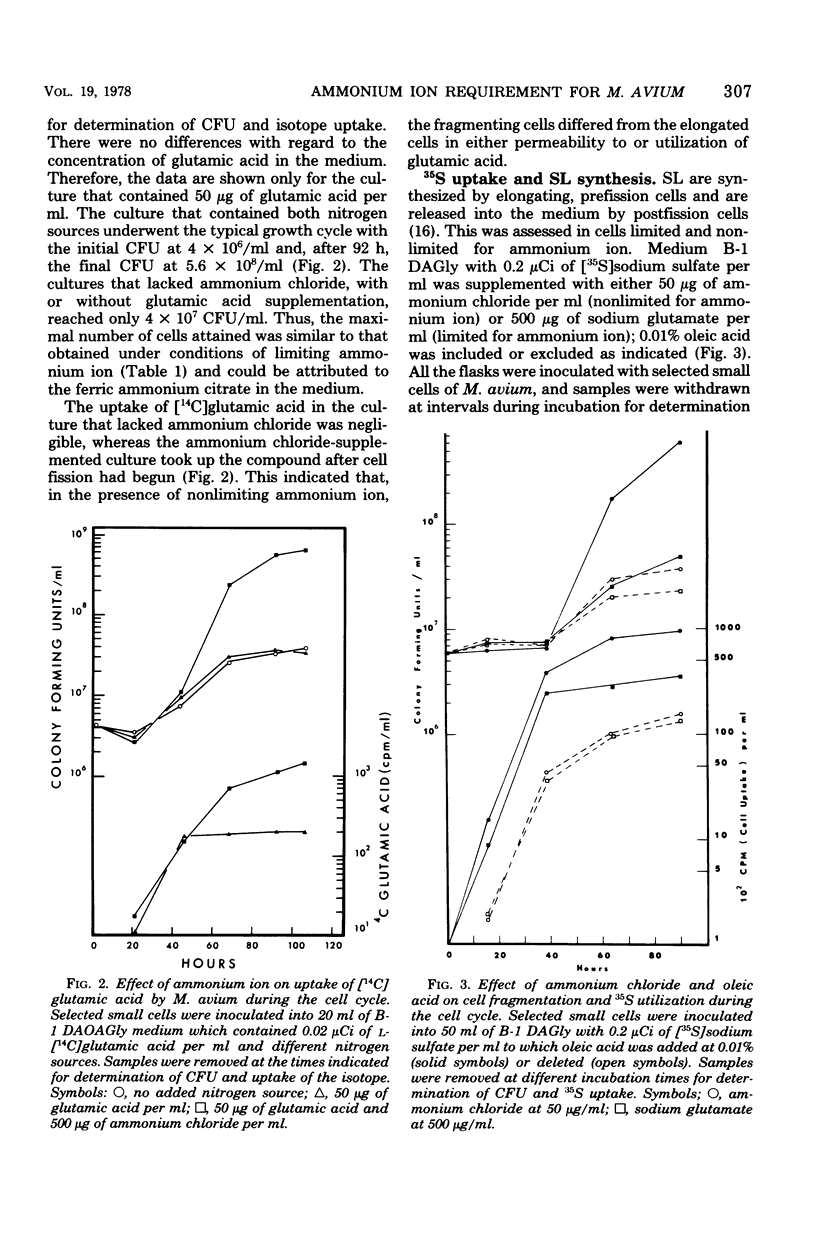
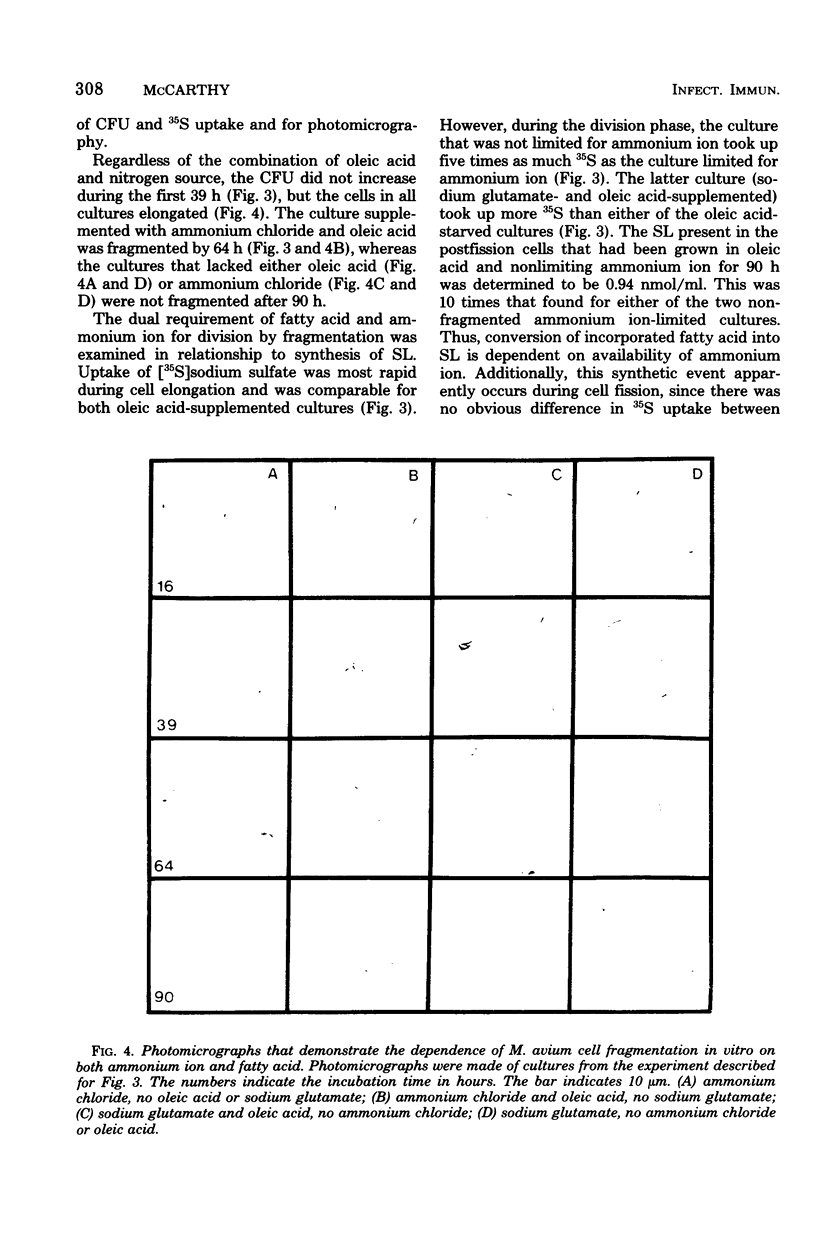
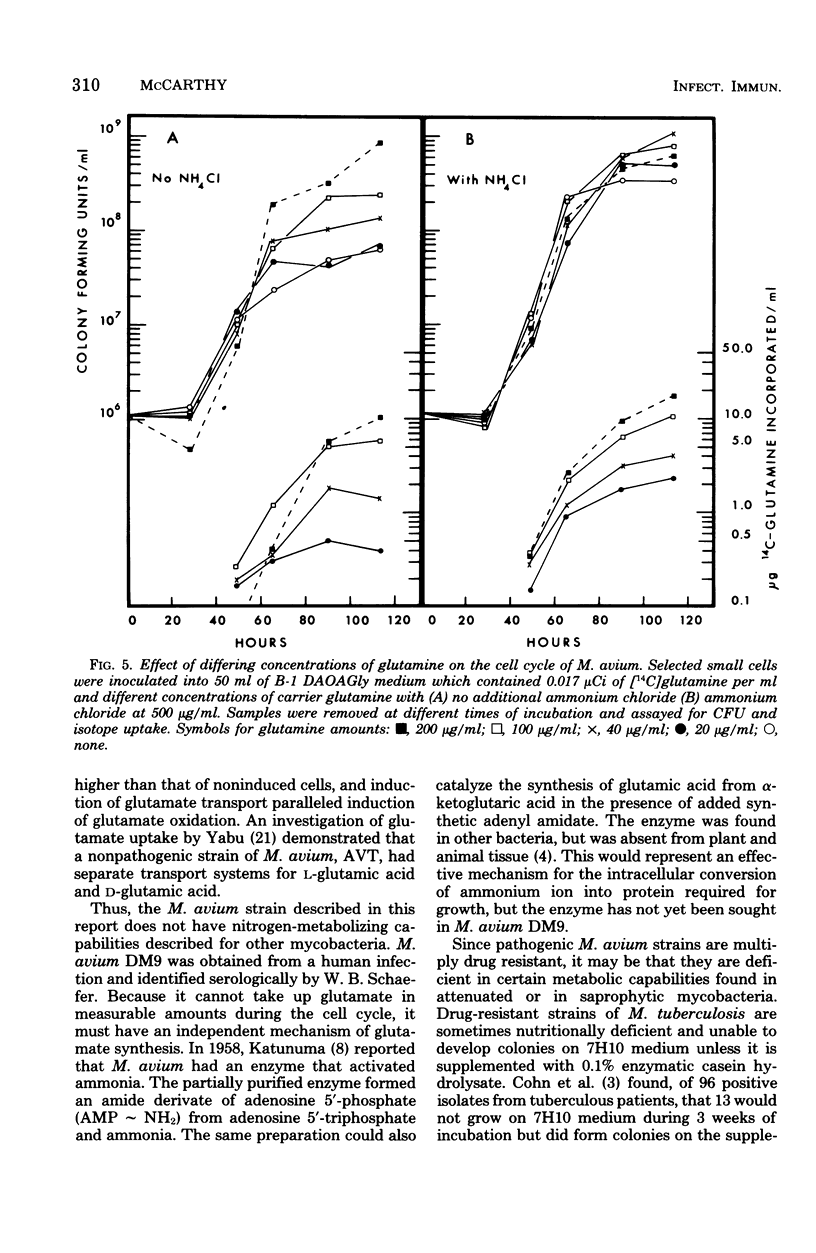
Mycobacterium avium has a defined cell cycle in which small cells elongate to about five times their original length and then divide by fragmentation. The nitrogen requirement for production of maximal number of colony-forming units was assessed by varying concentrations and kinds of nitrogen source in the medium. Ferric ammonium citrate at a concentration in 7H10 medium of 0.17 μmol/ml or ammonium chloride at 0.25 μmol/ml as the nitrogen source permitted the cells to elongate and to undergo limited division, with the final culture at 4 × 107 colony-forming units per ml. Ammonium chloride at 2.5 μmol/ml or glutamine at 1.37 μmol/ml supported completion of the cell cycle with final colony-forming units at about 5 × 108/ml. Other amino acids, including glutamic acid, at 2.5 μmol/ml did not support completion of the cell cycle, although in most cases an intermediate number of colony-forming units per milliliter were formed. Limited uptake of [14C]glutamic acid and uptake of [14C]glutamine were not detectable until cell fission began. Cells not limited for nitrogen took up five times as much 35S during fission as limited cells did during the same time. The nonlimited cells contained 10 times as much sulfolipid as the nitrogen-limited cells at the end of the cell cycle. These results demonstrate that rapidly dividing cells of M. avium utilize amino acids and sulfur and also synthesize sulfolipids in events that are apparently separable from metabolic functions of elongating cells. The results are contrasted with those found for other mycobacteria in which no cell cycle has been demonstrated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canetti G. Biology of the mycobacterioses. Pathogenesis of tuberculosis in man. Ann N Y Acad Sci. 1968 Sep 5;154(1):13–18. doi: 10.1111/j.1749-6632.1968.tb16691.x. [DOI] [PubMed] [Google Scholar]
- Cohn M. L., Waggoner R. F., McClatchy J. K. The 7H11 medium for the cultivation of mycobacteria. Am Rev Respir Dis. 1968 Aug;98(2):295–296. doi: 10.1164/arrd.1968.98.2.295. [DOI] [PubMed] [Google Scholar]
- ELLFOLK N., KATUNUMA N. The occurrence of ammonia-activiating enzyme in various organisms. Arch Biochem Biophys. 1959 Apr;81(2):521–522. doi: 10.1016/0003-9861(59)90233-4. [DOI] [PubMed] [Google Scholar]
- Falk G. A., Hadley S. J., Sharkey F. E., Liss M., Muschenheim C. Mycobacterium avium infections in man. Am J Med. 1973 Jun;54(6):801–810. doi: 10.1016/0002-9343(73)90069-7. [DOI] [PubMed] [Google Scholar]
- Goren M. B., D'Arcy Hart P., Young M. R., Armstrong J. A. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2510–2514. doi: 10.1073/pnas.73.7.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATUNUMA N. Adenyl amidate as active intermediate in the fixation of the amino group of amino acid. Arch Biochem Biophys. 1958 Aug;76(2):547–548. doi: 10.1016/0003-9861(58)90179-6. [DOI] [PubMed] [Google Scholar]
- Kato M., Goren M. B. Synergistic action of cord factor and mycobacterial sulfatides on mitochondria. Infect Immun. 1974 Oct;10(4):733–741. doi: 10.1128/iai.10.4.733-741.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean E. L. Rapid, sensitive spectrophotometric method for quantitative determination of sulfatides. J Lipid Res. 1968 May;9(3):319–327. [PubMed] [Google Scholar]
- Lyon R. H., Hall W. H., Costas-Martinez C. Utilization of Amino Acids During Growth of Mycobacterium tuberculosis in Rotary Cultures. Infect Immun. 1970 Jun;1(6):513–520. doi: 10.1128/iai.1.6.513-520.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon R. H., Rogers P., Hall W. H., Lichtein H. C. Inducible glutamate transport in Mycobacteria and its relation to glutamate oxidation. J Bacteriol. 1967 Jul;94(1):92–100. doi: 10.1128/jb.94.1.92-100.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C. Effect of palmitic acid utilization on cell division in Mycobacterium avium. Infect Immun. 1974 Feb;9(2):363–372. doi: 10.1128/iai.9.2.363-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C. Electronic counting in growth studies of Mycobacterium avium. Appl Microbiol. 1971 Oct;22(4):546–551. doi: 10.1128/am.22.4.546-551.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C. Spontaneous and Induced Mutation in Mycobacterium avium. Infect Immun. 1970 Sep;2(3):223–228. doi: 10.1128/iai.2.3.223-228.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C. Synthesis and release of sulfolipid by Mycobacterium avium during growth andcell division. Infect Immun. 1976 Nov;14(5):1241–1252. doi: 10.1128/iai.14.5.1241-1252.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C. Utilization of palmitic acid by Mycobacterium avium. Infect Immun. 1971 Sep;4(3):199–204. doi: 10.1128/iai.4.3.199-204.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G., Schröder K. H., Amadio G. E., Anz W., Chaparas S., Engel H. W., Jenkins P. A., Käppler W., Kleeberg H. H., Kubala E. A co-operative numerical analysis of nonscoto- and nonphotochromogenic slowly growing mycobacteria. J Gen Microbiol. 1974 Aug;83(2):207–235. doi: 10.1099/00221287-83-2-207. [DOI] [PubMed] [Google Scholar]
- Runyon E. H. Pathogenic mycobacteria. Bibl Tuberc. 1965;21:235–287. [PubMed] [Google Scholar]
- Schaefer W. B., Davis C. L., Cohn M. L. Pathogenicity of transparent, opaque, and rough variants of Mycobacterium avium in chickens and mice. Am Rev Respir Dis. 1970 Oct;102(4):499–506. doi: 10.1164/arrd.1970.102.4.499. [DOI] [PubMed] [Google Scholar]
- Winder F. G., Rooney S. A. Effects of nitrogenous components of the medium on the carbohydrate and nucleic acid content of Mycobacterium tuberculosis BCG. J Gen Microbiol. 1970 Sep;63(1):29–39. doi: 10.1099/00221287-63-1-29. [DOI] [PubMed] [Google Scholar]
- YOUMANS G. P. THE PATHOGENIC "ATYPICAL" MYCOBACTERIA. Annu Rev Microbiol. 1963;17:473–494. doi: 10.1146/annurev.mi.17.100163.002353. [DOI] [PubMed] [Google Scholar]
- Yabu K. The uptake of D-glutamic acid by Mycobacterium avium. Biochim Biophys Acta. 1967 Feb 1;135(1):181–183. doi: 10.1016/0005-2736(67)90026-0. [DOI] [PubMed] [Google Scholar]



