Abstract
Antiviral drugs used to treat HIV and hepatitis C are common causes of delayed drug hypersensitivities for which many of the more severe reactions have been recently shown to be immunogenetically mediated such as abacavir hypersensitivity where HLA-B*57:01 is now used routinely as a screening test to exclude patients carrying this allele from abacavir prescription. Most antiviral drug allergies consist of mild to moderate delayed rash without other serious features (e.g. fever, mucosal involvement, blistering rash, organ impairment. In these cases treatment can be continued with careful observation and symptomatic management and the discontinuation rate is low.
Keywords: altered peptide repertoire, abacavir, nevirapine, antiretroviral, telaprevir pharmacogenomics, human leukocyte antigen, major histocompatibility complex
Introduction
Pharmacologically predictable adverse drug reactions and drug interactions have been commonly associated with anti-infective drugs and particularly antiretroviral agents. Immunologically-mediated adverse drug reactions have also been commonly described in patients receiving drugs used to treat viral infections including those used to treat human immunodeficiency virus and hepatitis C infection1-3. These hypersensitivity reactions (HSR) associated with antiviral drugs can be classified by their immunological mechanisms as well as their specific clinical manifestation or phenotype. Gell-Coombs types I-III (immediate drug allergy, antibody mediated and immune complex mechanisms) are not commonly associated with antiviral drugs. Gell-Coombs type IV (T-cell mediated) reactions, however, have been commonly associated with drugs used to treat viral infection2-4. For instance the antiretroviral drug abacavir (ABC) causes a hypersensitivity syndrome which has a distinct phenotype not shared by other drugs and is characterized by fever, malaise, gastrointestinal symptoms. Mild to moderate skin rash is a late manifestation ABC hypersensitivity which occurs in 70% of cases4. The antiretroviral drug nevirapine has been commonly associated with a delayed rash as well as more severe hypersensitivity reactions (drug reaction with eosinophilia and systemic symptoms (DRESS) also known as drug-induced hypersensitivity syndromes (DIHS), Stevens-Johnson Syndrome/toxic epidermal necrolysis (SJS/TEN) and drug-induced liver disease (DILI))5. Many drugs such as the non-nucleoside reverse transcriptase inhibitor efavirenz and the anti-HCV NS3.4A serine protease inhibitor telaprevir are commonly associated with a non-specific exanthem without systemic symptoms which is not treatment limiting and continued treatment is usually possible with symptom control 5-9. More severe reactions with fever and/or mucosal and/or severe cutaneous involvement and/or internal organ (e.g. liver) involvement warrant immediate discontinuation and careful clinical monitoring. A major advance in pharmacogenomics has been the discovery that many immunologically-mediated drug reactions are mediated through interactions with class I and/or class II human leukocyte antigen alleles (HLA) (Table 1). In the case of abacavir a strong association between HLA-B*57:01 was discovered by two independent groups in 2002 and HLA-B*57:01 is now utilized as a routine screening test with a proven 100% negative predictive value, prior to abacavir prescription to exclude those at risk10, 11. Abacavir patch testing was also a useful research tool in this context that was used in clinical trials to identify those with true immunologically mediated abacavir HSR21-23. ABC patch testing has a diagnostic sensitivity of 87% but there is currently no in vivo or ex vivo diagnostic test for antiviral drug hypersensitivity that has a 100% sensitivity or negative predictive value, and clinical diagnosis is used as the gold standard on which future recommendations are based. Drug hypersensitivity reactions associated with nevirapine have also been associated with class I and II HLA alleles which appear to be ethnicity and phenotype dependent, with associations that appear currently too complex to apply as a screening strategy in routine clinical practice24-26 (Table 1). A major recent advance has been the elucidation of the science of abacavir hypersensitivity which has defined the specific mechanism by which abacavir specifically interacts with HLA-B*57:01 and alters the repertoire of self-peptide ligands22, 27, 28. Based on this work the crystal structure of abacavir bound peptide-HLA-B*57:01 has been resolved and a new mechanistic paradigm for how drugs effect T-cell mediated reactions defined29. Future applications of this work could include pre-clinical strategies to determine drugs at high risk to cause immunologically-mediated adverse drug reactions and hence inform drug design and development.
Table 1. Pharmacogenomics of Antiviral Drugs.
| Clinical Phenotypes and Drug | Alleles | Populations |
|---|---|---|
| DRESS/DIHS | ||
| Abacavir | B*57:01 | European, African10, 11 |
| Nevirapine (hepatitis) | DRB1*01:01 (CD4%≥25) and DRB1*01:02 | Australian, European, South African 12-14 |
| Nevirapine (DRESS/DIHS with rash) | Cw*8 or Cw*8-B*14 haplotype Cw*4 B*35 B*35:05 B*35:01 CYP2B6 516 G→T +C*04 CYP2B6(rs2054675,rs3786547,rs3745274) |
Italian, Japanese15, 16 White, Black, Asian Han Chinese 13, 16 Asian13 Asian (Thai)15 European/Australia17 White, Black, Asian13 White, Black, Asian13 |
| Delayed Rash (non-systemic) | ||
| Efavirenz | DRB1*01 | French5 |
| Nevirapine | DRB1*01 Cw*04 B*35:05; rs1576*G CCHR1 status (GWAS) |
French5 African, Asian, European, Thai13, 18 Thai15, 16 |
| SJS/TEN | ||
| Nevirapine | HLA-C*04:01 ?CYP2B6 983 T→C |
African (Malawian)19 Mozambique20 |
Immunopathogenesis and Pharmacogenomics of Antiviral Drug Allergy
Models that have been proposed to define the immunopathogenesis of drug allergy and hypersensitivity syndromes include the pre-hapten/hapten hypothesis, the pharmacologic-interaction (PI) model and most recently the altered peptide repertoire model. The hapten hypothesis suggests that a drug or a reactive metabolite irreversibly bind to and covalent modifies self-proteins leading to a neo-antigen27. The PI model suggests that drugs induce T-cell activation by directly interacting with immune receptors on a pharmacological level and non-covalently binding with HLA alleles and/or T-cell receptors28, 30. New evidence now exists that many drugs, including the nucleoside reverse transcriptase inhibitor and antiretroviral drug abacavir mediate hypersensitivity through the altered peptide repertoire model whereby it is proposed that drugs bind non-covalently with HLA, occupy anchor sites within the antigen binding cleft and thereby alter the repertoire of self-peptide ligand bound and presenting to T-cell creating a form of altered T-cell immunity based on alteration of immunological self by a drug. Multiple groups have now reproduced evidence demonstrating that abacavir binds non-covalently to the F binding pocket of HLA-B*57:01 to specific residues that define this allele and shifts the repertoire of self-peptides presented and the crystal structure of abacavir bound to HLA-B*57:01 and peptide has been solved 31-33. Because the altered peptide model does not explain why only 55% of HLA-B*57:01 positive patients experience abacavir hypersensitivity additional models are needed21. Other models for drug hypersensitivity include the heterologous immune model suggesting that these reactions could be mediated by cross-reactive memory T-cell responses to chronic prevalent viruses such as human herpes viruses (HHV) and/or mediated through restricted T-cell receptor repertoire usage as recently demonstrated for carbamazepine31. Reactivation of chronic persistent HHV such as HHV 6/7, cytomegalovirus (CMV) and Epstein-Barr Virus (EBV) has also been described as complications of DRESS/DIHS however this has not been a described feature of hypersensitivity associated with abacavir or other antivirals34-36.
In 2002 two independent groups reported a strong association between the HLA class I allele, HLA-B*57:01 and abacavir hypersensitivity syndrome10, 11. The non-specific nature of the clinical syndrome of abacavir hypersensitivity characterized by fever, rash, gastrointestinal and sometimes respiratory symptoms led to a high rate of false positive clinical diagnosis and the suggestion that the HLA-B*57:01 allele lacked sensitivity and 100% negative predictive value for abacavir hypersensitivity particularly in races with a low prevalence of HLA-B*57:01 carriage. A randomized controlled clinical trial that compared prospective HLA-B*57:01 screening versus abacavir initiation without screening and used abacavir patch testing (Figure 1) as a co-primary endpoint to clinical diagnosis to identify true immunologically mediated abacavir hypersensitivity, clarified the 100% negative predictive value of HLA-B*57:01 for abacavir hypersensitivity and hence its utility in clinical practice21. A follow-on study in whites and African Americans also confirmed the generalizability of this 100% negative predictive value to non-white ethnicities 37. Since 2008 international guidelines have recommended the use of HLA-B*57:01 screening prior to abacavir prescription and HLA-B*57:01 is now one of the most widely used genetic screening tests utilized in clinical practice today24.
Figure 1.
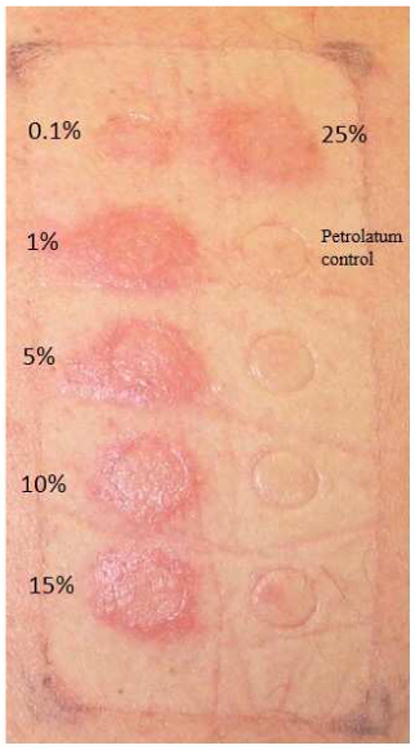
ABC patch test: 24-hour reading ABC in concentrations of 0.1, 1, 5, 10, 15, and 25% in petrolatum and petrolatum control are applied to the skin and left undisturbed for 24 hours.
Early in the post-marketing phase of abacavir's development abacavir hypersensitivity was defined as an HLA-B*57:01 restricted CD8+ T cell mediated reaction with long-lasting immunity as demonstrated by patch testing and ex vivo studies22, 38, 39. The PREDICT-1 study demonstrated that 55% of those carrying HLA-B*57:01 will develop abacavir hypersensitivity and although to-date no other HLA has been associated with abacavir hypersensitivity the mechanism of HLA-B*57:01 positive abacavir tolerance has not been determined21. Despite this incomplete positive predictive value abacavir specific CD8+ T cell responses can be reproduced in 10-12 day culture from 100% of HLA-B*57:01 positive abacavir naive healthy blood donors39.
Nevirapine is a non-nucleoside reverse transcriptase inhibitor (NNRTI) used to treat HIV whose major treatment limiting toxicity is a drug hypersensitivity syndrome. The phenotypes of nevirapine hypersensitivity vary from exanthem without other symptoms, DRESS/DIHS, hepatitis (DILI) and SJS/TEN (Figure 2.B.C.D). More recently each of these phenotypes has been associated with different class I and class II HLA associations across different ethnicities (Table 1)5, 12-18. An early study in a Western Australian cohort suggested that nevirapine hypersensitivity associated with rash and hepatitis was a CD4+ T cell dependent HLA-DRB1*01:01 restricted process12.
Figure 2.
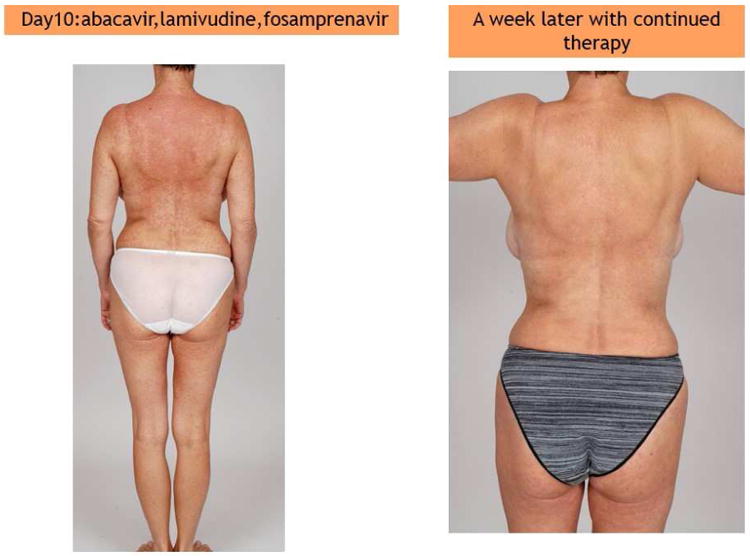
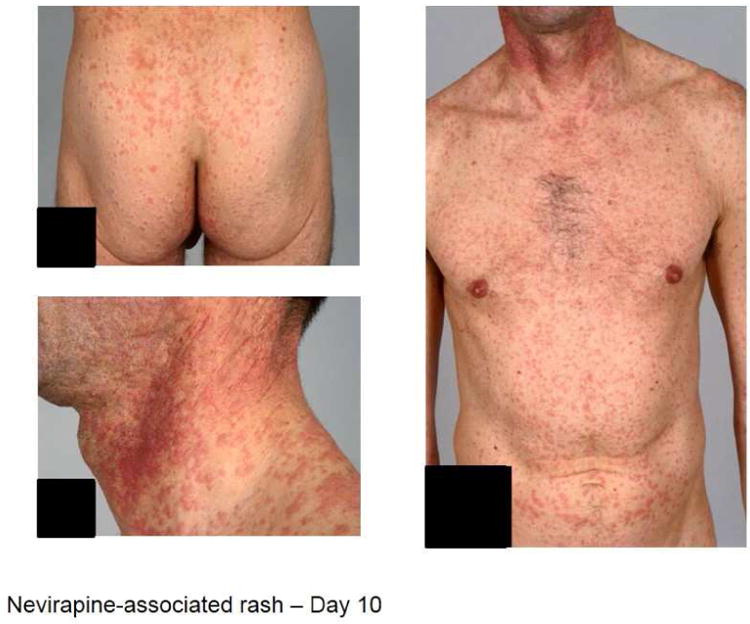
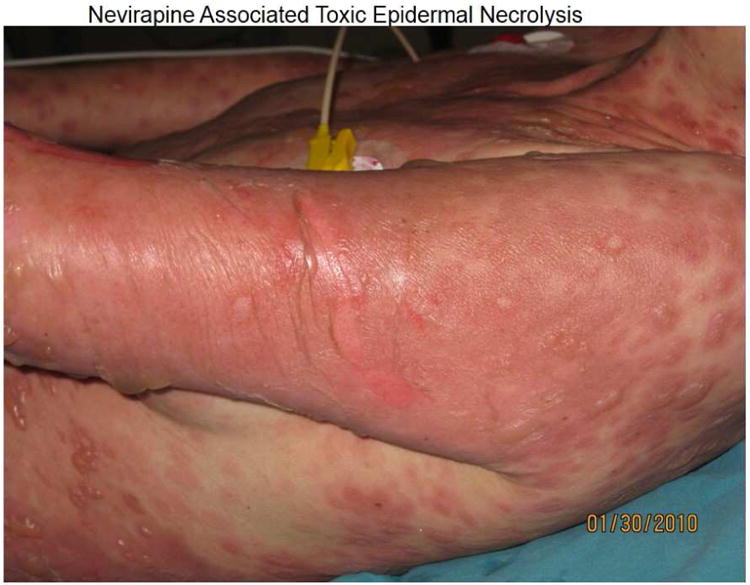
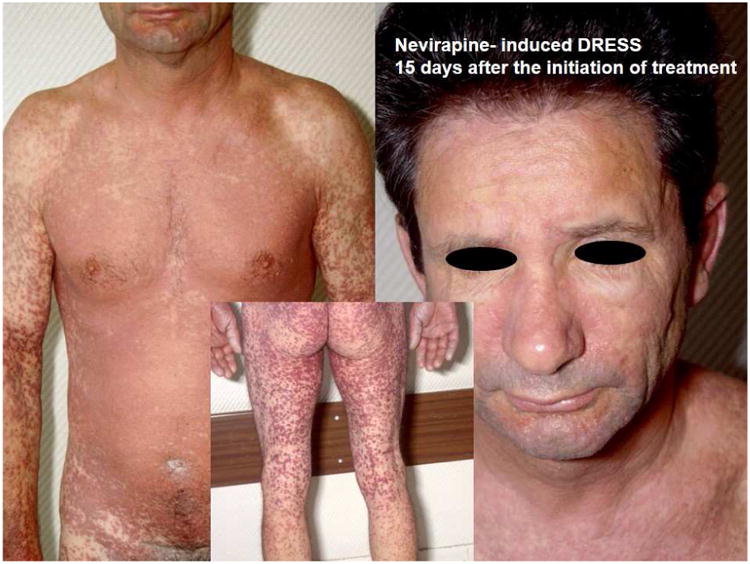
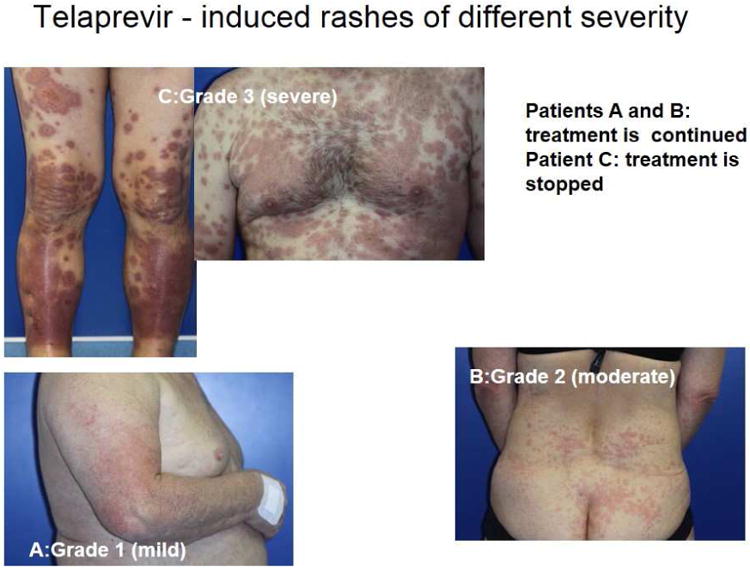
(A) (left) Patient on day 10: abacavir, lamivudine, fosamprenavir and (right) improvement a week later with continued therapy. (B) Patient displays Nevirapine-associated rash at Day 10. (C) Patient with Nevirapine-associated Toxic Epidermal Necrolysis. (D) Patient with Nevirapine-induced DRESS 15 days after the initiation of treatment. (E) Patients displaying Telaprevir-induced rashes of varying severity. Part A shows Grade 1 (mild): treatment is continued. Part B shows Grade 2 (moderate): treatment is continued. Part C shows Grade 3 (severe): treatment is stopped.
With regards to phenotype specific class I HLA associations and nevirapine hypersensitivity syndromes this includes HLA-B*35:05 and cutaneous hypersensitivity in Southeast Asians and HLA-Cw4 in association with cutaneous phenotypes across multiple ethnicitiets, but in particular, in association with CYB256 516 G>T in Blacks12. In a South African population, HLA-B*58:01 and HLA-DRB1*01:02 has recently been associated with Grade 3/4 hepatitis on nevirapine and HLA-C*04:01 was associated with SJS/TEN in a Malawian population. HLA-DRB1*01 was also previously reported with cutaneous hypersensitivity in association with nevirapine and another NNRTI efavirenz5. Severe drug-induced liver injury has was reported in 3.4% of an HIV Ethiopian population started on efavirenz and associated with higher efavirenz plasma levels and CYP2B6*6 as well as advanced HIV and pre-treatment liver disease. It is unclear from the report whether the hepatitis was toxic, immunologic or a mixture of both40. Rash/pruritus have been associated with up to 50% or more of patients receiving combination regimens including the direct acting hepatitis C antiviral (NS3/4A protease inhibitor) telaprevir resulting in treatment discontinuation in 6% of patients41. Severe hypersensitivity (DRESS/DIHS or severe cutaneous adverse reactions including SJS/TEN are uncommon occurring in <1% of patients42. A case control study of 187 telaprevir treated patients of whom 114 had developed rash (59 severe) was conducted to explore a potential association between telaprevir rash and HLA class I and II alleles. HLA-B*44:02 and HLA-DQB1*02:02 were the top ranked alleles associated with severe skin rash however these associations did not hold up after correction for multiple comparisons.41, 43
Hypersensitivity syndromes associated with antiretroviral treatment
Those living with HIV have both increased exposure to drugs more likely to cause drug hypersensitivity and immune dysregulation. Many delayed and likely T-cell mediated drug hypersensitivity reactions have been reported to occur more commonly in HIV-infected populations. In the first decade of HIV treatment, these mainly involved drugs used to treat HIV-related infections but now primarily include drugs used to treat HIV 44. The most common cutaneous drug reactions in HIV-infected patients are maculopapular exanthemas (MPE). These eruptions are characterized by widespread erythematous macules and papules that usually affect the trunk and proximal extremities; these rashes are often accompanied by pruritus without fever. Initial macules and papules can be increasing in size and leading to confluence or be followed by maculopapular, pustular or vesicular lesions. Maculopapular exanthema (MPE) usually appear between 2 and 10 weeks after primary exposure to antiretroviral therapy (ART) and within 1-2 days of rechallenge. Most of these exanthema are benign and resolve within 2 weeks without sequelae. The patient should be evaluated carefully for the presence of signs of severity such as edema, skin detachment, erosions, mucosal involvement, fever or systemic symptoms which alert to a more severe drug reaction such as drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome (DRESS/DIHS) or Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). Usually rash severity is graded in 4 levels (Table 2). Other allergic syndromes such as Gell-Coombs Type I (IgE-mediated) reactions or delayed urticarial eruptions are infrequently seen in association with ART.
Table 2. Grading of rash severity.
| Grade 1 (mild): localized skin eruption and/or limited skin eruption with or without pruritus |
| Grade 2 (moderate): diffuse eruption involving up to 50% of body surface area with or without superficial skin peeling, pruritus, or mucous membrane involvement and no ulceration |
Grade 3 (severe): generalized rash involving EITHER ≥ 50% of body surface area OR rash presenting with any of the following characteristics:
|
Grade 4 (Life-threatening reactions)
|
Stevens–Johnson syndrome/toxic epidermal necrolysis
SJS and TEN represent acute life-threatening conditions with high morbidity and mortality with an incidence estimated at 1–2 cases/million population/year. The histologic hallmark of these diseases is necrosis with detachment of the epidermis. Patients are classified according to the percentage of the body surface area exhibiting epidermal detachment (BSA%), which is less than 10% in SJS, more than 30% in TEN, and 10% to 30% in SJS/TEN overlap syndrome 45, 46. The mortality rate of SJS and TEN has recently been estimated to be about 22%47,correlating with the extent of detachment and age of the patient and thus elements of the prognostic score called SCORTEN48: seven independent risk factors: age above 40 y, malignancy, tachycardia above 120 per min, initial BSA% above 10%, serum urea above 10 mmol per liter, serum glucose above 14 mmol per liter, and bicarbonate below 20 mmol per litre. TEN usually develops 1–3 weeks after the administration of the responsible drug. The clinical diagnosis is based on the presence of characteristic eruptions of mucosal extensive erosions, typical targetoid lesions, erythematous confluent maculae and bullae, with a positive Nikolsky's sign (detachment of epidermis to finger pressure). Histopathology is characterized by keratinocyte apoptosis followed by necrosis, which creates the basis for the epidermal erosion and detachment. Infection is the most common acute complication and cause of death in SJS/TEN patients. Recovery may require 3 to 6 weeks and is often followed by pigmentation disorders and pathological scarring at mucosal sites. More than 50% of patients surviving SJS/TEN are suffering from long term complications primarily located on eyes such as vision loss in some cases44, 46. To-date, there is no established treatment for SJS/TEN. Symptomatic treatment (dressings, maintenance of thermoregulation, fluid and electrolyte balance, nutritional support, prevention of infections) and immediate cessation of the suspected causative drug is the key to the management of SJS/TEN. Patients benefit from being under the care of a dermatologist in an intensive care or burns unit.
Drug reaction with eosinophilia and systemic symptoms and drug-induced hypersensitivity syndrome (DRESS/DIHS)
DRESS/DIHS is a severe drug-induced adverse manifestation that occurs in most patients 2 to 6 weeks after drug administration. Clinical and biological manifestations of DRESS are characteristic: high fever, facial edema, erythroderma followed by an exfoliative dermatitis, diffuse lymphadenopathy, eosinophilia, atypical circulating lymphocytes, and abnormal results of liver function tests. Numerous systemic manifestations may occur (hepatitis, pneumonitis, pancreatitis, renal failure, neurologic symptoms, and many others). In contrast to SJS and TEN, involvement of the mucous membranes is rare45, 46. Reactivation of HHV, including EBV and HHV-6 and -7 have been described in association with many drug- induced DRESS/DIHS syndromes, however interestingly not with ART associated DRESS/DIHS. The detection of HHV-6 reactivation has even been recently proposed as a diagnostic marker for DRESS/DIHS27, 28, 30. To define more accurately the DRESS syndrome, a scoring system -the RegiSCAR scoring-has been designed to grade DRESS cases as “no,” “possible,” “probable,” or “definite” case48. This condition must be recognized early in order to immediately stop the suspected drugs, but the persistence or aggravation of symptoms despite the discontinuation of the culprit drug is possible. Topical high-potency corticosteroids or systemic corticosteroids are usually required for a full recovery. Cutaneous adverse drug reactions although reported with all ART are more prevalent with specific ART drugs and drug classes, particularly the non-nucleoside reverse transcriptase inhibitors (NNRTI) (Table 3).
Table 3. Clinical Manifestations and Incidence of Antiviral Allergic Syndromes.
| Class | Agent | Reaction | Incidence | Discontinuation |
|---|---|---|---|---|
| Antiretroviral Treatments | ||||
| Protease Inhibitors | Atazanavir | Rash | ≤6% | <1% |
| Darunavir | Rash SJS/TEN/DIHS/DRESS |
≤10% <1% |
<1% 100% |
|
| Fosamprenavir | Rash Moderate-Severe |
≤19 | <1% <1% |
|
| Lopinavir/Ritonavir | Rash | 2% | <1% | |
| Tipranavir | Rash | ≤10% | <1% | |
| NNRTIs | Efavirenz | Rash | 4.6%-20% | <2% |
| SJS/TEN DRESS/DIHS |
0.1% | 100% | ||
| Etravirine | Rash | ≤10% | <2% | |
| DRESS/DIHS, SJS/TEN | <0.1% | 100% | ||
| Nevirapine | Rash | 4-38% | 6% | |
| DRESS/DIHS SJS/TEN |
Up to 5% 0.3-1% |
100% | ||
| Rilpivirine | Rash | 2% | <1% | |
| Fusion Inhibitors |
Enfuvirtide | Hypersensitivity reaction | <1% | Mostly for subcutaneous reactions not HSR |
| NRTIs | Tenofovir | Rash | 5%-7% | <1% |
| Abacavir | Hypersensitivity reaction (fever, gastrointestinal symptoms, rash in 70%) Rash only |
5%-8% 3% |
100%, hypotension <1% |
|
| Emtricitabine | Pruritus, rash | 17%-30% | <1% | |
| Integrase Inhibitors |
Raltegravir | Pruritus, diaphoresis, rash | 2-7% | <1% |
| DRESS/DIHS/SJ S/TEN | <1% | 100% | ||
| CCR5 Inhibitors | Maraviroc | Pruritus | 3.8% | <1% |
| Direct-Acting Hepatitis C Treatments | Telaprevir | Mild to moderate eczematous rash | 50% | <10% |
| DRESS/DIHS, SJS/TEN | 5% <1% |
100% | ||
Protease inhibitors
All protease inhibitors have been associated with mild-to-moderate maculopapular rashes; these are typically observed within the first 2 weeks of treatment and are mild without need for treatment discontinuation (Figure 2.A). They are uncommonly associated with severe reactions such as DRESS/DIHS or SJS/TEN. The rate of rash in patients treated with a protease inhibitor has been recently estimated as around 5%. The newest protease inhibitors are more commonly responsible with variable rates, respectively up to 6%, 10%, 16%, 19% for atazanavir, tipranavir, daranavir and fosamprenavir 44. Darunavir and fosamprenavir are sulfa antimicrobials and there is concern for cross-reactivity with sulfamethoxazole and other sulfa antimicrobials. For darunavir patients with a history of sulfa antimicrobial hypersensitivity were not excluded from clinical trials.
Non-nucleoside analogue reverse transcriptase inhibitors (NNRTIs)
NNRTIs in clinical use include the first generation drugs nevirapine and efavirenz and second generation drugs etravirine and rilpivirine. Cutaneous problems and hepatotoxicity are the main side effects induced by NNRTIs. In most patients rashes are mild to moderate MPE that occur within the first 1-2 weeks of treatment and resolve with continued treatment (Figure 2.B). Rashes associated with nevirapine (4% -38%) and efavirenz (4.6%-20%) are frequent. (Table 3). SJS/TEN has most frequently reported with nevirapine (0.3-1%)(Figure 2.C) and less frequent with efavirenz (0·1%) 48 DRESS/DIHS syndrome, is well documented with nevirapine occurring in up to 5% of patients(Figure 2.D), but is uncommonly attributed to efavirenz49. Female sex, ethnicity (Hispanic, Chinese, and African), and individuals with higher CD4+ T cell count and uncontrolled HIV viremia in early studies appeared to be at higher risk for nevirapine-related rash51. An initial 2-week lead-in period at half the recommended dose has been shown to reduce the risk of skin rashes with nevirapine by at least 50%. The prophylactic use of corticosteroids or antihistamines to prevent hypersensitivity reactions to nevirapine has not been shown to be of benefit and may even increase risk52, 53. Risk factors for efavirenz-induced rash are less well described. Nevirapine-associated hypersensitivity syndromes have a pharmacogenomic basis and have been associated with various class I and II HLA alleles as described above.
Etravirine is the first of the second-generation NNRTIs that has showed efficacy in controlling HIV replication in treatment-experienced patients, in combination with other active agents. In clinical trials, rash was reported in 16.9% of the etravirine-treated patients compared with 9.3% in placebo (Table 3). Most rashes were of mild or moderate severity, occurring within the first few weeks of treatment (median 14 days) and resolved with continued treatment (only 2.2% of patients discontinued etravirine). In clinical trials women developed more rash than men. There was no relationship between rash and etravirine pharmacokinetic exposure, CD4+ T cell count, nor history of other NNRTI related rash. DRESS/DIHS and SJS/TEN were reported in <0.1% of the etravirine-treated patients during clinical trials. In 2009, the manufacturer issued a drug warning regarding the safety of etravirine in light of post-marketing reports of severe skin and hypersensitivity reactions. Cases included life-threatening and fatal reports of DRESS/DIHS and SJS/TEN54. Another second generation NNRTI, rilpivirine, has been associated with a low incidence (2%) of mild to moderate rash in clinical trials 55 (Table 3).
Fusion inhibitors
Enfuvirtide was the first of a class of antiretroviral medications called fusion inhibitors and the only one currently available as an injectable formulation. The majority of cutaneous side effects are injection site reactions. Hypersensitivity reaction has an incidence rate of <1% of patients in clinical trials56.
Nucleoside reverse transcriptase inhibitors (NRTIs)
NRTIs were the first medication approved for the treatment of HIV. Currently, the nucleoside analogues commonly used as part of combination ART include lamivudine (3TC), emtricitabine (3TC), tenofovir (nucleotide analogue) and abacavir. Due to inconvenience of dosing and long-term toxicities zidovudine (AZT), didanosine (ddI) and stavudine (d4T) are currently much less commonly used in the developed world. Rash has been less commonly associated with tenofovir and a hypersensitivity syndrome has been rarely reported (see Table 2).44
Abacavir (ABC)
Abacavir (ABC) can be associated with a drug hypersensitivity syndrome which is distinct from DRESS/DIHS. In early studies ABC hypersensitivity was reported to occur within the first 6 weeks of exposure, although most cases occur in the second week of first exposure at a median of 8-9 days. It is characterized by fever, malaise, gastrointestinal symptoms and internal organ involvement in approximately 5-8% of patients who begin therapy with ABC4. The syndrome can be accompanied by a mild-to-moderate rash in 70% of patients with ABC hypersensitivity and is associated with severe hypotension and possible death upon re-challenge, in contrast to the complete abrogation of symptoms 72 h after withdrawal of the drug. Once the hypersensitivity syndrome reaction has been diagnosed, abacavir therapy should be discontinued immediately and permanently. Corticosteroids, given prophylactically, do not appear to reduce the severity or frequency of the ABC hypersensitivity syndrome53. There is a strong association with ABC hypersensitivity and the presence of the HLA-B*57:01 allele7,8,57 and genetic testing to prevent abacavir HSR is currently one of the best examples of integrating pharmacogenetic testing into clinical practice. Mild to moderate rash without other symptoms of ABC hypersensitivity is known to occur in 3% of those starting ABC. This MPE is not known to be associated with HLA-B*57:01 and is not treatment limiting. SJS/TEN has only rarely been suspected in association with ABC therapy.58 ABC patch testing (Figure 1) has been used as a research tool to identify true immunologically mediated ABC hypersensitivity, however it has a diagnostic sensitivity of 87% and in view of the severe rechallenge reactions (hypotension, shock) that have occurred in patients with ABC hypersensitivity on second exposure to ABC a negative patch test alone should never be used as the sole basis for ABC reintroduction23, 24, 59.
Emtricitabine
Emtricitabine is used to prevent the replication of HIV and hepatitis B virus. The skin rashes most commonly reported in emtricitabine trials were pruritus, maculopapular rash, urticaria, vesiculobullous and pustular rash, observed in 17% to 30% of the patients. These reactions were mild or moderate; only 1% of the patients discontinued treatment because of rash44 (Table 3).
Integrase inhibitors
The majority of the rash events in raltegravir-treated subjects in the clinical trials were mild to moderate in intensity, and no discontinuations were reported. However, many of the rash events have been confounded by use of concomitant medications associated such as NNRTIs, ABC and protease inhibitors. More recently there have been cases of raltegravir associated DRESS/DIHS and SJS/TEN with three separate case publications of raltegravir associated DRESS/DIHS syndrome all in women of African descent60-62. A newly approved integrase inhibitor, dolutegravir has not been associated with rash.
Inhibitors of the CCR5 chemokine receptor
Maraviroc is the first human chemokine receptor 5 (CCR5) co-receptor antagonist approved for HIV treatment. Information about the cutaneous adverse events and hypersensitivity syndromes associated with maraviroc is scarce however pruritus occurred in 3.8% of the patients receiving maraviroc in clinical trials 63 (Table 3).
Hypersensitivity syndromes associated with treatment of hepatitis C virus
Hepatitis C virus (HCV) is a major global health problem with an estimated 170 million people chronically infected64. It may lead to chronic liver disease, cirrhosis and hepatocellular carcinoma, with high morbidity and mortality65. Standard combination treatment with pegylated interferon-alpha (PegIFN) and ribavirin (RBV) results in a virological response in only about 45% of patients66. New direct-acting antiviral drugs such as telaprevir and boceprevir that are inhibitors of the HCV NS3.4A serine protease have thus become of great interest for improving the efficacy of anti-HCV treatment. Telaprevir, has been recently developed for the treatment of chronic genotype 1 HCV infection in combination with RBV and PegIFN67. Telaprevir- associated rash has occurred in up to 50% or more of the patients on combination treatment with RBV and PegIFN since its first evaluation in clinical studies (Figure 2.E) (Table 3). More than 90% of these eruptions are mild and moderate, eczematous dermititis (grades 1 and 2) that does not require telaprevir discontinuation and are usually well controlled by antihistamine therapy, emollients and topical steroids2(Figure 2.E). Approximately 5% of these skin eruptions are potentially severe with cases of DRESS/DIHS and SJS/TEN reported8, 9, 41. There has been no association between the pharmacokinetics of PegIFN, ribavirin and telaprevir and incidence of rash41. In The safety data of the combination of boceprevir with PegIFN and RBV was assessed in Phase 2 and 3 placebo-controlled clinical trials. The most common adverse events observed were anemia, dysgeusia, and cutaneous side effects. Rashes were noted in 14 to 22% of cases and dry skin in 18 to 22% of cases, which was noted more significantly compared to PegIFN and RBV alone in treatment experienced patients, however, no patient receiving boceprevir discontinued the treatment because of skin eruption68. Severe cutaneous syndromes are uncommon with boceprevir, with one recently published case of DRESS/DIHS associated with boceprevir69. For mild to moderate skin rash without systemic symptoms or mucosal involvement, appropriate skin-care management usually facilitates continuation of antiviral therapy70 (Table 3). This emphasizes the importance of close collaboration between dermatologists and hepatologists.
Diagnosis and Treatment
The diagnosis and treatment of drug hypersensitivity to antivirals, as per any drugs, is still largely based on clinical assessment of the specific syndrome involved. As per other drugs the presence of mild to moderate rash without systemic symptoms, internal organ or mucosal involvement is commonly associated with many antiviral drugs. Given the need to continue uninterrupted antiretroviral and hepatitis C treatment it is reasonable in these mild cases to continue treatment with careful observation with the expectation that the rash will remit although the mechanism of immunological tolerance is not clear.
Desensitization or graded re-introduction of drug has also been used to reintroduce an antiviral where the original reaction consisted of an isolated mild to moderate skin rash8. ABC patch testing showed great utility as a research tool in the PREDICT-1 and SHAPE studies to define the specific syndrome of immunologically-mediated abacavir hypersensitivity11, 21. From the PREDICT-1 study the diagnostic sensitivity of ABC patch testing was 87% giving it one of the highest sensitivities of patch testing for drug hypersensitivity. However, given the severity and potential for morbidity and mortality associated with re-challenge with abacavir in patients who have already experienced abacavir hypersensitivity syndrome patch testing alone should never be used as the basis for re-challenge and clinical judgment should always take precedent71. In general patch testing has shown diagnostic utility for other drug hypersensitivity syndromes such as DRESS/DIHS, acute generalized exanthematous pustulosis (AGEP) and fixed drug eruption and less so for SJS/TEN, however in general the sensitivity of patch testing for drugs other than abacavir has been <50% and is very drug specific72,73. Patch testing for syndromes such as DRESS/DIHS/SJS/TEN for specific antivirals such as nevirapine appears to have low sensitivity and utility59, 72. For efavirenz, a type of photoallergic dermatitis has been described and there have been case reports of positive photopatch, where the drug is applied for 48 hours followed by low dose UVA application for 24 hours73-75. More recently direct acting drugs against hepatitis C such as telaprevir have been associated with a very high incidence of delayed rash however treatment is largely supportive and most patients do not have associated systemic symptoms or signs and can continue treatment.41 Specific ex vivo tests such as ELISpot have reproducibly shown gamma-interferon responses to the parent drug for several years after the acute ABC hypersensitivity reaction, but appear less reliable for other drugs such as nevirapine where responses have been shown to wane quickly over months to years. In general the management of antiviral drug hypersensitivity is similar to other drugs and includes use of prednisone or other immunosuppressants in cases of DRESS/DIHS with severe organ involvement of SJS/TEN respectively. The long half-life of nevirapine is a risk factor for poor outcome associated with SJS/TEN and nevirapine should be stopped immediately after onset of symptoms/signs suggestive of SJS/TEN76. HLA-B*57:01 has had great utility as a screening test with 100% negative predictive value generalizable across different ethnicities to identify patients at risk to develop abacavir hypersensitivity21, 77, 78. The complexity of HLA associations across different phenotypes and ethnicities with other drugs such as nevirapine are such that currently HLA testing has limited utility as a screening strategy to prevent nevirapine hypersensitivity syndromes prior to nevirapine prescription.
Conclusions and Future Directions
Antiviral drugs are common causes of delayed T-cell mediated drug reactions for which the immunopathogenesis has been more recently defined to be largely HLA class I and/or class II restricted and T-cell mediated. Fortunately most of these reactions are benign and consist of a mild to moderate delayed skin rash which is not treatment limiting and resolved with continued dosing. Although these drugs may share structure, such as a shared sulfa antimicrobial group in the case of darunavir and fosamprenavir, or shared mechanism of action and propensity to develop skin rash, as for the HIV non-nucleoside reverse transcriptase inhibitors, clinical and immunological cross-reactivity between antiviral drugs is uncommon in clinical practice. The association between HLA-B*57:01 and abacavir hypersensitivity represents an successful example of where a genetic marker with 100% negative predictive value and generalizable across different ethnicities has been utilized as a screening test in the routine clinical setting to prevent a specific antiviral drug hypersensitivity syndrome. Many other HLA class I and II associations have been described between various phenotypes of nevirapine hypersensitivity which have shed further light on the immunopathogenesis of antiviral hypersensitivity syndromes, however, the complexity of these associations and the lack of generalizability across all ethnicities make it unlikely they these will be utilized in the routine clinical setting. A paradigm shift has occurred in the science of drug hypersensitivity with definition of the crystal structure of abacavir bound to peptide-HLA-B*57:01 and evidence for an altered peptide model of drug hypersensitivity. From recent research is likely that this altered peptide repertoire model applies to many other phenotypes of severe T-cell mediated drug reactions including DIHS/DRESS and SJS/TEN24,27. The altered peptide repertoire model does not explain why in general only a small fraction of patients carrying a specific HLA class I and/or II risk allele will develop a drug hypersensitivity syndrome and other models are needed. Some evidence now exists to suggest that hypersensitivity reactions to drugs like ABC, where symptoms can occur within 1-2 days of first exposure, may occur secondary to a pre-existing memory T-cell response. This pre-existing memory T-cell response may be targeted against a prevalent persistent viral pathogen such a human herpes virus representing a heterologous immune model of drug hypersensitivity. The convergence of structural, biochemical and functional immunological approaches to define interactions between antiviral drugs and HLA molecules offer future promise for being able to predict drug hypersensitivity risk in the pre-clinical stage of drug development and positively impact the efficacy, safety and efficiency of antiviral drug development and design.
Key Points.
Antiviral drugs successful in suppressing replication of HIV and hepatitis C (HCV) are common causes of delayed drug hypersensitivities for which an increasing number have more recently been shown to be HLA class I and/or II restricted and T-cell mediated.
HLA-B*57:01 screening prior to abacavir prescription to prevent abacavir hypersensitivity is an example of a translational success story whereby a marker with 100% negative predictive value has now been implemented into guideline-based routine HIV clinical practice.
Ancillary in vivo and ex vivo laboratory tests have been useful to define the true immunologically-mediated phenotype of antiviral drug hypersensitivity (e.g. patch testing for abacavir), however the lower sensitivity of these tests for severe drug hypersensitivity syndromes mean that clinical diagnosis remains the gold standard to guide management.
Most allergic syndromes associated with antiviral medications consist of mild to moderate delayed rash without other serious manifestations (e.g. fever, mucosal involvement, blistering rash, organ impairment). In these cases treatment can be continued with careful observation and symptomatic management and the discontinuation rate is low.
Footnotes
Disclosures/conflict of interest: none related to the content of this article
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vilar FJ, Naisbitt DJ, Park BK, et al. Mechanisms of drug hypersensitivity in HIV-infected patients: the role of the immune system. J HIV Ther. 2003;8:42–47. [PubMed] [Google Scholar]
- 2.Cacoub P, Bourliere M, Lubbe J, et al. Dermatological side effects of hepatitis C and its treatment: patient management in the era of direct-acting antivirals. J Hepatol. 2012;56:455–463. doi: 10.1016/j.jhep.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Phillips E, Mallal S. Drug hypersensitivity in HIV. Curr Opin Allergy Clin Immunol. 2007;7:324–330. doi: 10.1097/ACI.0b013e32825ea68a. [DOI] [PubMed] [Google Scholar]
- 4.Clay PG. The abacavir hypersensitivity reaction: a review. Clin Ther. 2002;24:1502–1514. doi: 10.1016/s0149-2918(02)80057-1. [DOI] [PubMed] [Google Scholar]
- 5.Vitezica ZG, Milpied B, Lonjou C, et al. HLA-DRB1*01 associated with cutaneous hypersensitivity induced by nevirapine and efavirenz. AIDS. 2008;22:540–541. doi: 10.1097/QAD.0b013e3282f37812. [DOI] [PubMed] [Google Scholar]
- 6.Sherman KE. Managing adverse effects and complications in completing treatment for hepatitis C virus infection. Top Antivir Med. 2012;20:125–128. [PMC free article] [PubMed] [Google Scholar]
- 7.Torii H, Sueki H, Kumada H, et al. Dermatological side-effects of telaprevir-based triple therapy for chronic hepatitis C in phase III trials in Japan. J Dermatol. 2013;40:587–595. doi: 10.1111/1346-8138.12199. [DOI] [PubMed] [Google Scholar]
- 8.Phillips EJ, Kuriakose B, Knowles SR, et al. Efavirenz-induced skin eruption and successful desensitization. Ann Pharmacother. 2002;36:430–432. doi: 10.1345/aph.1A287. [DOI] [PubMed] [Google Scholar]
- 9.Roujeau JC, Mockenhaupt M, Tahan SR, et al. Telaprevir-related dermatitis. JAMA Dermatol. 2013;149:152–158. doi: 10.1001/jamadermatol.2013.938. [DOI] [PubMed] [Google Scholar]
- 10.Hetherington S, Hughes AR, Mosteller M, et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002;359:1121–1122. doi: 10.1016/S0140-6736(02)08158-8. [DOI] [PubMed] [Google Scholar]
- 11.Mallal S, Nolan D, Witt C, et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002;359:727–732. doi: 10.1016/s0140-6736(02)07873-x. [DOI] [PubMed] [Google Scholar]
- 12.Martin AM, Nolan D, James I, et al. Predisposition to nevirapine hypersensitivity associated with HLA-DRB1*0101 and abrogated by low CD4 T-cell counts. AIDS. 2005;19:97–99. doi: 10.1097/00002030-200501030-00014. [DOI] [PubMed] [Google Scholar]
- 13.Yuan J, Guo S, Hall D, et al. Toxicogenomics of nevirapine-associated cutaneous and hepatic adverse events among populations of African, Asian, and European descent. AIDS. 2011;25:1271–1280. doi: 10.1097/QAD.0b013e32834779df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips EB, Sanne I, Lederman M, et al. Associations between HLA-DRBA*0102, HLA-B*5801 and hepatotoxicity in patients who initiated nevirapine containing regimens in South Africa; 18th Conference on retroviruses and opportunistic infections; Boston. February 27–March 1, 2011; Paper #949. [Google Scholar]
- 15.Chantarangsu S, Mushiroda T, Mahasirimongkol S, et al. HLA-B*3505 allele is a strong predictor for nevirapine-induced skin adverse drug reactions in HIV-infected Thai patients. Pharmacogenet Genomics. 2009;19:139–146. doi: 10.1097/FPC.0b013e32831d0faf. [DOI] [PubMed] [Google Scholar]
- 16.Chantarangsu S, Mushiroda T, Mahasirimongkol S, et al. Genome-wide association study identifies variations in 6p21.3 associated with nevirapine-induced rash. Clin Infect Dis. 2011;53:341–348. doi: 10.1093/cid/cir403. [DOI] [PubMed] [Google Scholar]
- 17.Phillips E, Lucas M, Keane N, et al. HLA-B*35 is associated with nevirapine hypersensitivity in the contemporary western Australian HIV cohort study. Eur Ann Allergy Clin Immunol. 2010;42:48. [Google Scholar]
- 18.Likanonsakul S, Rattanatham T, Feangvad S, et al. HLA-Cw*04 allele associated with nevirapine-induced rash in HIV-infected Thai patients. AIDS Res Ther. 2009;6:22. doi: 10.1186/1742-6405-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr DF, Chaponda M, Jorgensen AL, et al. Association of human leukocyte antigen alleles and nevirapine hypersensitivity in a Malawian HIV-infected population. Clin Infect Dis. 2013;56:1330–1339. doi: 10.1093/cid/cit021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciccacci C, Di Fusco D, Marazzi MC, et al. Association between CYP2B6 polymorphisms and Nevirapine-induced SJS/TEN: a pharmacogenetics study. Eur J Clin Pharmacol. 2013;69:1909–1916. doi: 10.1007/s00228-013-1549-x. [DOI] [PubMed] [Google Scholar]
- 21.Mallal S, Phillips E, Carosi G, et al. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568–579. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- 22.Phillips EJ, Wong GA, Kaul R, et al. Clinical and immunogenetic correlates of abacavir hypersensitivity. AIDS. 2005;19:979–981. doi: 10.1097/01.aids.0000171414.99409.fb. [DOI] [PubMed] [Google Scholar]
- 23.Phillips EJ, Sullivan JR, Knowles SR, et al. Utility of patch testing in patients with hypersensitivity syndromes associated with abacavir. AIDS. 2002;16:2223–2225. doi: 10.1097/00002030-200211080-00017. [DOI] [PubMed] [Google Scholar]
- 24.Pavlos R, Mallal S, Phillips E, et al. HLA and pharmacogenetics of drug hypersensitivity. Pharmacogenomics. 2012;13:1285–1306. doi: 10.2217/pgs.12.108. [DOI] [PubMed] [Google Scholar]
- 25.Rive CM, Bourke J, Phillips EJ, et al. Testing for drug hypersensitivity syndromes. Clin Biochem Rev. 2013;34:15–38. [PMC free article] [PubMed] [Google Scholar]
- 26.Pavlos R, Mallal S, Ostrov D, et al. Fever, rash, and systemic symptoms: understanding the role of virus and HLA in severe cutaneous drug allergy. J Allergy Clin Immunol Pract. 2014;2:21–33. doi: 10.1016/j.jaip.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pichler W, Yawalkar N, Schmid S, et al. Pathogenesis of drug-induced exanthems. Allergy. 2002;57:884–893. doi: 10.1034/j.1398-9995.2002.02161.x. [DOI] [PubMed] [Google Scholar]
- 28.Adam J, Pichler WJ, Yerly D, et al. Delayed drug hypersensitivity: models of T-cell stimulation. Br J Clin Pharmacol. 2011;71:701–707. doi: 10.1111/j.1365-2125.2010.03764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pompeu YA, Stewart JD, Mallal S, et al. The structural basis of HLA-associated drug hypersensitivity syndromes. Immunol Rev. 2012;250:158–166. doi: 10.1111/j.1600-065X.2012.01163.x. [DOI] [PubMed] [Google Scholar]
- 30.Pichler WJ, Beeler A, Keller M, et al. Pharmacological interaction of drugs with immune receptors: the p-i concept. Allergol Int. 2006;55:17–25. doi: 10.2332/allergolint.55.17. [DOI] [PubMed] [Google Scholar]
- 31.Illing PT, Vivian JP, Dudek NL, et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature. 2012;486:554–558. doi: 10.1038/nature11147. [DOI] [PubMed] [Google Scholar]
- 32.Norcross MA, Luo S, Lu L, et al. Abacavir induces loading of novel self-peptides into HLA-B*57: 01: an autoimmune model for HLA-associated drug hypersensitivity. AIDS. 2012;26:F21–F29. doi: 10.1097/QAD.0b013e328355fe8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ostrov DA, Grant BJ, Pompeu YA, et al. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc Natl Acad Sci U S A. 2012;109:9959–9964. doi: 10.1073/pnas.1207934109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kano Y, Shiohara T. The variable clinical picture of drug-induced hypersensitivity syndrome/drug rash with eosinophilia and systemic symptoms in relation to the eliciting drug. Immunol Allergy Clin North Am. 2009;29:481–501. doi: 10.1016/j.iac.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Shiohara T, Inaoka M, Kano Y, et al. Drug-induced hypersensitivity syndrome (DIHS): a reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergol Int. 2006;55:1–8. doi: 10.2332/allergolint.55.1. [DOI] [PubMed] [Google Scholar]
- 36.Tohyama M, Hashimoto K, Yasukawa M, et al. Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br J Dermatol. 2007;157:934–940. doi: 10.1111/j.1365-2133.2007.08167.x. [DOI] [PubMed] [Google Scholar]
- 37.Saag M, Balu R, Phillips E, et al. High sensitivity of human leukocyte antigen-b*5701 as a marker for immunologically confirmed abacavir hypersensitivity in white and black patients. Clin Infect Dis. 2008;46:1111–1118. doi: 10.1086/529382. [DOI] [PubMed] [Google Scholar]
- 38.Martin AM, Nolan D, Gaudieri S, et al. Predisposition to abacavir hypersensitivity conferred by HLA-B*5701 and a haplotypic Hsp70-Hom variant. Proc Natl Acad Sci U S A. 2004;101:4180–4185. doi: 10.1073/pnas.0307067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chessman D, Kostenko L, Lethborg T, et al. Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity. 2008;28:822–832. doi: 10.1016/j.immuni.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 40.Yimer G, Amogne W, Habtewold A, et al. High plasma efavirenz level and CYP2B6*6 are associated with efavirenz-based HAART-induced liver injury in the treatment of naive HIV patients from Ethiopia: a prospective cohort study. Pharmacogenomics J. 2012;12:499–506. doi: 10.1038/tpj.2011.34. [DOI] [PubMed] [Google Scholar]
- 41.Chen ST, Wu PA. Severe cutaneous eruptions on telaprevir. J Hepatol. 2012;57:470–472. doi: 10.1016/j.jhep.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Teixeira R, Nascimento YD, Crespo D, et al. Safety aspects of protease inhibitors for chronic hepatitis C: adverse events and drug-to-drug interactions. Braz J Infect Dis. 2013;17:194–204. doi: 10.1016/j.bjid.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Advisory Committee Briefing Document for NDA 201-917 Telaprevir 375 mg tablets In: Members APAC, ed.
- 44.Borras-Blasco J, Navarro-Ruiz A, Borras C, et al. Adverse cutaneous reactions associated with the newest antiretroviral drugs in patients with human immunodeficiency virus infection. J Antimicrob Chemother. 2008;62:879–888. doi: 10.1093/jac/dkn292. [DOI] [PubMed] [Google Scholar]
- 45.Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med. 1994;331:1272–1285. doi: 10.1056/NEJM199411103311906. [DOI] [PubMed] [Google Scholar]
- 46.Bastuji-Garin S, Rzany B, Stern RS, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92–96. [PubMed] [Google Scholar]
- 47.Mockenhaupt M, Viboud C, Dunant A, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol. 2008;128:35–44. doi: 10.1038/sj.jid.5701033. [DOI] [PubMed] [Google Scholar]
- 48.Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149–153. doi: 10.1046/j.1523-1747.2000.00061.x. [DOI] [PubMed] [Google Scholar]
- 49.Fagot JP, Mockenhaupt M, Bouwes-Bavinck JN, et al. Nevirapine and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. AIDS. 2001;15:1843–1848. doi: 10.1097/00002030-200109280-00014. [DOI] [PubMed] [Google Scholar]
- 50.Colebunders R, Vanwolleghem T, Meurrens P, et al. Efavirenz-associated Stevens-Johnson syndrome. Infection. 2004;32:306–307. doi: 10.1007/s15010-004-4034-8. [DOI] [PubMed] [Google Scholar]
- 51.de Maat MM, ter Heine R, Mulder JW, et al. Incidence and risk factors for nevirapine-associated rash. Eur J Clin Pharmacol. 2003;59:457–462. doi: 10.1007/s00228-003-0613-3. [DOI] [PubMed] [Google Scholar]
- 52.Knobel H, Miro JM, Domingo P, et al. Failure of a short-term prednisone regimen to prevent nevirapine-associated rash: a double-blind placebo-controlled trial: the GESIDA 09/99 study. J Acquir Immune Defic Syndr. 2001;28:14–18. doi: 10.1097/00042560-200109010-00003. [DOI] [PubMed] [Google Scholar]
- 53.Wit FW, Wood R, Horban A, et al. Prednisolone does not prevent hypersensitivity reactions in antiretroviral drug regimens containing abacavir with or without nevirapine. AIDS. 2001;15:2423–2429. doi: 10.1097/00002030-200112070-00010. [DOI] [PubMed] [Google Scholar]
- 54.Croxtall JD. Etravirine: a review of its use in the management of treatment-experienced patients with HIV-1 infection. Drugs. 2012;72:847–869. doi: 10.2165/11209110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 55.Molina JM, Clumeck N, Redant K, et al. Rilpivirine vs. efavirenz in HIV-1 patients with baseline viral load 100,000 copies/ml or less: week 48 phase III analysis. AIDS. 2013;27:889–897. doi: 10.1097/QAD.0b013e32835e1554. [DOI] [PubMed] [Google Scholar]
- 56.Emerson CR, Post JJ, Workman C, et al. A delayed hypersensitivity reaction to enfuvirtide after rechallenge. Int J STD AIDS. 2009;20:288–289. doi: 10.1258/ijsa.2008.008331. [DOI] [PubMed] [Google Scholar]
- 57.Phillips E, Keane N, Blyth C, et al. Both HLA Class I restricted CD8+ and Class II restricted CD4+T Cells are implicated in the pathogenesis of nevirapine hypersensitivity; 51st Interscience Conference of Antimicrobial Agents and Chemotherapy; Chicago. 2011. [DOI] [PubMed] [Google Scholar]
- 58.Karlin E, Phillips E. Genotyping for severe drug hypersensitivity. Curr Allergy Asthma Rep. 2014;14:418. doi: 10.1007/s11882-013-0418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phillips EJ, Mallal SA. Pharmacogenetics of drug hypersensitivity. Pharmacogenomics. 2010;11:973–987. doi: 10.2217/pgs.10.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perry ME, Almaani N, Desai N, et al. Raltegravir-induced Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) syndrome—implications for clinical practice and patient safety. Int J STD AIDS. 2013;24:639–642. doi: 10.1177/0956462413481528. [DOI] [PubMed] [Google Scholar]
- 61.Loulergue P, Mir O. Raltegravir-induced DRESS syndrome. Scand J Infect Dis. 2012;44:802–803. doi: 10.3109/00365548.2012.689850. [DOI] [PubMed] [Google Scholar]
- 62.Zhang KS, Modi GM, Hsu S, et al. DRESS syndrome associated with raltegravir. Dermatol Online J. 2011;17:14. [PubMed] [Google Scholar]
- 63.Babiker ZO, Douthwaite ST, Collier LE, et al. Real-life outcomes of maraviroc-based regimens in HIV-1-infected individuals. J Int Assoc Provid AIDS Care. 2013;12:12–14. doi: 10.1177/1545109712462454. [DOI] [PubMed] [Google Scholar]
- 64.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 65.Lee MH, Yang HI, Lu SN, et al. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469–477. doi: 10.1093/infdis/jis385. [DOI] [PubMed] [Google Scholar]
- 66.Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355:2444–2451. doi: 10.1056/NEJMct061675. [DOI] [PubMed] [Google Scholar]
- 67.Hezode C, Forestier N, Dusheiko G, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839–1850. doi: 10.1056/NEJMoa0807650. [DOI] [PubMed] [Google Scholar]
- 68.Ascione A. Boceprevir in chronic hepatitis C infection: a perspective review. Ther Adv Chronic Dis. 2012;3:113–121. doi: 10.1177/2040622312441496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Samain A, Duval-Modeste AB, Joly P, et al. First case of drug rash eosinophilia and systemic symptoms due to boceprevir. J Hepatol. 2014;60:891–893. doi: 10.1016/j.jhep.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 70.Dupin N, Mallet V, Carlotti A, et al. Severe skin rash in case of readministration of telaprevir in a patient who previously experienced a non severe rash. Hepatology. 2012;55:2042–2043. doi: 10.1002/hep.25574. [DOI] [PubMed] [Google Scholar]
- 71.Weiss ME. Recognizing drug allergy. How to differentiate true allergy from other adverse drug reactions. Postgrad Med. 2005;117:32–36. 39. doi: 10.3810/pgm.2005.05.1629. [DOI] [PubMed] [Google Scholar]
- 72.Shear NH, Milpied B, Bruynzeel DP, et al. A review of drug patch testing and implications for HIV clinicians. AIDS. 2008;22:999–1007. doi: 10.1097/QAD.0b013e3282f7cb60. [DOI] [PubMed] [Google Scholar]
- 73.Furue M. Photosensitive drug eruption induced by efavirenz in a patient with HIV infection. Intern Med. 2004;43:533. doi: 10.2169/internalmedicine.43.533. [DOI] [PubMed] [Google Scholar]
- 74.Yoshimoto E, Konishi M, Takahashi K, et al. The first case of efavirenz-induced photosensitivity in a Japanese patient with HIV infection. Intern Med. 2004;43:630–631. doi: 10.2169/internalmedicine.43.630. [DOI] [PubMed] [Google Scholar]
- 75.Treudler R, Husak R, Raisova M, et al. Efavirenz-induced photoallergic dermatitis in HIV. AIDS. 2001;15:1085–1086. doi: 10.1097/00002030-200105250-00029. [DOI] [PubMed] [Google Scholar]
- 76.Pollard RB, Robinson P, Dransfield K, et al. Safety profile of nevirapine, a nonnucleoside reverse transcriptase inhibitor for the treatment of human immunodeficiency virus infection. Clin Ther. 1998;20:1071–1092. doi: 10.1016/s0149-2918(98)80105-7. [DOI] [PubMed] [Google Scholar]
- 77.Hughes DA, Vilar FJ, Ward CC, et al. Cost-effectiveness analysis of HLA B*5701 genotyping in preventing abacavir hypersensitivity. Pharmacogenetics. 2004;14:335–342. doi: 10.1097/00008571-200406000-00002. [DOI] [PubMed] [Google Scholar]
- 78.Schackman BR, Scott CA, Walensky RP, et al. The cost-effectiveness of HLA-B*5701 genetic screening to guide initial antiretroviral therapy for HIV. AIDS. 2008;22:2025–2033. doi: 10.1097/QAD.0b013e3283103ce6. [DOI] [PMC free article] [PubMed] [Google Scholar]


