Background: hMps1 and CHK2 are both required to safeguard the mitotic checkpoint, and yet the relationship between the two proteins is unclear.
Results: hMps1 Thr-288 mutation causes misaligned chromosomes and polyploidy as a result of defective kinetochore localization.
Conclusion: Phosphorylation of hMps1 Thr-288 by CHK2 facilitates kinetochore localization of hMps1.
Significance: The study provides insights on the involvement of CHK2 in SAC through hMps1.
Keywords: Checkpoint Control, Chromosomes, Kinetochore, Mitosis, Phosphorylation, CHK2, HEC1, Mps1, Mitotic Checkpoint
Abstract
Human Mps1 (hMps1) is a mitotic checkpoint kinase responsible for sensing the unattached and tensionless kinetochore. Despite its importance in safeguarding proper chromosome segregation, how hMps1 is recruited to the kinetochore remains incompletely understood. Here, we demonstrate that phosphorylation at Thr-288 by the cell cycle checkpoint kinase CHK2 is involved in this process. We discovered that the phosphorylation-deficient T288A mutant has an impaired ability to localize to the kinetochore and cannot reestablish the mitotic checkpoint in hMps1-depleted cells. In support, we found that nocodazole induced hMps1 phosphorylation at the previously identified CHK2 site Thr-288 and that this could be detected at the kinetochore in a CHK2-dependent manner. Mechanistically, phosphorylation at Thr-288 promoted the interaction with the KMN (KNL1-Mis12-Ndc80 network) protein HEC1. Forced kinetochore localization corrected the defects associated with the T288A mutant. Our results provide evidence of a newly identified hMps1 phosphorylation site that is involved in the mitotic checkpoint and that CHK2 contributes to chromosomal stability through hMps1.
Introduction
Faithful and even segregation of sister chromatids in mitosis is pivotal for maintenance of chromosome stability. In mammals, this is controlled by the spindle assembly checkpoint (SAC),3 which is activated by the unattached kinetochore, and an error correction mechanism triggered by the tensionless kinetochore to correct erroneous kinetochore-microtubule attachments (1). Among the mitotic checkpoint regulators, Mps1 protein kinase functions downstream of Aurora B and HEC1 and is responsible for the activation of downstream events such as the recruitment of BUB1, BUB3, MAD1, and MAD2, and subsequent activation of the mitotic checkpoint complex, which sequesters the anaphase-promoting complex cofactor Cdc20, resulting in mitotic arrest (2). Exactly how Mps1 is recruited to the kinetochore is not fully understood. Evidence has been presented that HEC1 phosphorylation by Aurora B is involved (3); however, a model of indirect regulation was also proposed because Aurora B does not appear to phosphorylate HEC1 or Mps1 directly (4).
CHK2 is a serine/threonine protein kinase involved in cell cycle checkpoint control in the response to DNA damage and in DNA repair via phosphorylation of substrates such as Cdc25, p53, and BRCA1 (5–7). Our understanding of its function was further expanded by the discovery that the kinase is required for proper progression of mitosis and chromosomal stability through phosphorylation of BRCA1 (8). Thus, CHK2 depletion is associated with an abnormal SAC and chromosome mis-segregation (8). In addition to the upstream kinase ATM, we have shown previously that CHK2 can be phosphorylated at threonine 68 and can be activated by the SAC kinase hMps1 (human Mps1) in response to DNA damage (9). Interestingly, CHK2 can also phosphorylate hMps1 at threonine 288 and can regulate the stability of the kinase, thus forming a positive feedback loop (10).
In this report, we explored the role of the CHK2-hMps1 circuitry in mitosis. We demonstrate the involvement of CHK2-mediated hMps1 Thr-288 phosphorylation in the regulation of hMps1 kinetochore localization and in the maintenance of chromosome stability during mitosis.
EXPERIMENTAL PROCEDURES
Cell Culture
HeLa and 293T cells were maintained in Dulbecco's modified Eagle's medium (HyClone, Logan, UT) supplemented with 10% fetal bovine serum (HyClone), 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Cells were treated with nocodazole (50 ng/ml) overnight for 16 to 18 h and then harvested as indicated. The HeLa Tet-Off cell lines WT-r-9 and T288A-r-16, which express siRNA-resistant WT or T288A hMps1, were grown and maintained as described previously (10).
Immunoblotting
Cell lysates were prepared by lysing the cells in TEGN buffer (10 mm Tris-HCl, pH 7.5, 1 mm EDTA, 400 mm NaCl, 0.5% Nonidet P-40, and 1 mm DTT) supplemented with protease and phosphatase inhibitors. The antibodies used were anti-actin (A2066, Sigma-Aldrich), anti-CHK2 (K0087, MBL, Nagoya, Japan), anti-hMps1 (9), and anti-HA (HA-11, Covance, Berkeley, CA).
Immunofluorescence
For kinetochore protein immunostaining, HeLa cells grown on coverslips were extracted and fixed in 4% formaldehyde buffer containing 0.5% Triton X-100, 20 mm PIPES, 10 mm EGTA, and 1 mm MgCl2 for 10 min. Cells were blocked in 3% bovine serum albumin in PBS at room temperature for 45 min and then incubated with primary antibodies for 2 h at room temperature. The antibodies used for immunofluorescence were mouse anti-HA (HA-11, Covance), rabbit anti-hMps1 (9), mouse anti-BubR1 (K0169-3, MBL), rabbit anti-CENP-B (sc-22788, Santa Cruz Biotechnology, Dallas, TX), mouse anti-CENP-B (sc-32285, Santa Cruz Biotechnology), and rabbit anti-phospho-threonine 288 (10). An FITC-conjugated goat anti-rabbit IgG antibody and a TRITC-conjugated goat anti-mouse IgG antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) or a DyLight 488-conjugated goat anti-mouse IgG antibody and a TRITC-conjugated goat anti-rabbit IgG antibody (Jackson ImmunoResearch Laboratories) were used as secondary antibodies. DNA was counterstained with DAPI. Images were obtained using a Carl Zeiss LSM700 confocal microscope (Carl Zeiss, Inc., Oberkochen, Germany).
Plasmids and Transfection
Plasmids expressing hMps1 WT or T288A and the corresponding siRNA-resistant constructs have been described previously (10). To generate Mis12 fusion protein, the cDNA encoding Mis12 was cloned into the BamHI site of the pXJ-HA-hMps1 vectors to express HA-tagged Mis12-hMps1. Turbofect transfection reagent (Thermo, Rockford, IL) was used to transfect HA-hMps1 and its derivatives.
siRNA Transfection
All siRNAs were synthesized by and purchased from Sigma-Aldrich. The sequences targeted by hMps1 and CHK2 siRNA were 5′-TGAACAAAGTGAGAGACAT-3′ (TK1), and 5′-TGTGTGAATGACAACTACT-3′ (CHK2–3) or 5′-GTTGTTGGTAGTGGATCCA-3′ (CHK2–4), respectively. Transfection of siRNA was performed using Oligofectamine (Invitrogen) in HeLa cells plated at 5 × 104 cells per 35-mm dish on the day before transfection.
Flow Cytometry
For cell cycle analysis, cells were detached using trypsin and collected in culture medium by centrifugation. Cells were fixed in cold methanol and stored at −20 °C for at least 2 h. Cells were rehydrated with cold PBS and resuspended in PBS containing 60 μg/ml propidium iodide and 50 μg/ml RNase A. Samples were analyzed using a BD FACSCanto flow cytometer (BD Biosciences), and the data were analyzed using CellQuest Pro (BD Biosciences).
RESULTS
Cells Expressing the hMps1 T288A Mutant Exhibit Defective Mitotic Arrest and Increased Polyploidy
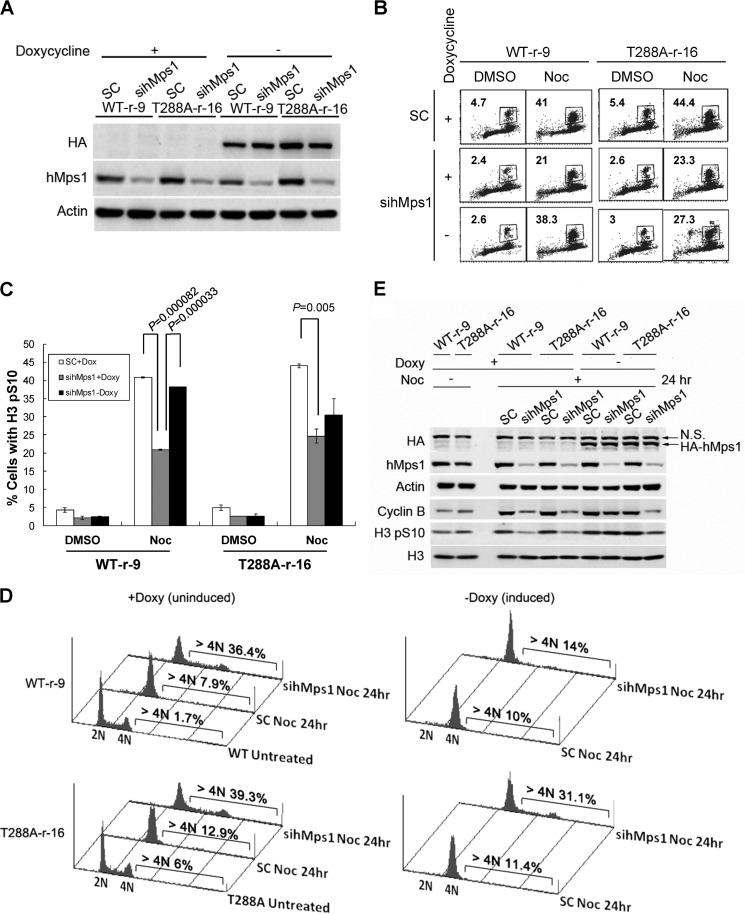
To determine whether Thr-288 phosphorylation is involved in the spindle checkpoint function of hMps1, we utilized the stable Tet-Off HeLa cells inducibly express the WT or the T288A mutant of hMps1; in the latter, Thr-288 has been changed to alanine (10). We first down-regulated endogenous hMps1 with a specific small interfering RNA (siRNA) and then induced the expression of HA-tagged WT hMps1 or the T288A mutant by withdrawing doxycycline. Immunoblot analysis confirmed that the expression of hMps1 was regulated as expected (Fig. 1A). These cells were then subjected to nocodazole treatment, and the mitotic arrest was analyzed by flow cytometry for histone H3 Ser-10 phosphorylation (H3 pSer-10), an M phase marker. Depletion of hMps1 abrogated the SAC, as evidenced by a reduced H3 pSer-10-positive population. Reexpression of WT but not the T288A mutant effectively rescued the checkpoint arrest (Fig. 1, B and C).
FIGURE 1.
Defective mitotic checkpoint and increased polyploidy in HeLa cells expressing the hMps1 T288A mutant. A, HeLa Tet-Off cells expressing siRNA-resistant WT or T288A hMps1 upon withdrawal of doxycycline (Doxy or Dox). Cells were transfected with control (SC) or hMps1 siRNA in the presence or absence of doxycycline. Lysates were collected 2 days later and subjected to immunoblot analysis using the antibodies indicated. B and C, impaired mitotic checkpoint in hMps1 T288A-expressing HeLa cells. Cells transfected with siRNA as described in A were treated with nocodazole (Noc) overnight, stained with anti-histone H3 pSer-10 antibody (H3 pS10) and then subjected to flow cytometry analysis. Results from a representative assay are shown in B. The mean ± S.D. of the positive-staining population from three independent experiments is displayed in C. D and E, increased polyploidy in nocodazole-treated T288A cells. Cells transfected as above were treated with nocodazole for 24 h and then collected for cell cycle analysis (D). Cell lysates were also prepared for immunoblot analysis using the antibodies indicated (E). Shown are results from one representative experiment. Similar results were obtained in two additional independent experiments. DMSO, dimethyl sulfoxide.
A dysfunctional SAC has been shown to result in polyploidy, which is linked to aneuploidy and tumorigenesis (11, 12). Because the T288A mutant was unable to restore the SAC (Fig. 1C), we then tested whether the ploidy of these cells would be affected after challenge with nocodazole. Consistent with the defective mitotic arrest, more of the T288A-expressing cells acquired greater than 4 n DNA content compared with the WT hMps1-expressing cells (Fig. 1D). These cells also accumulated less cyclin B and H3 pSer-10 compared with cells restored by WT hMps1 (Fig. 1E), further indicative of a failed SAC. Taken together, these results strongly suggest a role of Thr-288 phosphorylation in the proper execution of the checkpoint upon spindle disruption.
Chromosome Alignment Is Impaired in hMps1 T288A-expressing Cells
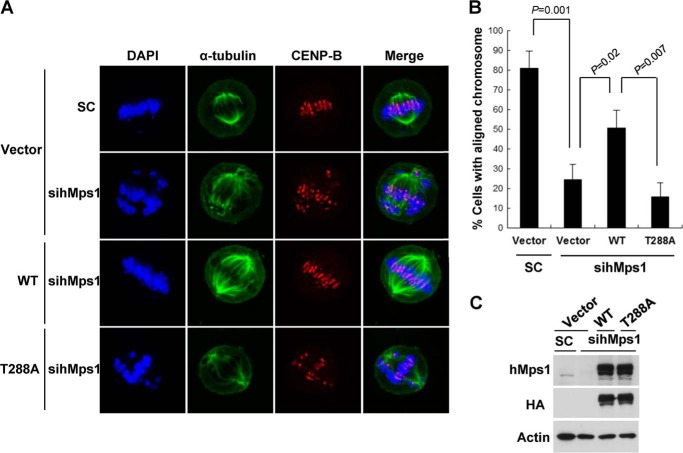
Because hMps1 is involved in sensing kinetochore attachment of the spindle and consequently in chromosome alignment and segregation (2), we sought to determine whether the T288A-expressing cells, shown above to be defective in mitotic arrest, exhibit any abnormality in chromosome arrangement. The MG132-arrested cells were costained with CENP-B (to identify the kinetochore) and α-tubulin (to identify the spindle) in addition to 4,6-diamidino-2-phenylindole (DAPI), which stains the condensed chromosomes. As reported previously (13), down-regulation of hMps1 increased the number of abnormal spindles and misaligned chromosomes (Fig. 2, A and B). This defect was reversed by expression of siRNA-resistant WT hMps1 but not by the T288A mutant, although both were expressed in equivalent amounts (Fig. 2C). These results confirm the importance of Thr-288 phosphorylation in normal spindle function and chromosome alignment.
FIGURE 2.
Re-expression of WT hMps1 but not of the T288A mutant rescues the chromosome alignment defects in hMps1-depleted cells. A and B, the chromosome alignment defect in hMps1 down-regulated cells was rescued by WT but not by T288A hMps1. HeLa cells were first transfected with siRNA then the next day with plasmid DNA expressing HA-hMps1. To arrest cells in mitosis, cells were treated with 10 μg/ml MG132 for 1 h before fixation. Anti-α-tubulin, anti-CENP-B, and DAPI were used to stain spindle microtubules, the kinetochore, and chromosomes, respectively (A). The percent of mitotic cells with properly aligned chromosomes is shown in B as mean ± S.D. from three independent experiments. C, expression of HA-hMps1 WT or T288A in hMps1-depleted HeLa cells.
hMps1 Thr-288 Phosphorylation Regulates Kinetochore Localization
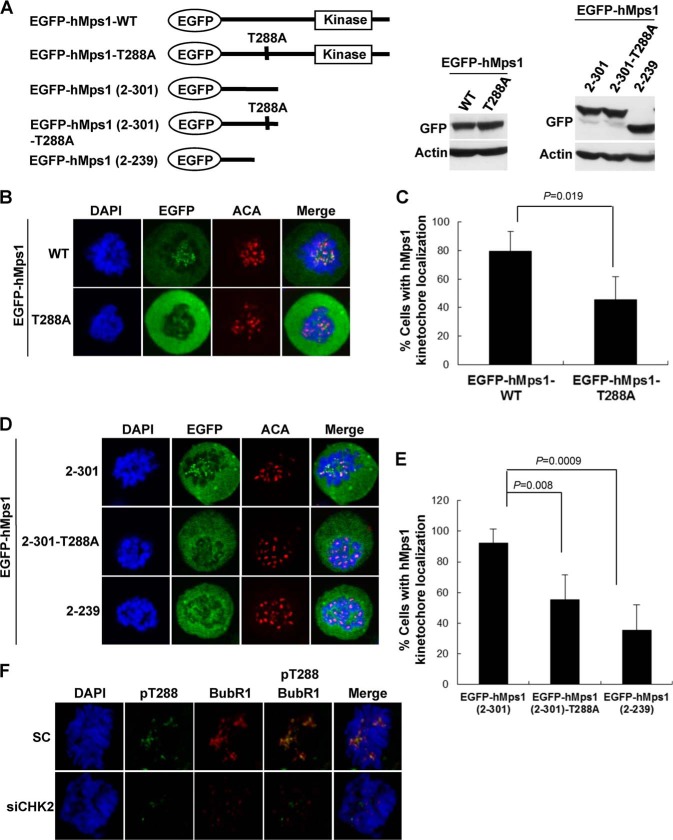
Human Mps1 is known to be recruited to the kinetochore when cells are exposed to a spindle poison (14). To determine whether the abnormalities in T288A-expressing cells described above originated from defective kinetochore localization, we used confocal microscopy to compare the kinetochore localization of EGFP-hMps1 WT and EGFP-hMps1 T288A. As shown in Fig. 3A, both constructs were expressed efficiently. Whereas the EGFP-hMps1 WT protein colocalized with the kinetochore marker ACA, EGFP-hMps1 T288A was inefficient in doing so (Fig. 3B). As shown in Fig. 3C, the EGFP-hMps1 T288A mutant was ∼50% as efficient as the EGFP-hMps1 WT protein in localizing to the kinetochore in this overexpression system.
FIGURE 3.
Kinetochore localization of hMps1 is disrupted by T288A mutation. A, schematic representations and expression of EGFP-hMps1 fusion constructs in HeLa cells. B and C, the hMps1 T288A mutant is defective in kinetochore localization. HeLa cells transfected with either EGFP-hMps1 WT or EGFP-hMps1 T288A were fixed, and their colocalization with the kinetochore marker was assessed by confocal microscopy following staining with the anti-centromere antibodies (ACA) (B). The percent of mitotic cells with EGFP colocalized with the staining of the anti-centromere antibodies is shown in C as mean ± S.D. from three independent experiments. D and E, T288A mutation in the context of the N-terminal kinetochore localization domain (residues 2–301) attenuates kinetochore localization. F, kinetochore-localized hMps1 is phosphorylated at Thr-288 in a CHK2-dependent manner. HeLa cells were transfected with control or CHK2-targeting siRNA for ∼30 h and then treated with nocodazole (Noc) for 16 h. Cells were costained with anti-phospho-Thr-288 (pT288) and anti-BubR1 antibodies and examined by confocal microscopy. EGFP, enhanced GFP.
The kinetochore localization of hMps1 is mediated by the N-terminal domain (residues 1–300) (15, 16). We next used the EGFP-hMps1 (2–301) construct to test whether Thr-288 phosphorylation is also important in the context of the minimal domain. Similar to what we observed with the full-length construct, the EGFP-hMps1 T288A (2–301) protein also exhibited impaired kinetochore localization compared with its WT counterpart (Fig. 3, D and E). This result suggests that the effect of modification at Thr-288 is independent of the kinase domain of hMps1.
The impairments we observed with the T288A mutant suggested that hMps1 must be phosphorylated at Thr-288 for correct kinetochore localization. To demonstrate directly that kinetochore hMps1 is phosphorylated at Thr-288, we used a phospho-Thr-288-specific Mps1 antibody that we had raised previously in rabbits (10). Confocal microscopy showed the colocalization of pThr-288 staining with BubRI, a checkpoint protein localized at the kinetochore (Fig. 3F). Down-regulation of CHK2 greatly reduced the pThr-288 staining concomitant with decreased BubRI kinetochore localization (Fig. 3F), suggesting that CHK2 acts as an hMps1 Thr-288 kinase during mitosis in addition to its action in the DNA damage response (10).
Forced Localization of the T288A Mutant to the Kinetochore Corrects the Chromosome Alignment Defect
Spindle attachment to the kinetochore requires the KNL1-Mis12-Ndc80 (KMN) network complex, including proteins KNL-1, Mis12, and HEC1/Ndc80 (Fig. 4A). Because HEC1 is required for Mps1 kinetochore localization (17), we first determined whether the T288A mutation would affect the interaction with HEC1. HA-tagged WT or T288A hMps1 was coexpressed with Myc-tagged HEC1 in 293T cells, and the interactions were assessed by coimmunoprecipitation using anti-hMps1 antibody. The results in Fig. 4B show that the interaction between hMps1 and HEC1 was increased by nocodazole treatment and that the interaction was diminished significantly by T288A mutation. By contrast, interaction of hMps1 with the inner kinetochore protein CENP-B was not affected (Fig. 4C). These results suggest that the kinetochore localization defect of the T288A mutant can be attributed at least in part to reduced HEC1 interaction.
FIGURE 4.
The hMps1 T288A mutant is defective in interaction with the kinetochore protein HEC1. A, schematic representation of the kinetochore-microtubule KNL1-Mis12-Ndc80 (KMN) network. B, the interaction between hMps1 and HEC1 is compromised by the T288A mutation. HA-hMps1 and Myc-HEC1 were coexpressed in 293T cells, and immunoprecipitation was performed with anti-hMps1. Coimmunoprecipitated Myc-HEC1 was detected with anti-Myc antibody. C, T288A mutation does not affect the interaction between hMps1 and the inner kinetochore protein CENP-B. Coimmunoprecipitation was performed as in (B) but using Myc-CENP-B. D, expression of the fusion proteins Mis12-hMps1 WT and T288A and HA-hMps1 T288A in HeLa cells. E and F, kinetochore localization defect of the T288A mutant can be corrected by fusion with the KMN network protein Mis12. HeLa cells were transfected with the constructs indicated, and kinetochore localization was evaluated by costaining with anti-HA and anti-CENP-B antibodies. Quantitative results from two independent experiments were averaged and are shown in F. G and H, forced kinetochore localization rescues the chromosome segregation defect of the T288A mutant. Transfected cells were arrested with 100 μm monastrol for 8 h and then released. Cells were fixed 1 h later for chromosome alignment (G) or 1.5 h later for chromosome segregation (H). The misaligned population was counted and the mean ± S.D. from three independent experiments is shown. *, p < 0.05; **, p < 0.01. Noc, nocodazole.
If the defects observed with the T288A mutant were due to its inability to localize to the kinetochore, we would expect that forced kinetochore localization would correct these defects. We therefore generated a chimera protein with Mis12 fused to the N terminus of hMps1. As shown in Fig. 4D, the proteins expressed were of the correct size. Confocal microscopy (Fig. 4E) showed that the Mis12-hMps1 T288A protein localized to the kinetochore almost as efficiently as the Mis12-WT hMps1 protein and more efficiently than the T288A hMps1 protein (Fig. 4F), even though a similar amount of the latter was expressed (Fig. 4D).
Next, we examined chromosome alignment in Mis12-hMps1 T288A-expressing cells. Cells expressing various hMps1 constructs were arrested with monastrol and then released to form bipolar spindles. Consistent with the kinetochore localization results, expression of Mis12-hMps1 T288A in hMps1-depleted cells corrected the chromosome misalignment defect, whereas the hMps1 T288A protein could not correct the defect (Fig. 4G). Similar results were obtained when lagging chromosomes were examined in anaphase (Fig. 4H). Taken together, our data strongly suggest that the mitotic checkpoint defect associated with the T288A mutant reflects its inability to localize to the kinetochore.
CHK2-mediated Phosphorylation Promotes Localization of hMps1 to the Kinetochore
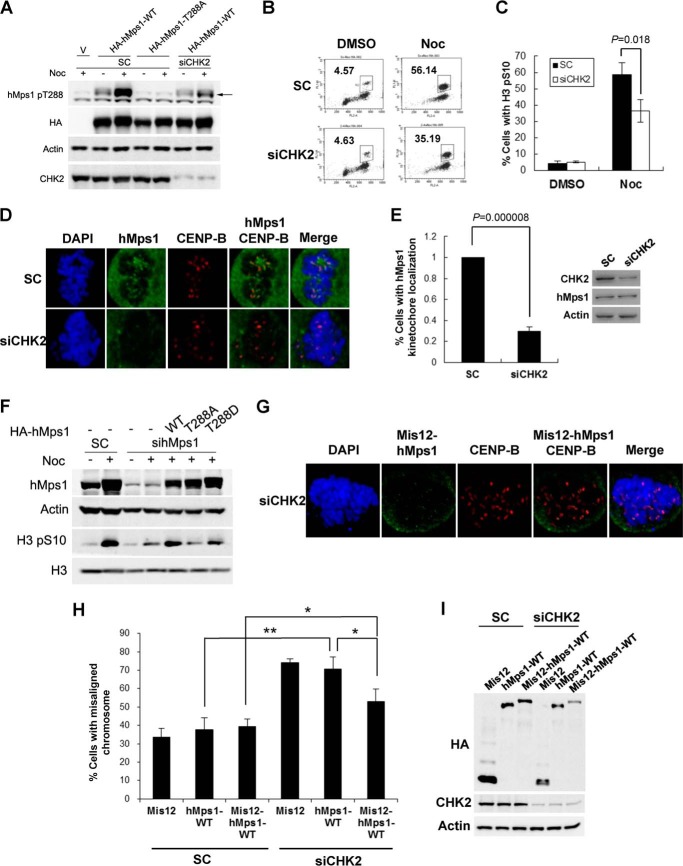
We demonstrated previously that CHK2 phosphorylates hMps1 at Thr-288 during the DNA damage response (10). Because CHK2 is activated in mitosis and upon spindle disruption (8), we wondered whether CHK2 is the kinase responsible for hMps1 Thr-288 phosphorylation in M phase. To address this issue, we first used specific siRNA to down-regulate endogenous CHK2 in HeLa cells. As shown in Fig. 5A, nocodazole treatment increased hMps1 Thr-288 phosphorylation, and this increase was attenuated in CHK2-depleted cells, suggesting that CHK2 is involved in Thr-288 phosphorylation in mitosis-arrested cells. Furthermore, CHK2 knockdown caused defective mitotic arrest (Fig. 5B), as demonstrated by reduced H3 pSer-10 staining (Fig. 5, B and C). Importantly, kinetochore localization of hMps1 was also reduced markedly in CHK2 knockdown cells (Fig. 5, D and E); this finding is consistent with the requirement for Thr-288 phosphorylation shown in the T288A mutant (Fig. 3, B and C). Additionally, we compared the activity of WT hMps1 and the phospho-mimicking mutant T288D in restoring the mitotic checkpoint in hMps1-depleted cells. Our data indicated that the T288D mutant was not as efficient as the WT protein, as evidenced by the levels of H3 pSer-10 (Fig. 5F). This result suggests that increased negative charge may not represent fully the impact of phosphorylation.
FIGURE 5.
CHK2-dependent Thr-288 phosphorylation facilitates kinetochore localization of hMps1. A, nocodazole (Noc)-induced hMps1 Thr-288 phosphorylation is diminished in CHK2-depleted HeLa cells. Cells were transfected with control or CHK2 siRNA and then hMps1 the next day. Cells were treated with nocodazole for 16 h, and lysates were prepared and analyzed by Western blotting using the antibodies indicated. B and C, CHK2 down-regulation impairs mitotic arrest. HeLa cells transfected with control or CHK2 siRNA were synchronized with thymidine and then released into the medium containing nocodazole overnight. Cells were collected, stained with the antibody against the M phase marker H3 pSer-10, and analyzed by flow cytometry (B). The percent positive staining population from three experiments is shown as mean ± S.D. in C. D and E, CHK2 depletion attenuates hMps1 kinetochore localization. HeLa cells transfected with control (sc) or CHK2 siRNA were treated with nocodazole overnight. Cells were then fixed and stained with anti-hMps1 and anti-CENP-B antibodies, and examined by confocal microscopy (D). Levels of colocalization were normalized to the small interfering control (SC) and are shown in E as mean ± S.D. from three independent experiments. Also shown in E are Western blots of whole cell lysates using the antibodies indicated. F, the activity of the phospho-mimicking mutant T288D in reestablishing the mitotic checkpoint. HeLa cells were first transfected with hMps1 siRNA then with WT, T288A, or T288D hMps1 on the next day. Cells were either treated with or without nocodazole, and lysates were analyzed by Western blotting using the antibodies indicated. G–I, forced kinetochore localization of hMps1 by Mis12 fusion partially rescues the chromosome alignment defect in CHK2-depleted cells. Hela cells were first depleted of CHK2 by CHK2 siRNA then transfected with HA-tagged Mis12, hMps1-WT, or Mis12-hMps1-WT fusion. Localization of Mis12-hMps1 was verified by costaining with anti-CENP-B followed by confocal microscopy (G). Chromosome alignment in transfected cells was examined as in Fig. 4G, and the results are shown in (H) as mean ± S.D. from three independent experiments. *, p < 0.05; **, p < 0.01. Relative expression of the constructs was examined by Western blotting (I). DMSO, dimethyl sulfoxide.
To determine whether forced localization of hMps1 to the kinetochore could rescue the chromosome alignment defect observed in CHK2 knockdown cells, we transiently expressed Mis12, hMps1 WT, or Mis12-hMps1 WT in these cells. Neither Mis12 nor hMps1 WT could restore chromosome alignment to the level as in small interfering control cells. However, the defect in CHK2-depleted cells was partially rescued by Mis12-hMps1 fusion (Fig. 5, G and I), suggesting that CHK2 regulates the SAC at least in part through modulation of hMps1 kinetochore localization.
DISCUSSION
Collectively, our data show the importance of hMps1 Thr-288 phosphorylation in hMps1 kinetochore localization, maintenance of the SAC, and chromosome segregation. Our data are consistent with a model in which, during mitosis, CHK2 phosphorylates hMps1 at Thr-288 to promote its interaction with HEC1 on the unattached kinetochore, thus facilitating its localization to the kinetochore where hMps1 activates the SAC and safeguards chromosome attachment. Failure to do so, for example, in the T288A mutant, would lead to chromosome misalignment and impaired mitotic arrest, culminating in polyploidy and genome instability (12). In addition to being a CHK2 site (10), Thr-288 was previously reported to be an hMps1 autophosphorylation site (18). We do not think that autophosphorylation is involved here because this would contradict a recent finding by Thebault et al. (19) that inhibition of hMps1 kinase activity promotes its kinetochore localization. In addition, our data with the EGFP-hMps1 (2–301) protein (Fig. 3, D and E) also suggest that the C-terminal kinase domain is dispensable for Thr-288 phosphorylation.
Although the role of CHK2 was first defined as a replication checkpoint regulator in yeast (20), its functions have since multiplied in mammals to include various DNA damage checkpoints (21). More recently, its involvement in the mitotic checkpoint was unveiled. Stolz et al. (8) showed that CHK2 participates in maintaining chromosome stability through phosphorylation of BRCA1. Our findings here add another layer of complexity by linking CHK2 directly to the spindle checkpoint kinase hMps1, making CHK2 an essential component of the SAC machinery. Our observations that CHK2 down-regulated mitotic cells exhibited not only reduced hMps1 Thr-288 phosphorylation but also the checkpoint defects similar to those of the T288A mutant cells suggest that CHK2 likely affects hMps1 directly by phosphorylation. Additionally, our data suggest that hMps1 may not be the only effector downstream of CHK2, as forced localization of hMps1 to the kinetochore reduced but not completely rescued the chromosome misalignment defect (Fig. 5H). How CHK2 is activated in mitosis remains unclear. ATM has been shown to be activated by default or by Aurora B in mitosis (22, 23) and therefore might phosphorylate and activate CHK2. Alternatively, hMps1 itself may act as the CHK2 activator in mitotic cells, as we have shown previously that hMps1 can phosphorylate and activate CHK2 (9). In this scenario, initial activation of hMps1 may drive the activation of CHK2, which in turn reinforces and stabilizes hMps1 function by phosphorylating the kinase on Thr-288. Much work is needed to test this autoregulatory circuitry and its relationship with other regulators such as Aurora B, which is known to also affect hMps1 kinetochore localization.
This work was supported by Academia Sinica and National Science Council Taiwan.
- SAC
- spindle assembly checkpoint
- hMps1
- human monopolar spindle 1
- CHK2
- checkpoint kinase 2
- TRITC
- tetramethyl rhodamine isothiocyanate
- KMN
- KNL1-Mis12-Ndc80.
REFERENCES
- 1. Foley E. A., Kapoor T. M. (2013) Microtubule attachment and spindle assembly checkpoint signaling at the kinetochore. Nat. Rev. Mol. Cell Biol. 14, 25–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu X., Winey M. (2012) The MPS1 family of protein kinases. Annu. Rev. Biochem. 81, 561–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu T., Dou Z., Qin B., Jin C., Wang X., Xu L., Wang Z., Zhu L., Liu F., Gao X., Ke Y., Wang Z., Aikhionbare F., Fu C., Ding X., Yao X. (2013) Phosphorylation of microtubule-binding protein Hec1 by mitotic kinase Aurora B specifies spindle checkpoint kinase Mps1 signaling at the kinetochore. J. Biol. Chem. 288, 36149–36159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nijenhuis W., von Castelmur E., Littler D., De Marco V., Tromer E., Vleugel M., van Osch M. H., Snel B., Perrakis A., Kops G. J. (2013) A TPR domain-containing N-terminal module of MPS1 is required for its kinetochore localization by Aurora B. J. Cell Biol. 201, 217–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahn J., Urist M., Prives C. (2004) The Chk2 protein kinase. DNA Repair 3, 1039–1047 [DOI] [PubMed] [Google Scholar]
- 6. Zhang J., Willers H., Feng Z., Ghosh J. C., Kim S., Weaver D. T., Chung J. H., Powell S. N., Xia F. (2004) Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair. Mol. Cell. Biol. 24, 708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang H. C., Chou W. C., Shieh S. Y., Shen C. Y. (2006) Ataxia telangiectasia mutated and checkpoint kinase 2 regulate BRCA1 to promote the fidelity of DNA end-joining. Cancer Res. 66, 1391–1400 [DOI] [PubMed] [Google Scholar]
- 8. Stolz A., Ertych N., Kienitz A., Vogel C., Schneider V., Fritz B., Jacob R., Dittmar G., Weichert W., Petersen I., Bastians H. (2010) The CHK2-BRCA1 tumour suppressor pathway ensures chromosomal stability in human somatic cells. Nat. Cell Biol. 12, 492–499 [DOI] [PubMed] [Google Scholar]
- 9. Wei J. H., Chou Y. F., Ou Y. H., Yeh Y. H., Tyan S. W., Sun T. P., Shen C. Y., Shieh S. Y. (2005) TTK/hMps1 participates in the regulation of DNA damage checkpoint response by phosphorylating CHK2 on Threonine 68. J. Biol. Chem. 280, 7748–7757 [DOI] [PubMed] [Google Scholar]
- 10. Yeh Y. H., Huang Y. F., Lin T. Y., Shieh S. Y. (2009) The cell cycle checkpoint kinase CHK2 mediates DNA damage-induced stabilization of TTK/hMps1. Oncogene 28, 1366–1378 [DOI] [PubMed] [Google Scholar]
- 11. Fang X., Zhang P. (2011) Aneuploidy and tumorigenesis. Semin. Cell Dev. Biol. 22, 595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ganem N. J., Storchova Z., Pellman D. (2007) Tetraploidy, aneuploidy and cancer. Curr. Opin. Genet. Dev. 17, 157–162 [DOI] [PubMed] [Google Scholar]
- 13. Jelluma N., Brenkman A. B., van den Broek N. J., Cruijsen C. W., van Osch M. H., Lens S. M., Medema R. H., Kops G. J. (2008) Mps1 phosphorylates Borealin to control Aurora B activity and chromosome alignment. Cell 132, 233–246 [DOI] [PubMed] [Google Scholar]
- 14. Stucke V. M., Silljé H. H., Arnaud L., Nigg E. A. (2002) Human Mps1 kinase is required for the spindle assembly checkpoint but not for centrosome duplication. EMBO J. 21, 1723–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu S. T., Chan G. K., Hittle J. C., Fujii G., Lees E., Yen T. J. (2003) Human MPS1 kinase is required for mitotic arrest induced by the loss of CENP-E from kinetochores. Mol. Biol. Cell 14, 1638–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stucke V. M., Baumann C., Nigg E. A. (2004) Kinetochore localization and microtubule interaction of the human spindle checkpoint kinase Mps1. Chromosoma 113, 1–15 [DOI] [PubMed] [Google Scholar]
- 17. Martin-Lluesma S., Stucke V. M., Nigg E. A. (2002) Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science 297, 2267–2270 [DOI] [PubMed] [Google Scholar]
- 18. Xu Q., Zhu S., Wang W., Zhang X., Old W., Ahn N., Liu X. (2009) Regulation of kinetochore recruitment of two essential mitotic spindle checkpoint proteins by Mps1 phosphorylation. Mol. Biol. Cell 20, 10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thebault P., Chirgadze D. Y., Dou Z., Blundell T. L., Elowe S., Bolanos-Garcia V. M. (2012) Structural and functional insights into the role of the N-terminal Mps1 TPR domain in the SAC (spindle assembly checkpoint). Biochem. J. 448, 321–328 [DOI] [PubMed] [Google Scholar]
- 20. Branzei D., Foiani M. (2006) The Rad53 signal transduction pathway: replication fork stabilization, DNA repair, and adaptation. Exp. Cell Res. 312, 2654–2659 [DOI] [PubMed] [Google Scholar]
- 21. Stracker T. H., Usui T., Petrini J. H. (2009) Taking the time to make important decisions: The checkpoint effector kinases Chk1 and Chk2 and the DNA damage response. DNA Repair 8, 1047–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oricchio E., Saladino C., Iacovelli S., Soddu S., Cundari E. (2006) ATM is activated by default in mitosis, localizes at centrosomes and monitors mitotic spindle integrity. Cell Cycle 5, 88–92 [DOI] [PubMed] [Google Scholar]
- 23. Yang C., Tang X., Guo X., Niikura Y., Kitagawa K., Cui K., Wong S. T., Fu L., Xu B. (2011) Aurora-B mediated ATM serine 1403 phosphorylation is required for mitotic ATM activation and the spindle checkpoint. Mol. Cell 44, 597–608 [DOI] [PMC free article] [PubMed] [Google Scholar]