Abstract
We examined the relationship between plasma adipokine concentrations and ultrasound measures of vascular health in 100 HIV-infected adults on antiretroviral therapy. Leptin was positively correlated with flow-mediated dilation of the brachial artery and negatively with carotid intima–media thickness. These relationships were independent of traditional risk factors and trunk fat in women but not men. Neither adiponectin nor resistin was associated with either measure of vascular health.
Changes in body fat composition in HIV-infected patients on antiretroviral therapy (ART) are associated with adverse cardiometabolic risk factors [1] and altered levels of circulating adipocytokines [2,3]. Adipokines are known to exert direct effects on endothelial cells and vascular function in vitro [4–7], although larger epidemiologic studies have failed to demonstrate a consistent relationship between plasma adipokine concentrations and cardiovascular disease (CVD) in humans [8,9]. Little is known about the relationship between adipokines and CVD risk in HIV. In this study, we sought to examine the relationship between adipokines and measures of vascular health among HIV-infected patients on stable ART.
Plasma concentrations of leptin, adiponectin, and resistin were measured by ELISA in 100 HIV-infected adults, on stable ART, with HIV-1 RNA less than 1000 copies/ml and low-density lipoprotein (LDL)-cholesterol 130 mg/dl or less. The study was approved by the Institutional Review Board of University Hospitals Case Medical Center (Cleveland, Ohio, USA) and written informed consent was obtained from each subject. Scatter plots, t-tests, Spearman correlations, and multivariable linear regression were used to examine the association of adipokines with regional fat distribution (trunk and limb fat), pericardial and periaortic fat volumes, common carotid artery intima–media thickness (CIMT), and endothelial function measured by brachial artery flow-mediated dilation (FMD). Fat distribution was measured by dual-energy X-ray absorptiometry (DEXA) in the anteroposterior view using Lunar Prodigy Advance (GE Healthcare, Waukesha, Wisconsin, USA). Pericardial and thoracic periaortic fat volumes were quantified as described previously [10] from a computed tomography scan of the chest (Somatom Sensation 64; Siemens Medical Solutions, USA). Semiautomated edge detection software (Medical Imaging Applications LLC, Coralville, lowa, USA) was used to measure mean–mean common carotid artery far wall CIMT [11]. Flow-dependent endothelial function testing was performed by brachial artery ultrasound using a 5-min forearm occlusion method as previously described [12]. All vascular ultrasound and perivascular fat measurements were performed by a single blinded reader (C.T.L.). Non-normally distributed variables (including the adipokine concentrations, FMD, CIMT, and perivascular fat volumes) were log-transformed for all analyses. All statistical tests were two-sided with a 0.05 significance level.
Overall, the study population was 77% men and 70% African–American. Median [interquartile range (IQR)] age and BMI were 47 (42–53) years and 27 (24–31) kg/m2, respectively; although BMI was higher in women compared with men [32 (26–37) kg/m2 versus 26 (23–29) kg/m2, P < 0.001]. Compared with men, women had 2.2-fold higher limb fat and 1.6-fold higher trunk fat (P < 0.001) by DEXA. Overall, median (IQR) CD4 cell count of 633 (453–854) cells/μl and 81% had HIV-1 RNA less than 50 copies/ml. Fifty percent were on a protease inhibitor; however, only 4% were currently taking a thymidine analog nucleoside reverse transcriptase inhibitor. One quarter of participants had metabolic syndrome [13]. Thirty percent of women were treated with antihypertensive medication, 4% were taking aspirin, and 83% had HIV-1 RNA less than 50 copies/ml. Of the women with detectable viremia (n = 4), all had less than 200 copies/ml.
Leptin concentration was much higher in women compared with men [median (IQR) 55 (21–73) versus 7.0 (2.9–13)ng/ml, P < 0.001], whereas adiponectin and resistin concentrations were similar (P = 0.844 and P = 0.345, respectively). Leptin concentration was strongly and linearly correlated with both trunk fat (r = 0.836, P < 0.0001) and limb fat (r = 0.862, P < 0.0001), although correlations with epicardial and periaortic fat were weaker (r = 0.399, P < 0.0001 and r = 0.267, P = 0.007, respectively). Adiponectin was negatively correlated with trunk fat (r = −0.336, P = 0.0006), epicardial fat (r = −0.300, P = 0.047), and periaortic fat (r = −0.200, P = 0.0008); but not limb fat (P = 0.108). No correlation was observed between resistin concentration and regional or perivascular fat volumes (P > 0.20).
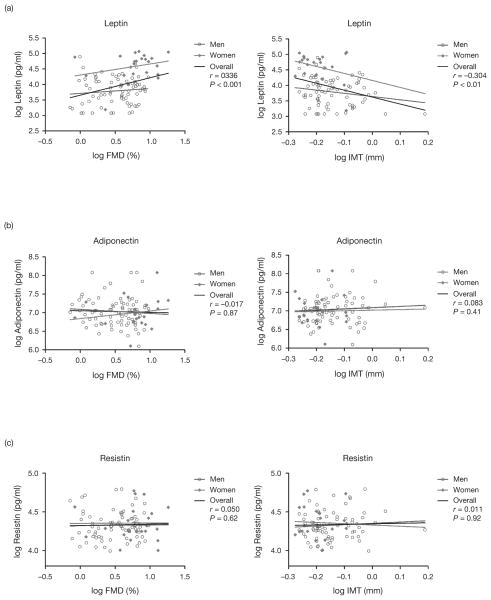
Leptin concentration was positively correlated with FMD (r = 0.336, P = 0.0006) and negatively with CIMT (r = −0.304, P = 0.002). These relationships were stronger in women compared with men (Fig. 1a). In multivariable models that adjusted for age, smoking, trunk fat, and baseline brachial artery diameter (for FMD only), leptin remained positively associated with FMD and negatively associated with CIMT in women (P = 0.018 and P = 0.022, respectively) but not men (P = 0.565 and P = 0.748, respectively). Neither adiponectin nor resistin was associated with FMD or CIMT (Fig. 1b and c).
Fig. 1. Relationship between three adipokines and markers of vascular structure and function.
(a) Leptin was positively correlated with flow-mediated dilation (FMD) of the brachial artery and negatively with common carotid artery intima–media thickness (IMT). (b) Adiponectin was not correlated with FMD or IMT. (c) Resistin was not correlated with FMD or IMT.
In this contemporary cohort of HIV-infected men and women on ART without clinical lipoatrophy and with limited use of thymidine analogs, higher plasma leptin concentration appears to be associated with healthier arterial structure and function measured by carotid ultrasound and brachial artery FMD. In women, these relationships were independent of several traditional CVD risk factors and visceral adiposity. These results suggest the possibility of an obesity paradox in this population of HIV-infected women with regards to CVD risk.
Although obesity is generally associated with poor vascular health and CVD risk in the general population [14,15], examples of CVD obesity paradoxes do exist [16,17]. Some prior studies of HIV-infected patients have reported similarly paradoxical results. In the Women’s Interagency Health Study, higher BMI was associated with lower prevalence of CIMT more than 1.5 mm despite a positive association with overall mean CIMT [18]. The Study of Fat Redistribution and Metabolic Change in HIV infection did not report associations of IMT with measures of adiposity [19]; however, in separate analyses, higher subcutaneous adipose tissue (SAT) was associated with higher leptin, paradoxically higher adiponectin [2], and lower Framingham risk [20]. In a study of endothelial function that included a small number of women (n = 27), both SAT and leptin concentration were positively correlated with FMD, although the relationship was attenuated in multivariable models [21]. Finally, we have previously described a positive correlation between BMI and FMD independent of other risk factors in a majority male population [22].
This study is limited by a relatively small sample size and cross-sectional design, although these limitations are shared by most studies of endothelial function in HIV. Therefore, our study was not powered to explore all possible confounders. Our participants had little clinical lipoatrophy and favorable LDL cholesterol levels, which reflects current trends in the HIV-infected population; however, this makes comparisons with older studies more difficult.
In conclusion, previous studies in HIV have linked SAT to higher leptin and lower cardiovascular risk [2,20]. In this ultrasound study of vascular health, these relationships appear to persist in an obese HIV-infected population with a low prevalence of clinical lipoatrophy, particularly among women. This unexpected observation merits further investigation in longitudinal studies.
Acknowledgments
This project was supported by an award from the National Institutes of Health NR012642. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health. The study is registered with clinicaltrials.gov, number NCT01218802.
Footnotes
Conflicts of interest
C.T.L. has received a virology fellow research grant from Bristol-Myers Squibb. S.M.D. currently serves on a DSMB of a Johnson and Johnson study. G.A.M. has served as a scientific advisor or speaker for Bristol-Myers Squibb, GlaxoSmithKline, Tibotec, and Merck, has received research grants from Bristol-Myers Squibb, GlaxoSmithKline, and Gilead Sciences, and is currently serving as the DSMB Chair for aPfizer-sponsored study. W.D. and Y.J. have no disclosures.
References
- 1.Gandhi RT, Sax PE, Grinspoon SK. Metabolic and cardiovascular complications in HIV-infected patients: new challenges for a new age. J Infect Dis. 2012;205 (Suppl 3):S353–S354. doi: 10.1093/infdis/jis202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosmiski LA, Bacchetti P, Kotler DP, Heymsfield SB, Lewis CE, Shlipak MG, et al. Relationship of fat distribution with adipokines in human immunodeficiency virus infection. J Clin Endocrinol Metab. 2008;93:216–224. doi: 10.1210/jc.2007-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veloso S, Escote X, Ceperuelo-Mallafre V, Lopez-Dupla M, Peraire J, Vilades C, et al. Leptin and adiponectin, but not IL18, are related with insulin resistance in treated HIV-1-infected patients with lipodystrophy. Cytokine. 2012;58:253–260. doi: 10.1016/j.cyto.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Knudson JD, Dincer UD, Zhang C, Swafford AN, Koshida R, Picchi A, et al. Leptin receptors are expressed in coronary arteries, and hyperleptinemia causes significant coronary endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H48–H56. doi: 10.1152/ajpheart.01159.2004. [DOI] [PubMed] [Google Scholar]
- 5.Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, et al. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56:1387–1394. doi: 10.2337/db06-1580. [DOI] [PubMed] [Google Scholar]
- 6.Ouchi N, Kihara S, Arita Y, Kazuhisa M, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Wang Y, Li Q, Chen Y, Sun SZ, Zhang WD, et al. Effect of resistin on vascular endothelium secretion dysfunction in rats. Endothelium. 2007;14:207–214. doi: 10.1080/10623320701617225. [DOI] [PubMed] [Google Scholar]
- 8.Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf) 2006;64:355–365. doi: 10.1111/j.1365-2265.2006.02474.x. [DOI] [PubMed] [Google Scholar]
- 9.Koh KK, Park SM, Quon MJ. Leptin and cardiovascular disease: response to therapeutic interventions. Circulation. 2008;117:3238–3249. doi: 10.1161/CIRCULATIONAHA.107.741645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yun CH, Lin TY, Wu YJ, Liu CC, Kuo JY, Yeh HI, et al. Pericardial and thoracic periaortic adipose tissues contribute to systemic inflammation and calcified coronary atherosclerosis independent of body fat composition, anthropometric measures and traditional cardiovascular risks. Eur J Radiol. 2012;81:749–756. doi: 10.1016/j.ejrad.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 11.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. quiz 89–90. [DOI] [PubMed] [Google Scholar]
- 12.Longenecker CT, Hileman CO, Carman TL, Ross AC, Seydafkan S, Brown TT, et al. Vitamin D supplementation and endothelial function in vitamin D deficient HIV-infected patients: a randomized placebo-controlled trial. Antivir Ther. 2012;17:613–621. doi: 10.3851/IMP1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 14.Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, et al. Body weight and mortality among women. N Engl J Med. 1995;333:677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 15.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 16.Uretsky S, Messerli FH, Bangalore S, Champion A, Cooper-Dehoff RM, Zhou Q, et al. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med. 2007;120:863–870. doi: 10.1016/j.amjmed.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EE, et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39:578–584. doi: 10.1016/s0735-1097(01)01802-2. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan RC, Kingsley LA, Gange SJ, Benning L, Jacobson LP, Lazar J, et al. Low CD4+T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22:1615–1624. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grunfeld C, Delaney JA, Wanke C, Currier JS, Scherzer R, Biggs ML, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009;23:1841–1849. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lake JE, Wohl D, Scherzer R, Grunfeld C, Tien PC, Sidney S, et al. Regional fat deposition and cardiovascular risk in HIV infection: the FRAM study. AIDS Care. 2011;23:929–938. doi: 10.1080/09540121.2010.543885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dube MP, Shen C, Mather KJ, Waltz J, Greenwald M, Gupta SK. Relationship of body composition, metabolic status, antiretroviral use, and HIV disease factors to endothelial dysfunction in HIV-infected subjects. AIDS Res Hum Retroviruses. 2010;26:847–854. doi: 10.1089/aid.2010.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hileman CO, Longenecker CT, Carman TL, Milne G, Labbato DE, Storer NJ, et al. Elevated D-dimer is independently associated with endothelial dysfunction: a cross-sectional study in HIV-infected adults on antiretroviral therapy. Antivir Ther. 2012;17:1345–1349. doi: 10.3851/IMP2297. [DOI] [PubMed] [Google Scholar]