Background: Cochaperones are important for the folding and activation of steroid hormone receptors.
Results: The androgen receptor-associated cochaperone SGTA binds both Hsp70 and Hsp90 and regulates progesterone and glucocorticoid receptors.
Conclusion: SGTA is a receptor-specific cochaperone that regulates distinct steps in the receptor chaperoning cycle.
Significance: SGTA is a relevant factor in diseases that depend on androgens, progestins and/or glucocorticoids.
Keywords: Androgen Receptor, Glucocorticoid Receptor, Heat Shock Protein 90 (Hsp90), Progesterone, Prostate Cancer, Steroid Hormone Receptor, FKBP52, Hsp70, SGTA, Cochaperone
Abstract
Steroid hormone receptors are ligand-dependent transcription factors that require the ordered assembly of multichaperone complexes for transcriptional activity. Although heat shock protein (Hsp) 90 and Hsp70 are key players in this process, multiple Hsp70- and Hsp90-associated cochaperones associate with receptor-chaperone complexes to regulate receptor folding and activation. Small glutamine-rich tetratricopeptide repeat-containing protein alpha (SGTA) was recently characterized as an Hsp70 and Hsp90-associated cochaperone that specifically regulates androgen receptor activity. However, the specificity of SGTA for additional members of the steroid hormone receptor superfamily and the mechanism by which SGTA regulates receptor activity remain unclear. Here we report that SGTA associates with and specifically regulates the androgen, glucocorticoid, and progesterone receptors and has no effect on the mineralocorticoid and estrogen receptors in both yeast and mammalian cell-based reporter assays. In both systems, SGTA knockdown/deletion enhances receptor activity, whereas SGTA overexpression suppresses receptor activity. We demonstrate that SGTA binds directly to Hsp70 and Hsp90 in vitro with similar affinities yet predominately precipitates with Hsp70 from cell lysates, suggesting a role for SGTA in early, Hsp70-mediated folding. Furthermore, SGTA expression completely abrogates the regulation of receptor function by FKBP52 (52-kDa FK506-binding protein), which acts at a later stage of the chaperone cycle. Taken together, our data suggest a role for SGTA at distinct steps in the chaperone-dependent modulation of androgen, glucocorticoid, and progesterone receptor activity.
Introduction
Steroid hormone receptors are ligand-dependent transcription factors that require the dynamic, ordered assembly of the heat shock protein 70 (Hsp70)3 and Hsp90 chaperone machinery to reach a functional conformation. Although Hsp70 and Hsp90 are key factors in this process, a number of Hsp70- and Hsp90-associated cochaperones associate with receptor-chaperone complexes to functionally affect a wide variety of steps within the receptor folding process (1, 2). Receptors associate with Hsp40 and Hsp70 early in the folding process (3). At the intermediate stage of receptor folding, an adaptor protein, Hop (Hsp organizing protein), facilitates the transfer of the partially folded receptor to Hsp90 (4–6). A conformational change in Hsp90 to the closed conformation is then accompanied by the release of Hsp70 and Hop and recruitment of the Hsp90-associated cochaperone p23 and one of a family of prolyl isomerases including large members of FK506-binding proteins (FKBP51 and FKBP52), Cyp40 (cyclophilin 40), and PP5 (protein phosphatase 5) (7–9). Hsp90 and associated cochaperones stabilize the high affinity hormone binding conformation of the receptors and prevent the receptors from facilitating changes in gene expression in the absence of hormone. In addition, evidence suggests that at least some of the Hsp90-associated cochaperones continue to have significant roles in the receptor signaling pathways after hormone binding and/or receptor release from the Hsp90 heterocomplex (9, 10).
A common feature shared by a majority of the receptor-associated cochaperones is the highly conserved tetratricopeptide repeat (TPR) motif (11), which facilitates interaction with an EEVD motif in the extreme C terminus of Hsp90, and/or an EEVD-like sequence in the C terminus of Hsp70 (12, 13). The repertoire of TPR-containing cochaperones known to regulate steroid hormone receptor signaling pathways include Hip (Hsc70-interacting protein), Hop, FKBP51, FKBP52, cyclophilin 40, protein phosphatase 5, FKBPL (FK506-binding protein like protein), and GCUNC-45 (1, 2, 14, 15). Additionally, the more recent identification of small glutamine-rich TRP-containing protein alpha (SGTA) as a functional modulator of the androgen receptor adds to the growing diversity of TPR-containing cochaperones involved in steroid hormone receptor signaling (16).
SGTA was first identified in association with nonstructural protein NS1 of parvovirus H-1 and found to be located both in the cytoplasm and nucleus (17). SGTA is a 34-kDa protein containing an N-terminal dimerization domain, three central tandem TPR motifs, and a C-terminal glutamine-rich domain (17–20). SGTA is conserved in almost all vertebrate and invertebrate species (18, 21, 22) and is ubiquitously present in nearly all tissues (17). The reported effects of SGTA on androgen receptor (AR) activity are variable and conflicting in some cases. SGTA is a cochaperone that has been shown to interact with Hsp70 and Hsp90 by coimmunoprecipitation (13, 23–25) and to down-regulate androgen receptor signaling in mammalian reporter assays by promoting cytoplasmic retention of the receptor (16) and/or regulating AR ligand sensitivity (20). SGTA expression is reduced during prostate cancer progression and has been implicated as a contributor to the sensitization of prostate tumor cells to hormone signaling (16). In contrast, knockdown of SGTA in C4–2B prostate cancer cells leads to the suppression of AR-mediated gene expression and cell proliferation (26).
Although SGTA may have a role in prostate cancer disease progression (16, 20, 26) and polycystic ovary syndrome (27, 28) through the regulation of AR signaling, the ability of SGTA to affect additional members of the steroid hormone receptor superfamily remains to be elucidated. In addition to steroid hormone receptor specificity, the precise manner by which SGTA regulates receptor function is still unclear. Thus, we elected to assess a panel of steroid hormone receptors for SGTA regulation and to further investigate the mechanism by which SGTA regulates receptor function.
EXPERIMENTAL PROCEDURES
Yeast Strains and β-Galactosidase Reporter Assays
The β-galactosidase reporter assays that were used as a quantitative measure of receptor activity were described previously (29, 30). The BY4742 parental strain (MATα his3Δ1, leu2Δ0, lys2Δ0, met3Δ0, ura3Δ0) and the sgt2 deletion strain (MATα his3Δ1, leu2Δ0, lys2Δ0, met3Δ0, ura3Δ0, sgt2::Kanr) used in the deletion complementation experiments described in Fig. 2 were obtained from Open Biosystems (Huntsville, AL). W303a (MATa leu2-112 ura3-1 trp1-1 his3-11, 15 ade2-1 can1-100 GAL SUC2) was the parental strain used for all other yeast-based assays. With the exception of the estrogen receptor (ER) alpha expression plasmid, the parent plasmids for all yeast expression vectors were described previously. pUCΔs-26X was the hormone-inducible reporter plasmid used for all AR, progesterone receptor (PR), glucocorticoid receptor (GR), and mineralocorticoid receptor (MR) assays (kind gift from Brian Freeman, University of Illinois, Urbana-Champaign, IL). The ER expression vector and the estrogen-responsive reporter plasmid (kind gifts from Didier Picard, University of Geneva, Switzerland) were described previously (31, 32). The galactose-inducible β-galactosidase reporter used to control for general effects on transcription, translation, and protein stability was kindly provided by Charles Miller (Tulane University School of Public Health and Tropical Medicine, New Orleans, LA). For assays with the galactose-inducible reporter, yeast carrying the reporter in the presence or absence of an SGTA expression vector were grown in galactose-containing medium for 2 h prior to measuring β-galactosidase expression. For hormone-responsive reporter assays, the indicated strains were cotransformed with three to four plasmids: a hormone-inducible β-galactosidase reporter plasmid, a plasmid constitutively expressing the indicated steroid hormone receptor from a glyceraldehyde phosphate dehydrogenase (GPD) promoter, and high copy number plasmids expressing yeast SGT2, human SGTA, and/or human FKBP52 where indicated. All hormones were obtained from Sigma and were stored as 10 mm stock solutions in ethanol. Hormone dilutions were set up so that the ethanol vehicle never exceeded 1% in the yeast cultures. The hormone concentrations were optimized to maximize the difference between cells carrying an empty vector versus cells carrying an SGTA expression vector by performing dose-response curves. Hormone-induced reporter activity was measured from yeast extracts as described previously with a single 2-h time point measurement. All assays were performed at least in triplicate, and the data shown are representative of at least three independent experiments that produced consistent results. Statistical significance was determined by one-way analysis of variance followed by pair-wise comparisons using Bonferonni's multiple comparisons test; p values less than or equal to 0.05 determine significant differences.
FIGURE 2.
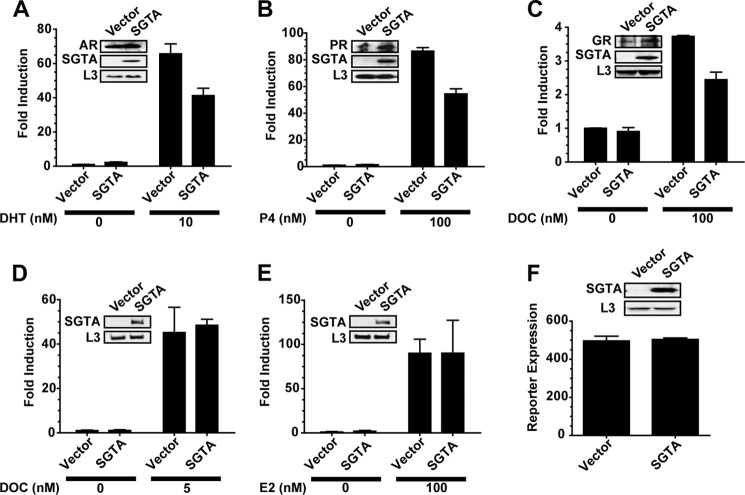
Receptor-specific effects of SGTA in yeast. Yeast reporter strains for AR (A), PR (B), GR (C), MR (D), and ER (E) in a W303a genetic background were transformed with empty plasmid vector or a plasmid expressing human SGTA. The cells were treated with or without the indicated concentration of hormone and assessed for receptor-mediated β-galactosidase expression. SGTA expression significantly reduced (p value < 0.05) the activity of AR (A), PR (B), and GR (C) but had no effect on MR (D) and ER (E). SGTA expression also had no effect on a galactose-inducible β-galactosidase reporter (F). In all experiments (A–F, insets) yeast lysates were prepared and immunoblotted for the appropriate steroid receptor, SGTA, or the yeast ribosomal protein L3 as a loading control. The hormones used were as follows: DHT, dihydrotestosterone; DOC, deoxycorticosterone; P4, progesterone; E2, 17β-estradiol.
Yeast Two-hybrid Analysis
Yeast two-hybrid assays were performed according to the manufacturer's recommendation (Matchmaker GAL4 two-hybrid system 3). Briefly, SGTA was used as bait, and GR or PR were used as prey. The yeast two-hybrid vectors pGADT7 (encoding Gal4 activation domain) and pGBKT7 (encoding Gal4 DNA-binding domain) were obtained from Clontech. SGTA was N-terminally fused with the Gal4 DNA-binding domain (pGBKT7:SGTA). The progesterone receptor in pGADT7 vector (pGADT7:PR, N-terminal fusion) was a kind gift from Dr. Miguel Beato (Centre de Regulació Genòmica, Universitat Pompeu Fabra), and the GR in pGADT7 vector (pGADT7:GR, N-terminal fusion) was kindly provided by Dr. Kaju Yanai (Department of Biomolecular Science Faculty of Sciences, Toho University). The resulting plasmids were cotransformed in combinations as indicated into the yeast strain AH109 and plated on synthetic complete medium lacking leucine and tryptophan (SC-LW). For interaction analysis, cotransformants were grown on synthetic complete medium lacking leucine, tryptophan, and histidine (SC-LWH) to select for expression of the HIS3 reporter.
Generation of Stable SGTA Knockdown HeLa Cells
To obtain SGTA knockdown (SGTAKD) HeLa cells, shRNA sequences targeting SGTA (sense, ATAAATAGGCGTTGTGACCTC; antisense, GAGGTCACAACGCCTATTTAT) separated by a loop (TTCGAGACG) were designed. The oligonucleotides targeting SGTA were designed as a synthetic duplex with overhanging sites for restriction digestion (BamHI-5′ end and HindIII-3′ end). The oligonucleotides were heated to 90 °C for 3 min and then cooled to 37 °C for 1 h for annealing reaction. The ligation reaction was performed with the annealed shRNA and BamHI- and HindII-digested pSilencer 2.1-U6 vector (Applied Biosystems, Foster City, CA) at room temperature for 2 h. The ligation product was transformed into DH5α competent cells. Positive clones were confirmed by sequencing. A scrambled shRNA was also constructed to exclude any shRNA specific effects. A blast search was performed to ensure that the scrambled construct had no significant homology with any known functional mRNA sequence. A second shRNA construct targeting SGTA was also made in a similar way (sense, ACTTTGAAGCTGCCGTGCA; antisense, TGCACGGCAGCTTCAAAGT; loop TTCGAGACG) to rule out any off target effects. For stable transfection, HeLa cells were plated in Petri dishes in Hyclone minimum essential medium/Earl's balanced salt solution media (Thermo Scientific, Waltham, MA) containing 10% FBS 24 h prior to transfection. At 80% confluence, shRNA targeting SGTA or control shRNA with a scrambled sequence were transfected by the Lipofectamine 2000 method according to the manufacturer's instructions (Invitrogen) followed by selection with hygromycin (Thermo Scientific). Individual colonies were isolated and screened for effective knockdown of endogenous SGTA protein levels by Western blot. The selected clone was used to perform the luciferase reporter assays. Also, the scrambled shRNA construct was stably transfected into HeLa cells to demonstrate that the effects were not due to nonspecific knockdown of cellular proteins in general.
Mammalian Cell Lines and Assays
Wild type HeLa cells, SGTAKD cells, and cells with stably transfected scrambled shRNA were cultured at 5% CO2 in minimum Eagle's medium supplemented with 10% charcoal-stripped FBS and essential amino acids. Cells were plated at a cell density of 6 × 105 cells/well in 6-well plates. At 80% confluence, the cells were transfected for 4 h using Lipofectamine 2000 (Invitrogen) at a DNA (μg):Lipofectamine (μl) ratio of 1:3 in minimum Eagle's medium lacking FBS according to the manufacturer's instructions. For the reporter activity, three plasmids (800 ng of each plasmid/well) were transfected: a hormone-responsive firefly luciferase reporter, a mammalian expression vector expressing the indicated steroid hormone receptor, and an empty mammalian expression vector or a mammalian expression vector expressing SGTA. To control for transfection efficiency, each well was also transfected with 50 ng of a constitutive β-galactosidase expression plasmid. For the PR assays, a mammalian expression vector expressing the progesterone receptor was also transfected. Given that HeLa cells express GR endogenously, no GR expression vector was needed. The parent plasmid for all mammalian expression vectors was pCI-neo (Promega, Madison, WI). At 24 h post-transfection, cells were treated with the indicated concentration of the appropriate hormone or vehicle control (ethanol) in medium containing charcoal-treated FBS. The cells were lysed 48 h after transfection in M-PER (Pierce). Luciferase activity was measured by adding 40 μl of cell lysate with 100 μl of luciferase reagent (Promega) in an opaque 96-well plate. β-Galactosidase activity was assayed by adding 20 μl of the cell lysate with 100 μl of Tropix Gal-Screen assay reagent (Applied Biosystems) in an opaque 96-well plate. The 96-well plates were incubated at room temperature until maximum luminescence developed (5 min for the luciferase assays and 2 h for the β-galactosidase assays). After normalizing for transfection efficiency (relative light units/β-galactosidase activity), the data were plotted as fold induction of luciferase activity over background activity observed in the absence of hormone. All assays were performed at least in triplicate, and the data presented are representative of at least three assays with consistent results.
Recombinant Protein Expression and Purification
Purified recombinant human Hsp90α and Hsp70 were previously purified in the Buchner lab (Department Chemie, Technische Universität München, Garching, Germany). SGTA was cloned into pET28a(+) vector with a C-terminal His6 tag and into pGEX-4T-1 (a kind gift from Dahai Zhu, Chinese Academy of Medical Sciences) with an N-terminal GST tag. The His6-tagged and GST-tagged SGTA were both shown to be functional in the yeast reporter assays (data not shown). For purification, the His6-tagged SGTA was expressed in Escherichia coli BL21(DE3). 30 ml of each overnight culture were grown in 1 liter of medium in a shaking incubator at 37 °C until the A600 reached 0.6. Protein expression was induced with isopropyl β-d-1-thiogalactoside to a final concentration of 1 mm. The cultures were grown for an additional 4 h and centrifuged at 6,000 rpm for 20 min. The pellets were resuspended in 12 ml of lysis buffer (20 mm Tris-HCl, 300 mm NaCl, 10 mm imidazole, pH 8) and divided into three 4-ml aliquots. 40 μl of 100 mg/ml lysozyme was then added to each 4-ml cell suspension and incubated for 20 min at 30 °C. To lyse the cells, each of the four samples were then sonicated and kept on ice. The four aliquots were pooled together and centrifuged at 15,000 rpm for 20 min at 4 °C. To purify the protein, the 12-ml lysate was loaded onto the nickel-nitrilotriacetic acid column pre-equilibrated with lysis buffer and mixed for 1 h at 4 °C. The resin was then washed with buffer (20 mm Tris-HCl, 300 mm NaCl, 20 mm imidazole, pH 8) and transferred to a 2-ml gravity flow column. 10 ml of elution buffer (20 mm Tris-HCl, 300 mm NaCl, 250 mm imidazole, pH 8) was used to elute the proteins. All proteins were dialyzed extensively against 50 mm HEPES, 50 mm KCl, 10 mm MgCl2, 1 mm DTT (pH 7.4) prior to sample concentration and storage at −80 °C. GST-tagged SGTA was purified in a similar manner, except a glutathione resin was used and the buffer formulations were as follows: lysis and wash buffer (50 mm Tris-HCl, 150 mm NaCl, 0.05% Nonidet P-40, pH 7.5), elution buffer (10 mm reduced glutathione, 50 mm Tris-HCl, pH 7.5), and dialysis buffer (100 mm NaCl, 20 mm Tris-HCl, 1 mm EDTA, pH 7.4).
PR-Chaperone Complex Reconstitution and Hormone Binding Assays
Purified PR was adsorbed onto PR22-protein A-Sepharose resin beads and reconstituted into multiprotein complexes as described previously (33). Briefly, ∼0.05 μm of PR was incubated with 1 μm each of Hsp90β, Hsp70, Hop, Hsp40, p23, and indicated concentrations of His6-tagged SGTA in 200 μl of reaction buffer A (20 mm Tris-HCl, pH 7.5, 5 mm MgCl2, 2 mm DTT, 0.01% Nonidet P-40, 50 mm KCl, and 5 mm ATP) for 30 min at 30 °C, resuspending beads every 3–4 min. 0.1 μm [3H]progesterone (American Radiolabeled Chemicals, Inc.) was then added, and the reaction mixture was incubated for 3 h at 4 °C on a gentle shaker. PR multiprotein complexes were washed with 1 ml of reaction buffer B (20 mm Tris-HCl, pH 7.5, 0.01% Nonident P-40, 50 mm KCl) three times and assessed for bound progesterone using Microbeta plate reader (PerkinElmer Life Sciences). The remaining samples were incubated with SDS sample buffer, and protein complexes were resolved using SDS-PAGE (10% gel) and Coomassie Blue staining. One-fifth fraction of each sample was run on SDS-PAGE (10% gel) and transferred to PVDF membrane.
Western Blot
Yeast cells growing overnight were diluted to an A600 of ∼0.2 and grown until the A600 reached 0.8. For Western blot to detect receptors, which tend to precipitate in yeast lysates, yeast cells were pelleted, resuspended in 2× SDS-PAGE sample buffer, and vortexed vigorously in the presence of glass beads. For all other Western blots yeast cells were pelleted, resuspended in extract buffer (20 mm Tris, pH 7.5, 100 mm NaCl, 5% glycerol supplemented with protease inhibitors), and vortexed vigorously in the presence of glass beads. Lysates were then clarified at 13,200 rpm for 20 min at 4 °C. For mammalian cell lysis, the cells were washed with 1× PBS and lysed with mammalian protein extraction reagent (M-PER; Pierce) supplemented with protease inhibitors (Complete mini EDTA-free; Roche). Protein concentrations for both yeast and mammalian extracts were determined by Coomassie Plus protein assay (Pierce). Typically 20 μg of total cellular protein was separated on a 10–20% Criterion gels (Bio-Rad) and transferred to polyvinylidene difluoride membranes. The human tissue blot was purchased (ProSci Inc., catalogue number 1541N/T). The following antibodies were used: rabbit α-human SGTA (Protein Tech Group, Chicago, IL), mouse monoclonal α-FKBP52 (HI52D), rabbit α-human AR (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit α-human GR (Santa Cruz Biotechnology), and mouse anti-chicken PR (PR-22). Antibodies detecting yeast ribosomal protein L3 (a gift from Jonathan Warner, Albert Einstein College of Medicine) and glyceraldehyde-3-phosphate dehydrogenase (6C5; Biodesign International, Saco, MN) were used as loading controls. The secondary antibodies were alkaline phosphatase-conjugated goat anti-mouse or anti-rabbit antibodies, and the bands were visualized with Immune-Star AP substrate (Bio-Rad) and exposed to x-ray film.
Isothermal Titration Calorimetry
Isothermal titration calorimetry was performed using a MicroCal VP-ITC instrument (Microcal Inc., Northampton, MA). For the binding of human SGTA to Hsp90 or Hsp70, recombinant human GST-SGTA was titrated from the syringe into the cell containing human Hsp90 or human Hsp70. The concentration of SGTA was 270 μm and the Hsp90 or Hsp70 concentration was 40 μm. In the case of Hsp90, the nucleotide dependence of SGTA binding was tested by measuring the binding under the same conditions but in the presence of 2 mm AMP-PNP (Roche) in the cell. The buffer used in the syringe and the cell was 40 mm HEPES (pH 7.5), 20 mm KCl, 5 mm MgCl2 at 25 °C. 40 injections of ligand solution were performed to fully saturate the protein in the cell. Data analysis was performed using Origin software (OriginLab Corporation, Northampton, MA).
Isolation of His-Cpr6 and Ura2-TAP Complexes
Plasmids expressing wild type Sgt2 or Cpr6 containing an N-terminal His6 tag and Xpress epitope expressed under the strong constitutive GPD promoter were transformed into an hsc82hsp82 strain expressing untagged WT Hsc82 or Hsc82ΔMEEVD. His-Sgt2 or His-Cpr6 complexes were isolated as described (1). Briefly, cells were grown overnight and harvested at an A600 of 1.2–2.0. Cell pellets were resuspended in lysis buffer (20 mm Tris, pH 7.5, 100 mm KCl, 5 mm MgCl2, 5 mm imidazole containing a protease inhibitor tablet (Roche Applied Science)) and were disrupted in the presence of glass beads with eight 30-s pulses. Cell lysates were incubated with nickel resin (1 h with rocking at 4 °C) followed by washes with lysis buffer plus 35 mm imidazole and 0.1% Tween 20. Proteins were eluted from nickel resin by boiling in SDS-PAGE sample buffer, and protein complexes were separated by gel electrophoresis (10% acrylamide) followed by Coomassie Blue staining. Alternatively, proteins were transferred to nitrocellulose, and chemiluminescence immunoblots were performed according to the manufacturer's suggestions (Pierce). Anti-Xpress antibody was obtained from Invitrogen. Anti-Hsp70 (Ssa isoform) was a gift from Dr. Elizabeth Craig.
RESULTS
Functional Comparison of Yeast Sgt2 and Human SGTA
We performed a comparative study of human SGTA and yeast Sgt2 to establish the budding yeast, S. cerevisiae, as a relevant model system for the study of functional interactions between SGTA and the steroid hormone receptors. Although yeast Sgt2 is considered to be a homologue of human SGTA (34), functional homology with respect to the regulation of androgen receptor function has not been demonstrated. An amino acid sequence alignment revealed that yeast Sgt2 and human SGTA share ∼30% amino acid identity. SGTA contains previously characterized N-terminal dimerization, middle TPR, and C-terminal glutamine-rich domains (19). Although significant conservation exists between the two proteins within all domains, the C terminus of yeast Sgt2 is significantly less glutamine-rich. The functional consequences of this difference with respect to steroid receptor regulation are unknown.
To assess functional homology between yeast Sgt2 and human SGTA, we performed deletion/complementation experiments. Androgen-responsive β-galactosidase reporter strains were constructed in the wild type (SGT2) and deletion (Δsgt2) strains in a BY4742 genetic background and assessed for AR function in the presence of empty vector, an SGT2 expression vector, or an SGTA expression vector (Fig. 1A). The deletion of SGT2 resulted in a significant enhancement (50–60%) in AR activity, and the overexpression of either SGT2 or human SGTA was able to restore AR activity to wild type levels. In addition to the effects observed in the deletion strain we consistently observed a significant decrease in AR activity in the wild type SGT2 strain upon SGTA overexpression. This effect was not likely the result of nonlimiting Sgt2 protein levels in the yeast because SGT2 overexpression did not produce this effect.
FIGURE 1.
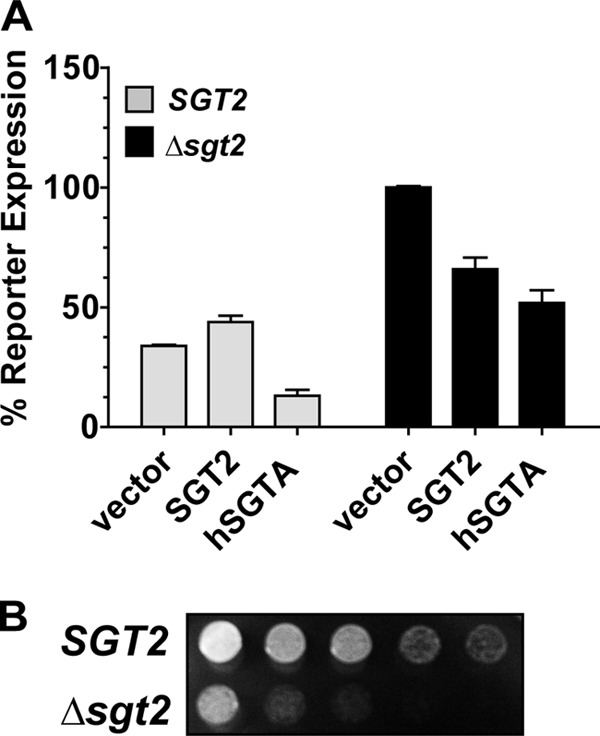
Deletion-complementation analysis of SGTA and Sgt2 in yeast. A, the wild type (SGT2) and sgt2 deletion (Δsgt2) strains (BY4742 genetic background) carrying an AR-responsive reporter and AR expression vector were transformed with an empty plasmid vector or plasmid expressing yeast SGT2 or human SGTA. The yeast were induced with hormone and assessed for β-galactosidase expression. AR activity in the Δsgt2 strain carrying empty vector was significantly increased (p value < 0.0001) as compared with the wild type control, and both human SGTA and yeast SGT2 restored activity to wild type levels (p value < 0.001). AR activity in the wild type strain was significantly reduced (p value < 0.001) in the presence of human SGTA as compared with the wild type strain in the presence of empty vector or plasmid expressing SGT2. B, the wild type (SGT2) and deletion strain (Δsgt2) were serially diluted, spotted onto selective medium, and grown at 30 °C for 2 days. The Δsgt2 strain displays a growth defect at optimal growth temperatures.
The wild type SGT2 and sgt2 deletion strains were obtained commercially. After several rounds of plasmid transformation and a few experiments, the sgt2 deletion strain displayed a growth defect at optimal growth temperature (Fig. 1B) that was not observed initially, and this growth defect was not rescued by exogenous expression of SGT2 or SGTA (data not shown). We purchased the strains a second time, and these cells also began to display growth defects over time. Thus, although we were able to produce consistent data during the time it took to complete three or four experiments, continued long term experimentation in the sgt2 deletion strain in a BY4742 genetic background was difficult. It is important to note that we have constructed an sgt2 deletion strain in a w303a genetic background, and this progressive growth defect was not observed in this strain after multiple rounds of plasmid transformation. Thus, this phenotype appears to be specific to the BY4742 genetic background.
SGTA Receptor Specificity
Previous studies in mammalian cells suggested that SGTA is an AR-specific cochaperone based solely on the lack of an effect on ER activity (16). However, ER is often an outlier with respect to cochaperone regulation. Thus, we assessed a panel of steroid hormone receptors for SGTA regulation. We observed consistent effects on AR activity in the wild type strain upon SGTA overexpression and reasoned that overexpression in a wild type strain could serve as a reliable measure of SGTA function. To assess SGTA receptor specificity, we constructed AR-, PR-, GR-, MR-, and ER-responsive β-galactosidase reporter strains in a W303a genetic background and assayed for receptor function in the presence of empty vector or an SGTA expression vector (Fig. 2, A–E). SGTA overexpression significantly reduced AR, PR, and GR activity as compared with the empty vector controls (Fig. 2, A–C), and these reductions in activity were not the result of receptor destabilization (Fig. 2, A–C, insets). SGTA overexpression had no effect on the activity of MR and ER (Fig. 2, D and E). Finally, SGTA overexpression had no effect on the galactose-inducible expression of a β-galactosidase reporter gene (Fig. 2F), indicating that the effects observed on AR, PR, and GR were not likely due to general toxic effects on transcription, translation, or protein stability.
SGTA was previously reported to associate with and functionally affect AR in a mammalian cell system (16). Our data are the first to report a role for SGTA in the PR and GR signaling pathways. To corroborate these results in a higher vertebrate model system, we constructed HeLa cells stably expressing a 19-base pair shRNA targeting SGTA mRNA. To control for the specificity of the knockdown, we also constructed a HeLa cell line stably expressing a scrambled shRNA construct. We were able to achieve ∼50–60% knockdown of SGTA protein levels as compared with wild type HeLa cells or the scrambled shRNA control, and SGTA knockdown had no consistent effect on GR and PR stability (Fig. 3A). Although the Western blots in Fig. 3A show a reduction of PR protein levels, this effect was not consistent, and the blots shown are representative. To assess the effects of SGTA knockdown on PR and GR function, we performed receptor-mediated luciferase assays in the presence of either an empty vector or SGTA expression vector (Fig. 3, B and C). In both cases, reductions in SGTA protein levels resulted in a significant increase in receptor activity as compared with activity in the wild type and control shRNA cells and with exogenous expression of SGTA in the knockdown cells restored receptor activity to at or below wild type levels. To control for off target effects, we produced SGTA knockdown cells using an independent 19-base pair oligonucleotide and performed luciferase assays as described above (data not shown). These data directly correlated with the results presented in Fig. 3 (B and C) (data not shown).
FIGURE 3.
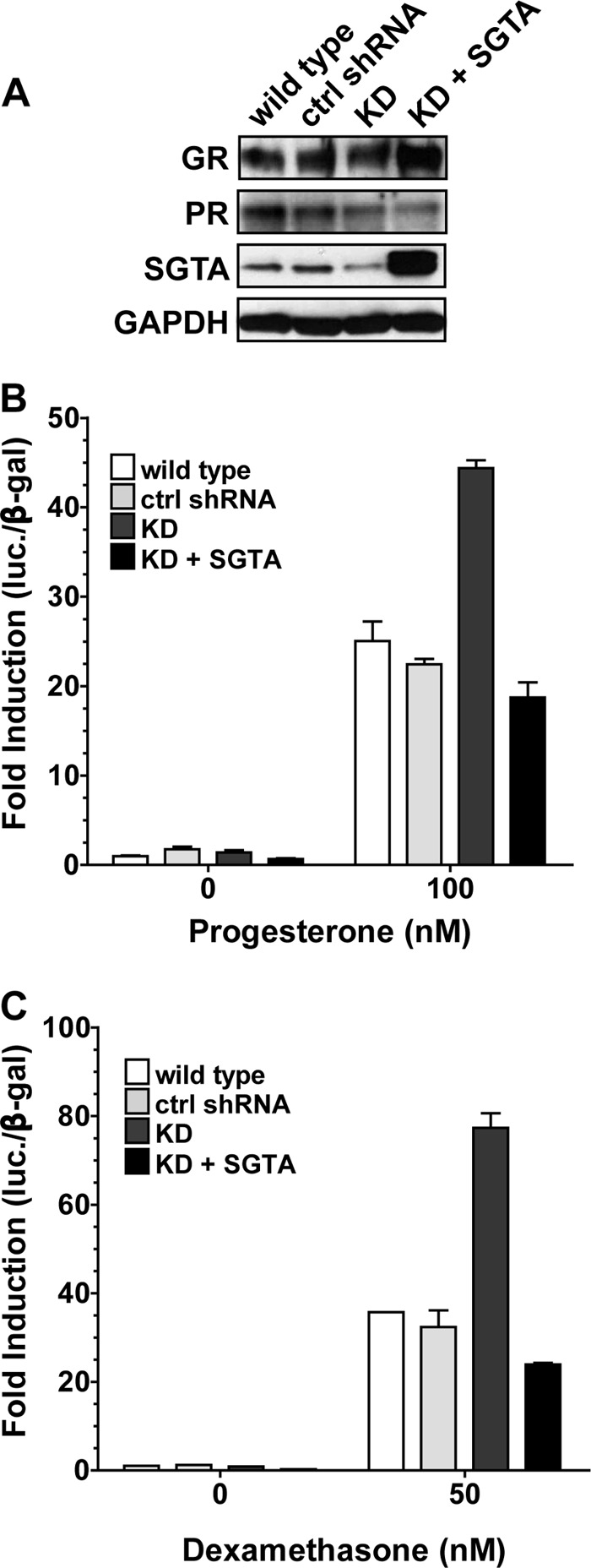
SGTA regulation of receptor function in mammalian cells. HeLa cells were stably transfected with an shRNA expression vector targeting SGTA, and cells were selected for stable knockdown of SGTA expression levels (KD). To control for specific knockdown, a stable cell line expressing a scrambled shRNA construct was also made (control shRNA). A, lysates were prepared from the indicated control or SGTA knockdown cells and immunoblotted for GR, PR, SGTA, and the loading control GAPDH. B and C, wild type, control, or SGTA knockdown HeLa cells carrying a receptor-responsive luciferase reporter and expressing either PR (B) or GR (C) were transfected with an empty plasmid vector or plasmid expressing human SGTA and assessed for receptor-mediated luciferase expression. In the experiments in B and C, receptor activity in the SGTA knockdown cells in the absence of exogenous SGTA expression (dark gray bars) is significantly increased (p value < 0.001) as compared with all other conditions. ctrl, control.
Human SGTA Associates with PR and GR Complexes
The data described in Figs. 1–3 establish SGTA as a functional modulator of AR, PR, and GR activity. However, association of SGTA with PR and GR heterocomplexes has not been shown. Previous in silico based approaches suggested that SGTA interacts with an EEVD-like sequence in the hinge region on AR, which is shaded in Fig. 4A. However, as demonstrated by an amino acid sequence alignment, this region is quite variable between the receptors, and PR and GR lack anything resembling an EEVD sequence. To demonstrate SGTA association with PR and GR, we set up a yeast two-hybrid analysis using GAL4 activation domain and DNA-binding domain fusions and a yeast strain containing a stable GAL4-inducible HIS3 reporter (Fig. 4B). Growth on histidine-lacking media was representative of an interaction. A positive interaction in this system does not specifically indicate direct interaction with the receptor but does indicate at least an association with the receptor-chaperone complex. GR and PR were expressed as fusions with the activation domain because the steroid receptors, when fused with the DNA-binding domain, can activate the reporter by way of their activation functions. We coexpressed all possible combinations of activation domain and DNA-binding domain constructs, including the individual domains, to control for nonspecific activation of the reporter. Only yeast containing the positive control interactors (SV40 large T antigen and p53) or the indicated steroid hormone receptor and SGTA grew on histidine-lacking medium. Thus, although they lack an EEVD-like sequence in the hinge region, PR and GR can associate with SGTA in a yeast two-hybrid analysis (Fig. 4B).
FIGURE 4.
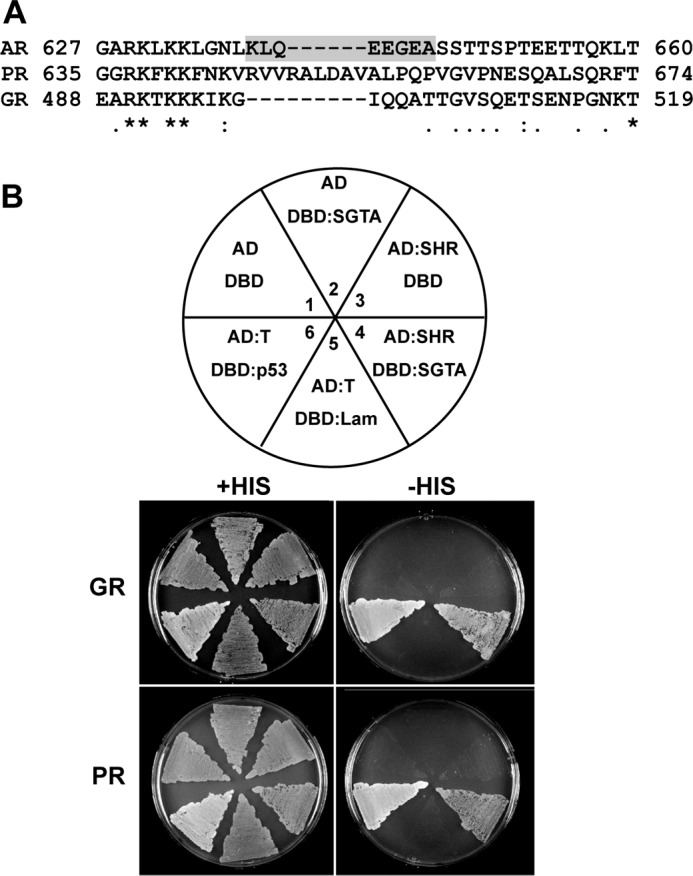
Yeast two-hybrid analysis of SGTA interactions with GR and PR complexes. A, amino acid sequence alignment of a portion of the hinge region of human AR, PR, and GR encompassing the previously proposed EEVD-like SGTA interaction site on AR (shaded). B, a yeast strain carrying a stable GAL4-inducible HIS3 reporter was transformed with various combinations of Gal4 activation domain (AD) and GAL4 DNA-binding domain (DBD) constructs and plated on histidine-containing or histidine-lacking media as outlined in the diagram (upper panel). Growth on histidine-lacking media is taken to represent an interaction. Plate regions 1–3 control for nonspecific activation of the histidine reporter. p53 and the SV40 large T antigen (T) represent a known positive interaction and served as the positive control (plate region 6). Lamin C (Lam) and SV40 large T antigen do not interact and served as a negative control (plate region 5). Only those cells containing the indicated steroid hormone receptor (SHR) in combination with SGTA (plate region 4) or p53 in combination with SV40 T antigen (plate region 6) grew on the histidine-lacking media (lower panel).
SGTA Effects on Receptor-Chaperone Complex Formation and Receptor Hormone Binding
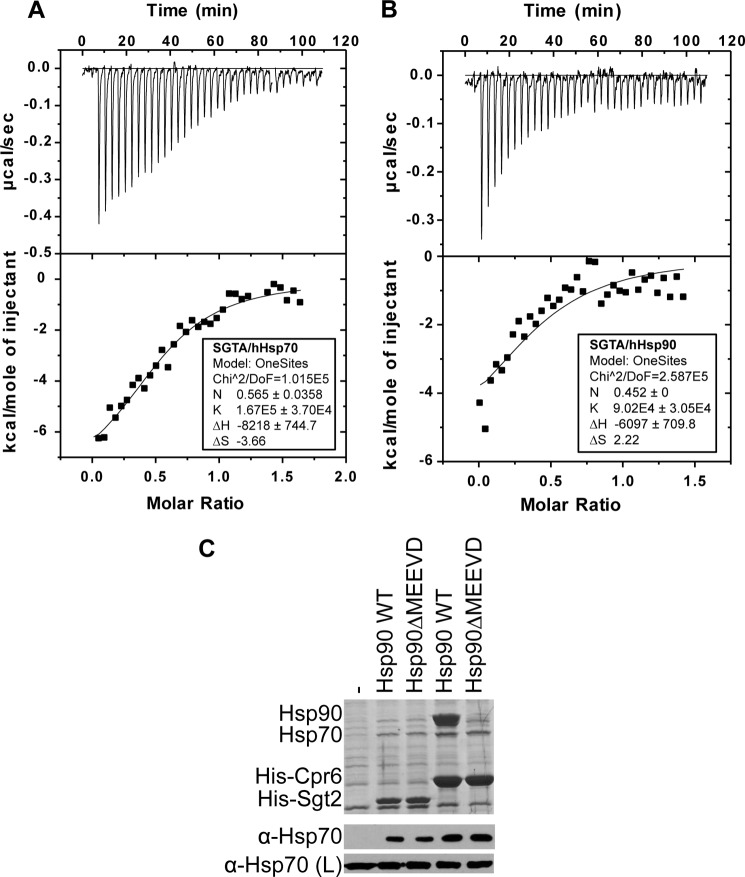
Our data establish SGTA as a functional modulator of GR and PR, in addition to the previously characterized role for SGTA in AR signaling. However, the mechanism by which SGTA functionally regulates receptor activity remains to be elucidated. Previous studies demonstrated that SGTA interacts with Hsp70 and weakly with Hsp90 in coimmunoprecipitation experiments, and these interactions were mapped to the three tandem TPRs between residues 95 and 195 on SGTA (23). To get a more quantitative measure of SGTA binding to Hsp70 and Hsp90, we performed isothermal titration calorimetry with recombinant human SGTA, Hsp70, and Hsp90 (Fig. 5). These results indicate that SGTA binds Hsp70 with an affinity of 6.1 μm (Fig. 5A) and to Hsp90 with an affinity of 11.0 μm (Fig. 5B) in vitro. Despite the similar affinities in vitro, previously published data suggested that SGTA precipitates predominately with Hsp70 in coimmunoprecipitations from mammalian cell lysates (23). To further corroborate these findings, we isolated His-Sgt2 complexes to determine whether Sgt2 interacts predominantly with Hsp70 or Hsp90 in yeast extracts. For comparison, we also isolated His-Cpr6, which is known to interact with both Hsp70 and Hsp90, but not Hsp90ΔMEEVD because of the deletion of the TPR binding site (1). As shown in Fig. 5C, similar levels of Hsp70 bound His-Sgt2 and His-Cpr6. There was no noticeable effect of the Hsp90ΔMEEVD mutation, suggesting that little or no Sgt2 directly binds Hsp90 in yeast extracts.
FIGURE 5.
SGTA and Sgt2 interaction with Hsp70 and Hsp90. A and B, isothermal titration calorimetry was performed with recombinant SGTA (270 μm) and either human Hsp70 (A) or human Hsp90 (B) (40 μm each). Titrations were performed with 40 injections of 8 μl of SGTA in the injection syringe. C, His-Sgt2 or His-Cpr6 was isolated from hsc82hsp82 cells expressing WT Hsp90 or Hsp90ΔMEEVD. Cell extracts were incubated with nickel resin, and complexes were analyzed using SDS-PAGE and immunoblot analysis. The upper panel displays the Coomassie Blue-stained gel of the resin samples, whereas the lower panels show immunoblot analysis. Immunoblots of the lysate (L) are provided as a loading control. The band in the stained gel that migrates below His-Sgt2 is an unknown protein that binds nonspecifically to nickel resin.
Our data support the idea that SGTA acts at an early stage in receptor folding. Based on this idea, we sought to determine whether SGTA influences receptor-chaperone complex assembly and the folding of the receptor to a hormone-binding competent conformation. Thus, we assessed the ability of increasing concentrations of recombinant SGTA to affect the assembly of PR-chaperone complexes in a progesterone receptor reconstitution assay using the five purified proteins (Hsp90, Hsp70, Hop, Hsp40, and p23) known to be required for PR to reach a hormone-binding competent conformation in vitro (Fig. 6A). Although SGTA was able to associate with PR-chaperone complexes, SGTA supplemented into the assay up to 16 μm had no effect on PR-chaperone complex assembly. It is possible that additional endogenous factors are required for SGTA function. Thus, in addition to the “five purified protein system,” we also performed these assays in rabbit reticulocyte lysate (Fig. 6B). SGTA did not affect PR-chaperone complex assembly in both systems. In addition, SGTA had no effect on PR folding to the hormone-binding competent conformation in reticulocyte lysate (Fig. 6B, left panel) and in the five purified protein system (data not shown). Thus, although SGTA can associate with receptor chaperone complexes and functionally affect receptor activity, these effects are not due to the alteration of receptor folding and hormone binding.
FIGURE 6.
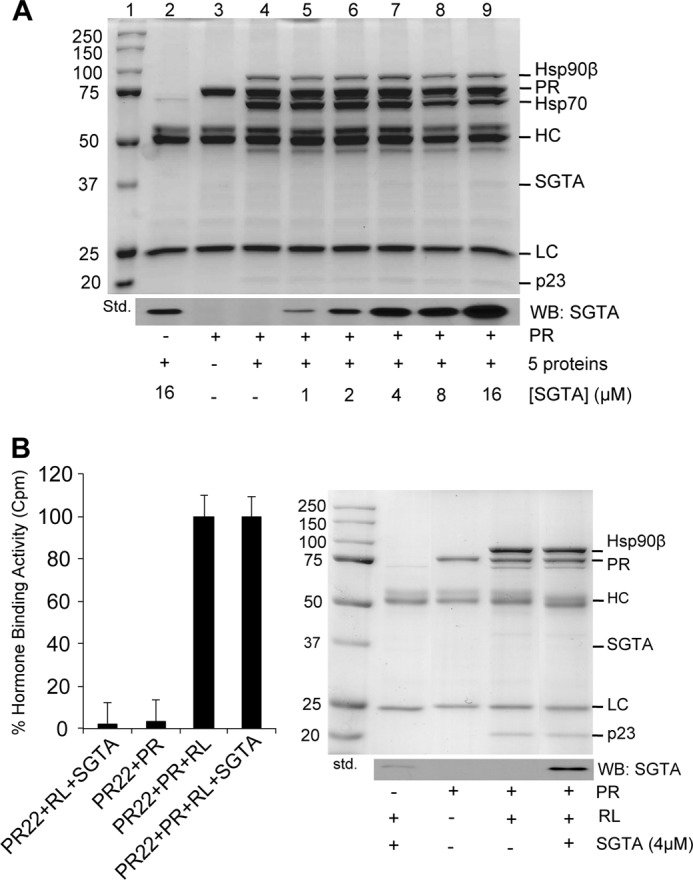
SGTA does not affect chaperone complex formation and receptor hormone binding in a cell-free system. A, an in vitro PR-chaperone complex reconstitution assay with serial SGTA protein concentrations. The five purified proteins known to be required for PR to reach a hormone-binding competent conformation in vitro include Hsp90, Hsp70, Hop, Hsp40, and p23. Binding buffer was supplemented with or without the indicated recombinant proteins and immunoprecipitated with the PR-specific antibody, PR22. The upper panel is the Coomassie dye-stained gel, and the lower panel is a Western blot (WB) for SGTA. Lane 1 is the molecular weight standard and lanes 2–4 are negative controls with or without PR, the five proteins, and/or SGTA. Increasing concentrations of SGTA from 1 to 16 μm (lanes 5–9) had no effect on PR-chaperone complex assembly. B, an in vitro PR-chaperone complex reconstitution assay in reticulocyte lysate, which contains the five proteins known to be required for PR to reach a hormone-binding competent conformation. The right panel shows the Coomassie-stained gel and the Western blot for SGTA. PR within the reconstituted complexes was assessed for hormone binding using [3H]progesterone (left panel).
SGTA Antagonizes FKBP52 Potentiation of Receptor Function
The data indicate that SGTA is a specific negative regulator of AR, PR, and GR activity. This pattern of receptor specificity is shared by the receptor-associated cochaperone FKBP52, which is a well characterized positive regulator of receptor function (35). For a general comparison of SGTA and FKBP52 expression patterns in human tissues, we immunoblotted for SGTA and FKBP52 using extracts from a variety of human tissues chosen based on their relevance to AR, PR, and GR function (Fig. 7A). Given that different antibodies were used to detect SGTA and FKBP52, a quantitative comparison of expression levels was not possible. However, the general level of each protein and its pattern of expression across tissues can be compared. The data in Fig. 7A reveal that SGTA and FKBP52 follow a similar pattern of expression in human tissues. Each protein is expressed the highest in the testis, brain, and liver and is expressed the lowest in the ovary.
FIGURE 7.
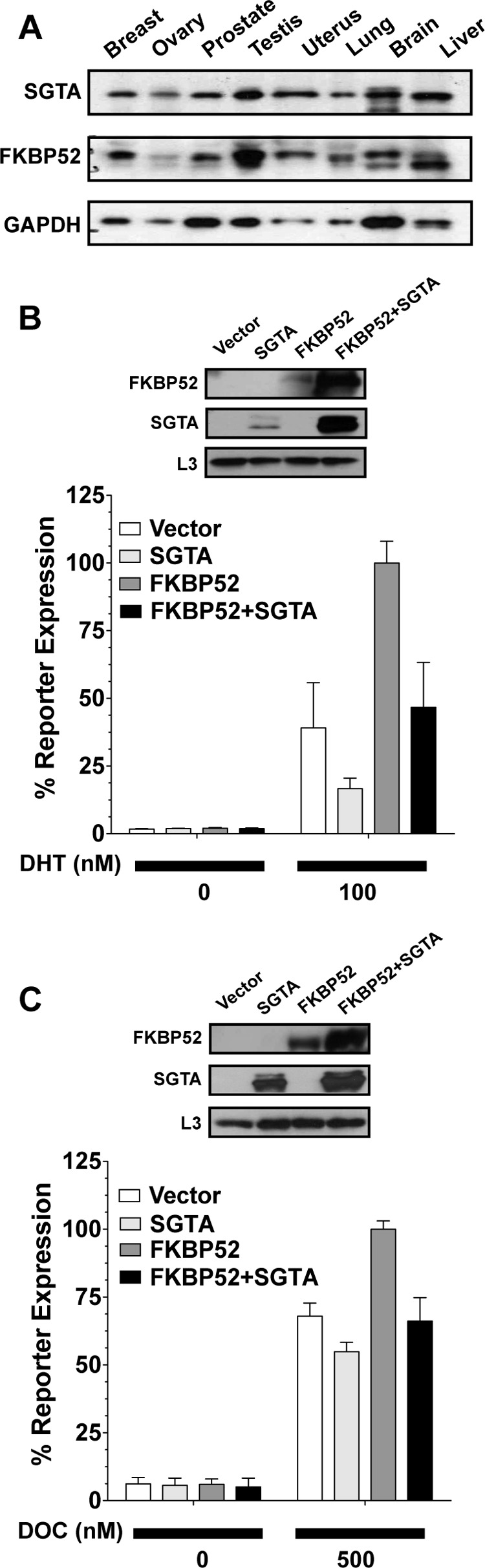
Effects of SGTA on FKBP52 regulation of receptor function. A, Western blot analysis of SGTA and FKBP52 protein levels in lysates (16 μg of total protein/lane) from various human tissues chosen based on their relevance to androgen, progesterone, and glucocorticoid receptor signaling. B and C, yeast reporter strains for AR (B) and GR (C) were transformed with empty plasmid vector or plasmid expressing SGTA and FKBP52 either alone or in combination. Yeast lysates were prepared and immunoblotted (upper panels) for FKBP52, SGTA, or the yeast ribosomal protein L3 as a loading control. In parallel, yeast strains were induced with the indicated concentration of hormone and assayed for β-galactosidase activity. In all cases, receptor signaling in cells expressing FKBP52 alone (dark gray bars) was significantly higher (p value < 0.001) as compared with all other conditions. Receptor signaling in the presence of SGTA alone (light gray bars) was significantly reduced (p value < 0.05) as compared with all other conditions. Receptor signaling in the presence of FKBP52 and SGTA (black bars) was not significantly different from the vector control (white bars). The hormones used were as follows: DHT, dihydrotestosterone; DOC, deoxycorticosterone.
We also assessed the effects of FKBP52 and SGTA coexpression on receptor activity in yeast. Yeast lack homologues of the large FKBP proteins and serve as an FKBP negative background in which to assess the effects of FKBP52-SGTA coexpression. Receptor-mediated β-galactosidase reporter assays were performed in the presence of empty vector or plasmids expressing FKBP52 and SGTA either alone or in combination (Fig. 7, B and C). For both AR (Fig. 7B) and GR (Fig. 7C), SGTA expression alone significantly reduced receptor activity, FKBP52 expression alone significantly enhanced receptor activity, and in the presence of both SGTA and FKBP52, receptor activity was unchanged. Thus, in the presence of SGTA, FKBP52-mediated potentiation was completely abrogated. The effect of SGTA alone on GR activity in Fig. 7C, although significant, appears smaller as compared with the data discussed above. However, this was the result of the hormone dose used, which was chosen based on maximizing the FKBP52 effect and the antagonistic effects of SGTA on FKBP52. Immunoblots for FKBP52, SGTA, and the loading control L3 were performed on the same samples used in the luciferase assays (Fig. 7, B and C, insets). We found that coexpression of FKBP52 and SGTA in yeast consistently resulted in a significant increase in FKBP52 and SGTA protein levels as compared with the levels present when each protein was expressed individually. The degree of stabilization varied, as can be seen from a comparison of the Western blots in Fig. 7 (B and C), but was always significant. No changes in Hsp90 levels were observed in yeast coexpressing SGTA and FKBP52 (data not shown).
DISCUSSION
Studies in the budding yeast, S. cerevisiae, have contributed much to the current knowledge regarding cochaperone regulation of steroid hormone receptor function, and our data firmly establish the relevance of the yeast system for the study of functional SGTA-steroid receptor interactions (Figs. 1 and 2). Although the data presented in Fig. 1A are in direct agreement with previously published data demonstrating that SGTA is a negative regulator of AR function in mammalian cells (16), the sgt2 deletion strain in a BY4742 genetic background displays a growth defect at optimal temperatures over time that makes continued experimentation difficult (Fig. 1B). This slow growth phenotype may result from the inability of the sgt2 null strain to recover from multiple rounds of plasmid transformation given that the sgt2 null mutant has been reported to display abnormal cell cycle progression, decreased competitive fitness, decreased chronological lifespan, increased heat sensitivity, and a small defect in vacuolar fragmentation (36–40). Regardless of the cause, this slow growth phenotype appears to be strain-specific, because the sgt2 deletion in a w303a genetic background does not display this growth phenotype. Human SGTA, but not Sgt2, consistently reduced AR activity when overexpressed in wild type cells (Fig. 2A). This effect is not due to general toxicity, because SGTA overexpression had no effect on MR and ER activity nor on the activity of a constitutive β-galactosidase reporter (Fig. 2). Although the reason for this functional difference is unclear, this effect can serve as a reliable measure of SGTA function in yeast.
The reporter assays in both yeast and mouse embryonic fibroblasts (Figs. 1–3) extend the repertoire of SGTA-regulated steroid hormone receptors to include AR, GR, and PR. In both systems, knockdown or deletion enhanced receptor activity, whereas overexpression reduced receptor activity. In addition, the data presented in Figs. 4 and 6 provide the first direct evidence of SGTA interaction with GR and PR heterocomplexes in a yeast two-hybrid system and SGTA interaction with PR heterocomplexes by coimmunoprecipitation. Previous in silico based studies suggested that SGTA directly interacts with an EEVD-like sequence upstream of the AR hormone-binding domain (16). Given the inability to purify the steroid hormone receptors for in vitro studies, we were only able to make conclusions based on interactions with the receptor-chaperone complexes rather than direct interaction with the receptors. The data in Figs. 4 and 6 do not demonstrate direct interaction of SGTA with GR and PR but do demonstrate at least an interaction with the receptor-chaperone complexes. In addition, GR and PR both lack an EEVD-like sequence analogous to that found in AR. Thus, it is more likely that SGTA interacts with GR and PR indirectly through interaction with Hsp70 and/or Hsp90.
The data presented in Fig. 5 demonstrate that SGTA binds Hsp70 and Hsp90 with similar affinities in vitro. However, SGTA predominately precipitates with Hsp70 in coimmunoprecipitations from mammalian cells (23) and yeast cells (Fig. 5C). Additionally, SGTA modulates Hsp70 ATPase activity (23, 41). SGTA modulation of Hsp90 ATPase activity has not been reported, and we were unable to observe SGTA effects on Hsp90 ATPase activity (data not shown). Our data suggest that SGTA does not have a role in the assembly of receptor-Hsp90 complexes and the folding of the receptor to the hormone binding conformation in a cell free system (Fig. 6). Philp et al. (42) proposed that SGTA may have a more predominant role in early receptor folding events mediated by Hsp70. Buchanan et al. (16) demonstrated that SGTA overexpression inhibits hormone-induced translocation of the androgen receptor to the nucleus and propose a model in which SGTA acts to retain the receptor in the cytoplasm. The fact that SGTA shows the same pattern of receptor specificity (Figs. 1–3) and tissue expression (Fig. 7A) as FKBP52 suggests that SGTA may act as a negative regulator of receptor function through simple competition for binding Hsp90. Although SGTA antagonized FKBP52 potentiation of receptor function in yeast (Fig. 7, B and C), competition with FKBP52 for binding Hsp90 is not likely the sole mechanism of action, given that SGTA abrogates receptor function in yeast lacking FKBP52 (Figs. 1–3).
The reported effects of SGTA on receptor function are variable. Buchanan et al. (16) demonstrated that SGTA negatively regulates AR in prostate cancer cells. Our data in mammalian and yeast-based reporter assays are consistent with these findings (Figs. 1–3). However, SGTA knockdown was recently shown to suppress AR-mediated gene expression in prostate cancer cells (26), which is opposite of what would be expected based on data from reporter assays. Buchanan et al. (16) also demonstrated that SGTA affects hormone-independent AR signaling in prostate cancer cells, suggesting that down-regulation of SGTA expression in late stage prostate cancer could contribute to the hormone refractory state. Although we did observe effects on basal AR activity in yeast and mammalian cell-based reporter assays, these effects were inconsistent, and no conclusions can be made regarding the effects of SGTA on hormone-independent receptor function from these studies. The nature and degree to which SGTA affects receptor activity could be dependent on the level of SGTA expression and/or on additional factors, the presence of which could vary between experimental systems.
Understanding the precise mechanism by which SGTA regulates receptor signaling pathways remains to be determined. SGTA can bind Hsp90, yet available evidence suggests that it is a more relevant factor in Hsp70 complexes. SGTA does inhibit hormone-induced receptor translocation to the nucleus and a model has been proposed in which the main role of SGTA is to prevent receptor from reaching the mature state where it can bind hormone and translocate to the nucleus (16). Much of our data would support this model. However, the role of SGTA in protein triage is intriguing. SGTA was recently shown to antagonize BAG6-mediated protein triage by promoting deubiquitination of substrates leading to the stabilization of mislocalized proteins (43). Although the role of SGTA in BAG6-mediated protein triage is independent of the TPR domain and interaction with Hsp70 and Hsp90, these findings provide a direct link between SGTA and a role in the stabilization of proteins. It is also important to note that SGTA is known to interact with ubiquitin-like domain (UBL)-containing proteins, including Get5/UBL4 and BAG6, leading to a direct role in the targeting of tail-anchored proteins to the endoplasmic reticulum in both yeast and mammalian cells (44, 45). In addition, yeast Sgt2 is known to interact with the J-domain protein Ydj1 indirectly through interaction with the UBL-containing protein Mdy2 (34). Based on this evidence, it is possible that SGTA affects the ubiquitination and recycling of the receptor and/or receptor regulatory proteins in the early stages of receptor folding in which Hsp70 is the central chaperone. Angeletti et al. (23) note that SGTA effects on Hsp70 most closely resemble those of CHIP, including sharing the same C-terminal binding site on Hsp70. However, SGTA lacks the U-box motif required for the protein degradation function of CHIP. Intriguingly, it is suggested that SGTA may act as a competitive inhibitor of CHIP-mediated proteolysis. We did consistently observe stabilized SGTA and FKBP52 protein levels when both proteins were coexpressed (Fig. 7, B and C), which provides the first evidence linking SGTA overexpression with the stability of receptor regulatory proteins. Taken as a whole, our data suggest a role for SGTA in early, Hsp70-mediated receptor folding events and suggest that SGTA may affect the stability of components of the receptor-chaperone complex leading to effects specifically on AR, GR, and PR signaling.
Acknowledgments
We thank Miguel Beato, Kaju Yanai, Chuck Miller, Chung Wang, Jonathan Warner, Didier Picard, Brian Freeman, and Elizabeth Craig for providing reagents and/or helpful advice. We thank the Border Biomedical Research Center's Biomolecule Analysis Core Facility, the Tissue Culture Core Facility, and the DNA Analysis Core Facility for the use of the instruments.
This work was supported, in whole or in part, by National Institutes of Health Grants 5 G12 RR008124, 8 G12 MD007592, SC1GM084863 (to M. B. C.), and R01 GM102443-01 (to A. C.).
- Hsp
- heat shock protein
- SGTA
- small glutamine-rich tetratricopeptide repeat-containing protein alpha
- TPR
- tetratricopeptide repeat
- GPD
- glyceraldehyde phosphate dehydrogenase
- AR
- androgen receptor
- PR
- progesterone receptor
- GR
- glucocorticoid receptor
- MR
- mineralocorticoid receptor
- ER
- estrogen receptor
- AMP-PNP
- adenosine 5′-(β,γ-imino)triphosphate.
REFERENCES
- 1. Echeverria P. C., Picard D. (2010) Molecular chaperones, essential partners of steroid hormone receptors for activity and mobility. Biochim. Biophys. Acta 1803, 641–649 [DOI] [PubMed] [Google Scholar]
- 2. Smith D. F., Toft D. O. (2008) The intersection of steroid receptors with molecular chaperones: observations and questions. Mol. Endocrinol. 22, 2229–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hernández M. P., Chadli A., Toft D. O. (2002) HSP40 binding is the first step in the HSP90 chaperoning pathway for the progesterone receptor. J. Biol. Chem. 277, 11873–11881 [DOI] [PubMed] [Google Scholar]
- 4. Chen S., Smith D. F. (1998) Hop as an adaptor in the heat shock protein 70 (Hsp70) and hsp90 chaperone machinery. J. Biol. Chem. 273, 35194–35200 [DOI] [PubMed] [Google Scholar]
- 5. Johnson B. D., Schumacher R. J., Ross E. D., Toft D. O. (1998) Hop modulates Hsp70/Hsp90 interactions in protein folding. J. Biol. Chem. 273, 3679–3686 [DOI] [PubMed] [Google Scholar]
- 6. Morishima Y., Kanelakis K. C., Silverstein A. M., Dittmar K. D., Estrada L., Pratt W. B. (2000) The Hsp organizer protein hop enhances the rate of but is not essential for glucocorticoid receptor folding by the multiprotein Hsp90-based chaperone system. J. Biol. Chem. 275, 6894–6900 [DOI] [PubMed] [Google Scholar]
- 7. Pratt W. B., Toft D. O. (2003) Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. (Maywood) 228, 111–133 [DOI] [PubMed] [Google Scholar]
- 8. Riggs D. L., Cox M. B., Cheung-Flynn J., Prapapanich V., Carrigan P. E., Smith D. F. (2004) Functional specificity of co-chaperone interactions with Hsp90 client proteins. Crit. Rev. Biochem. Mol. Biol. 39, 279–295 [DOI] [PubMed] [Google Scholar]
- 9. Storer C. L., Dickey C. A., Galigniana M. D., Rein T., Cox M. B. (2011) FKBP51 and FKBP52 in signaling and disease. Trends Endocrinol. Metab. 22, 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Echtenkamp F. J., Freeman B. C. (2012) Expanding the cellular molecular chaperone network through the ubiquitous cochaperones. Biochim. Biophys. Acta 1823, 668–673 [DOI] [PubMed] [Google Scholar]
- 11. Smith D. F. (2004) Tetratricopeptide repeat cochaperones in steroid receptor complexes. Cell Stress Chaperones 9, 109–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scheufler C., Brinker A., Bourenkov G., Pegoraro S., Moroder L., Bartunik H., Hartl F. U., Moarefi I. (2000) Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell 101, 199–210 [DOI] [PubMed] [Google Scholar]
- 13. Liu F. H., Wu S. J., Hu S. M., Hsiao C. D., Wang C. (1999) Specific interaction of the 70-kDa heat shock cognate protein with the tetratricopeptide repeats. J. Biol. Chem. 274, 34425–34432 [DOI] [PubMed] [Google Scholar]
- 14. McKeen H. D., McAlpine K., Valentine A., Quinn D. J., McClelland K., Byrne C., O'Rourke M., Young S., Scott C. J., McCarthy H. O., Hirst D. G., Robson T. (2008) A novel FK506-like binding protein interacts with the glucocorticoid receptor and regulates steroid receptor signaling. Endocrinology 149, 5724–5734 [DOI] [PubMed] [Google Scholar]
- 15. Chadli A., Graham J. D., Abel M. G., Jackson T. A., Gordon D. F., Wood W. M., Felts S. J., Horwitz K. B., Toft D. (2006) GCUNC-45 is a novel regulator for the progesterone receptor/hsp90 chaperoning pathway. Mol. Cell Biol. 26, 1722–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buchanan G., Ricciardelli C., Harris J. M., Prescott J., Yu Z. C., Jia L., Butler L. M., Marshall V. R., Scher H. I., Gerald W. L., Coetzee G. A., Tilley W. D. (2007) Control of androgen receptor signaling in prostate cancer by the cochaperone small glutamine rich tetratricopeptide repeat containing protein alpha. Cancer Res. 67, 10087–10096 [DOI] [PubMed] [Google Scholar]
- 17. Cziepluch C., Kordes E., Poirey R., Grewenig A., Rommelaere J., Jauniaux J. C. (1998) Identification of a novel cellular TPR-containing protein, SGT, that interacts with the nonstructural protein NS1 of parvovirus H-1. J. Virol. 72, 4149–4156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kordes E., Savelyeva L., Schwab M., Rommelaere J., Jauniaux J. C., Cziepluch C. (1998) Isolation and characterization of human SGT and identification of homologues in Saccharomyces cerevisiae and Caenorhabditis elegans. Genomics 52, 90–94 [DOI] [PubMed] [Google Scholar]
- 19. Liou S. T., Wang C. (2005) Small glutamine-rich tetratricopeptide repeat-containing protein is composed of three structural units with distinct functions. Arch. Biochem. Biophys. 435, 253–263 [DOI] [PubMed] [Google Scholar]
- 20. Trotta A. P., Need E. F., Butler L. M., Selth L. A., O'Loughlin M. A., Coetzee G. A., Tilley W. D., Buchanan G. (2012) Subdomain structure of the co-chaperone SGTA and activity of its androgen receptor client. J. Mol. Endocrinol. 49, 57–68 [DOI] [PubMed] [Google Scholar]
- 21. Ommen G., Chrobak M., Clos J. (2010) The co-chaperone SGT of Leishmania donovani is essential for the parasite's viability. Cell Stress Chaperones 15, 443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Worrall L. J., Wear M. A., Page A. P., Walkinshaw M. D. (2008) Cloning, purification and characterization of the Caenorhabditis elegans small glutamine-rich tetratricopeptide repeat-containing protein. Biochim. Biophys. Acta 1784, 496–503 [DOI] [PubMed] [Google Scholar]
- 23. Angeletti P. C., Walker D., Panganiban A. T. (2002) Small glutamine-rich protein/viral protein U-binding protein is a novel cochaperone that affects heat shock protein 70 activity. Cell Stress Chaperones 7, 258–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu S. J., Liu F. H., Hu S. M., Wang C. (2001) Different combinations of the heat-shock cognate protein 70 (hsc70) C-terminal functional groups are utilized to interact with distinct tetratricopeptide repeat-containing proteins. Biochem. J. 359, 419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Young J. C., Obermann W. M., Hartl F. U. (1998) Specific binding of tetratricopeptide repeat proteins to the C-terminal 12-kDa domain of hsp90. J. Biol. Chem. 273, 18007–18010 [DOI] [PubMed] [Google Scholar]
- 26. Trotta A. P., Need E. F., Selth L. A., Chopra S., Pinnock C. B., Leach D. A., Coetzee G. A., Butler L. M., Tilley W. D., Buchanan G. (2013) Knockdown of the cochaperone SGTA results in the suppression of androgen and PI3K/Akt signaling and inhibition of prostate cancer cell proliferation. Int. J. Cancer 133, 2812–2823 [DOI] [PubMed] [Google Scholar]
- 27. Goodarzi M. O., Xu N., Cui J., Guo X., Chen Y. I., Azziz R. (2008) Small glutamine-rich tetratricopeptide repeat-containing protein alpha (SGTA), a candidate gene for polycystic ovary syndrome. Hum. Reprod. 23, 1214–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Butler M. S., Yang X., Ricciardelli C., Liang X., Norman R. J., Tilley W. D., Hickey T. E. (2013) Small glutamine-rich tetratricopeptide repeat-containing protein alpha is present in human ovaries but may not be differentially expressed in relation to polycystic ovary syndrome. Fertil. Steril. 99, 2076–2083 [DOI] [PubMed] [Google Scholar]
- 29. Balsiger H. A., Cox M. B. (2009) Yeast-based reporter assays for the functional characterization of cochaperone interactions with steroid hormone receptors. Methods Mol. Biol. 505, 141–156 [DOI] [PubMed] [Google Scholar]
- 30. Cox M. B., Riggs D. L., Hessling M., Schumacher F., Buchner J., Smith D. F. (2007) FK506-binding protein 52 phosphorylation: a potential mechanism for regulating steroid hormone receptor activity. Mol. Endocrinol. 21, 2956–2967 [DOI] [PubMed] [Google Scholar]
- 31. Liu J. W., Picard D. (1998) Bioactive steroids as contaminants of the common carbon source galactose. FEMS Microbiol. Lett. 159, 167–171 [DOI] [PubMed] [Google Scholar]
- 32. Picard D., Khursheed B., Garabedian M. J., Fortin M. G., Lindquist S., Yamamoto K. R. (1990) Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature 348, 166–168 [DOI] [PubMed] [Google Scholar]
- 33. Patwardhan C. A., Fauq A., Peterson L. B., Miller C., Blagg B. S., Chadli A. (2013) Gedunin inactivates the co-chaperone p23 protein causing cancer cell death by apoptosis. J. Biol. Chem. 288, 7313–7325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liou S. T., Cheng M. Y., Wang C. (2007) SGT2 and MDY2 interact with molecular chaperone YDJ1 in Saccharomyces cerevisiae. Cell Stress Chaperones 12, 59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sivils J. C., Storer C. L., Galigniana M. D., Cox M. B. (2011) Regulation of steroid hormone receptor function by the 52-kDa FK506-binding protein (FKBP52). Curr. Opin. Pharmacol. 11, 314–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Niu W., Li Z., Zhan W., Iyer V. R., Marcotte E. M. (2008) Mechanisms of cell cycle control revealed by a systematic and quantitative overexpression screen in S. cerevisiae. PLoS Genet. 4, e1000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Laschober G. T., Ruli D., Hofer E., Muck C., Carmona-Gutierrez D., Ring J., Hutter E., Ruckenstuhl C., Micutkova L., Brunauer R., Jamnig A., Trimmel D., Herndler-Brandstetter D., Brunner S., Zenzmaier C., Sampson N., Breitenbach M., Fröhlich K. U., Grubeck-Loebenstein B., Berger P., Wieser M., Grillari-Voglauer R., Thallinger G. G., Grillari J., Trajanoski Z., Madeo F., Lepperdinger G., Jansen-Dürr P. (2010) Identification of evolutionarily conserved genetic regulators of cellular aging. Aging Cell 9, 1084–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Breslow D. K., Cameron D. M., Collins S. R., Schuldiner M., Stewart-Ornstein J., Newman H. W., Braun S., Madhani H. D., Krogan N. J., Weissman J. S. (2008) A comprehensive strategy enabling high-resolution functional analysis of the yeast genome. Nat. Methods 5, 711–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kohl C., Tessarz P., von der Malsburg K., Zahn R., Bukau B., Mogk A. (2011) Cooperative and independent activities of Sgt2 and Get5 in the targeting of tail-anchored proteins. Biol. Chem. 392, 601–608 [DOI] [PubMed] [Google Scholar]
- 40. Michaillat L., Mayer A. (2013) Identification of genes affecting vacuole membrane fragmentation in Saccharomyces cerevisiae. PLoS One 8, e54160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tobaben S., Thakur P., Fernández-Chacón R., Südhof T. C., Rettig J., Stahl B. (2001) A trimeric protein complex functions as a synaptic chaperone machine. Neuron 31, 987–999 [DOI] [PubMed] [Google Scholar]
- 42. Philp L. K., Butler M. S., Hickey T. E., Butler L. M., Tilley W. D., Day T. K. (2013) SGTA: a new player in the molecular co-chaperone game. Horm. Cancer 4, 343–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leznicki P., High S. (2012) SGTA antagonizes BAG6-mediated protein triage. Proc. Natl. Acad. Sci. U.S.A. 109, 19214–19219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu Y., Cai M., Yang Y., Huang L., Ye Y. (2012) SGTA recognizes a noncanonical ubiquitin-like domain in the Bag6-Ubl4A-Trc35 complex to promote endoplasmic reticulum-associated degradation. Cell Rep. 2, 1633–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Leznicki P., Roebuck Q. P., Wunderley L., Clancy A., Krysztofinska E. M., Isaacson R. L., Warwicker J., Schwappach B., High S. (2013) The association of BAG6 with SGTA and tail-anchored proteins. PLoS One 8, e59590. [DOI] [PMC free article] [PubMed] [Google Scholar]